ABSTRACT
The hypothalamic-pituitary-gonadal axis is central to normal reproductive function. This pathway begins with the release of gonadotropin-releasing hormone in systematic pulses by the hypothalamus. Gonadotropin-releasing hormone is bound by receptors on gonadotroph cells in the anterior pituitary gland and stimulates the synthesis and secretion of luteinizing hormone and, to some extent, follicle-stimulating hormone. Once stimulated by these glycoprotein hormones, the gonads begin gametogenesis and the synthesis of sex hormones. In humans, mutations of the forkhead transcription factor, FOXP3, lead to an autoimmune disorder known as immunodysregulation, polyendocrinopathy, and enteropathy, X-linked syndrome. Mice with a mutation in the Foxp3 gene have a similar autoimmune syndrome and are infertile. To understand why FOXP3 is required for reproductive function, we are investigating the reproductive phenotype of Foxp3 mutant mice (Foxp3sf/Y). Although the gonadotroph cells appear to be intact in Foxp3sf/Y mice, luteinizing hormone beta (Lhb) and follicle-stimulating hormone beta (Fshb) expression are significantly decreased, demonstrating that these mice exhibit a hypogonadotropic hypogonadism. Hypothalamic expression of gonadotropin-releasing hormone is not significantly decreased in Foxp3sf/Y males. Treatment of Foxp3sf/Y males with a gonadotropin-releasing hormone receptor agonist does not rescue expression of Lhb or Fshb. Interestingly, we do not detect Foxp3 expression in the pituitary or hypothalamus, suggesting that the infertility seen in Foxp3sf/Y males is a secondary effect, possibly due to loss of FOXP3 in immune cells. Pituitary expression of glycoprotein hormone alpha (Cga) and prolactin (Prl) are significantly reduced in Foxp3sf/Y males, whereas the precursor for adrenocorticotropic hormone, pro-opiomelanocortin (Pomc), is increased. Human patients diagnosed with IPEX often exhibit thyroiditis due to destruction of the thyroid gland by autoimmune cells. We find that Foxp3sf/Y mice have elevated expression of thyroid-stimulating hormone beta (Tshb), suggesting that they may suffer from thyroiditis as well. Expression of the pituitary transcription factors, Pitx1, Pitx2, Lhx3, and Egr1, is normal; however, expression of Foxl2 and Gata2 is elevated. These data are the first to demonstrate a defect at the pituitary level in the absence of FOXP3, which contributes to the infertility observed in mice with Foxp3 loss of function mutations.
Keywords: forkhead, FOXP3, gonadotropins, pituitary
The forkhead transcription factor, FOXP3, is indirectly required for pituitary gonadotropin expression.
INTRODUCTION
The Pituitary Gland
Normal reproductive function depends on the hypothalamic-pituitary-gonadal axis. Pulsatile secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus stimulates GnRH receptors (GnRHRs) on the surface of pituitary gonadotroph cells, increasing the number of GnRHRs and stimulating synthesis and secretion of luteinizing hormone (LH) and, to some extent, follicle-stimulating hormone (FSH). FSH secretion is dependent on the presence of activin. The degree of GnRH stimulation depends on both the quantity of GnRH released by the hypothalamus and the concentration of GnRHRs on the surface of gonadotroph cells. LH and FSH are dimeric hormones comprised of a common glycoprotein hormone α subunit (αGSU), but it is the unique β subunits that render their unique functions. FSH acts on Sertoli cells, structures that form a blood-testis barrier functioning as a filter that permits only certain substances to reach spermatocytes [1]. In males, LH stimulation of Leydig cells results in secretion of testosterone that is essential for spermatogenesis. In females, a surge of LH initiates ovarian follicle luteinization and meiotic maturation of the oocyte [2, 3]. Transcriptional regulation of the LH β (Lhb) gene involves steroidogenic factor 1 (SF1, Nr5a1), early growth response factor 1 (EGR1), and pituitary transcription factor 1 (PITX1) [4]. Gonadal steroids can then negatively feed back at the levels of the pituitary and the hypothalamus.
Infertility in the United States
Male infertility is a component in half of infertile couples, and in approximately one third of cases male infertility is the sole cause [5]. Epidemiological studies suggest that approximately 80 million people worldwide are infertile [6]. Although the etiology is largely unknown, some necessary genes have been identified. LH and its receptor are among these. LH acts on the gonads to facilitate production of testosterone, estrogen, and progesterone [4, 7] and is essential for spermatogenesis and ovulation. Low LH levels can cause reduced libido, bone density, and muscle mass, whereas overexpression of LH increases the risk of ovarian and testicular tumors [7]. Thus, understanding how LH production is regulated is key for treating a number of human pathologies.
The Forkhead Transcription Factor, FOXP3
The “forkhead” family is classified based on a conserved DNA-binding domain or forkhead domain. This family has over 100 members and is present in organisms as simple as yeast and as complex as humans [8]. The forkhead factor, FOXP3, is constitutively expressed in CD4+CD25+ regulatory T (Treg) cells. FOXP3 plays important roles in the differentiation and function of Treg cells [9]. The gene encoding FOXP3 is located on the X chromosome in humans and mice. Mutations in the human FOXP3 gene result in an autoimmune syndrome referred to as immunodysregulation, polyendocrinopathy, and enteropathy, X-linked (IPEX). Symptoms include diarrhea, eczema, hemolytic anemia, diabetes mellitus, and thyroid autoimmunity leading to hypothyroidism [10]. Death often occurs during the first years of life [10].
Mutations in the Murine Foxp3 Gene
Foxp3 is expressed in Treg cells as well as in thymic, breast, and prostate epithelial cells [11, 12]. A spontaneously occurring mutation, referred to as scurfy (sf), results in an IPEX-like syndrome in mice. This mutation has been mapped to the Foxp3 gene and consists of a 2-bp insertion causing a frame shift. This codes for a premature stop codon, producing a truncated, nonfunctional protein [13]. Interestingly, affected males (Foxp3sf/Y) are infertile [14, 15].
MATERIALS AND METHODS
Mice
Foxp3 mutant mice were purchased from the Jackson Laboratory (www.jax.org) and maintained on a C57BL/6J background. Foxp3sf/Y male mice were left with dams to increase survival time. Mice were maintained in a 12L:12D cycle. To genotype mice, we used a Custom Taqman SNP Genotyping Assay (Applied Biosystems, www.appliedbiosystems.com) according to manufacturer's instructions. Male mice were used for all studies.
All procedures using mice were approved by the University of Michigan Committee on Use and Care of Animals or the Southern Illinois University Animal Care and Use Committee. All experiments were conducted in accord with the principles and procedures outlined in the NIH Guidelines for the Care and Use of Experimental Animals.
Histology and Immunohistochemistry
Pituitaries were dissected and fixed for 20 min in 4% paraformaldehyde in PBS (pH 7.2). All samples were washed in PBS, dehydrated in a graded series of ethanol, and embedded in paraffin. Sections (5 μm) were deparaffinized in xylene, rehydrated through a series of graded ethanol washes, and stained in hematoxylin (Fisher Scientific, www.fishersci.com) and eosin (Sigma, www.sigmaaldrich.com) or used for immunohistochemistry. To visualize LHB, and αGSU in the pituitary, slides were deparaffinized in xylene and incubated for 1 h at room temperature with an antibody directed against LHB (1:500; NHPP, www.humc.edu/hormones) or αGSU (1:150; NHPP). Slides were then incubated with an anti-guinea pig or anti-rabbit secondary antibody, respectively, and conjugated to fluorescein (FITC; 1:100 dilution; Jackson ImmunoResearch Laboratories, Inc., www.jacksonimmuno.com) for 30 min at room temperature. Following a 5-min incubation with water, sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (167 nM; Molecular Probes, www.invitrogen.com). Digital images of pituitary sections were captured with a Leica DM 5000B fluorescent microscope and Retiga 2000R digital camera. FITC and DAPI section pictures were merged using Adobe Photoshop CS3.
RT-PCR
Pituitaries were dissected from mice at and stored in RNAlater (Ambion, Inc., www.ambion.com) at −20°C. Hypothalami from C57BL/6 mice at 6 wk of age were excised from 1 mm rostral of the optic chiasm to 1 mm caudal of the optic chiasm and stored in RNAlater at −20°C. Total RNA was isolated with the RNAqueous-Micro kit (Ambion, Inc.) according to manufacturer's directions. RNA concentrations were determined by spectrophotometry. RNA was treated with DNase I and DNase inactivating reagent from the TURBO DNase-free kit (Ambion, Inc.) as per manufacturer's instructions. We synthesized cDNA using ImPromII reagents and random primers (Promega, www.promega.com).
Real-time RT-PCR was performed on a CFX96 Real Time System (BioRad, www.bio-rad.com). Amplification of Lhb was accomplished with 0.2 μM primers (5′-ATC ACC TTC ACC ACC AGC ATC TGT, 5′-TGA GGG CTA CAG GAA AGG AGA CTA) and SYBR green master mix (BioRad). The internal control used was β-globin (Hbb-b1; 5′-ACATTGGCATGGCTTTGTTT, 5′-GTTTGCTCCAACCAACTGCT) because it is not changed significantly in wild-type as compared to scurfy animals. Ten nanograms of cDNA was used in a 15-μl reaction volume. Samples and controls were run in triplicate. No-template controls and no-reverse transcriptase controls were used to assure the absence of contamination and efficacy of the DNase treatment, respectively. Amplification was achieved by the following protocol: 95°C for 3 min, 40 cycles of 95°C for 10 sec, 57°C for 1 min, plate read. Melt curve analysis was performed to ensure that no primer-dimer amplification occurred. Amplification of all other amplicons was performed using Taqman Gene Expression Assays (Applied Biosystems) as per manufacturer's instructions. For all real-time experiments, at least three individuals were included in each group. All experiments were performed in triplicate. Data were analyzed by the ΔΔCT method [16, 17]. The values for ΔΔCT were calculated by subtracting the average ΔCT of controls (either wild-type for Figs. 1, 2, 4, and 5, A and B, or NaCl-treated wild-type controls for Fig. 5, C and D) from the ΔCT for each sample.
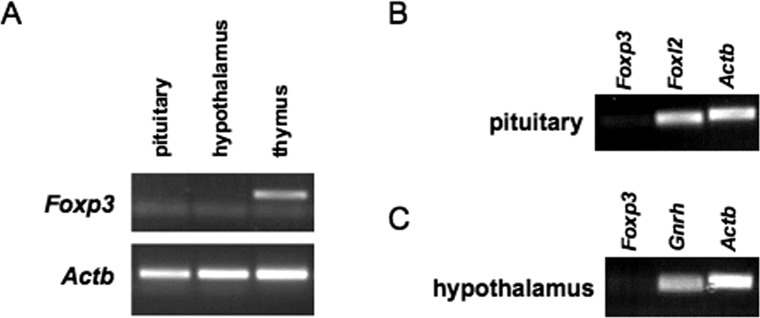
To visualize Foxp3 expression in pituitary, hypothalamus, and thymus, quantitative PCR was performed using primers for Foxp3 (5′-ATC TCC TGG ATG AGA AAG GCA AGG-3′, 5′-AGA GCT CTT GTC CAT TGA GGC CA -3′) or Actb (5′-ACA TTG GCA TGG CTT TGT TT-3′, 5′-GTT TGC TCC AAC CAA CTG CT-3′) at 0.2 μM (see Fig. 3A). Ten nanograms of cDNA was used in a 25 μl reaction volume with Go Taq Green Master Mix (Promega). Amplification was achieved by the following protocol: 95°C for 3 min, 40 cycles of 95°C for 10 sec, 57°C for 1 min. Products were visualized by gel electrophoresis (see Fig. 3A). Taqman probes were used in real-time RT-PCR to demonstrate the integrity of the pituitary and hypothalamic RNA. We measured Gnrh as a positive control for the integrity of hypothalamic RNA and Foxl2 as a positive control for the integrity of pituitary RNA. We measured β-actin (Actb) as an internal control. The resulting products were visualized by gel electrophoresis (see Fig. 3, B and C).
FIG. 3. .
Foxp3 mRNA is not detected in adult mouse pituitary or hypothalamus. Foxp3 expression levels were measured in pituitary (A, B) and hypothalamus (A, C) from adult C57BL/6J male mice. Foxp3 expression in thymus was used as a positive control for our Foxp3 assay (A). Foxl2 and Gnrh expression was used as a positive control for the integrity of the RNA from pituitary (B) and hypothalamus (C), respectively. Products from PCR (A) and real-time PCR (B, C) were visualized by agarose gel electrophoresis.
D-ala-GnRH Treatment
Mice were injected with 1 ng of the GnRH analog (D-Ala-6-GnRH) i.p. in 100 μl 0.15 M NaCl every 2 h for 48 h [18]. Two hours after the last injection, mice were euthanized and pituitary was collected. Total RNA was isolated and real-time RT-PCR was performed to measure Lhb expression as described above.
Statistical Analysis
All results are expressed as mean ± SEM. Data were analyzed by Student t-test using Microsoft Excel. P values less than 0.05 were considered significant (*). P values less than 0.01 were considered very significant (**).
RESULTS
Pituitary Hormone Expression Levels in Foxp3sf/Y Males
Foxp3sf/Y mice are hypogonadal and infertile [14, 15]. To begin to understand why Foxp3sf/Y mice are infertile, we analyzed LH production in these mice. The Foxp3 gene is on the X chromosome and Foxp3sf/Y males are infertile; thus, it is difficult to obtain Foxp3sf/sf females. For these reasons, the following studies used only male mice. Using real-time RT-PCR, we found that mRNA levels of Lhb were reduced in 6- to 9-wk-old Foxp3sf/Y mice (Fig. 1A). Lhb expression was also reduced at 3 wk of age, but not at the day before birth (data not shown). We performed immunohistochemistry on pituitary from 6-wk-old Foxp3sf/Y and Foxp3+/Y mice. We found that LHB protein was not detected in the pituitaries of Foxp3sf/Y mice (Fig. 1, B and C). These data suggested that the hypogonadism these mice exhibited was hypogonadotropic in nature.
FIG. 1. .
Several pituitary hormones are misexpressed in Foxp3sf/Y mice. A) Expression levels of Lhb, Cga, Fshb, and Prl were significantly reduced in Foxp3sf/Y mice as compared to wild-type male littermate controls. Tshb and Pomc expression were significantly increased. Real-time RT-PCR experiments were performed using pituitary from 6- to 9- (Lhb) or 6- (Cga, Fshb, Prl, Tshb, Pomc) wk-old male mice. Expression level was calculated by the ΔΔCT method and represents expression relative to the average ΔCT of wild-type samples. Data are expressed as mean ± SEM of four animals per group. The data were analyzed by Student t-test to determine significant difference between wild-type and Foxp3sf/Y mice (*P < 0.05; **P < 0.01). B–E) Pituitary from 6-wk-old Foxp3sf/Y mice and wild-type littermates was sectioned coronally and analyzed by immunohistochemistry. B, C) Cytoplasmic LHB immunoreactivity (green) is apparent in the wild-type pituitary (B), but is not detected in pituitary from Foxp3sf/Y mice (C). All cell nuclei are stained with DAPI (blue). D, E) Cytoplasmic αGSU immunoreactivity is apparent in the wild-type pituitary (D), but is reduced in pituitary from Foxp3sf/Y mice (E). Original magnification ×630 (B, C) and ×400 (D, E); bars = 100 μm.
We next investigated whether hormones other than Lhb were misexpressed in Foxp3sf/Y male mice. Using real-time RT-PCR to measure expression levels in 6-wk-old male mice, we found that the gonadotropin subunits, Fshb, and the common α subunit (Cga) were significantly decreased in Foxp3sf/Y male mice as compared to normal male littermates (Fig. 1A). This likely contributes to the hypogonadotropic hypogonadism exhibited by these mice. Prolactin (Prl) expression was also significantly reduced (Fig. 1A), whereas growth hormone (Gh) expression was unchanged (data not shown) in Foxp3sf/Y male mice as compared to normal male littermates. Pomc expression was significantly increased, possibly because of the stress these animals experienced as a result of their illness. Finally, Tshb expression was significantly increased (Fig. 1A). Human patients with IPEX often exhibit thyroiditis due to destruction of the thyroid gland by autoimmune cells [19]. Thus, the elevated Tshb expression in Foxp3sf/Y male mice is consistent with a lack of negative feedback from thyroid hormone due to possible thyroiditis. Immunoreactivity for αGSU was reduced in Foxp3sf/Y as compared to wild-type male littermates (Fig. 1, D and E). We did not detect any cells in the pituitary that exhibit normal immunoreactivity for αGSU. However, immunohistochemistry for TSHB showed no obvious difference in the number of thyrotrope cells in Foxp3sf/Y mice (data not shown). Together these data suggested that thyrotrope cells were not producing normal amounts of αGSU or biologically active TSH.
Pituitary Transcription Factors
Several transcription factors are important for pituitary hormone expression. For example, PITX1 and EGR1 are important for stimulating expression of Lhb [20–23]. Mice homozygous for a hypomorphic allele of Pitx2 lack gonadotroph cells, have a decreased number of somatotrope and thyrotrope cells, and have little or no expression of the gonadotroph transcription factors Gata2 and Egr1 [24, 25]. Loss of LHX3 results in an inability to produce all anterior lobe pituitary hormones except ACTH [26, 27]. Finally, FOXL2 stimulates expression of GnRH receptor (Gnrhr), Cga, and Fshb [28–32]. We found that expression of Pitx1, Pitx2, Lhx3, and Egr1 was not different in Foxp3sf/Y male mice at 6 wk of age compared to normal male littermate controls (Fig. 2). Gata2 and Foxl2 expression was significantly increased in Foxp3sf/Y male mice as compared to normal male littermates, possibly in an attempt to compensate for decreased gonadotropin production (Fig. 2).
FIG. 2. .
Pituitary expression of some transcription factors is altered in Foxp3sf/Y mice. Pituitary expression levels of the transcription factors Pitx1, Pitx2, Lhx3, and Egr1 is not significantly different in Foxp3sf/Y mice as compared to wild-type littermates. However, expression of Gata2 and Foxl2 is significantly increased in Foxp3sf/Y mice. Real-time RT-PCR was performed on pituitary from 6-wk-old male mice. Expression level was calculated by the ΔΔCT method and represents expression relative to the average ΔCT of samples from Foxp3+/Y mice. Data are expressed as mean ± SEM of four animals per group. The data were analyzed by Student t-test to determine significant difference between wild-type and Foxp3sf/Y mice (*P < 0.05; **P < 0.01).
Foxp3 Expression in Pituitary
We next sought to understand why Lhb expression was reduced in Foxp3sf/Y male mice as compared to normal mice. The simplest explanation is that FOXP3 directly stimulates Lhb expression, which would require FOXP3 to be present in the adult pituitary. To determine if Foxp3 is expressed in the adult male mouse pituitary, we performed RT-PCR. We isolated pituitary from male mice at 6 wk of age. Real-time RT-PCR was performed to measure Foxp3 expression levels, using Foxp3 expression in thymus as a positive control for the Foxp3 primer/probe. Ct values for Foxp3 in pituitary were consistently at background levels ranging from 36 to 40 or were undetectable (data not shown). Products were then visualized by agarose gel electrophoresis. Foxp3 expression was detectable in thymus, but not in pituitary (Fig. 3A). Foxl2 expression in each pituitary sample served as a positive control for RNA/cDNA integrity. Although Foxl2 expression was detected in pituitary, Foxp3 was not (Fig. 3B). Based on these data, we concluded that Foxp3 was not expressed in the pituitary and that the reduction in Lhb expression seen in Foxp3sf/Y mice was a secondary effect due to loss of Foxp3 in some other organ.
Gonadotroph Cells in Foxp3sf/Y Mice
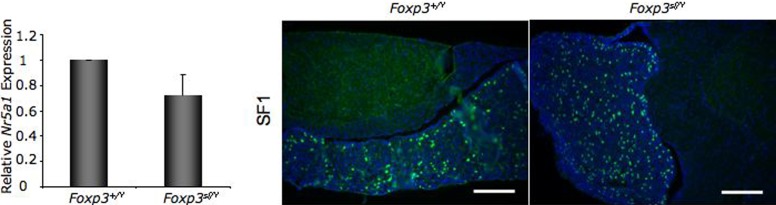
Foxp3sf/Y mice suffer from autoimmunity. In the pancreas this results in destruction of β cells, leading to diabetes [33]. We investigated whether gonadotroph cells are intact in Foxp3sf/Y mice to determine if the loss of Lhb production is due to destruction of gonadotroph cells. To determine if gonadotroph cells are intact in pituitary glands from adult Foxp3sf/Y mice, we performed RT-PCR for SF1 (Nr5a1), which is expressed in gonadotroph cells but not in other pituitary cell types. Expression of Nr5a1 was not different in Foxp3sf/Y male mice as compared to normal male littermates (Fig. 4). We confirmed this result with immunohistochemistry. Many SF1-positive cells were present in pituitary from Foxp3sf/Y mice (Fig. 4). These data demonstrated that gonadotroph cells were present in pituitary glands of Foxp3sf/Y mice and had not been phagocytized by the immune system.
FIG. 4. .
Gonadotroph cells are present in pituitary from Foxp3sf/Y mice. Expression of the gene encoding SF1, Nr5a1, is not significantly reduced in Foxp3sf/Y mice at 6 wk of age. Expression level was calculated by the ΔΔCT method and represents expression relative to the average ΔCT of Foxp3+/Y mouse pituitary. Data are expressed as mean ± SEM of at least four animals per group and were analyzed by Student t-test. Pituitary sections were analyzed by immunohistochemistry for SF1 (green), which will mark gonadotroph cells, but not other pituitary cell types. All cell nuclei are stained with DAPI (blue). Pituitary from Foxp3sf/Y mice contains many SF1-positive cells, suggesting that gonadotroph cells in Foxp3sf/Y mice are intact. Original magnification ×200; bars = 100 μm.
Foxp3 Expression in Hypothalamus
Pituitary production of LH is intimately tied to hypothalamic function. One possible explanation is that loss of LH production is due to a hypothalamic defect. To determine if Foxp3 is expressed in the hypothalamus, we performed RT-PCR for Foxp3 with hypothalamic tissue. We excised hypothalami from C57BL/6 mice at 6–9 wk of age, 1 mm rostral of the optic chiasm to 2 mm caudal of the optic chiasm, to obtain GnRH neurons. Using RT-PCR, we measured Foxp3 mRNA levels. Thymus RNA was used as a positive control for the Foxp3 Taqman primer/probe and Gnrh RNA as a positive control for the integrity of the hypothalamic RNA. Hypothalamic expression of Foxp3 was at background levels with Ct values ranging from 35 to undetectable (data not shown). Products were visualized by agarose gel electrophoresis. We did not detect Foxp3 expression in hypothalamic tissue, indicating that FOXP3 did not directly affect hypothalamic function (Fig. 3, A and C).
Hypothalamic Gnrh Expression in Foxp3sf/Y Mice
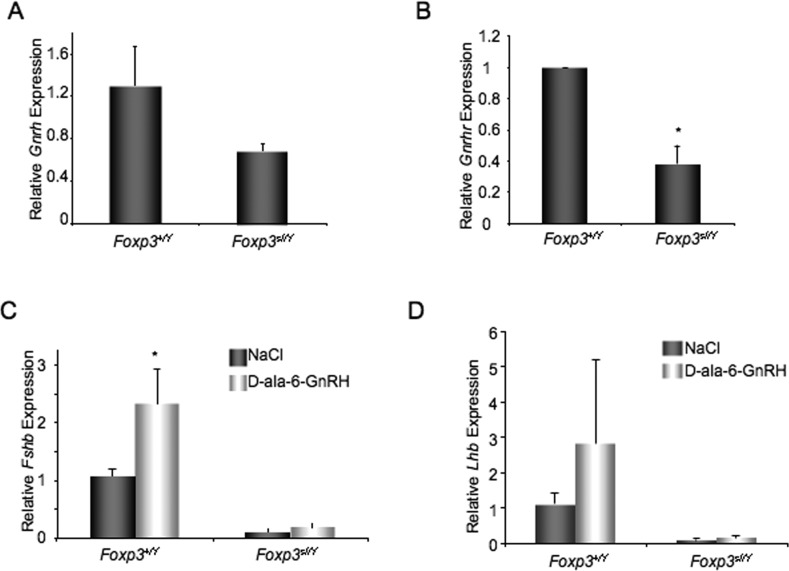
LH production by the pituitary is dependent on GnRH from the hypothalamus. To determine if Gnrh expression in the hypothalamus is decreased, we collected hypothalami from wild-type and scurfy mice at 6–9 wk of age and performed real-time RT-PCR to measure Gnrh mRNA levels. We found that Gnrh levels were not significantly decreased in scurfy mice as compared to wild-type male littermates (Fig. 5A). However, this did not rule out the possibility of a hypothalamic defect. We did detect a significant decrease in pituitary expression of Gnrhr, suggesting that the ability of the pituitary gland to respond to GnRH signaling was impaired (Fig. 5B).
FIG. 5. .
GnRH treatment does not rescue Lhb expression in Foxp3sf/Y mice. A) Hypothalamic Gnrh expression is not significantly reduced in 6- to 9-wk-old Foxp3sf/Y mice as compared to wild-type male littermates. B) Expression of pituitary Gnrhr is significantly reduced (*P < 0.05) in 6- to 9-wk-old Foxp3sf/Y mice. C) Treatment of 6- to 9-wk-old male mice with the GnRH receptor agonist D-ala-6-GnRH significantly increases Fshb expression in wild-type male controls (*P < 0.05), but does not significantly increase Fshb expression in Foxp3sf/Y mice. D) Treatment of 6- to 9-wk-old male mice with D-ala-6-GnRH does not significantly increase Lhb expression in Foxp3sf/Y mice. Expression level was calculated by the ΔΔCT method. Data are expressed as mean ± SEM of at least four animals per group and were analyzed by Student t-test. Values represent expression relative to the average ΔCT of samples from Foxp3+/y pituitary (A, B), or average ΔCT of NaCl-treated Foxp3+/Y (C, D).
Treatment with GnRH Receptor Agonist
We next sought to determine if we could stimulate gonadotropin production in scurfy mice by activating GnRH receptor signaling with pulsatile GnRH analog treatments. Foxp3sf/Y mice and wild-type male littermates at 6 wk of age were treated with D-Ala-6-GnRH every 2 h for 48 h. Pituitaries were collected 2 h after the last injection and Fshb and Lhb mRNA levels were measured. We found that pulsatile GnRH analog treatments significantly increased Fshb expression in wild-type males (Fig. 5C). Although there was a trend toward increased Lhb expression, the difference was not significant. This could be because the wild-type animals had an intact hypothalamic-pituitary-gonadal axis, and therefore Lhb expression may already have been maximally stimulated. In Foxp3sf/Y male mice, neither Fshb nor Lhb expression was significantly increased with D-Ala-6-GnRH treatment (Fig. 5, C and D).
DISCUSSION
FOXP3 is essential for normal immune function [13, 34] because of its role in proper development and function of regulatory T cells [11, 35]. Without FOXP3, humans and mice develop severe autoimmunity, characterized by hypothyroidism, diabetes, and failure to thrive [34, 36]. The gene encoding FOXP3 resides on the X chromosome in mice and humans; thus, males are primarily affected by loss of FOXP3 [13, 19, 34, 35]. Previous reports have indicated that mice with a mutation in the Foxp3 gene, Foxp3sf/Y, are infertile [14, 15, 37]. Sharma et al. demonstrated that submandibular gland development, which is sexually dimorphic and dependent on testosterone in males, is inhibited in Foxp3sf/Y males, suggesting that they have low testosterone levels, although testosterone levels have never been directly measured [37]. We find that Foxp3sf/Y mice have severely reduced Lhb and Fshb expression, which can explain the hypogonadism seen in these mice and would contribute to low testosterone.
Several systems are out of balance in Foxp3sf/Y mice in consequence of their autoimmunity. Pancreatic β cells are destroyed by immune cells, resulting in type 1 diabetes due to loss of insulin production [33, 34]. Pituitary-specific insulin receptor knockout female mice do have decreased Lhb levels as compared to wild-type littermates in the context of obesity [38]. When mice were not obese, no differences in Lhb levels were observed; however, the effects of eliminating pituitary insulin signaling in male mice has not been addressed [38]. Humans with mutations in FOXP3 exhibit hypothyroidism due to destruction of the thyroid gland [39]. Although, to our knowledge, the thyroid hormone status of Foxp3sf/Y mice has not been examined, loss of thyroid hormone could negatively affect gonadotroph function [40]. Finally, Foxp3sf/Y mice have high cytokine levels because of the decreased action of regulatory T cells [41]. Cytokines have been shown to inhibit gonadotropin production [42–44]. The loss of insulin, loss of thyroid hormone, and increase in cytokine levels could act individually or in combination to inhibit gonadotroph function.
LH, FSH, and TSH are all dimeric hormones of the glycoprotein hormone family. We find that expression of Lhb, Fshb, and Cga is reduced in Foxp3sf/Y mice as compared to wild-type male littermates, whereas expression of Tshb is increased. Immunohistochemistry for TSHβ shows no obvious difference in the number of thyrotrope cells (data not shown). Immunohistochemistry for αGSU does not detect normal levels of staining in any pituitary cells, demonstrating that αGSU is reduced in thyrotropes as well as gonadotrophs. This suggests that Foxp3sf/Y mice have reduced levels of biologically active TSH, which could lead to hypothyroidism. Thus, the increased expression levels of Tshb may be due to lack of negative feedback, which is ultimately caused by reduced αGSU production in the pituitary.
The hpg mice, which have a mutation in the Gnrh gene, are infertile because of hypogonadotropic hypogonadism. Treatment of adult hpg mice with pulsatile GnRH increased pituitary LH content, but did not increase plasma LH to detectable levels [45]. Hamernik et al. demonstrated that 2-h pulses of GnRH stimulated transcription of Lhb in ewes after hypothalamic-pituitary disconnect [46]. Spratt and Crowley found that pituitary and gonadal responsiveness to exogenous GnRH was reduced in men who were deficient in GnRH. Treatment with pulsatile GnRH for a period of 6 mo enhanced pituitary and gonadal responsiveness to physiological doses of exogenous GnRH, suggesting that the pituitary gland and gonads require hypothalamic stimulation in order to mature before they can respond to GnRH appropriately [47]. We find that treating 6-wk-old mutant animals with the GnRH agonist D-ala-6-GnRH does not significantly increase Lhb or Fshb expression. Although we cannot rule out the possibility that treating Foxp3sf/Y mice with GnRH for a longer period of time or at a different pulse frequency may have some effect, failure of the GnRH agonist to stimulate Lhb and Fshb expression suggests that the gonadotroph cells are not capable of responding normally to GnRH stimulation.
We have marked gonadotroph cells by performing immunohistochemistry for steroidogenic factor-1 (Fig. 4). We do not observe an obvious difference in gonadotroph cell number or expression of the gene encoding SF1 (Nr5a1), suggesting that the decrease in gonadotropin production is at the level of Fshb and Lhb expression rather than gonadotroph cell number. Pituitary from Foxp3sf/Y mice does exhibit a decrease in expression of GnRH receptor, suggesting a decrease in gonadotroph responsiveness to GnRH. Consistent with this, Fshb, Lhb, Cga, and Gnrhr are all GnRH-responsive genes [4, 46, 48–51]. However, this does not rule out the possibility that other factors, such as low insulin or high cytokines, are contributing to diminished gonadotroph function.
When the immune and endocrine systems become unbalanced, for example, cases of increased immune function such as autoimmunity or decreased endocrine function like pituitary hormone deficiency, neither system functions properly. For example, many autoimmune diseases result in subfertility in males and females [52, 53]. Studies of mink with spontaneous autoimmune orchitis find that these animals have high levels of anti-sperm antibodies, high levels of tumor necrosis factor (TNF) α and interleukin (IL) 6, and low levels of testosterone [53]. Pituitary hormone levels were not analyzed in these animals.
The balance between the immune and reproductive systems is maintained, in part, because these systems are modulated by many of the same factors, including GnRH, estradiol, and cytokines. In fact, Gnrh is expressed in the thymus as well as in the hypothalamus and is important for thymic maturation and differentiation [54]. Estrogens can promote or inhibit inflammation [55, 56]. Several cytokines, including IL1, IL2, IL6, IFNG, and TNF, are produced in the hypothalamus and/or pituitary [43]. GH, PRL, and LH are also produced in the thymus. In addition, receptors for several cytokines and hormones are expressed in both the thymus and the hypothalamic/pituitary axis [43]. There are many examples that demonstrate cytokine regulation of reproductive function; for example, IL2 has been shown to stimulate Pomc expression and inhibit LH, FSH, and GH release [43]. Central administration of IL1 drastically inhibits GnRH production; however, systemic IL1 appears to have little effect [42]. TNF has been shown to inhibit release of GH, LH, and PRL [44]. Thus, one can see how an imbalance in the immune system, such as elevated cytokines, can have a huge effect on reproductive function.
We do not detect Foxp3 expression above background in the pituitary or hypothalamus, suggesting that the loss of gonadotropin production in Foxp3sf/Y mice is not due to a primary defect of the hypothalamus or pituitary, but rather is secondary to loss of functional FOXP3 protein in some other tissue. Gonadotroph cells are readily detected by immunohistochemistry for SF1, suggesting that they have not been destroyed by the immune system. One possibility is that Foxp3 is required for maintaining the balance between the immune system and reproductive system and loss of Foxp3 disrupts this balance, resulting in infertility. Interestingly, another forkhead factor that is important for regulating immune function is FOXN1. A spontaneously occurring recessive mutation in the murine Foxn1 gene, referred to as nude (nu), causes the mice (Foxn1nu/nu) to be immunocompromised and athymic. Congenitally athymic Foxn1nu/nu female mice are subfertile, exhibiting low gonadotropin levels. Although Foxn1nu/nu male mice are fertile, they too exhibit significantly reduced gonadotropin levels [57]. Injecting wild-type mice with antibodies against thymulin, a thymic hormone, can recapitulate the reduction in gonadotropin levels seen in athymic animals [58].
Breeding the sf mutation onto a nude (Foxn1nu/nu) mouse background (Foxp3sf/Y; Foxn1nu/nu) rescues both the Foxp3sf/Y autoimmunity and infertility; however, Foxp3sf/Y; Foxn1nu/nu mice retain the Foxn1nu/nu immunodeficiency [15]. In other words, breeding the sf mutation on a nude background rescues the scurfy phenotype, including infertility, but does not rescue the nude phenotype. The fact that fertility in Foxp3sf/Y males is rescued when the mice are congenitally athymic (by breeding them to nude mice) suggests that the infertility in Foxp3sf/Y mice is secondary to their autoimmunity, which is dependent on the presence of the thymus. Lack of Foxp3 expression in the pituitary and hypothalamus, together with the fact that fertility is rescued by loss of thymic function, suggests that infertility in Foxp3sf/Y mice is secondary to their autoimmunity.
Together, these data demonstrate that FOXP3 is required for fertility. We find that LH production is suppressed in Foxp3sf/Y mice even though Foxp3 is not expressed in the pituitary, suggesting that the loss of LH production is secondary to loss of FOXP3 in another tissue. Godfrey et al. demonstrated that breeding Foxp3sf/Y mice onto a Foxn1nu/nu background rescued their autoimmunity and infertility, suggesting that their immune defects are the primary cause of their infertility [15]. Thus, in the absence of FOXP3, Treg cells fail to develop and immune activity escalates, ultimately resulting in suppression of the endocrine system. Although Foxp3 is not expressed in the pituitary, we find that it is essential for normal pituitary function.
ACKNOWLEDGMENT
We thank A.F. Parlow and the National Hormone and Pituitary Program, the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Institute of Child Health and Human Development for providing antibodies for LHβ, αGSU, and PRL, and Ken-ichirou Morohashi, Kyushu University, Japan, for generously providing antibodies for SF1. We thank Sally A. Camper (University of Michigan, Ann Arbor, Michigan) for financial support during initiation of the project.
Footnotes
Supported by startup funds from Southern Illinois University School of Medicine (B.S.E.) and partially supported by National Institutes of Health (NIH) grant RO1-HD34283 (to Sally A. Camper, University of Michigan, Ann Arbor, Michigan).
REFERENCES
- Genuth SM. Physiology. St. Louis, MO: Mosby Year Book; 1993. [Google Scholar]
- Conti M, Andersen CB, Richard FJ, Shitsukawa K, Tsafriri A. Role of cyclic nucleotide phosphodiesterases in resumption of meiosis. Mol Cell Endocrinol 1998; 145: 9 14 [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Robker RL, Dajee M, Alliston TN. Molecular mechanisms of ovulation and luteinization. Mol Cell Endocrinol 1998; 145: 47 54 [DOI] [PubMed] [Google Scholar]
- Jorgensen JS, Quirk CC, Nilson JH. Multiple and overlapping combinatorial codes orchestrate hormonal responsiveness and dictate cell-specific expression of the genes encoding luteinizing hormone. Endocr Rev 2004; 25: 521 542 [DOI] [PubMed] [Google Scholar]
- Brugh VM, III, Lipshultz LI. Male factor infertility: evaluation and management. Med Clin North Am 2004; 88: 367 385 [DOI] [PubMed] [Google Scholar]
- Nachtigall RD. International disparities in access to infertility services. Fertil Steril 2006; 85: 871 875 [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I, Zhang FP, Kero J, Hamalainen T, Poutanen M. Transgenic and knockout mouse models for the study of luteinizing hormone and luteinizing hormone receptor function. Mol Cell Endocrinol 2002; 187: 49 56 [DOI] [PubMed] [Google Scholar]
- Wijchers PJ, Burbach JP, Smidt MP. In control of biology: of mice, men and Foxes. Biochem J 2006; 397: 233 246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Greene MI. FOXP3 actively represses transcription by recruiting the HAT/HDAC complex. Cell Cycle 2007; 6: 1432 1436 [PubMed] [Google Scholar]
- Powell BR, Buist NR, Stenzel P. An X-linked syndrome of diarrhea, polyendocrinopathy, and fatal infection in infancy. J Pediatr 1982; 100: 731 737 [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299: 1057 1061 [PubMed] [Google Scholar]
- Chen GY, Chen C, Wang L, Chang X, Zheng P, Liu Y. Cutting edge: broad expression of the FoxP3 locus in epithelial cells: a caution against early interpretation of fatal inflammatory diseases following in vivo depletion of FoxP3-expressing cells. J Immunol 2008; 180: 5163 5166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet 2001; 27: 68 73 [DOI] [PubMed] [Google Scholar]
- Lyon MF. Hypogonadism in scurfy (sf) males. Mouse News Lett 1986; 74: 93 [Google Scholar]
- Godfrey VL, Wilkinson JE, Rinchik EM, Russell LB. Fatal lymphoreticular disease in the scurfy (sf) mouse requires T cells that mature in a sf thymic environment: potential model for thymic education. Proc Natl Acad Sci U S A 1991; 88: 5528 5532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402 408 [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008; 3: 1101 1108 [DOI] [PubMed] [Google Scholar]
- Duval DL, Farris AR, Quirk CC, Nett TM, Hamernik DL, Clay CM. Responsiveness of the ovine gonadotropin-releasing hormone receptor gene to estradiol and gonadotropin-releasing hormone is not detectable in vitro but is revealed in transgenic mice. Endocrinology 2000; 141: 1001 1010 [DOI] [PubMed] [Google Scholar]
- van der Vliet HJ, Nieuwenhuis EE. IPEX as a result of mutations in FOXP3. Clin Dev Immunol 2007; 2007: 89017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe MW, Call GB. Early growth response protein 1 binds to the luteinizing hormone-beta promoter and mediates gonadotropin-releasing hormone-stimulated gene expression. Mol Endocrinol 1999; 13: 752 763 [DOI] [PubMed] [Google Scholar]
- Halvorson LM, Ito M, Jameson JL, Chin WW. Steroidogenic factor-1 and early growth response protein 1 act through two composite DNA binding sites to regulate luteinizing hormone beta-subunit gene expression. J Biol Chem 1998; 273: 14712 14720 [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Drouin J. Egr-1 is a downstream effector of GnRH and synergizes by direct interaction with Ptx1 and SF-1 to enhance luteinizing hormone beta gene transcription. Mol Cell Biol 1999; 19: 2567 2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk CC, Lozada KL, Keri RA, Nilson JH. A single Pitx1 binding site is essential for activity of the LHbeta promoter in transgenic mice. Mol Endocrinol 2001; 15: 734 746 [DOI] [PubMed] [Google Scholar]
- Gage PJ, Suh H, Camper SA. Dosage requirement of Pitx2 for development of multiple organs. Development 1999; 126: 4643 4651 [DOI] [PubMed] [Google Scholar]
- Suh H, Gage PJ, Drouin J, Camper SA. Pitx2 is required at multiple stages of pituitary organogenesis: pituitary primordium formation and cell specification. Development 2002; 129: 329 337 [DOI] [PubMed] [Google Scholar]
- Ellsworth BS, Butts DL, Camper SA. Mechanisms underlying pituitary hypoplasia and failed cell specification in Lhx3-deficient mice. Dev Biol 2008; 313: 118 129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng HZ, Zhadanov AB, Mosinger B, Jr, Fujii T, Bertuzzi S, Grinberg A, Lee EJ, Huang SP, Mahon KA, Westphal H. Specification of pituitary cell lineages by the LIM homeobox gene Lhx3. Science 1996; 272: 1004 1007 [DOI] [PubMed] [Google Scholar]
- Ellsworth BS, Burns AT, Escudero KW, Duval DL, Nelson SE, Clay CM. The gonadotropin releasing hormone (GnRH) receptor activating sequence (GRAS) is a composite regulatory element that interacts with multiple classes of transcription factors including Smads, AP-1 and a forkhead DNA binding protein. Mol Cell Endocrinol 2003; 206: 93 111 [DOI] [PubMed] [Google Scholar]
- Ellsworth BS, Egashira N, Haller JL, Butts DL, Cocquet J, Clay CM, Osamura RY, Camper SA. FOXL2 in the pituitary: molecular, genetic, and developmental analysis. Mol Endocrinol 2006; 20: 2796 2805 [DOI] [PubMed] [Google Scholar]
- Coss D, Mellon PL, Thackray VG. A. FoxL in the Smad house: activin regulation of FSH. Trends Endocrinol Metab 2010; 21: 562 568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran S, Lamba P, Wang Y, Bernard DJ. SMADs and FOXL2 synergistically regulate murine FSHbeta transcription via a conserved proximal promoter element. Mol Endocrinol 2011; 25: 1170 1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice NJ, Blount AL, Pelosi E, Schlessinger D, Vale W, Bilezikjian LM. Impaired FSH{beta} expression in the pituitaries of Foxl2 mutant animals. Mol Endocrinol 2011; 25: 1404 1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, Hamann A, Wagner H, Huehn J, Sparwasser T. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med 2007; 204: 57 63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol 2006; 24: 209 226 [DOI] [PubMed] [Google Scholar]
- Ochs HD, Gambineri E, Torgerson TR. IPEX. FOXP3 and regulatory T-cells: a model for autoimmunity. Immunol Res 2007; 38: 112 121 [DOI] [PubMed] [Google Scholar]
- Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G. et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet 2001; 27: 18 20 [DOI] [PubMed] [Google Scholar]
- Sharma R, Deshmukh US, Zheng L, Fu SM, Ju ST. X-linked Foxp3 (Scurfy) mutation dominantly inhibits submandibular gland development and inflammation respectively through adaptive and innate immune mechanisms. J Immunol 2009; 183: 3212 3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers KJ, Wu S, DiVall SA, Messmer MR, Kahn CR, Miller RS, Radovick S, Wondisford FE, Wolfe A. Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor. Cell Metab 2010; 12: 295 305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildin RS, Freitas A. IPEX. and FOXP3: clinical and research perspectives. J Autoimmun 2005; 25 (suppl): 56 62 [DOI] [PubMed] [Google Scholar]
- Jannini EA, Ulisse S, D'Armiento M. Thyroid hormone and male gonadal function. Endocr Rev 1995; 16: 443 459 [DOI] [PubMed] [Google Scholar]
- Lin W, Truong N, Grossman WJ, Haribhai D, Williams CB, Wang J, Martin MG, Chatila TA. Allergic dysregulation and hyperimmunoglobulinemia E in Foxp3 mutant mice. J Allergy Clin Immunol 2005; 116: 1106 1115 [DOI] [PubMed] [Google Scholar]
- Wu S, Wolfe A. Signaling of cytokines is important in regulation of GnRH neurons. Mol Neurobiol 2012; 45: 119 125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino W, Arzt E, Dardenne M. Immunoneuroendocrine connectivity: the paradigm of the thymus-hypothalamus/pituitary axis. Neuroimmunomodulation 1999; 6: 126 136 [DOI] [PubMed] [Google Scholar]
- Gaillard RC, Turnill D, Sappino P, Muller AF. Tumor necrosis factor alpha inhibits the hormonal response of the pituitary gland to hypothalamic releasing factors. Endocrinology 1990; 127: 101 106 [DOI] [PubMed] [Google Scholar]
- Charlton HM, Halpin DM, Iddon C, Rosie R, Levy G, McDowell IF, Megson A, Morris JF, Bramwell A, Speight A, Ward BJ, Broadhead J. et al. The effects of daily administration of single and multiple injections of gonadotropin-releasing hormone on pituitary and gonadal function in the hypogonadal (hpg) mouse. Endocrinology 1983; 113: 535 544 [DOI] [PubMed] [Google Scholar]
- Hamernik DL, Nett TM. Gonadotropin-releasing hormone increases the amount of messenger ribonucleic acid for gonadotropins in ovariectomized ewes after hypothalamic-pituitary disconnection. Endocrinology 1988; 122: 959 966 [DOI] [PubMed] [Google Scholar]
- Spratt DI, Crowley WF., Jr Pituitary and gonadal responsiveness is enhanced during GnRH-induced puberty. Am J Physiol 1988; 254: E652 657 [DOI] [PubMed] [Google Scholar]
- Gharib SD, Wierman ME, Shupnik MA, Chin WW. Molecular biology of the pituitary gonadotropins. Endocr Rev 1990; 11: 177 199 [DOI] [PubMed] [Google Scholar]
- Mason AJ, Hayflick JS, Zoeller RT, Young WS, III, Phillips HS, Nikolics K, Seeburg PH. A deletion truncating the gonadotropin-releasing hormone gene is responsible for hypogonadism in the hpg mouse. Science 1986; 234: 1366 1371 [DOI] [PubMed] [Google Scholar]
- McCue JM, Quirk CC, Nelson SE, Bowen RA, Clay CM. Expression of a murine gonadotropin-releasing hormone receptor-luciferase fusion gene in transgenic mice is diminished by immunoneutralization of gonadotropin-releasing hormone. Endocrinology 1997; 138: 3154 3160 [DOI] [PubMed] [Google Scholar]
- Clayton RN, Channabasavaiah K, Stewart JM, Catt KJ. Hypothalamic regulation of pituitary gonadotropin-releasing hormone receptors: effects of hypothalamic lesions and a gonadotropin-releasing hormone antagonist. Endocrinology 1982; 110: 1108 1115 [DOI] [PubMed] [Google Scholar]
- Hubert FX, Kinkel SA, Crewther PE, Cannon PZ, Webster KE, Link M, Uibo R, O'Bryan MK, Meager A, Forehan SP, Smyth GK, Mittaz L. et al. Aire-deficient C57BL/6 mice mimicking the common human 13-base pair deletion mutation present with only a mild autoimmune phenotype. J Immunol 2009; 182: 3902 3918 [DOI] [PubMed] [Google Scholar]
- Pelletier RM, Yoon SR, Akpovi CD, Silvas E, Vitale ML. Defects in the regulatory clearance mechanisms favor the breakdown of self-tolerance during spontaneous autoimmune orchitis. Am J Physiol Regul Integr Comp Physiol 2009; 296: R743 R762 [DOI] [PubMed] [Google Scholar]
- Maier CC, Marchetti B, LeBoeuf RD, Blalock JE. Thymocytes express a mRNA that is identical to hypothalamic luteinizing hormone-releasing hormone mRNA. Cell Mol Neurobiol 1992; 12: 447 454 [DOI] [PubMed] [Google Scholar]
- Marchetti B, Gallo F, Farinella Z, Tirolo C, Testa N, Caniglia S, Morale MC. Gender, neuroendocrine-immune interactions and neuron-glial plasticity. Role of luteinizing hormone-releasing hormone (LHRH). Ann N Y Acad Sci 2000; 917: 678 709 [DOI] [PubMed] [Google Scholar]
- Morale MC, Gallo F, Tirolo C, L'Episcopo F, Gennuso F, Testa N, Caniglia S, Spina-Purrello V, Avola R, Scoto GM, Marchetti B. The reproductive system at the neuroendocrine-immune interface: focus on LHRH, estrogens and growth factors in LHRH neuron-glial interactions. Domest Anim Endocrinol 2003; 25: 21 46 [DOI] [PubMed] [Google Scholar]
- Goya RG, Console GM, Sosa YE. Gomez Dumm CL, Dardenne M. Altered functional responses with preserved morphology of gonadotrophic cells in congenitally athymic mice. Brain Behav Immun 2001; 15: 85 92 [DOI] [PubMed] [Google Scholar]
- Camihort G, Luna G, Vesenbeckh S, Ferese C, Dardenne M, Goya R, Console G. Morphometric assessment of the impact of serum thymulin immunoneutralization on pituitary cell populations in peripubertal mice. Cells Tissues Organs 2006; 184: 23 30 [DOI] [PubMed] [Google Scholar]