ABSTRACT
Uterine glands and their secretions are required for conceptus (embryo/fetus and associated placenta) survival and development. In most mammals, uterine gland morphogenesis or adenogenesis is a uniquely postnatal event; however, little is known about the mechanisms governing the developmental event. In sheep, progestin treatment of neonatal ewes permanently ablated differentiation of the endometrial glands. Similarly, progesterone (P4) inhibits adenogenesis in neonatal mouse uterus. Thus, P4 can be used as a tool to discover mechanisms regulating endometrial adenogenesis. Female pups were treated with sesame vehicle alone as a control or P4 from Postnatal Day 2 (PD 2) to PD 10, and reproductive tracts were examined on PD 5, 10, or 20. Endometrial glands were fully developed in control mice by PD 20 but not in P4-treated mice. All other uterine cell types appeared normal. Treatment with P4 stimulated proliferation of the stroma but suppressed proliferation of the luminal epithelium. Microarray analysis revealed that expression of genes were reduced (Car2, Fgf7, Fgfr2, Foxa2, Fzd10, Met, Mmp7, Msx1, Msx2, Wnt4, Wnt7a, Wnt16) and increased (Hgf, Ihh, Wnt11) by P4 in the neonatal uterus. These results support the idea that P4 inhibits endometrial adenogenesis in the developing neonatal uterus by altering expression of morphoregulatory genes and consequently disrupting normal patterns of cell proliferation and development.
Keywords: early development; mechanisms of hormone action; rodents (guinea pigs, mice, rats, voles); steroid hormones; steroid hormone receptors; uterus
Progesterone inhibition of uterine gland development involves effects on morphoregulatory gene expression and cell proliferation.
INTRODUCTION
Development of the uterus is initiated in the fetus with formation, patterning, and fusion of the Müllerian ducts [1, 2]. However, establishment of tissue-specific histoarchitecture is completed only after birth in laboratory rodents and domestic animals [3–5]. At birth, the mouse uterus lacks endometrial glands and consists of a simple luminal epithelium (LE) supported by undifferentiated mesenchyme [6]. During the next 3 days of postnatal life, the mesenchyme segregates into radially oriented endometrial stroma and inner circular and prospective outer longitudinal myometrial layers. Between birth (Postnatal Day [PD] 0) and PD 6, glandular epithelial (GE) cells differentiate and bud from the LE [7]. By PD 12, GE buds extend from the LE into the surrounding endometrial stroma, and the inner and outer layers of the myometrium are fully organized [6]. By PD 15, the histoarchitecture of the uterus resembles that of the adult [5, 8].
Development of the endometrial glands or adenogenesis is a critical event in uterine morphogenesis because it determines, in part, the embryotrophic potential of the adult uterus [4, 9]. The endometrial glands of the uterus produce substances that are critical for conceptus (embryo/fetus and associated placenta) survival, development, and implantation [5, 10, 11]. For example, leukemia inhibitory factor (Lif) is produced and secreted exclusively by endometrial glands of the mouse uterus [12], and Lif null mice are infertile due to a failure of blastocyst implantation [13]. Studies of postnatal uterine development, as well as the regenerating endometrium of the adult, implicate cell proliferation and epithelium-stroma interactions in uterine development and endometrial adenogenesis [3, 5, 14, 15]. Communication between the epithelium and stroma appears to be mediated by Wnt and Hox genes, intrinsic growth factor systems, and changes in the composition and distribution of the extracellular matrix [3, 16, 17]. Mutant mouse models have been used to identify a number of genes involved in uterine gland development including Foxa2, Hoxa10, Hoxa11, Wnt4, Wnt5a, and Wnt7a [1–5, 5, 18–22].
One approach to discover novel regulatory genes and pathways is to disrupt uterine morphogenesis by using steroid hormones, given that the initial stages of postnatal uterine development are ovary- and steroid-independent [5, 23]. For example, treatment of newborn ewe lambs with progestin from birth to PD 56 permanently ablated endometrial gland development, resulting in an adult uterine gland knockout ewe that exhibited recurrent early pregnancy loss due to defects in peri-implantation conceptus survival and growth [24]. In neonatal mice, progesterone receptor (PGR) protein can be detected in the uterine LE by PD 3 and in the stroma by PD 6 [25, 26]. Recently, exposure of neonatal mice to P4 from birth to PD 5 was found to inhibit but not permanently ablate endometrial adenogenesis [27]. However, exposure of neonatal mice to P4 from PD 3 to PD 10 permanently ablated endometrial adenogenesis [28]. The objective here was to use neonatal P4 exposure as a tool to discover new cellular and molecular mechanisms governing postnatal endometrial adenogenesis and uterine development in mice.
MATERIALS AND METHODS
Animals and Tissue Collection
All animal procedures were approved by the Institutional Animal Care and Use Committee and were conducted according to Guide for the Care and Use of Laboratory Animals. In Study One, a total of 24 litters of C57BL/6J mice were randomly assigned to one of two treatment groups at birth (defined as PD 0). All female pups within a litter received daily subcutaneous injections of sesame oil vehicle alone as a control or P4 (50 μg/g of body weight) dissolved in sesame oil from PD 2 to 10. Female pups were necropsied on PD 5, 10, or 20 (n ≥ 4 litters per PD and treatment). One uterine horn was fixed in 4% paraformaldehyde in PBS (pH 7.2) at room temperature for 24 h and embedded in paraffin for histology. The remaining uterine horn was snap frozen in liquid nitrogen and stored at −80°C. In Study Two, female pups were treated with vehicle alone or with P4 from PD 2 to 10 as described above, and uteri were collected at 8 wk of age (n = 6 females/treatment) for histological analysis.
Transcriptional Profiling Using Agilent Microarrays
Total RNA was extracted from frozen uteri (n = 4 PD-10 pups per treatment) by using a Qiagen RNeasy mini-kit (Valencia, CA) and then treated with DNase I (Qiagen). The integrity and quantity of total RNA were determined using an Agilent Bioanalyzer 2100 Lab-on-chip system (Agilent Technologies, Palo Alto, CA). Total RNA (400 ng) was reverse transcribed to cDNA, during which a T7 sequence was introduced into the cDNA. T7 RNA polymerase-driven RNA synthesis was used for preparation and labeling of RNA with Cy3 (or Cy5) dye. Fluorescent cRNA probes were purified using a Qiagen RNeasy mini-kit, and equal amounts (825 ng) of Cy3- and Cy5-labeled cRNA probes were hybridized to a 44K whole-mouse genome Agilent array (catalog no. G2519F-014868). The hybridized slides were washed using Agilent reagents and then scanned using a Genepix 4100A scanner (Molecular Devices Corp., Sunnyvale, CA) with a tolerance of saturation setting of 0.005%. For each channel, the median of the signal intensity and local background values were used. A locally weighted linear regression normalization was applied to remove signal intensity-dependent dye bias for each array by using R software [29]. The Student t-test was used to identify differentially expressed genes, and a P value of 0.01 or less was considered significant.
Database for Annotation, Visualization, and Integrated Discovery Analysis
Database for annotation, visualization, and integrated discovery (DAVID) version 6.7 software (http://david.abcc.ncifcrf.gov/) enables using microarray gene lists to generate specific functional annotations of biological processes affected by treatment in microarray experiments [30, 31] and was used to annotate biological themes in the uterus affected by P4 treatment. All differentially expressed genes identified as both significantly (P < 0.01) and numerically (1.5-fold change) different between control and P4-treated mice were used in the DAVID analysis.
Semi-Quantitative Real-Time RT-PCR Analysis
Semi-quantitative real-time RT-PCR (qPCR) analysis was conducted using total RNA extracted from the uteri (n = 4 pups per PD and treatment) and by methods described by our laboratory [20]. The primers used for PCR analysis are provided in Supplemental Table S1 (all Supplemental Data are available online at www.biolreprod.org). Mouse Rpl13a was used as a reference gene. Foxa2 was determined by TaqMan analysis with TaqMan gene expression Master Mix (Applied Biosystems, Carlsbad, CA) and normalized against Rn18s. Real-time probes and primers for Foxa2 and Rn18s were purchased from Applied Biosystems.
Immunohistochemistry
Paraffin-embedded uteri (n = 4 pups per PD and treatment) were sectioned at a thickness of 5 μm and mounted on slides, deparaffinized, and rehydrated in a graded alcohol series. Sections were subjected to antigen retrieval using boiling citrate buffer, preincubated with 10% normal goat serum in PBS (pH 7.5), and then incubated with primary antibody or nonimmune normal immunoglobulin G (IgG; Sigma-Aldrich) at the same final concentration as the negative control. Primary antibodies were diluted in 1% BSA in PBS (pH 7.5) as follows: anti-MKI67 at 1:750 dilution (catalog no. ab66155; Abcam, Cambridge, MA) and anti-FOXA2 at 1:7000 dilution (catalog no. WRAB-FOXA2; Seven Hills Bioreagents, Cincinnati, OH). Sections were then washed in PBS and incubated with biotinylated secondary antibody (5 μg/ml; Vector Laboratories, Burlingame, CA). Immunoreactive protein was visualized using a Vectasin ABC kit (Vector Laboratories) and diaminobenzidine tetrahydrochloride as the chromagen. Some sections were counterstained with hematoxylin before affixing coverslips with Permount (Fisher Scientific, Fairlawn, NJ).
Statistical Analyses
All quantitative data was subjected to least-squares ANOVA using general linear model procedures of Statistical Analysis System (SAS Institute Inc., Cary, NC). In all analyses, error terms used in tests of significance were identified according to the expectation of the mean squares for error. For analysis of real-time PCR data, the Ct values of the target mRNA were analyzed for effects of day, treatment (control or P4), and their interaction with the Rpl13a or Rn18s values used as a covariate. Significance (P < 0.05) was determined by probability differences of least-squares means.
RESULTS
Neonatal Progesterone Exposure Ablates Endometrial Gland Development
The morphology of the uterus was not different between control and P4-treated mice on PD 5 (Fig. 1). Uteri contained a low columnar LE supported by stroma and myometrium. In control mice, endometrial glands were absent on PD 5, whereas budding and differentiating GE were observed in uteri on PD 10. By PD 20, uteri of control mice contained distinct glands in the stroma. Treatment of female mice with P4 from PD 2 to 10 inhibited endometrial adenogenesis, because no endometrial glands were observed in the uteri of mice treated with P4 (Fig. 1A). All other cell types appeared histologically normal in the uteri from P4-treated mice. Interestingly, the uteri of 8-wk old adult females treated with P4 from PD 2 to 10 had no uterine glands but did contain histologically normal LE, stroma, and myometrium (Fig. 1B).
FIG. 1. .
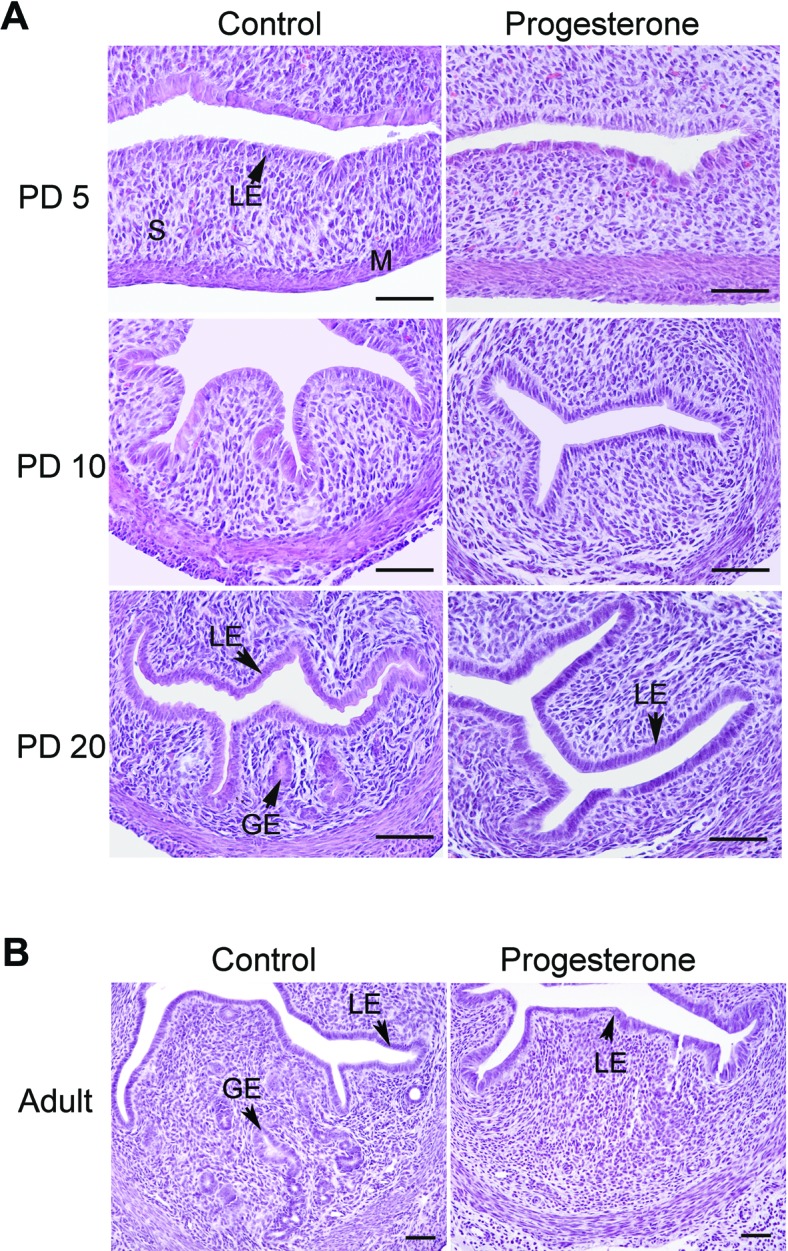
Neonatal progesterone treatment inhibits endometrial gland development in the mouse uterus. Mice were treated with sesame oil vehicle as a control or P4 in vehicle from PD 2 to 10 after birth. Histological examination of the uteri collected on PD 5, 10, and 20 and at 8 wk of age from control mice (left) and P4-treated mice (right). Sections of uteri were stained with hematoxylin and eosin. Note the absence of endometrial glands in the uteri of P4-treated mice as compared to control mice. LE, luminal epithelium; GE, glandular epithelium; M, myometrium; S, stroma. Bar = 50 μm.
Forkhead box A2 (FOXA2) protein was evaluated because it is a primary regulator of gland development in mice [19, 20]. As illustrated in Figure 2A, Foxa2 mRNA increased substantially in the uteri of control mice between PD 5 and 20. In contrast, no increase in Foxa2 mRNA was observed in uteri of P4-treated mice. On PD 5 and 10, immunoreactive FOXA2 protein was detected in the cytoplasm of LE and some stromal cells in uteri of both control and P4-treated mice (Fig. 2B). The FOXA2 protein was in the nuclei of some stromal cells on PD 5. In contrast, abundant FOXA2 protein was observed in the nuclei of GE cells of uteri from control mice on PD 20.
FIG. 2. .
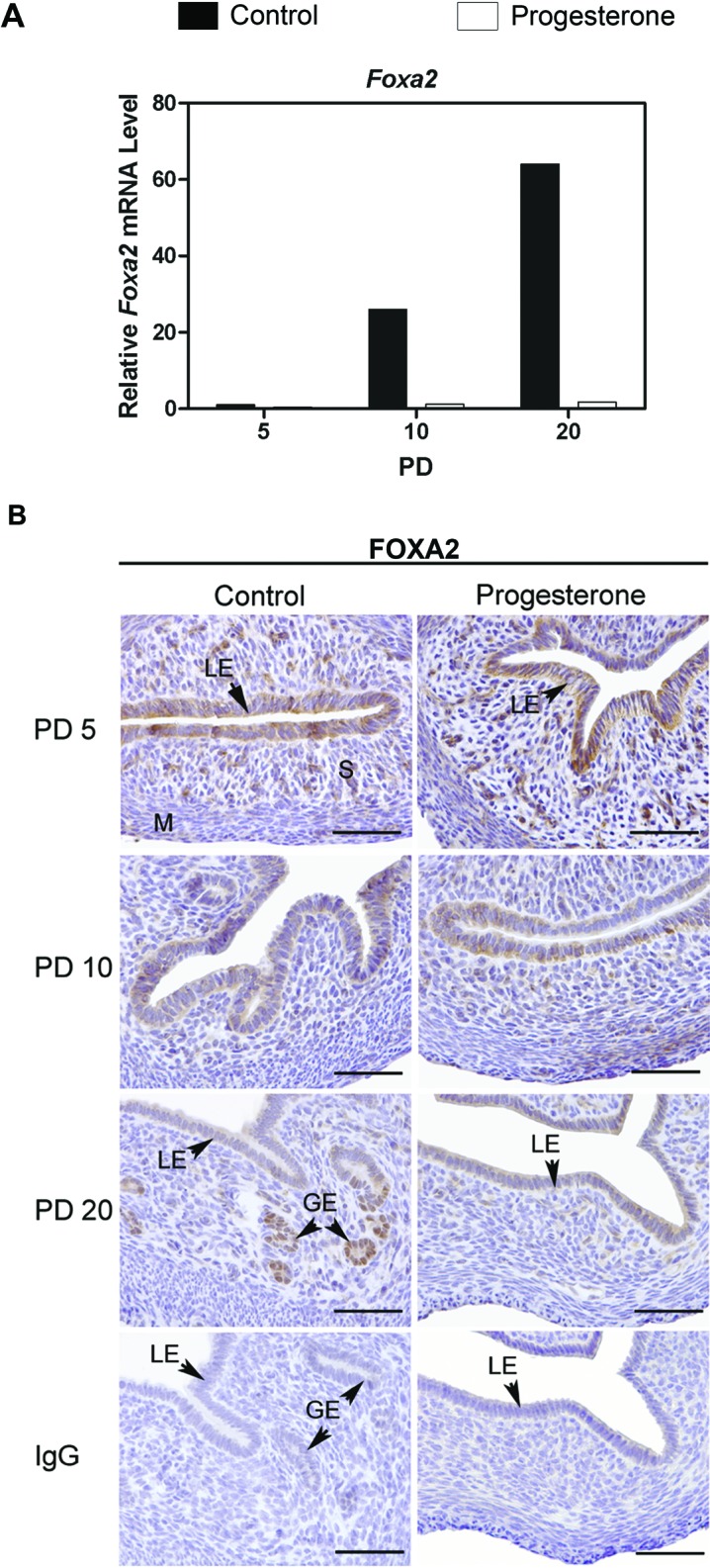
Expression and localization of FOXA2 in the uterus of control and progesterone-treated mice. Mice were treated with sesame oil vehicle as a control or P4 in vehicle from postnatal day (PD) 2 to 10 after birth. A) The relative mRNA levels of Foxa2 was measured in the uterus of control and P4-treated mice by real-time RT-PCR. Data are presented as fold change relative to the mRNA level on PD 5 in uteri from control mice. B) Immunohistochemical localization of FOXA2 protein in uteri of control and P4-treated mice on PD 5, 10, and 20. Sections were counterstained with hematoxylin after immunolocalization. Note the presence of FOXA2 protein in the nuclei of endometrial glands. LE, luminal epithelium; GE, glandular epithelium; M, myometrium; S, stroma. Bar = 50 μm.
Progesterone Alters Uterine Cell Proliferation
To investigate P4 effects on cell proliferation, MKI67, a cellular marker of proliferation [32], was evaluated in the uterus (Fig. 3). In control mice, the number of MKI67-positive cells in the stroma and myometrium was maximal on PD 5 and decreased thereafter. The number of MKI67-positive cells in the LE was high on PD 5 and 10. No MKI67-positive cells were observed in the LE of control mice on PD 20, and only a few stromal and myometrial cells were positive. Treatment with P4 increased the number MKI67-positive cells in the stroma on PD 5, 10, and 20 but decreased the number of MKI67-positive cells in the LE on PD 5 and 10 (Fig. 3).
FIG. 3. .
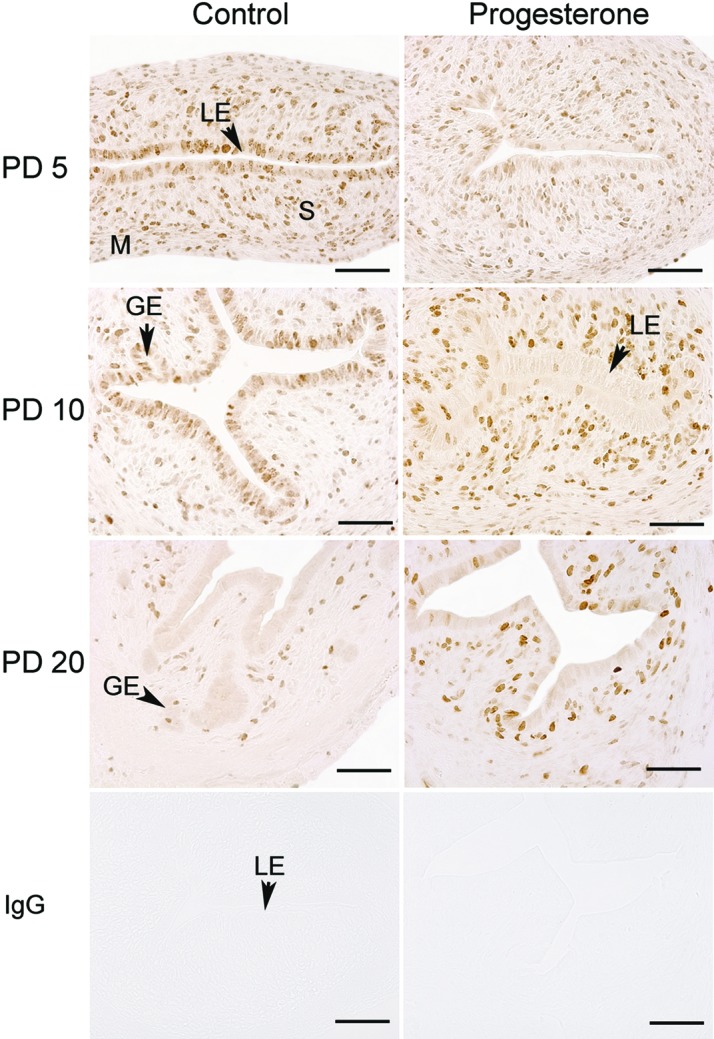
Immunohistochemical localization of MKI67 in the uteri of control and progesterone-treated mice. Mice were treated with sesame oil vehicle as a control or P4 in vehicle from PD 2 to 10 after birth. Cell proliferation was assessed by immunostaining uteri for MKI67. Sections were not counterstained with hematoxylin after immunolocalization. LE, luminal epithelium; GE, glandular epithelium; M, myometrium; S, stroma. Bar = 50 μm.
Progesterone Affects Gene Expression in the Neonatal Uterus
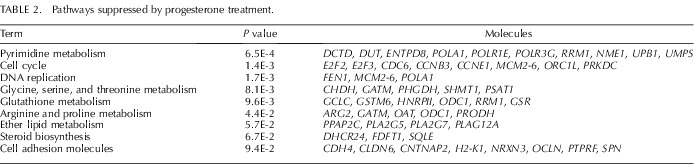
Microarray analysis found that 497 and 564 Agilent probe sets (each representing a gene transcript) were increased or decreased (P < 0.01 and ≥1.5-fold change), respectively, in the P4-treated uteri compared to control PD 10 uteri (Supplemental Tables S2 and S3). DAVID analysis revealed that P4 treatment increased expression of genes associated with extracellular matrix (ECM)-receptor interactions, focal adhesion, and transforming growth factor (TGF)-beta signaling pathways (Table 1). In contrast, P4 treatment decreased expression of genes associated with cell cycle, DNA replication, and cell adhesion pathways (Table 2).
TABLE 1. .
Pathways stimulated by progesterone treatment.
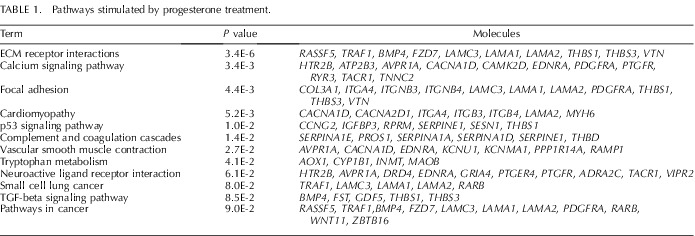
TABLE 2. .
Pathways suppressed by progesterone treatment.
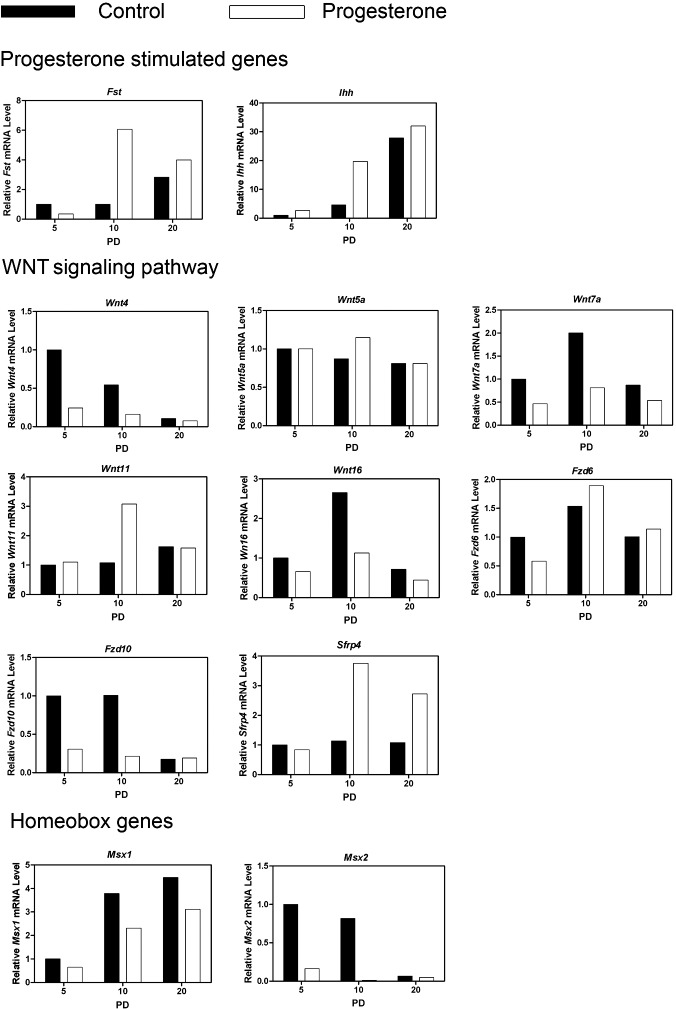
A selected number of differentially expressed genes were validated by qPCR with a focus on genes implicated in uterine development, including WNTs and their Frizzled (FZD) receptors; WNT signaling inhibitors; HOX transcription factors; growth factors and their receptors; and metalloproteinases and regulators of their activity. As presented in Figure 4, neonatal P4 treatment increased (day x treatment; P < 0.05) expression of both Fst and Ihh on PD 10, which are well known P4-stimulated genes in the mouse uterus [33]. Treatment with P4 decreased (P < 0.05) expression of Wnt4, Wnt7a, and Wnt16. In contrast, Wnt11 was increased by P4 on PD 10 (day x treatment, P < 0.05). Neonatal exposure to P4 did not affect (P > 0.10) Wnt5a. Fzd6 and Fzd10 are the most abundantly expressed WNT receptors in the neonatal mouse uterus [34]. Treatment with P4 decreased expression of Fzd6 on PD 5 and reduced Fzd10 expression on PD 5 and 10 (day x treatment, P < 0.05). Expression of the WNT signaling inhibitor Sfrp4 was increased on PD 10 and 20 by P4 (day x treatment; P < 0.05).
FIG. 4. .
Progesterone alters expression of progesterone stimulated, WNT signaling and homeobox genes in the neonatal mouse uterus. Mice were treated with sesame oil vehicle as a control or P4 in vehicle from PD 2 to 10 after birth. The relative mRNA levels of the indicated genes was measured in the uterus of control and P4-treated mice by real-time RT-PCR. Data are presented as fold change relative to the mRNA level on PD 5 in uteri from control mice.
Two msh homolog homeobox genes (Msx1 and Msx2) were expressed in the mouse uterus [35, 36]. As illustrated in Figure 4, Msx1 expression increased (P < 0.05), while Msx2 expression decreased (P < 0.05) between PD 5 and 20 in uteri from control mice. Treatment with P4 decreased (P < 0.05) expression of both Msx1 and Msx2.
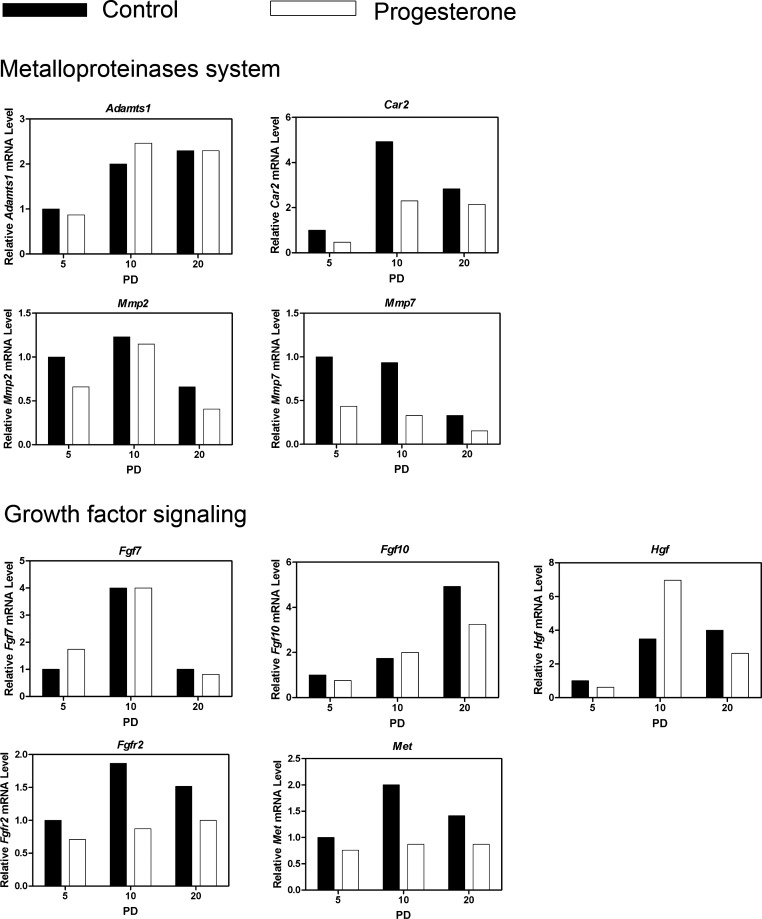
Metalloproteinases are involved in extracellular matrix remodeling, and Mmp2, Mmp7, and the metalloproteinase regulators Car2 and Adamts1 are expressed in the developing and adult mouse uterus [37, 38]. As illustrated in Figure 5, Adamts1 and Mmp2 expression were not affected (P > 0.10) by P4 treatment, whereas P4 decreased (P < 0.05) Mmp7 on all PDs and reduced (day x treatment, P < 0.05) Car2 expression on PD 5 and 10.
FIG. 5. .
Progesterone alters expression of the metallproteinase system and growth factor signaling genes in the neonatal mouse uterus. Mice were treated with sesame oil vehicle as a control or P4 in vehicle from PD 2 to 10 after birth. The relative mRNA levels of the indicated genes was measured in the uterus of control and P4-treated mice by real-time RT-PCR. Data are presented as fold change relative to the mRNA level on PD 5 in uteri from control mice.
A number of growth factors and their receptors are implicated in P4 inhibition of uterine adenogenesis in neonatal ewes [24]. As illustrated in Figure 5, expression of Met and Fgfr2 were decreased (day x treatment, P < 0.05) by P4 treatment, whereas Hgf was increased (day x treatment, P < 0.05) on PD 10 by P4 treatment. In contrast, Fgf7 and Fgf10 were not affected (P > 0.10) by P4 treatment.
DISCUSSION
This study supports the idea that P4 can be used as an effective tool with which to discover new factors and pathways regulating postnatal endometrial adenogenesis and uterine development in the mouse. In the present study, P4 treatment of neonatal C57BL/6J mice altered expression of morphoregulatory genes and epithelial cell proliferation, leading to a disruption of endometrial adenogenesis in the developing uterus. Histological analysis of adult uterine tissues revealed the absence of endometrial glands in neonatally progestinized mice. Similarly, Cooke et al. [28] recently reported that treatment of C57BL/6J mice with P4 from PD 3 to 10 permanently blocked endometrial adenogenesis and resulted in adult infertility. In contrast, treatment of Swiss Webster mice with P4 from PD 0 to 10 reduced but did not permanently ablate endometrial adenogenesis [27]. In that study, the uteri of neonatally P4-treated Swiss Webster mice had substantially reduced numbers of uterine glands at 3 and 8 wk of age. Similar to the present study, the observed differences in neonatal responses to P4 are not known, but could be due to strain differences in steroid receptor expression or steroid metabolism.
Microarray analysis revealed that P4 treatment suppressed cell cycle and DNA replication pathways in the neonatal mouse uterus. Immunostaining for MKI67 revealed that P4 treatment of neonatal mice decreased the number of proliferating cells in the LE and increased them in the stroma of the uterus, which is similar to a previous study using radioactive thymidine labeling [23]. Furthermore, the inhibitory effect of P4 on uterine epithelial DNA synthesis is mediated by stromal PGR, because epithelial PGR are neither necessary nor sufficient for P4 inhibition of estrogen-induced epithelial DNA synthesis [39]. Collectively, these results indicate that P4 ablation of endometrial adenogenesis in the neonatal mouse uterus may be attributed, in part, to negative effects on proliferation of the LE cells that serve as the progenitors of GE cells. Moreover, the induction of stromal cell proliferation would disrupt normal patterns of cell-cell communication that regulate uterine morphogenesis [3, 15, 25, 40].
In the current study, P4 treatment of neonatal mice markedly upregulated Ihh. IHH is a critical mediator of P4 signaling in the uterus during mouse embryo implantation that is expressed in the LE [41]. Overexpression of smoothened (SMO), a downstream effector of IHH expressed in the stroma, disrupted postnatal uterine morphogenesis leading to a hypertrophic uterus with reduced number of uterine glands [42]. Uteri of those mice overexpressing SMO contained increased levels of genes involved in chondrocyte differentiation, such as laminin alpha 2 (Lama2) and nidogen 1 (Nid1), as well as cartilage-specific genes including the hyaluronan synthase Has2. In the present study, P4 treatment of neonatal mice up-regulated those genes in the uterus. Indeed, P4 induction of IHH in the LE would activate SMO pathways in the stroma and presumably inhibit gland development [42].
Microarray analysis revealed that a number of genes involved in ECM-receptor interactions and focal adhesions (e.g., vitronectin, laminin alpha 1, integrin beta 4) were increased by P4 treatment. Endometrial gland morphogenesis involves cell-cell, epithelial-stromal and cell-ECM interactions [3, 5, 14, 15, 43]. In the neonatal mouse uterus, Wnt4, Wnt5a, and Wnt16 are expressed in the stroma, while Wnt7a and Wnt11 are expressed in the uterine epithelium [2, 34]. Thus, WNTs likely govern the epithelial-stromal interactions involved in prenatal and postnatal uterine morphogenesis. Recently, conditional deletion of Wnt4 and Wnt7a in the uterus after birth using the PGR-Cre mouse model confirmed their biological roles in postnatal uterine development [20, 44]. Mice deficient in Wnt4 and Wnt7a displayed reduced or completely absent endometrial glands, respectively. In the present study, P4 treatment downregulated Wnt7a and Wnt4 as well as Wnt16 expression in the uterus. Wnt16 is expressed in the endometrial stroma of the neonatal and adult mouse uterus [34, 45], but the effect of postnatal deletion of Wnt16 has not been reported. Of particular note, P4 treatment increased the expression of Sfrp4, a secreted inhibitor of Wnt signaling [46], in the present study. In the neonatal ewe lamb, estrogen upregulation of SFRP2 in the uterus was implicated in the effect of that steroid to inhibit endometrial gland development [47]. In the present study, Msx1 and Msx2 expression in the uterus were also decreased by neonatal P4 treatment. The ability of the mesenchyme to induce or maintain Msx1 expression in the Müllerian duct epithelium is thought to involve mesenchymal expression of Wnt5a [36], and Msx2 is required for expression of Wnt7a [48]. However, recent results indicate that postnatal deletion of MSX1 or MSX2 or both has no effect of endometrial adenogenesis [49]. Collectively, available results strongly support the idea that WNTs are important epithelial and stromal derived factors that regulate cell proliferation and differentiation in the developing neonatal uterus of the mouse and other species [5].
In addition to the secreted WNTs, other factors play important roles in epithelial proliferation, differentiation and branching morphogenesis in many developing organs including the uterus [3, 50, 51]. Hgf and Met were found to be expressed by endometrial epithelium and stroma in the adult mouse uterus, and their tissue-specific expression was tightly controlled by steroidal hormones throughout the estrous cycle [52]. Interestingly, P4 stimulated their expression in the stroma, whereas it inhibited their expression in the epithelia [52]. Similarly in the present study, P4 treatment increased Hgf expression and decreased expression of Met in the neonatal mouse uterus. Because Fgfr2 or Met null mice are embryonically lethal, it is difficult to draw conclusions on FGFs and HGF signaling role in the postnatal uterus. However, injections of FGF7 into neonatal mice stimulated proliferation of the uterine LE [16]. Recently, Li et al. [17] found that P4 inhibits expression of several FGFs (Fgf1, Fgf2, Fgf9, Fgf18) in the stroma of the adult uterus, thereby reducing LE cell proliferation. In the present study, microarray analysis found that P4 treatment of neonatal mice reduced Fgf9 and Fgf12 expression in the uterus along with LE cell proliferation. Thus, FGF and HGF signaling are also likely important for uterine development and adenogenesis as in other organs exhibiting branching morphogenesis such as the lung, mammary gland and kidney [53–55].
Matrix metalloproteinases (MMPs) and their inhibitors are involved in postnatal uterine morphogenesis in the mouse [56–58] by reorganizing the extracellular matrix necessary for endometrial gland development [37]. Hu et al. [57] implicated the MMP system (Mmp2, Mmp7, Adamts1) in the postnatal uterine morphogenesis in the mouse. In the present study, P4 disruption of endometrial adenogenesis was associated with a reduction Mmp7 and Mmp2 expression. Further, Car2 was reduced in P4-treated mice on PD 5 and 10. Carbonic anhydrases (CAR) are hypothesized to have a key role in regulating neonatal mouse uterine adenogenesis by affecting GE cell migration and MMP activity [38]. Interestingly, inhibition of CAR2 activity in neonatal mice reduced endometrial gland development [38]. Collectively, available results support the idea that P4 deregulates MMP expression and activity in the endometrium, which impairs ECM remodeling required for GE cell proliferation, migration and/or differentiation in the developing neonatal uterus.
A recent study found that conditional ablation of Foxa2 in the postnatal mouse uterus using the PGR-Cre model resulted in an absence of endometrial glands in the adult, suggesting that FOXA2 is a critical regulator of endometrial adenogenesis in the mouse uterus [19]. In the lung and prostate, Foxa2 also regulates epithelial budding and morphogenesis [59, 60]. The present study found that Foxa2 is a sensitive marker of GE differentiation and development, because Foxa2 expression increased with age in control but not P4-treated mice that lack endometrial glands. The nuclear localization of FOXA2 in the endometrial GE cells is similar to other reports that Foxa2 is predominantly expressed by GE cells [19, 20] and consistent with its function as transcription factor regulating organogenesis [61].
In summary, the present study illustrates the utility of P4 as a tool to discover mechanisms regulating postnatal uterine development and provides insights into P4 action within the uterus. Available results support the hypothesis that P4 inhibits endometrial adenogenesis in the developing neonatal uterus by altering expression of morphoregulatory genes and consequently disrupting normal patterns of cell proliferation and communication. Future mechanistic studies are needed to more precisely understand the novel factors and pathways involved in endometrial adenogenesis in the neonatal mouse uterus, which is important as endometrial glands are required for fertility of the adult.
Supplementary Material
ACKNOWLEDGMENT
We thank Dr. Kathrin Dunlap of Texas A&M University, College Station, TX, and Dr. Allison Stewart of the University of Texas MD Anderson Cancer Center, Houston, TX, for assistance and advice.
Footnotes
Supported by National Institutes of Health grant 1 R21 HD054679.
REFERENCES
- Kobayashi A, Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet 2003; 4: 969 980 [DOI] [PubMed] [Google Scholar]
- Masse J, Watrin T, Laurent A, Deschamps S, Guerrier D, Pellerin I. The developing female genital tract: from genetics to epigenetics. Int J Dev Biol 2009; 53: 411 424 [DOI] [PubMed] [Google Scholar]
- Cunha GR. Epithelial-stromal interactions in development of the urogenital tract. Int Rev Cytol 1976; 47: 137 194 [DOI] [PubMed] [Google Scholar]
- Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW, Spencer TE. Developmental biology of uterine glands. Biol Reprod 2001; 65: 1311 1323 [DOI] [PubMed] [Google Scholar]
- Spencer TE, Dunlap KA, Filant J. Comparative developmental biology of the uterus: Insights into mechanisms and developmental disruption. Mol Cell Endocrinol 2012; 354 (1–2): 34 53 [DOI] [PubMed] [Google Scholar]
- Brody JR, Cunha GR. Histologic, morphometric, and immunocytochemical analysis of myometrial development in rats and mice: I. Normal development. Am J Anat 1989; 186: 1 20 [DOI] [PubMed] [Google Scholar]
- Branham WS, Sheehan DM, Zehr DR, Ridlon E, Nelson CJ. The postnatal ontogeny of rat uterine glands and age-related effects of 17 beta-estradiol. Endocrinology 1985; 117: 2229 2237 [DOI] [PubMed] [Google Scholar]
- Hu J, Gray CA, Spencer TE. Gene expression profiling of neonatal mouse uterine development. Biol Reprod 2004; 70: 1870 1876 [DOI] [PubMed] [Google Scholar]
- Bartol FF, Wiley AA, Floyd JG, Ott TL, Bazer FW, Gray CA, Spencer TE. Uterine differentiation as a foundation for subsequent fertility. J Reprod Fertil Suppl 1999; 54: 287 302 [PubMed] [Google Scholar]
- Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab 2002; 87: 2954 2959 [DOI] [PubMed] [Google Scholar]
- Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev 2004; 25: 341 373 [DOI] [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 1992; 359: 76 79 [DOI] [PubMed] [Google Scholar]
- Chen JR, Cheng JG, Shatzer T, Sewell L, Hernandez L, Stewart CL. Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology 2000; 141: 4365 4372 [DOI] [PubMed] [Google Scholar]
- Okulicz WC. Regeneration. : Glasser SR, Aplin JD, Giudice LC, Tabibzadeh S. (eds.), The Endometrium. London: Taylor and Francis Inc.; 2002; 110 120 [Google Scholar]
- Cunha GR, Bigsby RM, Cooke PS, Sugimura Y. Stromal-epithelial interactions in adult organs. Cell Differ 1985; 17: 137 148 [DOI] [PubMed] [Google Scholar]
- Hom YK, Young P, Thomson AA, Cunha GR. Keratinocyte growth factor injected into female mouse neonates stimulates uterine and vaginal epithelial growth. Endocrinology 1998; 139: 3772 3779 [DOI] [PubMed] [Google Scholar]
- Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, Yamagishi H, Srivastava D, Bagchi MK, Bagchi IC. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science 2011; 331: 912 916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C, Sassoon DA. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development 1998; 125: 3201 3211 [DOI] [PubMed] [Google Scholar]
- Jeong JW, Kwak I, Lee KY, Kim TH, Large MJ, Stewart CL, Kaestner KH, Lydon JP, DeMayo FJ. Foxa2 is essential for mouse endometrial gland development and fertility. Biol Reprod 2010; 83: 396 403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap KA, Filant J, Hayashi K, Rucker EB, III, Song G, Deng JM, Behringer RR, DeMayo FJ, Lydon J, Jeong JW, Spencer TE. Postnatal deletion of wnt7a inhibits uterine gland morphogenesis and compromises adult fertility in mice. Biol Reprod 2011; 85: 386 396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta L, Sassoon D. Wnt7a is a suppressor of cell death in the female reproductive tract and is required for postnatal and estrogen-mediated growth. Biol Reprod 2004; 71: 444 454 [DOI] [PubMed] [Google Scholar]
- Miller C, Degenhardt K, Sassoon DA. Fetal exposure to DES results in de-regulation of Wnt7a during uterine morphogenesis. Nat Genet 1998; 20: 228 230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigsby RM, Cunha GR. Effects of progestins and glucocorticoids on deoxyribonucleic acid synthesis in the uterus of the neonatal mouse. Endocrinology 1985; 117: 2520 2526 [DOI] [PubMed] [Google Scholar]
- Gray CA, Taylor KM, Bazer FW, Spencer TE. Mechanisms regulating norgestomet inhibition of endometrial gland morphogenesis in the neonatal ovine uterus. Mol Reprod Dev 2000; 57: 67 78 [DOI] [PubMed] [Google Scholar]
- Kurita T, Cooke PS, Cunha GR. Epithelial-stromal tissue interaction in paramesonephric (Mullerian) epithelial differentiation. Dev Biol 2001; 240: 194 211 [DOI] [PubMed] [Google Scholar]
- Franco HL, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ. In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin Cell Dev Biol 2008; 19: 178 186 [DOI] [PubMed] [Google Scholar]
- Stewart CA, Fisher SJ, Wang Y, Stewart MD, Hewitt SC, Rodriguez KF, Korach KS, Behringer RR. Uterine gland formation in mice is a continuous process, requiring the ovary after puberty, but not after parturition. Biol Reprod 2011; 85: 954 964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke PS, Ekman GC, Kaur J, Davila J, Bagchi IC, Clark SG, Dziuk PJ, Hayashi K, Bartol FF. Brief exposure to progesterone during a critical neonatal window prevents uterine gland formation in mice. Biol Reprod 2012; 86 (3):63, 1 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, Speed TP. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucl Acids Res 2002; 30: e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol 2003; 4: P3 [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protocols 2008; 4: 44 57 [DOI] [PubMed] [Google Scholar]
- Starborg M, Gell K, Brundell E, Hoog C. The murine Ki-67 cell proliferation antigen accumulates in the nucleolar and heterochromatic regions of interphase cells and at the periphery of the mitotic chromosomes in a process essential for cell cycle progression. J Cell Sci 1996; 109 (Pt 1): 143 153 [DOI] [PubMed] [Google Scholar]
- Wetendorf M, Demayo FJ. The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Mol Cell Endocrinol 2012, published online ahead of print 17 November 2011 as DOI: 10.1016/j.mce.2011.10.028 [DOI] [PMC free article] [PubMed]
- Hayashi K, Yoshioka S, Reardon SN, Rucker EB, III, Spencer TE, Demayo FJ, Lydon JP, Maclean JA., II WNTs in the neonatal mouse uterus: potential regulation of endometrial gland development. Biol Reprod 2011; 84: 308 319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas R, Chen YP, Bei M, Woo I, Satokata I. The role of Msx genes in mammalian development. Ann N Y Acad Sci 1996; 785: 171 181 [DOI] [PubMed] [Google Scholar]
- Pavlova A, Boutin E, Cunha G, Sassoon D. Msx1 (Hox-7.1) in the adult mouse uterus: cellular interactions underlying regulation of expression. Development 1994; 120: 335 345 [DOI] [PubMed] [Google Scholar]
- Curry TE, Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev 2003; 24: 428 465 [DOI] [PubMed] [Google Scholar]
- Hu J, Spencer TE. Carbonic anhydrase regulate endometrial gland development in the neonatal uterus. Biol Reprod 2005; 73: 131 138 [DOI] [PubMed] [Google Scholar]
- Kurita T, Young P, Brody JR, Lydon JP, O'Malley BW, Cunha GR. Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology 1998; 139: 4708 4713 [DOI] [PubMed] [Google Scholar]
- Kurita T. Normal and abnormal epithelial differentiation in the female reproductive tract. Differentiation 2011; 82: 117 126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Jeong J, Kwak I, Yu CT, Lanske B, Soegiarto DW, Toftgard R, Tsai MJ, Tsai S, Lydon JP, DeMayo FJ. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet 2006; 38: 1204 1209 [DOI] [PubMed] [Google Scholar]
- Franco HL, Lee KY, Rubel CA, Creighton CJ, White LD, Broaddus RR, Lewis MT, Lydon JP, Jeong JW, DeMayo FJ. Constitutive activation of smoothened leads to female infertility and altered uterine differentiation in the mouse. Biol Reprod 2010; 82: 991 999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JW, Lee HS, Franco HL, Broaddus RR, Taketo MM, Tsai SY, Lydon JP. DeMayo FJ. beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene 2009; 28: 31 40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco HL, Dai D, Lee KY, Rubel CA, Roop D, Boerboom D, Jeong JW, Lydon JP, Bagchi IC, Bagchi MK, DeMayo FJ. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J 2011; 25: 1176 1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Erikson DW, Tilford SA, Bany BM, Maclean JA, II, Rucker EB, III, Johnson GA, Spencer TE. Wnt genes in the mouse uterus: potential regulation of implantation. Biol Reprod 2009; 80: 989 1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004; 20: 781 810 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Spencer TE. WNT pathways in the neonatal ovine uterus: potential specification of endometrial gland morphogenesis by SFRP2. Biol Reprod 2006; 74: 721 733 [DOI] [PubMed] [Google Scholar]
- Huang WW, Yin Y, Bi Q, Chiang TC, Garner N, Vuoristo J, McLachlan JA, Ma L. Developmental diethylstilbestrol exposure alters genetic pathways of uterine cytodifferentiation. Mol Endocrinol 2005; 19: 669 682 [DOI] [PubMed] [Google Scholar]
- Daikoku T, Cha J, Sun X, Tranguch S, Xie H, Fujita T, Hirota Y, Lydon J, Demayo F, Maxson R, Dey SK. Conditional deletion of MSX homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity. Developmental Cell 2011; 21: 1014 1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Chung LW, Shannon JM, Taguchi O, Fujii H. Hormone-induced morphogenesis and growth: role of mesenchymal-epithelial interactions. Recent Prog Horm Res 1983; 39: 559 598 [DOI] [PubMed] [Google Scholar]
- Cunha GR, Lung B. The importance of stroma in morphogenesis and functional activity of urogenital epithelium. In Vitro 1979; 15: 50 71 [DOI] [PubMed] [Google Scholar]
- Zhang X. Hepatocyte growth factor system in the mouse uterus: variation across the estrous cycle and regulation by 17-beta-estradiol and progesterone. Biol Reprod 2010; 82: 1037 1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates CM. Role of fibroblast growth factor receptor signaling in kidney development. Pediatr Nephrol 2007; 22: 343 349 [DOI] [PubMed] [Google Scholar]
- Spencer-Dene B, Dillon C, Fantl V, Kerr K, Petiot A, Dickson C. Fibroblast growth factor signalling in mouse mammary gland development. Endocr Relat Cancer 2001; 8: 211 217 [DOI] [PubMed] [Google Scholar]
- Ware LB, Matthay MA. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol 2002; 282: L924 940 [DOI] [PubMed] [Google Scholar]
- Zhou HE, Zhang X, Nothnick WB. Disruption of the TIMP-1 gene product is associated with accelerated endometrial gland formation during early postnatal uterine development. Biol Reprod 2004; 71: 534 539 [DOI] [PubMed] [Google Scholar]
- Hu J, Zhang X, Nothnick WB, Spencer TE. Matrix metalloproteinases and their tissue inhibitors in the developing neonatal mouse uterus. Biol Reprod 2004; 71: 1598 1604 [DOI] [PubMed] [Google Scholar]
- Nothnick WB. Disruption of the tissue inhibitor of metalloproteinase-1 gene in reproductive-age female mice is associated with estrous cycle stage-specific increases in stromelysin messenger RNA expression and activity. Biol Reprod 2001; 65: 1780 1788 [DOI] [PubMed] [Google Scholar]
- Besnard V, Wert SE, Hull WM, Whitsett JA. Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene Expr Patterns 2004; 5: 193 208 [DOI] [PubMed] [Google Scholar]
- Kaestner KH. The hepatocyte nuclear factor 3 (HNF3 or FOXA) family in metabolism. Trends Endocrinol Metab 2000; 11: 281 285 [DOI] [PubMed] [Google Scholar]
- Kaestner KH. The FoxA factors in organogenesis and differentiation. Curr Opin Genet Dev 2010; 20: 527 532 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.