Abstract
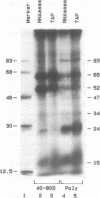
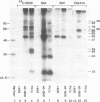
This laboratory previously detected by UV crosslinking a number of proteins associated with cytoplasmic mRNA in mammalian cells, and the data suggested that they are involved in translation. To find out which proteins are associated with caps we made use of reticulocyte mRNA specifically labeled in the cap with 32P together with a cell-free translation system and UV crosslinking. Approximately 8 bands corresponding to proteins crosslinked to the cap itself have been detected by polyacrylamide gel electrophoresis after UV crosslinking and digestion with RNases or tobacco pyrophosphatase. All but one were specific for methylated caps. One was similar in size and partial peptide map to a cap-binding protein, CBP I, previously identified in other laboratories, and most of the others corresponded to proteins previously known to be associated with mRNA but not known to be associated with caps. The results suggest that most mRNA-associated proteins are associated with caps or poly(A). Also, the number of cap-associated proteins may be greater than previously suspected.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonoff R. S., Ferguson J. J., Jr, Idelkope G. Direct photo-affinity labeling of cyclic nucleotide binding proteins with guanosine-3',5'-monophosphate. Photochem Photobiol. 1976 May;23(5):327–329. doi: 10.1111/j.1751-1097.1976.tb07256.x. [DOI] [PubMed] [Google Scholar]
- Asselbergs F. A., Peters W., Venrooij W. J., Bloemendal H. Diminished sensitivity of re-initiation of translation to inhibition by cap analogues in reticulocyte lysates. Eur J Biochem. 1978 Aug 1;88(2):483–488. doi: 10.1111/j.1432-1033.1978.tb12473.x. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K. 5'-terminal cap structure in eucaryotic messenger ribonucleic acids. Microbiol Rev. 1980 Jun;44(2):175–205. doi: 10.1128/mr.44.2.175-205.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G. A protein of molecular weight 78,000 bound to the polyadenylate region of eukaryotic messenger RNAs. Proc Natl Acad Sci U S A. 1973 Mar;70(3):924–928. doi: 10.1073/pnas.70.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan R. N., Hayashi M. Two proteins are bound to most species of polysomal mRNA. Nat New Biol. 1973 Aug 29;244(139):271–274. doi: 10.1038/newbio244271a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dreyfuss G., Adam S. A., Choi Y. D. Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol. 1984 Mar;4(3):415–423. doi: 10.1128/mcb.4.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edery I., Hümbelin M., Darveau A., Lee K. A., Milburn S., Hershey J. W., Trachsel H., Sonenberg N. Involvement of eukaryotic initiation factor 4A in the cap recognition process. J Biol Chem. 1983 Sep 25;258(18):11398–11403. [PubMed] [Google Scholar]
- England T. E., Bruce A. G., Uhlenbeck O. C. Specific labeling of 3' termini of RNA with T4 RNA ligase. Methods Enzymol. 1980;65(1):65–74. doi: 10.1016/s0076-6879(80)65011-3. [DOI] [PubMed] [Google Scholar]
- Green M. R., Maniatis T., Melton D. A. Human beta-globin pre-mRNA synthesized in vitro is accurately spliced in Xenopus oocyte nuclei. Cell. 1983 Mar;32(3):681–694. doi: 10.1016/0092-8674(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R., Carroll E., 3rd Reconstitution of functional mRNA-protein complexes in a rabbit reticulocyte cell-free translation system. Mol Cell Biol. 1985 Feb;5(2):342–351. doi: 10.1128/mcb.5.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. R. Proteins crosslinked to messenger RNA by irradiating polyribosomes with ultraviolet light. Nucleic Acids Res. 1980 Dec 11;8(23):5685–5701. doi: 10.1093/nar/8.23.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. R. The polyribosomal mRNA--protein complex is a dynamic structure. Proc Natl Acad Sci U S A. 1981 May;78(5):2923–2926. doi: 10.1073/pnas.78.5.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifo J. A., Tahara S. M., Morgan M. A., Shatkin A. J., Merrick W. C. New initiation factor activity required for globin mRNA translation. J Biol Chem. 1983 May 10;258(9):5804–5810. [PubMed] [Google Scholar]
- Hickey E. D., Weber L. A., Baglioni C. Inhibition of initiation of protein synthesis by 7-methylguanosine-5'-monophosphate. Proc Natl Acad Sci U S A. 1976 Jan;73(1):19–23. doi: 10.1073/pnas.73.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith G., Gilham P. T. Stepwise degradation of polyribonucleotides. Biochemistry. 1974 Aug 13;13(17):3601–3606. doi: 10.1021/bi00714a031. [DOI] [PubMed] [Google Scholar]
- Lamed R., Levin Y., Wilchek M. Covalent coupling of nucleotides to agarose for affinity chromatography. Biochim Biophys Acta. 1973 Apr 28;304(2):231–235. doi: 10.1016/0304-4165(73)90239-0. [DOI] [PubMed] [Google Scholar]
- Lischwe M. A., Ochs D. A new method for partial peptide mapping using N-chlorosuccinimide/urea and peptide silver staining in sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1982 Dec;127(2):453–457. doi: 10.1016/0003-2697(82)90203-2. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Alzner-Deweerd B., Heckman J. E., MacGee J., Tabor M. W., RajBhandary U. L. Sequence analysis of 5'[32P] labeled mRNA and tRNA using polyacrylamide gel electrophoresis. Nucleic Acids Res. 1978 Jan;5(1):37–56. doi: 10.1093/nar/5.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Rose J. K. Relative importance of 7-methylguanosine in ribosome binding and translation of vesicular stomatitis virus mRNA in wheat germ and reticulocyte cell-free systems. J Biol Chem. 1977 Feb 25;252(4):1181–1188. [PubMed] [Google Scholar]
- Martin S. A., Moss B. Modification of RNA by mRNA guanylyltransferase and mRNA (guanine-7-)methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9330–9335. [PubMed] [Google Scholar]
- Nath J. P., Eagle G. R., Himes R. H. Direct photoaffinity labeling of tubulin with guanosine 5'-triphosphate. Biochemistry. 1985 Mar 12;24(6):1555–1560. doi: 10.1021/bi00327a040. [DOI] [PubMed] [Google Scholar]
- Olsen A. S., Breckenridge B. M., Sanders M. M. Identification of cyclic guanosine monophosphate-binding proteins in Drosophila by direct photoaffinity labeling. Anal Biochem. 1982 Nov 1;126(2):306–311. doi: 10.1016/0003-2697(82)90520-6. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Photochemical cross-linking of cap binding proteins to eucaryotic mRNAs: effect of mRNA 5' secondary structure. Mol Cell Biol. 1985 Nov;5(11):3222–3230. doi: 10.1128/mcb.5.11.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath E., Randerath K. Ion-exchange thin-layer chromatography. XII. Quantitative elution and microdetermination of nucleoside monophosphates, ATP, and other nucleotide coenzymes. Anal Biochem. 1965 Jul;12(1):83–93. doi: 10.1016/0003-2697(65)90145-4. [DOI] [PubMed] [Google Scholar]
- Roman R., Brooker J. D., Seal S. N., Marcus A. Inhibition of the transition of a 40 S ribosome-Met-tRNA-i-Met complex to an 80 S ribosome-Met-tRNA-i-Met- complex by 7-Methylguanosine-5'-phosphate. Nature. 1976 Mar 25;260(5549):359–360. doi: 10.1038/260359a0. [DOI] [PubMed] [Google Scholar]
- Rychlik W., Domier L. L., Gardner P. R., Hellmann G. M., Rhoads R. E. Amino acid sequence of the mRNA cap-binding protein from human tissues. Proc Natl Acad Sci U S A. 1987 Feb;84(4):945–949. doi: 10.1073/pnas.84.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer B., Kemper W., Jagus R. Identification of a 48 S preinitiation complex in reticulocyte lysate. J Biol Chem. 1978 May 25;253(10):3384–3386. [PubMed] [Google Scholar]
- Setyono B., Greenberg J. R. Proteins associated with poly(A) and other regions of mRNA and hnRNA molecules as investigated by crosslinking. Cell. 1981 Jun;24(3):775–783. doi: 10.1016/0092-8674(81)90103-3. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Weinstein J. A., Safer B., Merrick W. C., Weber L. A., Hickey E. D., Baglioni C. Evidence for role of m7G5'-phosphate group in recognition of eukaryotic mRNA by initiation factor IF-M3. Nature. 1976 May 27;261(5558):291–294. doi: 10.1038/261291a0. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. mRNA cap binding proteins: essential factors for initiating translation. Cell. 1985 Feb;40(2):223–224. doi: 10.1016/0092-8674(85)90132-1. [DOI] [PubMed] [Google Scholar]
- Shinshi H., Miwa M., Sugimura T. Enzyme cleaving the 5'-terminal methylated blocked structure of messenger RNA. FEBS Lett. 1976 Jun 1;65(2):254–257. doi: 10.1016/0014-5793(76)80492-9. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Stability of HeLa cell mRNA in actinomycin. Nature. 1972 Nov 10;240(5376):100–102. doi: 10.1038/240100a0. [DOI] [PubMed] [Google Scholar]
- Sonenberg N. ATP/Mg++-dependent cross-linking of cap binding proteins to the 5' end of eukaryotic mRNA. Nucleic Acids Res. 1981 Apr 10;9(7):1643–1656. doi: 10.1093/nar/9.7.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Morgan M. A., Merrick W. C., Shatkin A. J. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5'-terminal cap in mRNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4843–4847. doi: 10.1073/pnas.75.10.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Rupprecht K. M., Hecht S. M., Shatkin A. J. Eukaryotic mRNA cap binding protein: purification by affinity chromatography on sepharose-coupled m7GDP. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4345–4349. doi: 10.1073/pnas.76.9.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Shatkin A. J. Reovirus mRNA can be covalently crosslinked via the 5' cap to proteins in initiation complexes. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4288–4292. doi: 10.1073/pnas.74.10.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H. Effect of 7-methylguanosine-5'-phosphate on rabbit globin synthesis. FEBS Lett. 1976 Dec 31;72(2):309–313. doi: 10.1016/0014-5793(76)80993-3. [DOI] [PubMed] [Google Scholar]
- Suzuki H. Effect of m7G5'ppp5'Nm on the rabbit globin synthesis. FEBS Lett. 1977 Jul 1;79(1):11–14. doi: 10.1016/0014-5793(77)80339-6. [DOI] [PubMed] [Google Scholar]
- Thomas N. S., Arnstein H. R. Formation of a 22S mRNA X rRNA X protein complex during translation of globin messenger RNA. Eur J Biochem. 1984 Aug 15;143(1):27–33. doi: 10.1111/j.1432-1033.1984.tb08334.x. [DOI] [PubMed] [Google Scholar]
- Weber L. A., Hickey E. D., Nuss D. L., Baglioni C. 5'-Terminal 7-methylguanosine and mRNA function: influence of potassium concentration on translation in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3254–3258. doi: 10.1073/pnas.74.8.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. M., Cheung C. P., Suhadolnik R. J. Differential inhibition with partially purified and endogenous rabbit reticulocyte globin mRNA by 7-methylguanosine 5'-monophosphate. Biochem Biophys Res Commun. 1977 Oct 10;78(3):1079–1086. doi: 10.1016/0006-291x(77)90531-9. [DOI] [PubMed] [Google Scholar]