Abstract
Introduction
Febrile neutropenia is a common and potentially life-threatening complication of treatment for childhood cancer, which has increasingly been subject to targeted treatment based on clinical risk stratification. Our previous meta-analysis demonstrated 16 rules had been described and 2 of them subject to validation in more than one study. We aimed to advance our knowledge of evidence on the discriminatory ability and predictive accuracy of such risk stratification clinical decision rules (CDR) for children and young people with cancer by updating our systematic review.
Methods
The review was conducted in accordance with Centre for Reviews and Dissemination methods, searching multiple electronic databases, using two independent reviewers, formal critical appraisal with QUADAS and meta-analysis with random effects models where appropriate. It was registered with PROSPERO: CRD42011001685.
Results
We found 9 new publications describing a further 7 new CDR, and validations of 7 rules. Six CDR have now been subject to testing across more than two data sets. Most validations demonstrated the rule to be less efficient than when initially proposed; geographical differences appeared to be one explanation for this.
Conclusion
The use of clinical decision rules will require local validation before widespread use. Considerable uncertainty remains over the most effective rule to use in each population, and an ongoing individual-patient-data meta-analysis should develop and test a more reliable CDR to improve stratification and optimise therapy. Despite current challenges, we believe it will be possible to define an internationally effective CDR to harmonise the treatment of children with febrile neutropenia.
Introduction
Febrile neutropenia (FNP) is a common and potentially life-threatening complication of therapy for childhood cancer, which has increasingly been subject to targeted treatment based on clinical risk stratification [1]. For children this move towards risk-directed care is based upon evidence of the low incidence of death [2], the majority of patients being without identified significant infection or sepsis [3], and small randomised trials demonstrating the feasibility of out-patient based treatment for patients at low-risk of septic complications [4]. A large proportion of the evidence for risk stratifications has originated from adult oncology [5] It is acknowledged that children are not ‘little adults’ but distinct in the biology of their malignancies, treatment regimens, infections and psychosocial setting and therefore specific evidence for stratification of children with FNP is needed [6].
Since we undertook a systematic review and meta-analysis of risk stratification systems in 2008 [3], further studies have been published which address this issue [7]. Accordingly we have updated our review to summarise the most recent advances in our knowledge of evidence on the discriminatory ability and predictive accuracy of such risk stratification clinical decision rules (CDR) for children and young people with cancer.
Methods
This update review was conducted in accordance with “Systematic reviews: CRD's guidance for undertaking reviews in health care” [8] and registered on the PROSPERO Registry of systematic reviews: CRD42011001685. It sought studies which aimed to derive or validate a CDR in children or young people (aged 0–18 y) presenting with febrile neutropenia. Both prospective and retrospective cohorts were included, but those using a case-control (“two-gate”) approach were excluded as these have been previously shown to exaggerate diagnostic accuracy estimates [9].
Search strategy and selection criteria
The electronic search strategy [3] was reviewed and repeated on the following databases from February 2009 to September 2011:
MEDLINE
MEDLINE In-Process & Other Non-Indexed Citations
EMBASE
CINAHL
Literatura Latinoamericana y del Caribe en Ciencias de la Salud (LILACS)
Reference lists of relevant systematic reviews and included articles were reviewed for further relevant articles. Published and unpublished studies were sought and no language restrictions applied. Non-English language studies were translated. Two reviewers independently screened the title and abstract of studies for inclusion, and then the full text of retrieved articles. Disagreements were resolved by consensus.
Validity assessment and data extraction
The validity of each study was assessed as with our previous review using 11 of the 14 questions from the QUADAS assessment tool for diagnostic accuracy studies [10].
Data were extracted by one reviewer and checked by the other. The data extracted included age and sex distribution of the included participants, geographical location of the study, the participant inclusion/exclusion criteria, and the performance of the CDR as a 2*k table (where k refers to the number of strata described) or as sensitivity/specificity, as well as aspects of the methods used to derive the CDR (where applicable).
Methods of analysis/synthesis
Where possible, data from new publications were added to meta-analyses undertaken in the original review [3]. Quantitative synthesis was undertaken when more than 2 studies tested the same CDR, and where appropriate, was investigated for sources for heterogeneity. For this update review, only dichotomous test data were found. For CDR with 3 datasets, a univariate approach was used (pooling sensitivity and specificity separately) [11]. For those with 4 or more, a bivariate model was fitted using ‘metandi’ in STATA10 [12]. The protocol specified a random-effects meta-analysis was undertaken using WinBUGS 1.4.3 [13] for tests with 3 or more risk strata, but no data were found eligible for this analysis.
Heterogeneity between study results was explored through consideration of study populations, study design, CDR and outcomes chosen, although the small number of studies in each category limited this approach. Sensitivity analysis was undertaken by comparing results when the original (derivation) data set was included and excluded.
For those areas where a quantitative synthesis was not possible, a narrative approach was used.
Results
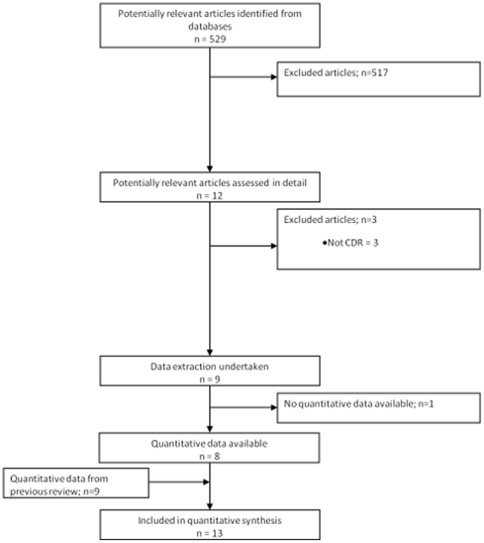
9 articles reporting on 8 studies were eligible for inclusion in the review (see Figure 1). The studies included patients from 2 month to 22 years old, with a wide range of malignancies, and a total of 2591 episodes of FNP describing four groups of outcomes: death, critical care requirement, serious medical complication, and bacteraemia. Six studies undertook prospective data collection, two retrospective. Details of the CDR included in this review are given in Table 1.
Figure 1. Flowchart of studies through the review.
Table 1. Clinical decision rules examined.
| Patient and disease related factors | Episode specific factors | Rule formulation | Outcome | Inclusion criteria | Exclusion criteria | |
| Klaassen [24] | None | Absolute monocyte count | Absolutely monocyte count >100/mm3 = low risk of bacteraemia | Significant bacterial infection | ANC <500cells/mm3 or ≤1000cells/mm3 and falling. Temperature >38.0°C ≥2 occasions in ≥12 h, or once >38.5°C, or localised infection | New malignant diagnosis; bone marrow or stem-cell transplantation in preceding 6 months. Another medical condition that independently required inpatient observation. Interstitial infiltrate or lobar consolidation on chest x-ray |
| Alexander [28] | AML, Burkitts lymphoma, induction ALL, progressive disease, relapsed with marrow involvement | Hypotension, tachypnea/hypoxia <94%, new CXR changes, altered mental status, severe mucositis, vomiting or abdominal pain, focal infection, other clinical reason for in-patient treatment | Absence of any risk factor = low risk of serious medical complication | Significant adverse outcome | ANC ≤500/mm3, temperature >38.5°C. Outpatient status. | Post stem cell transplant |
| Rondinelli [27] | 2 points for central venous catheter, 1 point for age >5 years | 4.5 points for clinical site of infection,2.5 points for no URTI, 1 point each for fever >38.5°C, hemoglobin ≤7 g/dL | Total score <6 = low risk of serious infectious complication | Serious infectious compllications | ANC <500cells/mm3 or ≤1000cells/mm3 and falling, temperature ≥37.8°C ≥3 occasions in ≥24 h, or once >38.0°C. First episode per patient (new or relapsed disease) | Second or subsequent episode. Episodes in progressive disease (<6 m from between completing therapy and relapse). History of BMT |
| PINDA [26] | Relapsed leukaemia, chemotherapy within 7 days of episode | CRP ≥90 mg/dL, hypotension, platelets ≤50 G/L | Zero risk factors or only low platelets or only <7 days from chemotherapy = low risk of invasive bacterial infection | Invasive bacterial infection | ANC ≤500cells/mm3, axillary temperature ≥38.0°C ≥2 occasions 1 h apart, or once ≥38.5°C | Not reported |
| Ammann [32] | Bone marrow involvement, central venous catheter, pre-B-cell leukemia | Absence of clinical signs of viral infection, CRP >50 mg/dL, white blood cell count <0.5 G/L, hemoglobin >10 g/dL | Three or fewer risk factors = low risk of significant infection | Significant infection | Age 1–18 y, episode following non-myleoablative chemotherapy, temperature >38.5°C or >38°C for >2 h, and ANC <500 cells/mm3 | High dose chemotherapy |
| SPOG (Adverse events rule) [7] | Applied after 24 hours: 4 points for chemotherapy more intensive than ALL maintenance | Applied after 24 hours: 5 points for hemoglobin >90 g/L, 3 points each for white blood cell count <0.3 G/L, platelet <50 G/L, any adverse event occurred | Total score <9 = low risk of adverse FN outcome | Significant adverse outcome | Age 1–18 y, episode following non-myleoablative chemotherapy, temperature >38.5°C or >38°C for >2 h, and ANC <500 cells/mm3 | High dose chemotherapy |
| SPOG (Bacteraemia rule) [18] | None | Applied after 24 hours: shaking chills ever observed, haemoglobin >90 g/L, platelet <50 G/L, any other need for IP treatment | No risk factors = low risk of bacteraemia presenting after 24 hours | Late bacteraemia (>24 h) | Age 1–18 y, episode following non-myleoablative chemotherapy, temperature >38.5°C or >38°C for >2 h, and ANC <500 cells/mm3 | High dose chemotherapy |
| Hakim [16] | Score from cancer diagnosis: AML = 20, ALL/lymphoma = 7, Solids = 0 | Clinical presentation of serious unwell or toxic = 14, fever at presentation: ≥39°C = 11, ANC <100/mm3 = 10 points | Total score <24 = low risk of serious infection or sepsis | Serious infection or sepsis | Outpatient, temperature >38.3°C or >38°C for >1 h, and ANC <500 cells/mm3 | Inpatients, stem cell transplant recipients |
| Hakim [16] | Score from cancer diagnosis: AML = 11, others = 0. Relapsed disease = 11. Non-white patient = 8 | Clinical presentation of serious unwell or toxic = 20 | Total score <20 = low risk of any medical complication | Any medical complication | Outpatient, temperature >38.3°C or >38°C for >1 h, and ANC <500 cells/mm3 | Inpatients, stem cell transplant recipients |
| Delebarre [14] (abstract only) | 1 point for hematological malignancy, chemotherapy at high-risk of prolonged neutropenia, | 1 point for clinical signs of local infection, fever >39°C, white cell count <500/mm3 or monocytes <100/mm3 and procalcitonin >0.3 ng/ml. TWO points for severe sepsis. | High risk >1 point. | Severe infection | Unclear | Unclear |
| Mian [15] (abstract only) | No clear rule – uses postive blood culture result and raised CRP | No clear rule | Intensive care admission | Unclear | Unclear | |
| Badiei [17] | Platelets <20 g/dL, temperature ≥39°C, ANC <100/mm3, mucositis, abnormal CXR on presentation | Risk of infection greater with more risk factors: no single threshold applied | Life threatening infection | Outpatient, temperature >38.5°C or >38°C for >1 h, and ANC <500 cells/mm3 | BMT, Fever with new diagnosis, inpatient status |
AML = acute myeloid leukaemia; ANC = Absolute neutrophil count; BMT = bone marrow transplant; CXR = chest radiograph; CRP = C-reactive protein; PINDA = Programa Infantil Nacional de Drogas Antineoplásticas; SPOG = Swiss Pediatric Oncology Group; URTI = upper respiratory tract infection.
Quality assessment
The studies varied in quality. Potential biases due to threats to independent outcome assessment were present in two studies [2], [14], verification bias in two [2], [7], and two were presented only as abstracts [14], [15]. Five definitions of febrile neutropenia were used, with five definitions of fever and two of neutropenia. However, all definitions were clinically similar, with variation was mainly in the duration of time for a lower temperature to be considered ‘prolonged’.
New CDR derivations
Five studies attempted to derive at least one CDR. Four studies examined rules to predict significant medical complications; a group of outcomes generally encompassing death, intensive care admission, significant bacterial or fungal infection, and need for organ support such as supplemental oxygen, inotropes or dialysis [7], [14], [16], [17]. Two examined rules to predict bacteraemia [16], [18], and one intensive care admission [15]. In one case a clear CDR could not be assessed [15]. The CDR used data from the initial/admission assessment, or from a later assessment after approximately 24 hours of observation. The new CDR generally had high sensitivity for the chosen outcome at the expense of poor specificity (see Table 2) and considered patient-disease, patient-episode and laboratory factors. Considerable imprecision in the estimates was seen, due mainly to the small numbers in individual studies (fewer than 350 patients).
Table 2. Diagnostic test accuracy of newly described CDR.
| Number of patients | Number of episodes | Age of patients | Outcome | Number with poor outcome | Proportion in low risk group | Sensitivity | Specificity | |
| Hakim [16] | 332 | 332 | Median 6 yrs, range 2.4 months to 21.6 years | Serious infection or sepsis | 47 | 69% | 74.5% (95% CI 60.5% to 84.7%) | 76.4% (95% CI 71.1% to 81.1%) |
| Medical complications | 40 | 63.7% | 77.5% (95% CI 62.5% to 87.7%) | 69.5% (95% CI 63.9% to 74.5%) | ||||
| Delebarre [14](abstract only) | 146 | 316 | Mean age 8 years, range 0.5 yrs to 17.5 yrs | 70 | 20.6% | 98.6% (95% CI 92.3% to 99.7%) | 26% (95% CI 20.9% to 31.8%) | |
| Badiei [17], Threshold value: 0 risk factors | 68 | 120 | Mean 5.9 years | Life threatening infection | 35 | 29.2% | 97.1% (95% CI 85.5% to 99.5%) | 40% (95% CI 30.2% to 50.6%) |
| 1 risk factor | 35 | 64.2% | 71.4% (95% CI 54.9% to 83.7%) | 78.8% (95% CI 69% to 86.2%) | ||||
| 2 risk factors | 35 | 75% | 62.9% (95% CI 46.3% to 76.8%) | 90.6% (95% CI 82.5% to 95.2%) | ||||
| 3 risk factors | 35 | 85% | 40% (95% CI 25.6% to 56.4%) | 95.3% (95% CI 88.5% to 98.2%) | ||||
| 4 risk factors | 35 | 98.3% | 5.7% (95% CI 1.6% to 18.6%) | 100% (95% CI 95.7% to 100%) | ||||
| SPOG [7] (Adverse events rule) | 206 | 423 | 6.9 years (IQR 3.8 years to 11.6 years) | Serious adverse medical outcome | 122 | 35% | 92% (IQR 91% to 93%, range 90% to 98%) | 45% (IQR, 38% to 49%, range 12% to 57%) |
| SPOG [18] (Bacteraemia rule) | Late bacteraemia | 67 | 36% | 93% (IQR 91% to 97%) | 41% (IQR 21% to 45%) |
CI = confidence intervals; IQR = interquartile range.
The newly derived CDR were subject to validation by internal statistical means (cross-validation) or in one alternative data set (see Table 3). In all except one case [15], multivariable regression analysis was used to build the model. One rule was built with a classification and regression tree (CART) approach [15].
Table 3. Validations of rules.
| Rule | Number of patients | Number of episodes | Age of patients | Outcome | Proportion in low risk group | Sensitivity | Specificity |
| Klaassen rule | |||||||
| Klaassen [24] (derivation) | 140 | 227 | Median 6.8 y (range 6 m to 17 y: derivation set) | Significant bacterial infection | 37% | 87.5% (95% CI 76.4% to 93.8%) | 41.2% (95% CI 35.9% to 46.6%) |
| Amman [7] | 206 | 423 | 6.9 years (IQR 3.8 years to 11.6 years) | Serious adverse medical outcome | 38% | 86.9% (95% CI 79.8% to 91.8%) | 48.5% (95% CI 42.9% to 54.1%) |
| Macher [20] | 167 | 377 | Median 6 y, range7 m to 19 y | Significant bacterial infection | 40% | 79.3% (95% CI 61.6% to 90.2%) | 45% (95% CI 35.9% to 54.3%) |
| Baorto [22] | 558 | 1171 | Mean 8.0 y (range 1 y to 23 y) | Bacteraemia | 21% | 88.9% (95% CI 83.6% to 92.6%) | 22.4% (95% CI 19.9% to 25.1%) |
| Madsen [23] | 76 | 157 | Mean 8 y (range 2 m to 18 y) | Microbiologically documented infection | 39% | 91.7% (95% CI 74.2% to 97.7%) | 41% (95% CI 35.5% to 46.8%) |
| Rackoff [21] | 72 | 172 | Range 9 m to 18 y: derivation set | Bacteraemia | 19% | 100% (95% CI 89.8% to 100%) | 23.2% (95% CI 16.9% to 30.9%) |
| Ammann 2003 rule | |||||||
| Ammann [32] (derivation) | 132 | 364 | Not reported | Severe bacterial infection | 14% | 100% (95% CI 95.9% to 100%) | 22.7% (95% CI 16.8% to 30%) |
| SPOG [7] | 206 | 423 | 6.9 years (IQR 3.8 years to 11.6 years) | Severe bacterial infection | 10% | 96.7% (95% CI 91.9% to 98.7%) | 12.3% (95% CI 9.1% to 16.5%) |
| Macher [20] | 167 | 377 | Median 6 y, range7 m to 19 y | Severe bacterial infection | 8% | 95.2% (95% CI 86.9% to 98.4%) | 9.1% (95% CI 6.4% to 12.8%) |
| Alexander rule | |||||||
| Alexander [28] (derivation) | 104 | 188 | Mean 8.9 y, SD 5.7 | Adverse medical complication | 58% | 84.6% (95% CI 57.8% to 95.7%) | 64.6% (95% CI 53.8% to 74.1%) |
| SPOG [7] | 206 | 423 | 6.9 years (IQR 3.8 years to 11.6 years) | Adverse medical complication | 8% | 94.3% (95% CI 88.6% to 97.2%) | 8.6% (95% CI 6% to 12.4%) |
| Dommett [2] | 368 | 762 | Median age 5 years 7 months (range 1 month to 17 years 6 months). | Adverse medical complication | 53% | 58.7% (95% CI 52.2% to 65%) | 57.9% (95% CI 53.7% to 62%) |
| Programa Infantil Nacional de Drogas Antineoplásticas (PINDA) Rule | |||||||
| Santolaya (derivation) [26] | 257 | 447 | Mean 7 y (range 6 m to 18 y) | Invasive bacterial infection | 43% | 85.4% (95% CI 79.5% to 89.8%) | 64.6% (95% CI 58.2% to 70.5%) |
| Santolaya (validation) [25] | 170 | 263 | Mean 7 y (range 7 m to 17 y) | Invasive bacterial infection | 40% | 92.1% (95% CI 86.5% to 95.6%) | 76.4% (95% CI 68.2% to 83.1%) |
| SPOG [7] | 206 | 423 | 6.9 years (IQR 3.8 years to 11.6 years) | Invasive bacterial infection | 15% | 93.4% (95% CI 87.6% to 96.6%) | 18.9% (95% CI 14.9% to 23.7%) |
| Macher [20] | 167 | 377 | Median 6 y, range7 m to 19 y | Invasive bacterial infection | 46% | 66.7% (95% CI 51% to 79.4%) | 48.1% (95% CI 41.4% to 54.8%) |
| Swiss Pediatric Oncology Group (SPOG) 2010 Rule | |||||||
| SPOG [7] (Adverse events rule derivation) | 206 | 423 | 6.9 years (IQR 3.8 years to 11.6 years) | Serious adverse medical outcome | 35% | 92% (IQR 91% to 93%, range 90% to 98%) | 45% (IQR, 38% to 49%, range 12% to 57%) |
| Miedeima [19] | 110 | 210 | Median 6.6 years (IQR 4.3 years to 10.8 years) | Serious adverse medical outcome | 50% | 82% (95% CI, 77% to 87% | 57% (95% CI, 50% to 64%) |
| Rondinelli Rule [27] (no derivation data) | |||||||
| Macher [20] | 167 | 377 | Median 6 y, range7 m to 19 y | Severe bacterial infection | N/A | 62% (36–82) | 43% (34–52) |
| SPOG [7] | 206 | 423 | 6.9 years (IQR 3.8 years to 11.6 years) | Severe bacterial infection | 35% | 84.4% (95% CI 77% to 89.8%) | 43.2% (95% CI 37.7% to 48.8%) |
Validation of CDR
Four studies [2], [7], [19], [20] were explicit in undertaking validations of 9 previously described CDR. These universally demonstrated poorer discriminatory ability when tested in alternative data sets (see Table 3). The geographical settings for validations of the rules varied from those where the rule had been derived.
Synthesis of CDR accuracy
Meta-analysis was undertaken for two CDR; the “Klaassen” rule and the “Ammann” rule. Two further CDR, the PINDA rule and the “Alexander” rule, have not been subject to meta-analysis as the results are too heterogeneous, these results are presented graphically. Two further CDR, the Rondellini rule and the SPOG2003 rule, have been assessed in two datasets, too few to perform meaningful meta-analysis. No data were available to update the three-stratum “Rackoff” rule meta-analysis of the previous study [3].
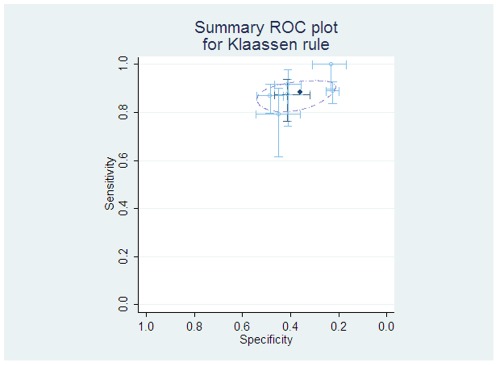
The “Klaassen” rule is based on a single feature: an absolute monocyte count of greater than 100/mm3 to predict patients less likely to have significant infection. Data were pooled from 4 studies from the previous review [21], [22], [23], [24] and two new sources [7], [20]. The results of this analysis give a pooled average sensitivity of 88% (95% CI 84 to 91%) and specificity of 36% (95% CI 27 to 45%), see Figure 2.
Figure 2. Individual and pooled diagnostic test accuracy of ‘Klaassen’ rule.
The ROC space plots show each study estimates of sensitivity and specificity as a marker at the point estimate, with 95% confidence intervals demonstrated by lines. In reading such graphs, tests with a better discriminatory ability fall in the top left corner of the plot, and non-discriminatory tests fall on a 45° line between the bottom left and top right. The light lines and circles represent individual studies, with the darker dashed lines showing the study from which the rule was derived. The dark circle is the pooled estimate of sensitivity and specificity, and the dashed ellipse represents the bivariate 95% confidence intervals of this result.
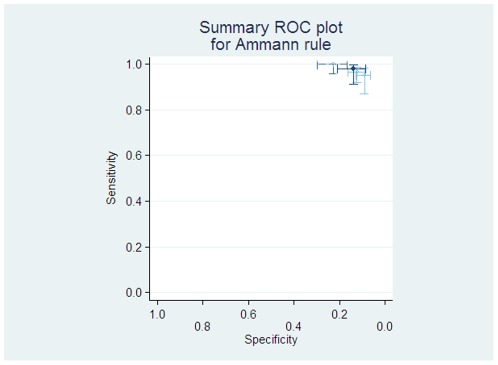
The “Ammann” rule describes patients at low risk of significant bacterial infection as from weighted factors including: bone marrow involvement, clinical signs of viral infection, serum C-reactive protein (CRP) level, leukocyte count, presence of a central venous catheter, high haemoglobin level, and diagnosis of pre-B-cell leukaemia (see Table 1 for details). Three studies provide data to test this rule [7], [18], [20]. The pooled average sensitivity was 98% (95%CI 91 to 99%) but pooled average specificity only 13% (95% CI 8% to 21%), see Figure 3.
Figure 3. Individual and pooled diagnostic test accuracy of ‘Ammann’ rule.
The ROC space plots here has the light lines and circles represent individual studies, with the darker dashed lines showing the study from which the rule was derived, and the heavy dark lines the pooled estimate of sensitivity and specificity, with the univariate 95% confidence intervals.
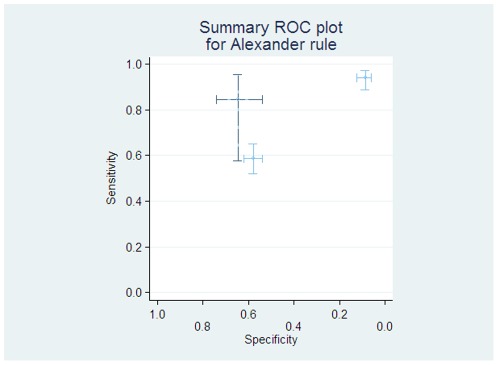
The “Alexander” rule examined adverse clinical consequences, using a combination of clinical features which predict prolonged neutropenia, and significant co-morbidities at presentation. This rule was assessed by two further studies [2], [18]. There was marked heterogeneity in the results of these three studies (see Figure 4). When used at reassessment after 48 hrs of hospitalisation, there was marked improvement in the discriminatory ability of the rule [2] (sensitivity = 100%, specificity = 39%).
Figure 4. Individual diagnostic test accuracy of ‘Alexander’ rule.
The light lines and circles represent individual studies, with the darker dashed lines showing the study from which the rule was derived.
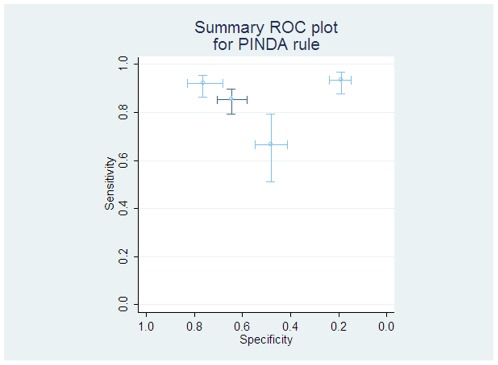
The PINDA rule again describes patients at low risk of significant bacterial infection as from weighted factors including laboratory and chemotherapy related parameters. This has been examined in two studies from the Santolya group [25], [26] and by two validations from European centres [7], [20]. There was marked heterogeneity (see Figure 5), potentially explained through geographical variation: the rule worked well applied in the population in Chile, but failed to differentiate patients in French and Swiss/German studies.
Figure 5. Individual and pooled diagnostic test accuracy of ‘PINDA’ rule.
The light lines and circles represent individual studies, with the darker dashed lines showing the study from which the rule was derived.
The rule of Rondellini [27] is a weighted score of clinical and haematological parameters (see Table 1 for details) and was assessed in two validation datasets. These demonstrated a sensitivity of 84% [7] and 62% [20] and both estimated specificity at 43%.
The SPOG2003 is a weighted score of haematological parameters with intensity of chemotherapy. It is applied after 8–24 hours of hospitalisation. This model was shown to have a sensitivity 92% and specificity of 45% [7]. A validation of this model demonstrated poorer sensitivity (82%) and slightly better specificity (57%) [19].
Discussion
This update systematic review builds on previous work to bring our knowledge of currently developed clinical decision rules for risk stratification in paediatric febrile neutropenia up to date. Now nine further models have been described, bringing the total to 25, and have included 10,000 episodes. It remains the case that no one rule is clearly better than any other, but we are now more clearly aware of the limitations of CDR which have not been subject to temporal and geographical validation.
The majority of CDR in this review focus upon defining a group at ‘low risk’ of complications. These rules once again have clinical and physiological similarities. The dominant themes are of a relationship between underlying diagnosis, chemotherapeutic regime, and clinical and laboratory parameters at the outset of the episode of fever. A further finding from this review is the demonstration that undertaking risk stratification at 24–48 hours after the onset of the episode leads to much greater discrimination, as many occult infections will have declared in this period.
Two rules have shown relative consistency of results. These are the simplest stratification of patients using the criteria of absolute monocyte count >100/mm3 to define a low risk group [24]. This has a pooled average sensitivity of 88% (95% CI 84 to 91%) and specificity of 36% (95% CI 27 to 45%), and if we assume serious infectious events occur in 30% of the group, the low-risk group has a 9% risk of serious infection, and accounts for approximately 29% of the total population. The high risk group has a 37% risk of infectious complications.
The Ammann 2003 rule [7] has much better sensitivity (estimated at 98%), leading to a risk of serious infectious complications in around 5% cases, but would only class 9% of patients as low risk, making it of little practical use.
Other further rules have shown marked heterogeneity: the Alexander rule [28] and the PINDA rule. The data support the use of the PINDA rule in Chile, where it has been successfully validated [25], but do not support its use in Europe. A similar situation exists with the Brazilian rule [27] which again was not successfully validated in European data sets. The Alexander rule did not successfully differentiate patients at admission in the UK and Europe, but its use at a 48 hour reassessment was associated with successful reductions in hospital stay. A further, newer, rule from the SPROG group requires more validation before a decision can be made on its usefulness.
These findings, that validation of CDR may be poor in comparison to derivation, and that geographical variation may mean CDR fail to work universally, have important clinical implications. There is a wealth of examples in the statistical and methodological literature regarding the over-optimism of newly derived CDR [29], [30]. The core concept is that rules derived from one dataset fit the idiosyncrasies and anomalies of the data collected, rather than reflecting the predictive power in the whole population of children experiencing FNP. However, these frequently equation-laden papers are uncommonly read by clinicians, and the complex approaches suggested to ‘shrinking’ the CDR values to increase their reproducibility are tricky to understand and to implement. The finding of geographical variation is potentially through different interpretations of similar findings; for example, how “unwell” should a child appear before they fall into this diagnostic category? There may also be subtle differences in the regimes used, as an example the use of steroid pulses in maintenance treatment for acute lymphoblastic leukaemia varies across Europe, and this may affect the CDR discriminatory ability.
This review has demonstrated there is an increasingly wide range of rules mainly for the prediction of an absence of adverse outcomes during episodes of febrile neutropenia in children, despite the existence of at least sixteen other applicable CDR [3]. Six rules have been subject to further verification, each demonstrating a variable degree of over-optimism in the original reports when the CDR is applied in different settings. The small size of these reports, with low ratios of events per variable examined may explain some of the variability in factors selected and poor reproducibility, as may undefined aspects of geographical differences between populations.
The practical application of these CDR requires it to be appropriate to the healthcare setting, and validated in the setting in which it is to be used. There remains a need for further research to reduce uncertainty around the efficiency of CDR, and potentially generate a very robust model on the basis of a much larger dataset, with well over 20 events per variable under examination. Importantly, rules should also identify a group at the highest risks of complications, to concentrate hopefully lifesaving early sepsis interventions in this group [31]. This project is already underway, with the PICNICC collaboration having collected data on around 5000 episodes of febrile neutropenia from 18 collaborating groups across North & South America, Europe and Asia.
Footnotes
Competing Interests: Sarah Alexander is the author of a paper assessed in this study, but was not involved with selection, extraction or analysis of the contents, and co-author Robert S. Phillips is a PLoS ONE Editorial Board member. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials. The other authors have declared that no competing interests exist.
Funding: RSP is supported by a Medical Research Council (United Kingdom) Research Training Fellowship G0800472. LS is supported by a New Investigator Award from the Canadian Institutes of Health Research. No other author has received specific funding for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Phillips B, Selwood K, Lane SM, Skinner R, Gibson F, et al. Variation in policies for the management of febrile neutropenia in United Kingdom Children's Cancer Study Group centres. Arch Dis Child. 2007;92:495–498. doi: 10.1136/adc.2006.102699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dommett R, Geary J, Freeman S, Hartley J, Sharland M, et al. Successful introduction and audit of a step-down oral antibiotic strategy for low risk paediatric febrile neutropaenia in a UK, multicentre, shared care setting. EJC. 2009;45:2843–2849. doi: 10.1016/j.ejca.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Phillips B, Wade R, Stewart LA, Sutton AJ. Systematic review and meta-analysis of the discriminatory performance of risk prediction rules in febrile neutropaenic episodes in children and young people. EJC. 2010;46:2950–2964. doi: 10.1016/j.ejca.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teuffel O, Ethier MC, Alibhai SM, Beyene J, Sung L. Outpatient management of cancer patients with febrile neutropenia: a systematic review and meta-analysis. Ann Oncol. 2011;22:2358–2365. doi: 10.1093/annonc/mdq745. [DOI] [PubMed] [Google Scholar]
- 5.Freifeld AG, Sepkowitz KA. No place like home? Outpatient management of patients with febrile neutropenia and low risk. J Clin Oncol. 2011;29:3952–3954. doi: 10.1200/JCO.2011.37.5758. [DOI] [PubMed] [Google Scholar]
- 6.Sung L, Phillips R, Lehrnbecher T. Time for paediatric febrile neutropenia guidelines – Children are not little adults. EJC. 2011;47:811–813. doi: 10.1016/j.ejca.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Ammann RA, Bodmer N, Hirt A, Niggli FK, Nadal D, et al. Predicting adverse events in children with fever and chemotherapy-induced neutropenia: the prospective multicenter SPOG 2003 FN study. J Clin Oncol. 2010;28:2008–2014. doi: 10.1200/JCO.2009.25.8988. [DOI] [PubMed] [Google Scholar]
- 8.Centre for Reviews and Dissemination. York: University of York; 2009. Systematic review: CRD's guidance for undertaking reviews in health care. [Google Scholar]
- 9.Lijmer JG, Mol BW, Heisterkamp S, Bonsel GJ, Prins MH, et al. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA. 1999;282:1061–1066. doi: 10.1001/jama.282.11.1061. [DOI] [PubMed] [Google Scholar]
- 10.Whiting P, Rutjes A, Reitsma J, Bossuyt P, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Medical Research Methodology. 2003;3:25–25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simel DL, Bossuyt PM. Differences between univariate and bivariate models for summarizing diagnostic accuracy may not be large. J Clin Epidemiol. 2009;62:1292–1300. doi: 10.1016/j.jclinepi.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Harbord R. S456932 ed: Department of Social Medicine, University of Bristol; 2008. METANDI: Stata module to perform meta-analysis of diagnostic accuracy. [Google Scholar]
- 13.Lunn D, Thomas A, Best N, Spiegelhalter D. WinBUGS – A Bayesian modelling framework: Concepts, structure, and extensibility. Statistics and Computing 10: 337, 325–337, 2000;325 [Google Scholar]
- 14.Delebarre M, Dubos F, Macher E, Garnier N, Mazingue F, et al. Identifying high-risk patients for severe infection in children with chemotherapy-induced febrile neutropenia: A new decision rule. Pediatr Blood Cancer. 2010;55:823. doi: 10.1002/pbc.22586. [DOI] [PubMed] [Google Scholar]
- 15.Mian A, Prodhan P, Bhutta A, Watkins B. Clinical variables and acute phase reactants at initial hospitalization as predictors of admission to intensive care unit (ICU) among febrile neutropenic children. Crit Care Med. 2009;37:A344. [Google Scholar]
- 16.Hakim H, Flynn PM, Srivastava DK, Knapp KM, Li C, et al. Risk prediction in pediatric cancer patients with fever and neutropenia. Pediatr Infect Dis J. 2010;29:53–59. doi: 10.1097/INF.0b013e3181c3f6f0. [DOI] [PubMed] [Google Scholar]
- 17.Badiei Z, Khalesi M, Alami MH, Kianifar HR, Banihashem A, et al. Risk factors associated with life-threatening infections in children with febrile neutropenia: a data mining approach. J Pediatr Hematol Oncol. 2011;33:e9–e12. doi: 10.1097/MPH.0b013e3181f6921a. [DOI] [PubMed] [Google Scholar]
- 18.Agyeman P, Aebi C, Hirt A, Niggli FK, Nadal D, et al. Predicting bacteremia in children with cancer and fever in chemotherapy-induced neutropenia: results of the prospective multicenter SPOG 2003 FN study. Pediatr Infect Dis J. 2011;30:e114–119. doi: 10.1097/INF.0b013e318215a290. [DOI] [PubMed] [Google Scholar]
- 19.Miedema KG, de Bont ES, Oude Nijhuis CS, van Vliet D, Kamps WA, et al. Validation of a new risk assessment model for predicting adverse events in children with fever and chemotherapy-induced neutropenia. J Clin Oncol 29: e182–184; author reply. 2011;e185 doi: 10.1200/JCO.2010.32.7767. [DOI] [PubMed] [Google Scholar]
- 20.Macher E, Dubos F, Garnier N, Delebarre M, De Berranger E, et al. Predicting the risk of severe bacterial infection in children with chemotherapy-induced febrile neutropenia. Pediatr Blood Cancer. 2010;55:662–667. doi: 10.1002/pbc.22586. [DOI] [PubMed] [Google Scholar]
- 21.Rackoff WR, Gonin R, Robinson C, Kreissman SG, Breitfeld PB. Predicting the risk of bacteremia in childen with fever and neutropenia. J Clin Oncol. 1996;14:919–924. doi: 10.1200/JCO.1996.14.3.919. [DOI] [PubMed] [Google Scholar]
- 22.Baorto EP, Aquino VM, Mullen CA, Buchanan GR, DeBaun MR. Clinical parameters associated with low bacteremia risk in 1100 pediatric oncology patients with fever and neutropenia. Cancer. 2001;92:909–913. doi: 10.1002/1097-0142(20010815)92:4<909::aid-cncr1400>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 23.Madsen K, Rosenman M, Hui S, Breitfeld PP. Value of electronic data for model validation and refinement: bacteremia risk in children with fever and neutropenia. J Pediatr Hematol Oncol. 2002;24:256–262. doi: 10.1097/00043426-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Klaassen RJ, Goodman TR, Pham B, Doyle JJ. “Low-risk” prediction rule for pediatric oncology patients presenting with fever and neutropenia. J Clin Oncol. 2000;18:1012–1019. doi: 10.1200/JCO.2000.18.5.1012. [DOI] [PubMed] [Google Scholar]
- 25.Santolaya ME, Alvarez AM, Avil's CL, Becker A, Cofr' J, et al. Prospective evaluation of a model of prediction of invasive bacterial infection risk among children with cancer, fever, and neutropenia. Clinical Infectious Diseases. 2002;35:678–683. doi: 10.1086/342064. [DOI] [PubMed] [Google Scholar]
- 26.Santolaya ME, Alvarez AM, Becker A, Cofre J, Enriquez N, et al. Prospective, multicenter evaluation of risk factors associated with invasive bacterial infection in children with cancer, neutropenia, and fever. J Clin Oncol. 2001;19:3415–3421. doi: 10.1200/JCO.2001.19.14.3415. [DOI] [PubMed] [Google Scholar]
- 27.Rondinelli PIP, Ribeiro KdCB, de Camargo B. A proposed score for predicting severe infection complications in children with chemotherapy-induced febrile neutropenia. J Pediatr Hematol Oncol. 2006;28:665–670. doi: 10.1097/01.mph.0000212996.94929.0b. [DOI] [PubMed] [Google Scholar]
- 28.Alexander SW, Wade KC, Hibberd PL, Parsons SK. Evaluation of risk prediction criteria for episodes of febrile neutropenia in children with cancer. J Pediatr Hematol Oncol. 2002;24:38–42. doi: 10.1097/00043426-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Janssen KJ, Moons KG, Kalkman CJ, Grobbee DE, Vergouwe Y. Updating methods improved the performance of a clinical prediction model in new patients. J Clin Epidemiol. 2008;61:76–86. doi: 10.1016/j.jclinepi.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Toll DB, Janssen KJM, Vergouwe Y, Moons KGM. Validation, updating and impact of clinical prediction rules: A review. J Clin Epidemiol. 2008;61:1085–1094. doi: 10.1016/j.jclinepi.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 32.Ammann RA, Hirt A, Luthy AR, Aebi C. Identification of children presenting with fever in chemotherapy-induced neutropenia at low risk for severe bacterial infection. Medical & Pediatric Oncology. 2003;41:436–443. doi: 10.1002/mpo.10320. [DOI] [PubMed] [Google Scholar]