Abstract
Early life adversity affects hypothalamus-pituitary-adrenal axis activity, alters cognitive functioning and in humans is thought to increase the vulnerability to psychopathology–e.g. depression, anxiety and schizophrenia- later in life. Here we investigated whether subtle natural variations among individual rat pups in the amount of maternal care received, i.e. differences in the amount of licking and grooming (LG), correlate with anxiety and prefrontal cortex-dependent behavior in young adulthood. Therefore, we examined the correlation between LG received during the first postnatal week and later behavior in the elevated plus maze and in decision-making processes using a rodent version of the Iowa Gambling Task (rIGT). In our cohort of male and female animals a high degree of LG correlated with less anxiety in the elevated plus maze and more advantageous choices during the last 10 trials of the rIGT. In tissue collected 2 hrs after completion of the task, the correlation between LG and c-fos expression (a marker of neuronal activity) was established in structures important for IGT performance. Negative correlations existed between rIGT performance and c-fos expression in the lateral orbitofrontal cortex, prelimbic cortex, infralimbic cortex and insular cortex. The insular cortex correlations between c-fos expression and decision-making performance depended on LG background; this was also true for the lateral orbitofrontal cortex in female rats. Dendritic complexity of insular or infralimbic pyramidal neurons did not or weakly correlate with LG background. We conclude that natural variations in maternal care received by pups may significantly contribute to later-life decision-making and activity of underlying brain structures.
Introduction
Psychiatric diseases such as schizophrenia, anxiety disorders and depression involve disruptions of higher executive processes, which are under control of the prefrontal cortex (PFC) [1], [2]. Exposure to stressful events is -in vulnerable individuals- an important risk factor for the development of these diseases and thought to affect the function and dendritic structure of the prefrontal cortex [3], [4], [5], [6], [7], [8]. Interestingly, regulation of the hypothalamo-pituitary-adrenal (HPA) axis is partly controlled by the PFC and disrupted in many psychiatric disorders [9], [10], [11] which suggests an iterative loop in which hormones secreted after HPA activation may further exacerbate dysfunction of the PFC.
The risk of developing psychopathology is particularly increased after early life adversity [12], [13], [14], [15]. The presumed influence of early life adversity on brain structure and neurotransmission can be addressed in detail in appropriate animal models. Indeed, some studies show that interfering with mother-pup interactions affects the development and function of the PFC. For instance, repeated neonatal maternal separation (MS) in rodents, a frequently used model for adverse early life experience, was reported to alter dendritic morphology and spine density in PFC pyramidal neurons [16], [17], [18].
Studies examining natural variations in maternal care during the early postnatal period reported that maternal licking and grooming (LG) of the pups affects neuroendocrine responses, cognitive performance and neuronal development. Pups from mothers providing very low amounts of LG (Low-LG) to their entire litter showed -in adulthood- enhanced HPA axis responses [19], increased anxiety [20] and emotional memories [21], impaired spatial memory performance [22] and reduced hippocampal dendritic complexity [21], [23], when compared to pups from mothers that spent a very high amount of time licking and grooming (High-LG) their litter.
Since early life experiences and alterations in prefrontal cortex function are relevant for psychopathology, we here examined whether variations in the amount of maternal care also correlate with decision making processes – which critically depend on an intact PFC [24] – as well as the associated neuronal activation and dendritic complexity in the PFC. Earlier studies on the impact of maternal care for behavior and neuronal development focused on entire litters from High-LG and Low-LG mothers [19], [20], [21], [22], [23]. However, there is considerable within-litter variation [25], [26], [27], [28]. In the current study we therefore investigated whether the amount of care that individual pups within a litter receive from their mother correlates with PFC behavior and function. This approach enables to directly study the relationship between maternal care and PFC-dependent behavioral processes, activation and PFC structure, with a minimal role of confounding genetic factors.
We first tested all animals for their behavior in an elevated plus maze, since this parameter was earlier found to predict later performance in a decision-making task. Next, the relationship between LG received by individual pups early in life and PFC-dependent decision-making performance in adulthood was examined in a rodent version of the Iowa Gambling Task (rIGT, [29]). We also assessed the underlying neural circuitry using the immediate early gene c-fos as a marker for neural activity in structures associated with IGT performance, i.e. prefrontal, striatal and limbic areas (see [30]). Finally, we tested putative LG-related differences in dendritic morphology of two prefrontal cortex subregions of interest, i.e. the infralimbic and insular cortex.
Materials and Methods
Maternal care
Male and female outbred Long Evans rats were purchased from Harlan (Indianapolis, US) at approximately 2.5 months of age and allowed to habituate to the animal facility in Amsterdam. Then, two females were housed with one male for one week to allow mating, and after another week of paired-housing, the females were placed separately in large observation cages (30 cm×55 cm×45 cm). Maternal care observations commenced on postnatal day 1 (PND1; with PND0 being the day of birth), after culling the litters by randomly selecting eight healthy-looking pups (preferably four males and four females). The observation procedure has been extensively described by Champagne et al. (2003) [31] and was comparable to what we used in our earlier studies [26], [27]. Briefly, maternal behavior was scored every three minutes during five one-hour observation sessions daily (7:00, 10:00, 13:00, 17:00 and 20:00 hrs) for seven days, resulting in a total of 700 observations for each litter. Maternal care observations are generally done during the light period because dams show less maternal care during the dark period; the amount of maternal care is evenly distributed over the light period [31]. Several specific maternal behaviors were scored, including licking and grooming (LG), particularly towards individual pups within each litter. In order to be able to identify the pup that underwent licking and grooming, all pups were uniquely marked every morning until weaning with a non-scenting, non-toxic surgical marker (Codman, Johnson & Johnson, Brunswick, NY). We were able to distinguish which pup was being licked and groomed in about 60% of the cases, and since this percentage varied slightly between litters, we corrected for this with the following equation: (% individual LG observed)/(% total LG identified) * 100%. The handling of the pups which is inevitably associated with marking their fur might elicit an increase in overall licking and grooming [19], [32]. However, both a pilot study carried out previously in our lab (C.A. Oomen, M. Joëls, unpublished data) and earlier observations by Champagne et al. (2003) did not show any differences in maternal licking and grooming behavior in response to pup marking. In addition, one could argue that even if the amount of maternal care towards the whole litter was somewhat shifted due to the experimental procedures, within-litter differences in pup-preference should not be affected.
The animals were kept on a 12 h light/dark schedule (lights on at 8:00 hrs) until PND24, when they were weaned and ear-punched. Then they were moved to a different room where the light cycle was reversed (lights on at 21:00 hrs), so that eventually they could be tested in the dark phase, when they are active. During the entire experiment, temperature and humidity were maintained at 20–22°C and 40–60% respectively, and food and water were available ad libitum, unless otherwise specified. Post-weaning, the rats were group-housed with same-sex non-littermates.
All rats were first exposed on PND35 to a protocol which determined the effect of differences in %LG received on play behavior, of which the results have been published elsewhere [28]. The latter manuscript also contains data on the within-litter variation of this cohort of rats (six litters, PND1–PND7: Figure 1 in [28]). More specifically, %LG received varied considerably between pups within litters (overall range 0.00% to 2.30% LG, n = 48). In general, males received significantly more LG than females [28], which has been described before and has been explained by the fact that male pups require more LG to be able to urinate and defecate than female pups (i.e. [33]). We cannot exclude that pups that are larger and stronger at birth can maneuver themselves easier and closer to their mother and thus receive more LG. Although we have no information on birth weights of our pups, we did determine weaning weights and did not find a correlation with %LG in this cohort of rats (data not shown).
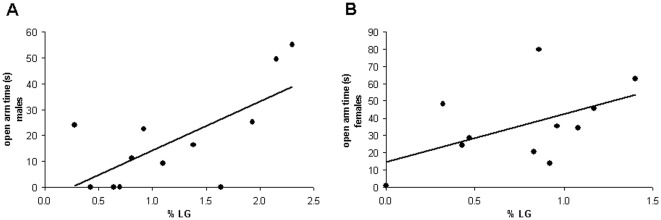
Figure 1. Elevated Plus Maze.
(A) In ten-week-old male offspring a positive correlation emerged between %LG and open arm time on the Elevated Plus Maze (n = 12, r = 0.690, p = 0.013), indicating reduced anxiety in animals with higher LG scores. (B) Similarly, in 8-week-old females, there was a positive trend between these parameters (n = 11, r = 0.511, p = 0.108).
A subset of rats (n = 24, 12 males/12 females) entered the decision-making experiment at around eight (females; PND56) to ten (males; PND70) weeks of age. Because the animals used were from the same breeding cohort, they were all born around the same time. Yet, only 12 animals can be tested in the rIGT concurrently. Instead of using a wide age-range for both sexes and also because it is known that both male and female behavior is affected by the presence and smell of the opposite sex, we decided to test males and females separately. Females were tested at a slightly younger age because they mature faster and thus can be considered to be at a comparable level of maturity at PND56 as males of a slightly later age.
Another group of 19 animals (10 males/9 females) were used to assess prefrontal dendritic morphology (ten weeks of age; PND70). All experimental procedures used in this study were approved by the animal ethical and welfare committee of the University of Amsterdam.
Elevated Plus Maze (EPM)
All 24 animals that were designated to participate in the decision-making experiment (see below) were first tested on the EPM. The maze was made of black PVC, consisted of four arms (50×10 cm) – two open and two enclosed by 30 cm high walls, forming a cross with the center area – and was elevated 60 cm above the ground. Animals were tested during the dark phase of their circadian light cycle, though under bright light conditions, to amplify putative maternal care related differences in anxiety level. Each rat was put on the center platform facing one of the open arms and allowed to freely explore the maze for 5 minutes. Between trials the maze was cleaned with ethanol and water, and dried thoroughly with clean paper towels. All test sessions were recorded for later analysis of spatiotemporal measures (i.e. the time spent in the open and closed arms and the number of entries into each arm) using the Observer 5.0 software (Noldus Information Technology B.V., Wageningen, The Netherlands).
Rodent Iowa Gambling Task (rIGT)
24 animals (12M/12F) along the entire range of LG scores (between 0.32% and 2.30% LG for males; between 0.00% and 1.40% LG for females) were randomly selected for testing in the rIGT. The same apparatus and procedure as previously described was used [29], [30], [34], [35], [36]. Before the start of the experiment, rats were habituated to the test apparatus in a 10 min free exploration trial. Two days later, they were mildly food restricted (approximately 95% of free feeding body weight) and tested during two 5-day periods. On weekend days food was available ad libitum. All testing occurred in red-light conditions during the dark phase of the day-night cycle, between 11:00 and 16:00 hrs.
The test apparatus was made of grey PVC and consisted of a start box, choice area and four arms. A trial started by lifting the slide door of the start box, allowing the rat to freely enter the choice area of the apparatus and choose one of the four arms. The chosen arm was closed when the rat had entered that arm with its full body, including its tail. At the end of each arm, rats could either obtain sucrose pellets or quinine-treated sucrose pellets (baited arms; see below) or no pellets at all (empty arms). Each trial had a maximum duration of 6 min, the inter-trial interval was 30 s, and each animal received a total of 120 trials (10 trials during the first 6 days; 15 trials on the final 4 days). Sucrose pellets (45 mg; Bio-Serv, Frenchtown, NJ, USA) - to which rats were familiarized prior to the start of the experiment - were used as a reward while quinine-treated sucrose pellets, that were unpalatable but not uneatable, were used as punishment. There was no significant correlation between the amount of maternal care received early in life and the speed of sugar-pellet consumption during the habituation phase (n = 19, r = −0.39, p = 0.1), indicating no LG-related anhedonic predisposition. As mentioned before, two of the four arms in the maze were empty. These were included to measure non-reward related exploration [29], [30], [34], [35], [36]. The two baited arms consisted of a ‘bad’ arm and a ‘good’ arm. In the ‘bad’ arm, the rats received occasional big rewards (three sucrose pellets in 1 out of 10 trials) among frequent punishments (three quinine-treated sucrose pellets in 9 out of 10 trials). In the ‘good’ arm, the rats received frequent small rewards (one sucrose pellet in 8 out of 10 trials) and infrequent punishments (one quinine-treated sucrose pellet in 2 out of 10 trials). This provided the same principle as in the human IGT: an option with a chance of a big reward (3 sucrose pellets), but with little long-term success (3 sucrose pellets per 10 trials; cf. decks A and B; [37]) and an option with a chance of a small reward (1 sucrose pellet), but with bigger long-term success (8 sucrose pellets per 10 trials; cf. decks C and D). The location of the baited and empty arms, as well as ‘good’ and ‘bad’ arms was counterbalanced across subjects.
To determine the performance of the rats, the number of choices for the most advantageous option were calculated and expressed as a fraction of the total number of trials per block. Choices were calculated in blocks of 10 trials. Scores during the last block of 10 trials (trial 111–120) were taken as a measure of final rIGT performance. The number of sucrose pellets collected during the entire task (trial 1–120) was used as a measure of overall task performance to reflect the final “budget” (cf. monetary budget in the human IGT, [38]. In addition we determined the total number of visits to either of the empty arms and the number of switches between different arms to assess exploratory behavior [29], [30], [34], [35], [36]. Finally, we measured win-stay/lose-shift behavior after encounters with the sucrose reward (win) or the quinine punishment (lose) in the advantageous arm. Win-stay and lose-shift were corrected for the total number of encounters with sugar and quinine respectively [30].
C-fos immunohistochemistry
Two hours after the last trial in the rIGT, the animals were rapidly decapitated. Their brains were removed, immediately snap-frozen on dry-ice and stored at −80°C. Coronal sections (20 µm) were cut on a cryostat (Leica CM3050S), mounted on Starfrost adhesive slides (Knittel Glaser, Waldemar Knittel, Germany) and stored at −20°C. For the immuno-histochemical detection of c-fos (at protein level), rabbit anti-c-fos (Calbiochem, Darmstadt, Germany) was used. During the staining procedure the sections were rinsed several times after every step in 0.01 M phosphate-buffered saline (PBS; pH 7.4). First, the sections were dehydrated. Endogenous peroxidase was blocked by treatment with H2O2 (0.1%) for 30 min. Sections were pre-incubated with 5% normal donkey serum (NDS) and 1% bovine serum albumin (BSA) in PBS (PBS-BSA 1%+NDS 5%) for 30 min before the rabbit anti-c-fos incubation (1∶4000 in PBS-BSA 1%+NDS 5%, 4°C, 24 h). Negative controls were incubated with the PBS-BSA 1%+NDS 5% solution. Next, the sections were incubated with donkey–anti-rabbit IgG Biotin SP conjugate (1∶400 in PBS-BSA 1%+NDS 5%, Jackson ImmunoResearch Laboratories, Inc., PA, USA) for 45 min. Subsequently, the sections were incubated with avidin-horseradish peroxidase solution (1∶400 in PBS-BSA 1%+NDS 5% VECTASTAIN® ELITE ABC, Brunswich Chemie, Amsterdam, The Netherlands) for 60 min. Then, slices were pre-incubated with inactive diaminobenzidine tetrahydrochloride (DAB, Sigma-Aldrich, St. Louis, MO, USA) solution containing nickel sulphate. To activate DAB for visualization of bound peroxidase complexes, the substrate H2O2 (30%, 1∶2000) was added to the DAB solution and incubated for 5 min. Afterwards the sections were dehydrated in alcohol and coverslipped.
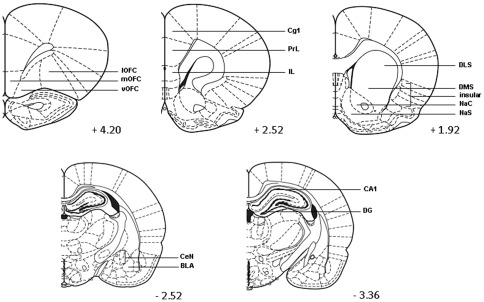
The images of brain sections were projected (10× magnification) and digitized using an Olympus BX 51 microscope (Olympus, Tokyo, Japan) with a high-resolution digital camera interfaced with a computer. The anatomical localization was aided by use of adjacent Nissl stained sections and illustrations in a stereotaxic atlas [39]. The following brain regions were investigated: orbitofrontal cortex (OFC; +4.20 from bregma), insular cortex (insular; +1.92 from bregma), medial prefrontal cortex, i.e. cingulate cortex (Cg1; +2.52 from bregma), prelimbic (PrL; +2.52 from bregma) and infralimbic cortex (IL; +2.52 from bregma), dorsolateral striatum (DLS; +1.92 from bregma), dorsomedial striatum (DMS; +1.92 from bregma), nucleus accumbens core (NaC; +1.92 from bregma), nucleus accumbens shell (NaS; 1.92 from bregma), basolateral amygdala (BLA; −2.52 from bregma), central nucleus of the amygdala (CeN; −2.52 from bregma), dentate gyrus (DG; −3.36 from bregma) and CA1 regio of the hippocampus (CA1; −3.36 from bregma). For each region at least two overt landmarks were used. For quantitative analysis of c-fos positive cells, the program Leica QWIN (image processing and analysis software, Cambridge, UK) was used. Only right hemispheres were analyzed, using two subsequent sections per animal. For all regions of interest, the number of positive cells was then averaged for each animal and expressed per mm2.
Morphology
For further morphological survey we selected two areas which exhibited clear c-fos immunoreactivity related to 1) rIGT performance or 2) LG background, i.e. the infralimbic cortex and the insular cortex respectively. To determine dendritic complexity of principal neurons in these areas, we used the Golgi-Cox method, as described previously [27], [40]. Ten males and 9 females were sacrificed at around 2.5 months of age, concurrent with the animals that were tested in the rIGT. We rapidly removed their brains and immediately put one of the hemispheres (right) in a vial containing Golgi-Cox solution (1% potassium dichromate, 1% mercuric chloride, 0.8% potassium chromate). The tissue remained immersed in this solution in the dark for 28 days, and was subsequently dehydrated and embedded in celloidine. The forebrain was cut in 200 µm thick slices using a vibratome, sections were stained as described by Boekhoorn et al. (2006) and mounted on glass slides.
For each animal, four cells that were randomly chosen from different slices were imaged and traced using ImagePro and NeuroDraw software. Only cells that i) were thoroughly filled, ii) were located in cortical layer 2/3, iii) had their soma in the middle plane of the slice, and iv) did not substantially interfere with neighboring cells or debris, were selected for analysis. Dendritic tracing was carried out by an experimenter blind to the background of the animals and several morphological parameters were analyzed, including total dendritic length, average branch length, number of branch points, and dendritic complexity index [DCI = (Σ branchtip orders+# of branch tips)/(# of primary dendrites) * (total dendritic length)].
Statistical analysis
Statistical analyses were conducted using SPSS 11.0 for Windows. All correlations were tested using linear regression with %LG as the independent (predictor) variable. Male and female data were only pooled for a certain correlation if i) the direction of that correlation was similar in both sexes and if ii) neither of the parameters in the correlation (e.g. %LG, IGT performance) differed significantly between sexes (analyzed by an independent Student's t-test). To determine the effect of maternal care on the relationship between rIGT performance and c-fos staining we used partial correlations, controlling for %LG. Furthermore, we examined whether differences existed between the learning curves of animals with different LG backgrounds using a split-half approach. Animals were divided into two equally sized groups: 12 animals with the lowest LG scores (5M/7F) and 12 animals with the highest LG scores (7M/5F). The number of advantageous choices was calculated in blocks of 10 trials and we used a repeated measures ANOVA to assess task progression in both groups (within-subjects factor: trial block; between-subjects factor: LG group).
Results
Elevated Plus Maze
All animals selected for the rIGT were first tested on the Elevated Plus Maze. The average time spent on the open arms of the maze differed between sexes (mean ± SEM: 17.71±5.42 sec in males versus 35.74±6.78 sec in females; t = 2.095, df = 21, p = 0.048) and therefore we analyzed them separately. In males, a significant positive correlation emerged between %LG and time spent on the open arms (n = 12, r = 0.690, p = 0.013; Figure 1A), whereas in females this was only a positive trend (n = 11, r = 0.511, p = 0.108; Figure 1B). Individual %LG scores did not correlate with the number of closed arm entries, neither in males (n = 12, r = 0.137, p = 0.672) nor in females (n = 12, r = −0.069, p = 0.830), suggesting that there was no difference in general activity between animals.
Rat Iowa Gambling Task (rIGT)
After testing animals in the Elevated Plus Maze they were studied at least 1 week later in the rIGT. Male and female rats did not differ in average rIGT performance (fraction of advantageous choices; mean ± SEM: 0.58±0.06, n = 12, for males and 0.58±0.07, n = 12 for females; t = 0.000, df = 22, p = 1.000). In addition, gonadal hormone status was previously shown not to affect decision-making in the IGT, neither in rats nor in humans [36], [41]. Therefore we pooled male and female data for further correlational analysis.
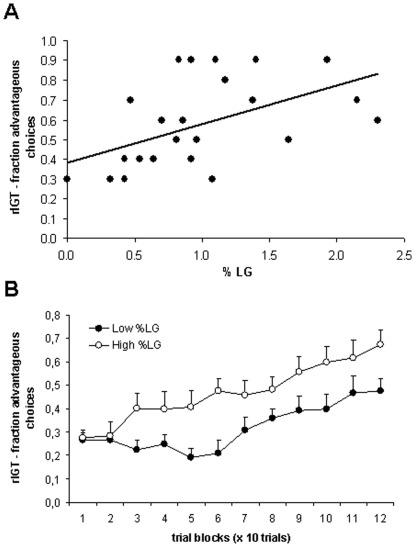
We observed a highly significant positive correlation between the %LG and the number of advantageous choices made in the rIGT during the last block of 10 trials (n = 24, r = 0.521, p = 0.009; Figure 2A). To examine task progression, a median split into ‘Low’ and ‘High’ LG animals was applied. This yielded a significant difference in improving performance across trial blocks between the two subgroups (repeated measures ANOVA, trial block, F(11, 242) = 14.544, p = 0.001; LG group * trial block interaction, F(11,242) = 1.807, p = 0.05; Figure 2B): rats that received relatively high maternal care showed a steady increase in the number of choices for the advantageous option from trials 11–20 onwards, while rats that received less maternal care tended to show a decrease in choosing for the advantageous option in the first trial blocks, and only showed a steady increase after trials 61–70. These differences in choice behavior between animals with high and low %LG were accompanied by a significant positive correlation between %LG and the total number of sugar pellets earned during the task (n = 24, r = 0.420, p = 0.02), indicating that rats that received more maternal care had a higher overall yield. A significant negative correlation existed between %LG and the number of visits to the empty arms during the task (n = 24, r = −0.421, p = 0.04), which points to increased non-reward related exploratory behavior in animals with lower LG scores. The total number of switches between arms did not correlate with %LG (n = 24, r = 0.017, p = 0.938). A positive trend was found for the relationship between %LG and win-stay behavior across all 120 trials (n = 24, r = 0.366, p = 0.08), while no significant effect or trend was observed for lose-shift behavior (n = 24, r = −0.132, p = 0.537). We did not observe a correlation between anxiety, as measured by open arm time on the Elevated Plus Maze, and rIGT performance measured in the last block of 10 trials (n = 21, r = 0.138, p = 0.551).
Figure 2. Performance on the rodent Iowa Gambling Task.
(A) In young-adult males and females (approx. 3 months old) there was a positive correlation between task performance on the rIGT, as indicated by the number of advantageous choices made during the last block of 10 trials, and %LG (n = 24, r = 0.521, p = 0.009). Thus, animals with higher LG scores perform better on a higher cognitive decision-making task. (B) When dividing the experimental group in ‘Low’ and ‘High’ LG animals by median split, a significant interaction effect between %LG and trial block emerged (10 trials per block; repeated measures ANOVA, main effect of group: F(1,22) = 6.485, p = 0.018; LG-group * trial block interaction: F(11,242) = 1.807, p = 0.05; trial block: F(11,242) = 14.544, p = 0.001). This suggests delayed learning in the ‘Low’ LG group, particularly in the first part of the task.
C-fos immunohistochemistry
Significant gender differences in levels of c-fos expression were found in the lateral orbitofrontal cortex (OFC; t = −2.444; df = 22; p = 0.027) but not in any other brain area. Therefore, we only refrained from pooling the male and female data for the lateral OFC.
First the correlation between rIGT performance and c-fos expression was established in a range of brain areas important for decision making (Table 1; Figure 3). The rIGT performance, expressed as the fraction of advantageous choices during the last block of 10 trials, showed a significant negative correlation with c-fos expression in the mPFC (prelimbic and infralimbic), the insular cortex and the lateral OFC (in females only; males: n = 12, r = −0.018, p = 0.952). There was a negative trend between rIGT performance and c-fos expression in both the cingulate cortex and nucleus accumbens shell. Next, correlations were calculated for %LG and c-fos expression after rIGT performance. Significant negative correlations were found between %LG received early in life and c-fos expression levels in the insular cortex and nucleus accumbens shell. There was a negative trend between %LG and c-fos expression in the lateral OFC (in females only; males: n = 12, r = −0.070, 0.828) and cingulate cortex, and a positive trend in the basolateral amygdala. Finally, partial correlations were calculated between rIGT and c-fos, controlling for %LG, to determine the effect of maternal care on the relationship between rIGT performance and c-fos staining. Table 2 shows that after controlling for %LG, the correlations between rIGT performance and c-fos staining in the cingulate cortex and medial PFC (both prelimbic and infralimbic cortex) became stronger, whereas correlations (significant or trend) between rIGT performance and c-fos expression in the lateral OFC, insular cortex and nucleus accumbens shell were now non-significant (p>0.1). This indicates that maternal care significantly contributes to the relationships between rIGT performance and c-fos labeling in the lateral OFC, insular cortex and nucleus accumbens shell.
Table 1. Correlations of c-fos expression with rIGT performance and individual %LG.
| Brain area | rIGT-advantageous choices | LG% | ||
| r = | p = | r = | p = | |
| Cortical | ||||
| Medial OFC | −.201 | .346 | −.299 | .156 |
| Ventral OFC | −.292 | .167 | −.330 | .115 |
| Lateral OFC (females only) | −.600 | .039* | −.554 | .062† |
| Cingulate (Cg1) | −.369 | .076† | −.360 | .084† |
| Prelimbic (PrL) | −.418 | .042* | −.344 | .100 |
| Infralimbic (IL) | −.460 | .024* | −.251 | .236 |
| Insular | −.456 | .025* | −.531 | .008* |
| Striatal | ||||
| Dorsolateral striatum (DLS) | −.222 | .308 | .136 | .536 |
| Dorsomedial striatum (DMS) | −.332 | .141 | −.273 | .214 |
| Nucleus accumbens core (NaC) | −.308 | .153 | −.269 | .214 |
| Nucleus accumbens shell (NaS) | −.373 | .080† | −.464 | .026* |
| Limbic | ||||
| Amygdala central nucleus (CeN) | .148 | .500 | .222 | .309 |
| Basolateral amygdala (BLA) | −.075 | .735 | .366 | .086† |
| Dentate gyrus (DG) | .151 | .493 | −.096 | .663 |
| CA1 region (CA1) | .015 | .944 | −.251 | .236 |
Correlations between c-fos expression and both rIGT performance and individual %LG (* p<0.05; † p<0.1). Male and female data were pooled (n = 24), except for the lateral OFC (n = 12).
Figure 3. Anatomical localization of brain regions used for analysis of c-fos expression.
Anterior-posterior coordinates relative to bregma are stated below each coronal section. Abbreviations: mOFC = medial orbital frontal cortex (OFC)), vOFC = ventral OFC, lOFC = lateral OFC, insular = insular cortex, Cg1 = cingulate cortex, PrL = prelimbic cortex, IL = infralimbic cortex, DLS = dorsolateral striatum, DMS = dorsomedial striatum, NaC = nucleus accumbens core, NaS = nucleus accumbens shell, BLA = basolateral amygdala, CeN = central nucleus of the amygdala, DG = dentate gyrus and CA1 = CA1 regio of the hippocampus. Based on Paxinos and Watson (2005).
Table 2. c-fos expression and rIGT performance when corrected for %LG.
| Brain area | rIGT-advantageous choices controlled for %LG | |
| r = | p = | |
| Cortical | ||
| Medial OFC | −.247 | .395 |
| Ventral OFC | −.419 | .135 |
| Lateral OFC (females only) | −.395 | .230 |
| Cingulate (Cg1) | −.552 | .041* |
| Prelimbic (PrL) | −.583 | .029* |
| Infralimbic (IL) | −.675 | .008* |
| Insular | −.470 | .090† |
| Striatal | ||
| Dorsolateral striatum (DLS) | −.229 | .430 |
| Dorsomedial striatum (DMS) | −.253 | .384 |
| Nucleus accumbens core (NaC) | −.430 | .124 |
| Nucleus accumbens shell (NaS) | −.262 | .366 |
| Limbic | ||
| Amygdala central nucleus (CeN) | .068 | .817 |
| Basolateral amygdala (BLA) | −.118 | .688 |
| Dentate gyrus (DG) | .280 | .332 |
| CA1 region (CA1) | .197 | .500 |
Partial correlations between c-fos expression and rIGT performance, corrected for the effect of LG (* p<0.05; † p<0.1). Male and female data were pooled (n = 24), except for the lateral OFC (n = 12).
Morphology
We next examined if individual differences in maternal care correlate with dendritic morphology in the forebrain. We selected one area which exhibited clear c-fos immunoreactivity related to rIGT performance (infralimbic cortex) and another area for which we observed a correlation with LG background (insular cortex; see typical example of stained neuron in Figure 4A).
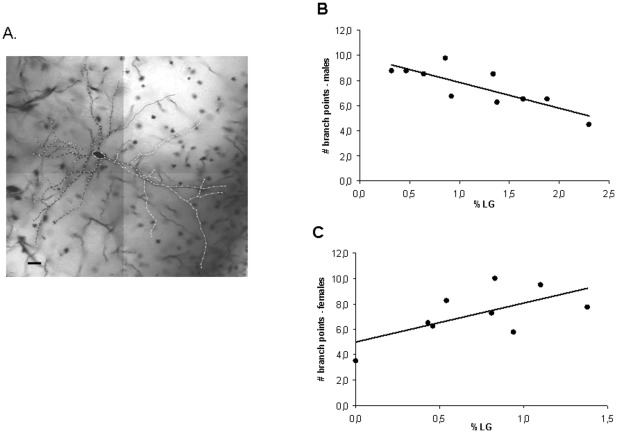
Figure 4. Dendritic morphology in 2.5 month old naïve animals.
(A) Typical example of a Golgi-stained pyramidal neuron in the insula and the corresponding dendritic reconstruction drawing. Calibration bar: 20 µm (B) In male infralimbic cortex, %LG correlated negatively and significantly with the number of branch-points in the basal dendrite (n = 10, r = −0.820, p = 0.004). (C) In females, this correlation was positive and just missed significance (n = 9, r = 0.635, p = 0.066).
Since the direction of the correlation coefficients generally differed between sexes we analyzed males and females separately. In the insular cortex no significant correlations appeared between %LG and pyramidal cell morphology, neither in males nor in females: both for the apical and the basal dendritic tree none of the parameters was affected by individual differences in maternal care (see Table 3). In the infralimbic part of the medial prefrontal cortex the effect of LG on dendritic morphology was different for males and females. In males, the number of branch points (n = 10, r = −0.820, p = 0.004; Figure 4B) in the basal tree showed a significant negative correlation with %LG. The total dendritic length (n = 10, r = −0.575, p = 0.08) and complexity of the basal dendritic tree as measured by the DCI (dendritic complexity index; n = 10, r = −0.557, p = 0.09) only showed a negative trend. No effects of maternal care on the apical dendritic tree were found. In general, these data suggest a more complex basal but not apical dendritic tree in male animals that received lower amounts of licking and grooming. In females, early life background correlated only marginally with morphological properties (Table 3): The correlation between maternal care and the number of branch points showed a positive trend (n = 9, r = 0.635, p = 0.066; Figure 4C), but all other correlations were not significant (see Table 3).
Table 3. Dendritic morphology in 2.5 month old naïve animals.
| Males | ||||
| Parameter | Infralimbic cortex | Insula | ||
| r = | p = | r = | p = | |
| Apical dendritic length | −.034 | .925 | −.052 | .887 |
| Apical # branch points | −.132 | .716 | −.058 | .874 |
| Apical DCI | −.088 | .808 | −.168 | .642 |
| Basal dendritic length | −.575 | .082† | .214 | .552 |
| Basal # branch points | −.820 | .004* | .032 | .929 |
| Basal DCI | −.557 | .094† | .091 | .803 |
Correlations between individual %LG and dendritic morphology in two forebrain areas, i.e. the infralimbic cortex and the insular cortex, in 2.5 months old naïve animals (* p<0.05; † p<0.1). Males (n = 10) and females (n = 9) were analyzed separately.
Discussion
In the present study, we investigated the effects of variations in %LG received by rats during the first postnatal week on decision-making and associated neuronal activity and structure in young adulthood.
Maternal care model
To address this question we first established the amount of care that individual pups within a litter receive from their mother (within-litter model) and used this as an index for correlations with behavior later in life. This approach differs from most studies (reviewed in [42]) which compare the entire offspring from dams that spend a relatively low amount of time LG (>1SD below the mean) with litters from dams that spend a relatively high amount of time LG (>1SD above the mean). The latter (between-litter) model focuses on the extremes of the normal distribution in maternal care, excluding approximately 70% of the litters, which receive moderate amounts of care. Moreover, by comparing entire litters one cannot exclude the contribution of genetic background unless this is ruled out by dedicated cross-fostering experiments. By examining animals over the entire range of LG and using the %LG that was received (regardless of the dam) as a predictor, the influence of these two potential drawbacks is minimized.
The %LG received early in life correlated negatively with the levels of anxiety as measured on the Elevated Plus Maze, which agrees with earlier findings using the more extreme between-litter maternal care model [43]. This effect appeared to be stronger for male than female rats, but it should be noted that in female rats the dwell-time in the open arm time on the Elevated Plus Maze may also reflect exploratory behavior [44]. The differences in EPM behavior between males and females should therefore be cautiously interpreted. Regardless, the findings support that, even within a litter, variations in maternal care can be reliably scored and correlated with anxiety related behavior for male and female offspring.
Behavioral performance in the rIGT
The correlational analysis indicated that rats receiving low amounts of LG as pup show poorer decision-making -as evident from a lower number of advantageous choices in the rIGT- than rats which receive high amounts of LG. In fact, the performance of some of the rats with a low LG background was hardly above chance level after 120 trials. The poor performance was associated with elevated non-reward related exploration, reflected by a higher number of choices for the empty arms, and a trend towards less win-stay behavior in the advantageous arm. We did not observe a correlation between the time rats needed to consume two sucrose pellets during familiarization and %LG received or the number of advantageous choices in the IGT (data not shown), suggesting that the association between %LG received and rIGT performance is not simply due to differences in sucrose sensitivity. Overall, these findings suggest a choice strategy that is still in an exploratory phase in rats that received little LG, as opposed to rats that received more LG which successfully changed their choice strategy from exploration to exploitation of the most advantageous option.
Earlier studies showed that high anxiety is related to impaired decision-making in the IGT, both in humans [45], [46] and in rats [30]. Although we did not find a direct correlation between anxiety and the number of advantageous choices in the rIGT in the current study, the correlation that emerged between %LG and rIGT performance was in fact inversed to that found between %LG and anxiety in both males and females, which indirectly supports a negative relationship between anxiety and decision-making.
Both male and female animals learned to differentiate the long-term advantageous arm from the long-term disadvantageous arm and the empty arm as the task progressed. Previous studies in rats [29], [36], [47], mice [29] and humans [41], [45], [47], [48], [49], [50] have shown clear differences in IGT performance between sexes. It should be noted that although on average IGT scores between males and females differ, both in humans [48] and rats (Van den Bos, unpublished observations), a strong overlap exists between the IGT scores of the two sexes, so that differences may not appear unless group sizes are large. Moreover, male Wistar rats outperform the currently used male Long-Evans rats [29], so that strain differences could contribute to the difference between the current and earlier studies.
Neurobiological substrate
Performance in the rIGT depends on frontostriatal functioning [30], [34], [35]. This prompted us to study frontostriatal activity in rats receiving different amounts of maternal care, using the immediate early gene c-fos as a marker. The areas that were selected are related to reward, punishment and motivation and implicated in IGT performance, both in humans and in rats [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], see [30] for review.
Strong correlations were observed between c-fos activity in the mPFC (prelimbic cortex and infralimbic cortex) and rIGT decision-making performance. These observations correspond to earlier findings in (male) Wistar rats not characterized for LG received early in life [30]. Both in the earlier and the current study, good rIGT performance was associated with decreased c-fos expression in the mPFC. Which population of neurons is responsible for the changes in c-fos expression cannot be discerned from these investigations, but indirect evidence points to a role of GABAergic interneurons. Thus, transient mPFC inactivation by muscimol (GABA-A agonist) or baclofen (GABA-B agonist) resulted in poorer IGT performance as well as higher anxiety levels [34].
A growing body of literature shows that the mPFC is critically involved in strategy shifting, behavioral flexibility and goal-directed learning behavior by encoding task-rules [66], [67], [68], [69], [70], [71], [72], [73]. Functional integrity of the mPFC may allow for the coupling of the history of the animal's choices and rewards as well as behavioral flexibility, to generate and implement an optimal decision-making strategy under conditions of uncertainty. Involvement of the mPFC in the human IGT has been especially associated with punishment processing [74] and risk anticipation [75]. In this scenario, the mPFC contributes to cognitive control over emotional influences on behavior, allowing the subject to maintain a long-term perspective and withhold responding to immediate rewards or losses [76], [77]. Interestingly, the correlation between rIGT performance and c-fos activity in the mPFC did not depend on the LG background, i.e. when data were corrected for the amount of LG that pups received the correlations still existed, and actually became stronger. This was also observed for the cingulate area, which in an earlier study [30] did not correlate with rIGT performance.
Conversely, correlations between rIGT performance and c-fos expression in other areas (lateral orbitofrontal cortex; nucleus accumbens shell) disappeared or became just a trend (insular cortex) when correcting for the amount of LG. This suggests that the rIGT-related activity in the lateral OFC, nucleus accumbens shell and insular cortex might critically depend on important factors in the early life environment such as LG. Recently, several studies have clearly indicated a role for the OFC in rodent versions of IGT decision-making tasks [64], [65], [78]. The degree of OFC involvement may depend on the level of ambiguity experienced by the subject, i.e. the OFC may be important when individuals are learning the reinforcement contingencies and ambiguity is high [79]. Once uncertainty is reduced, the OFC may play less of a role in maintaining the optimal choice strategy. Indeed, the OFC has been suggested to play a role in integrating potentially salient information about environmental contingencies [80], and to use this information to assign a value to a reward and signal outcome expectancies, thus influencing action selection [81], [82], [83], [84]. If so, (female) rats receiving little LG early in life may be less able to assess the expected values of the different options in the initial stages of the task. This agrees with the fact that they were still in the exploratory phase after 120 trials.
The insular cortex is involved in anticipation and receipt of risky, aversive stimuli, such as switches in a choice task [85], [86], [87]. In humans, damage to the insula is associated with a decrease in sensitivity to differences in expected value between choice options and with a concurrent decrease in the amount of risky choices they make [88]. In line with this, increased insula activation during risky decision-making was reported after choosing from a disadvantageous card deck and has been associated with switching choices after a loss as well as with harm avoidance and neuroticism in healthy humans [62], [89]. Also in rats, the insula has been shown to play a role in the anticipation of reward [90] and the memory of the incentive value of this reward [91]. The insular cortex is thought to be a key structure in integrating representations of the homeostatic state (interoceptive or bodily state) associated with prior experiences and using that to guide future motivated behaviors, including risky decision making [92], [93], [94]. Accordingly, differences in insular cortex activity due to differences in the amount of LG may translate into differences in the perception of risks associated with a choice and hence to differences in rIGT performance. Thus, high LG animals may have a lower insular cortex activity while performing the IGT, and thus are less sensitive to taking risks, which expresses itself as a good IGT performance.
Early life environment, including maternal care, is known to have lasting neuroendocrine consequences, with low amounts of LG generally being associated with impaired negative feedback regulation of the HPA axis, resulting in higher glucocorticoid exposure [19]. Increased glucocorticoid exposure (particularly when taking place over an extended period of time) was reported to change the dendritic complexity of pyramidal cells in the prefrontal cortex [8], [95], [96], [97]. We therefore considered the possibility that the influence of LG on the correlation between c-fos expression and rIGT decision-making was mediated by changes in dendritic complexity. However, our data give insufficient support for this thesis. With regard to morphology we did not observe any relationship between maternal care and dendritic properties in the insular cortex. With regard to infralimbic pyramidal cells, the amount of LG in males correlated negatively with the number of branch points in basal dendrites. In females no significant correlations were observed between the amount of LG and infralimbic basal or apical dendritic morphology, although this might in part be due to the low sample size. Overall, the correlation between rIGT performance and dendritic morphology was complex, with sex-dependent differences. Therefore, we interpreted these data conservatively. While our results suggest that rIGT performance and the associated c-fos activity might depend on structural properties of the principal neurons in the selected areas, we cannot exclude the possibility that other characteristics of these cells or structure/function of neurons other than pyramidal cells contribute to the overall outcome.
In conclusion, our data show that the amount of care received by an individual rat from its mother affects adult decision-making performance as well as the associated c-fos activity particularly in the lateral OFC (in females), insular cortex and nucleus accumbens shell. It is tempting to speculate that early life environment may impact on the development of these parts of the brain such that their functionality is lastingly altered. If so, this may bear relevance to the fact that many neuropsychiatric disorders that are associated with early life adversity, such as mood disorders, anxiety disorders and schizophrenia, involve aberrant function of the frontostriatal circuit (for reviews see [24], [98]).
Acknowledgments
The authors thank Noortje van der Knaap and Daniëlle de Jong for their help with maternal care observations.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by Hersenstichting (https://www.hersenstichting.nl/homepage/index.html) grant #15F07 to MJ and HJK and Human Frontiers Science Program (http://www.hfsp.org/) grant #RGP0039/2006 to MJ. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fossati P, Amar G, Raoux N, Ergis AM, Allilaire JF. Executive functioning and verbal memory in young patients with unipolar depression and schizophrenia. Psychiatry Res. 1999;89:171–187. doi: 10.1016/s0165-1781(99)00110-9. [DOI] [PubMed] [Google Scholar]
- 2.Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, et al. Executive and prefrontal dysfunction in unipolar depression: a review of neuropsychological and imaging evidence. Neurosci Res. 2004;50:1–11. doi: 10.1016/j.neures.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Bremner JD. Neuroimaging studies in post-traumatic stress disorder. Curr Psychiatry Rep. 2002;4:254–263. doi: 10.1007/s11920-996-0044-9. [DOI] [PubMed] [Google Scholar]
- 4.Mann JJ, McBride PA, Malone KM, DeMeo M, Keilp J. Blunted serotonergic responsivity in depressed inpatients. Neuropsychopharmacology. 1995;13:53–64. doi: 10.1016/0893-133X(95)00016-7. [DOI] [PubMed] [Google Scholar]
- 5.Nikolaus S, Antke C, Beu M, Muller HW. Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders–results from in vivo imaging studies. Rev Neurosci. 2010;21:119–139. doi: 10.1515/revneuro.2010.21.2.119. [DOI] [PubMed] [Google Scholar]
- 6.Nutt DJ. Neurobiological mechanisms in generalized anxiety disorder. J Clin Psychiatry 62 Suppl 11: 22–27; discussion. 2001;28 [PubMed] [Google Scholar]
- 7.Olvera-Cortes ME, Anguiano-Rodriguez P, Lopez-Vazquez MA, Alfaro JM. Serotonin/dopamine interaction in learning. Prog Brain Res. 2008;172:567–602. doi: 10.1016/S0079-6123(08)00927-8. [DOI] [PubMed] [Google Scholar]
- 8.Shansky RM, Morrison JH. Stress-induced dendritic remodeling in the medial prefrontal cortex: effects of circuit, hormones and rest. Brain Res. 2009;1293:108–113. doi: 10.1016/j.brainres.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology. 2002;27:99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- 12.Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, et al. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4:163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- 13.Breier A, Kelsoe, Kirwin PD, Beller SA, Wolkowitz OM, et al. Early parental loss and development of adult psychopathology. Arch Gen Psychiatry. 1988;45:987–993. doi: 10.1001/archpsyc.1988.01800350021003. [DOI] [PubMed] [Google Scholar]
- 14.Niwa M, Matsumoto Y, Mouri A, Ozaki N, Nabeshima T. Int J Neuropsychopharmacol; 2010. Vulnerability in early life to changes in the rearing environment plays a crucial role in the aetiopathology of psychiatric disorders. pp. 1–19. [DOI] [PubMed] [Google Scholar]
- 15.Pechtel P, Pizzagalli DA. Psychopharmacology (Berl); 2010. Effects of early life stress on cognitive and affective function: an integrated review of human literature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bock J, Gruss M, Becker S, Braun K. Experience-induced changes of dendritic spine densities in the prefrontal and sensory cortex: correlation with developmental time windows. Cereb Cortex. 2005;15:802–808. doi: 10.1093/cercor/bhh181. [DOI] [PubMed] [Google Scholar]
- 17.Monroy E, Hernandez-Torres E, Flores G. Maternal separation disrupts dendritic morphology of neurons in prefrontal cortex, hippocampus, and nucleus accumbens in male rat offspring. J Chem Neuroanat. 2010;40:93–101. doi: 10.1016/j.jchemneu.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Pascual R, Zamora-Leon SP. Effects of neonatal maternal deprivation and postweaning environmental complexity on dendritic morphology of prefrontal pyramidal neurons in the rat. Acta Neurobiol Exp (Wars) 2007;67:471–479. doi: 10.55782/ane-2007-1663. [DOI] [PubMed] [Google Scholar]
- 19.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 20.Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci U S A. 2006;103:3480–3485. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, et al. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 23.Bagot RC, van Hasselt FN, Champagne DL, Meaney MJ, Krugers HJ, et al. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol Learn Mem. 2009;92:292–300. doi: 10.1016/j.nlm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Claessens SE, Daskalakis NP, van der Veen R, Oitzl MS, de Kloet ER, et al. Development of individual differences in stress responsiveness: an overview of factors mediating the outcome of early life experiences. Psychopharmacology (Berl) 214:141–154. doi: 10.1007/s00213-010-2118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Hasselt FN, Boudewijns ZS, van der Knaap NJ, Krugers HJ, Joels M. Maternal care received by individual pups correlates with adult CA1 dendritic morphology and synaptic plasticity in a sex-dependent manner. J Neuroendocrinol. 2012;24:331–340. doi: 10.1111/j.1365-2826.2011.02233.x. [DOI] [PubMed] [Google Scholar]
- 27.van Hasselt FN, Cornelisse S, Zhang TY, Meaney MJ, Velzing EH, et al. Adult hippocampal glucocorticoid receptor expression and dentate synaptic plasticity correlate with maternal care received by individuals early in life. Hippocampus. 2012;22:255–266. doi: 10.1002/hipo.20892. [DOI] [PubMed] [Google Scholar]
- 28.van Hasselt FN, Tieskens JM, Trezza V, Krugers HJ, Vanderschuren LJ, et al. Physiol Behav; 2012. Within-litter variation in maternal care received by individual pups correlates with adolescent social play behavior in male rats. [DOI] [PubMed] [Google Scholar]
- 29.Van den Bos R, Lasthuis W, Den Heijer E, Van der Harst J, Spruijt B. Toward a rodent model of the Iowa gambling task. Behav Res Methods. 2006;38:470–478. doi: 10.3758/bf03192801. [DOI] [PubMed] [Google Scholar]
- 30.De Visser L, Baars AM, Lavrijsen M, van der Weerd CM, van den Bos R. Decision-making performance is related to levels of anxiety and differential recruitment of frontostriatal areas in male rats. Neuroscience. 2011;184:97–106. doi: 10.1016/j.neuroscience.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 31.Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 32.Pryce CR, Bettschen D, Feldon J. Comparison of the effects of early handling and early deprivation on maternal care in the rat. Dev Psychobiol. 2001;38:239–251. doi: 10.1002/dev.1018. [DOI] [PubMed] [Google Scholar]
- 33.Moore CL, Morelli GA. Mother rats interact differently with male and female offspring. J Comp Physiol Psychol. 1979;93:677–684. doi: 10.1037/h0077599. [DOI] [PubMed] [Google Scholar]
- 34.De Visser L, Baars AM, Van 't Klooster J, Van den Bos R. Transient inactivation of the medial prefrontal cortex affects both anxiety and decision-making in male wistar rats. Front Neurosci. 2011;5:102. doi: 10.3389/fnins.2011.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Visser L, Homberg JR, Mitsogiannis M, Zeeb FD, Rivalan M, et al. Rodent versions of the iowa gambling task: opportunities and challenges for the understanding of decision-making. Front Neurosci. 2011;5:109. doi: 10.3389/fnins.2011.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Homberg JR, van den Bos R, den Heijer E, Suer R, Cuppen E. Serotonin transporter dosage modulates long-term decision-making in rat and human. Neuropharmacology. 2008;55:80–84. doi: 10.1016/j.neuropharm.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 37.Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 38.Van den Bos R, Houx BB, Spruijt BM. The effect of reward magnitude differences on choosing disadvantageous decks in the Iowa Gambling Task. Biol Psychol. 2006;71:155–161. doi: 10.1016/j.biopsycho.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Paxinos G, Watson C. The rat brain in stereotaxic coordinates: Elsevier Academic Press. 2005. [DOI] [PubMed]
- 40.Boekhoorn K, Terwel D, Biemans B, Borghgraef P, Wiegert O, et al. Improved long-term potentiation and memory in young tau-P301L transgenic mice before onset of hyperphosphorylation and tauopathy. J Neurosci. 2006;26:3514–3523. doi: 10.1523/JNEUROSCI.5425-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Overman WH. Sex differences in early childhood, adolescence, and adulthood on cognitive tasks that rely on orbital prefrontal cortex. Brain Cogn. 2004;55:134–147. doi: 10.1016/S0278-2626(03)00279-3. [DOI] [PubMed] [Google Scholar]
- 42.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 43.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, et al. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernandes C, Gonzalez MI, Wilson CA, File SE. Factor analysis shows that female rat behaviour is characterized primarily by activity, male rats are driven by sex and anxiety. Pharmacol Biochem Behav. 1999;64:731–738. doi: 10.1016/s0091-3057(99)00139-2. [DOI] [PubMed] [Google Scholar]
- 45.De Visser L, Van der Knaap LJ, Van de Loo AJ, Van der Weerd CM, Ohl F, et al. Trait anxiety affects decision-making differently in healthy men and women: towards gender-specific endophenotypes of anxiety. Neuropsychologia. 2010;48:1598–1606. doi: 10.1016/j.neuropsychologia.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 46.Miu AC, Heilman RM, Houser D. Anxiety impairs decision-making: psychophysiological evidence from an Iowa Gambling Task. Biol Psychol. 2008;77:353–358. doi: 10.1016/j.biopsycho.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 47.Van den Bos R, De Visser L, Van de Loo AJAE, Mets MAJ, Van Willigenburg GM, et al. Moore KO, Gonzalez NP, editors. Sex differences in decision-making in adult normal volunteers are related to differences in the interaction of emotion and cognitive control. Handbook on Psychology of Decision-making: Nova Science Publisher Inc. 2012.
- 48.Overman W, Graham L, Redmond A, Eubank R, Boettcher L, et al. Contemplation of moral dilemmas eliminates sex differences on the Iowa gambling task. Behav Neurosci. 2006;120:817–825. doi: 10.1037/0735-7044.120.4.817. [DOI] [PubMed] [Google Scholar]
- 49.Reavis R, Overman WH. Adult sex differences on a decision-making task previously shown to depend on the orbital prefrontal cortex. Behav Neurosci. 2001;115:196–206. doi: 10.1037/0735-7044.115.1.196. [DOI] [PubMed] [Google Scholar]
- 50.van den Bos R, Harteveld M, Stoop H. Stress and decision-making in humans: performance is related to cortisol reactivity, albeit differently in men and women. Psychoneuroendocrinology. 2009;34:1449–1458. doi: 10.1016/j.psyneuen.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 51.Reynolds SM, Zahm DS. Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosci. 2005;25:11757–11767. doi: 10.1523/JNEUROSCI.3432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ragozzino ME, Kesner RP. The role of the agranular insular cortex in working memory for food reward value and allocentric space in rats. Behav Brain Res. 1999;98:103–112. doi: 10.1016/s0166-4328(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 54.Salamone JD. The behavioral neurochemistry of motivation: methodological and conceptual issues in studies of the dynamic activity of nucleus accumbens dopamine. J Neurosci Methods. 1996;64:137–149. doi: 10.1016/0165-0270(95)00125-5. [DOI] [PubMed] [Google Scholar]
- 55.Todtenkopf MS, Parsegian A, Naydenov A, Neve RL, Konradi C, et al. Brain reward regulated by AMPA receptor subunits in nucleus accumbens shell. J Neurosci. 2006;26:11665–11669. doi: 10.1523/JNEUROSCI.3070-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Sex-related differences in a gambling task and its neurological correlates. Cereb Cortex. 2004;14:1226–1232. doi: 10.1093/cercor/bhh083. [DOI] [PubMed] [Google Scholar]
- 58.Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- 59.Brand M, Labudda K, Markowitsch HJ. Neuropsychological correlates of decision-making in ambiguous and risky situations. Neural Netw. 2006;19:1266–1276. doi: 10.1016/j.neunet.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, et al. Decision-making in a risk-taking task: a PET study. Neuropsychopharmacology. 2002;26:682–691. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- 61.Grachev ID, Apkarian AV. Anxiety in healthy humans is associated with orbital frontal chemistry. Mol Psychiatry. 2000;5:482–488. doi: 10.1038/sj.mp.4000778. [DOI] [PubMed] [Google Scholar]
- 62.Lawrence NS, Jollant F, O'Daly O, Zelaya F, Phillips ML. Distinct roles of prefrontal cortical subregions in the Iowa Gambling Task. Cereb Cortex. 2009;19:1134–1143. doi: 10.1093/cercor/bhn154. [DOI] [PubMed] [Google Scholar]
- 63.Li X, Lu ZL, D'Argembeau A, Ng M, Bechara A The Iowa Gambling Task in fMRI images. Hum Brain Mapp. 31:410–423. doi: 10.1002/hbm.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rivalan M, Coutureau E, Fitoussi A, Dellu-Hagedorn F. Inter-individual decision-making differences in the effects of cingulate, orbitofrontal, and prelimbic cortex lesions in a rat gambling task. Front Behav Neurosci. 2011;5:22. doi: 10.3389/fnbeh.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zeeb FD, Winstanley CA. Lesions of the basolateral amygdala and orbitofrontal cortex differentially affect acquisition and performance of a rodent gambling task. J Neurosci. 2011;31:2197–2204. doi: 10.1523/JNEUROSCI.5597-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Balleine BW, O'Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 69.Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 70.Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sul JH, Kim H, Huh N, Lee D, Jung MW. Distinct roles of rodent orbitofrontal and medial prefrontal cortex in decision making. Neuron. 2010;66:449–460. doi: 10.1016/j.neuron.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tran-Tu-Yen DA, Marchand AR, Pape JR, Di Scala G, Coutureau E. Transient role of the rat prelimbic cortex in goal-directed behaviour. Eur J Neurosci. 2009;30:464–471. doi: 10.1111/j.1460-9568.2009.06834.x. [DOI] [PubMed] [Google Scholar]
- 73.Young JJ, Shapiro ML. Double dissociation and hierarchical organization of strategy switches and reversals in the rat PFC. Behav Neurosci. 2009;123:1028–1035. doi: 10.1037/a0016822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin CH, Chiu YC, Cheng CM, Hsieh JC. Brain maps of Iowa gambling task. BMC Neurosci. 2008;9:72. doi: 10.1186/1471-2202-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fukui H, Murai T, Fukuyama H, Hayashi T, Hanakawa T. Functional activity related to risk anticipation during performance of the Iowa Gambling Task. Neuroimage. 2005;24:253–259. doi: 10.1016/j.neuroimage.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 76.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 77.Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, et al. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat Neurosci. 2004;7:887–893. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- 78.Pais-Vieira M, Lima D, Galhardo V. Orbitofrontal cortex lesions disrupt risk assessment in a novel serial decision-making task for rats. Neuroscience. 2007;145:225–231. doi: 10.1016/j.neuroscience.2006.11.058. [DOI] [PubMed] [Google Scholar]
- 79.Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310:1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- 80.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 81.Mainen ZF, Kepecs A. Neural representation of behavioral outcomes in the orbitofrontal cortex. Curr Opin Neurobiol. 2009;19:84–91. doi: 10.1016/j.conb.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 82.Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Prog Neurobiol. 2008;86:216–244. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 83.Takahashi YK, Roesch MR, Stalnaker TA, Haney RZ, Calu DJ, et al. The orbitofrontal cortex and ventral tegmental area are necessary for learning from unexpected outcomes. Neuron. 2009;62:269–280. doi: 10.1016/j.neuron.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- 85.Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, et al. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131:1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28:2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu R, Mobbs D, Seymour B, Calder AJ. Insula and striatum mediate the default bias. J Neurosci. 2010;30:14702–14707. doi: 10.1523/JNEUROSCI.3772-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weller JA, Levin IP, Shiv B, Bechara A. The effects of insula damage on decision-making for risky gains and losses. Soc Neurosci. 2009;4:347–358. doi: 10.1080/17470910902934400. [DOI] [PubMed] [Google Scholar]
- 89.Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- 90.Kesner RP, Gilbert PE. The role of the agranular insular cortex in anticipation of reward contrast. Neurobiol Learn Mem. 2007;88:82–86. doi: 10.1016/j.nlm.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. The contribution of the amygdala, nucleus accumbens, and prefrontal cortex to emotion and motivated behaviour. International Congress Series. 2003;1250:347–370. [Google Scholar]
- 92.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xue G, Lu Z, Levin IP, Bechara A. The impact of prior risk experiences on subsequent risky decision-making: the role of the insula. Neuroimage. 2010;50:709–716. doi: 10.1016/j.neuroimage.2009.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318:655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- 95.Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lapiz-Bluhm MD, Bondi CO, Doyen J, Rodriguez GA, Bedard-Arana T, et al. Behavioural assays to model cognitive and affective dimensions of depression and anxiety in rats. J Neuroendocrinol. 2008;20:1115–1137. doi: 10.1111/j.1365-2826.2008.01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nagai M, Kishi K, Kato S. Insular cortex and neuropsychiatric disorders: a review of recent literature. Eur Psychiatry. 2007;22:387–394. doi: 10.1016/j.eurpsy.2007.02.006. [DOI] [PubMed] [Google Scholar]