Abstract
The parasitic protozoan Leishmania alternates between an invertebrate and a mammalian host. Upon their entry to mammalian macrophages, Leishmania promastigotes differentiate into amastigote forms within the harsh environment of the phagolysosomal compartment. Here, we provide evidence for the importance of translational control during the Leishmania differentiation process. We find that exposure of promastigotes to a combined elevated temperature and acidic pH stress, a key signal triggering amastigote differentiation, leads to a marked decrease in global translation initiation, which is associated with eIF2α phosphorylation. Interestingly, we show that amastigotes adapted to grow in a cell-free medium exhibit lower levels of protein synthesis in comparison to promastigotes, suggesting that amastigotes have to enter a slow growth state to adapt to the stressful conditions encountered inside macrophages. Reconversion of amastigotes back to promastigote growth results in upregulation of global translation and a decrease in eIF2α phosphorylation. In addition, we show that while general translation is reduced during amastigote differentiation, translation of amastigote-specific transcripts such as A2 is preferentially upregulated. We find that A2 developmental gene regulation is triggered by temperature changes in the environment and that occurs mainly at the level of translation. Upon elevated temperature, the A2 transcript is stabilized through its association with polyribosomes leading to high levels of translation. When temperature decreases during amastigote to promastigote differentiation, the A2 transcript is not longer associated with translating polyribosomes and is being gradually degraded. Overall, these findings contribute to our better understanding of the adaptive responses of Leishmania to stress during its development and highlight the importance of translational control in promastigote to amastigote differentiation and vice-versa.
Introduction
Leishmania spp. are the causative agents of leishmaniasis, the collective name for a number of important parasitic diseases affecting over 12 million people in 88 countries worldwide [1]. These parasites exist in two major developmental stages; free-living flagellated promastigotes in the midgut of the sand fly vector and non-motile amastigotes that can replicate in the phagolysosome of mammalian macrophages. During its intracellular development, Leishmania undergoes marked morphological and biochemical transformations that are essential for its adaptation and survival to the rapidly changing environments in the macrophage [2], [3], [4], [5]. These changes implicate dynamic alterations in the regulation of gene expression, mostly at the posttranscriptional level (reviewed in [6], [7]).
Environmental stimuli like elevated temperature and low pH have been shown to be crucial in triggering Leishmania promastigote to amastigote differentiation in vitro [8], [9], [10], [11]. Genome-wide transcriptomics and proteomics studies have shown that during amastigote differentiation, 3%–9% of genes in several Leishmania species are regulated at the level of individual mRNAs and up to 12%–18% are modulated at the protein level [12], [13], [14], [15], [16], [17], [18]. Posttranslational modifications (PTMs) are also likely to play a key role in the parasite’s development and in response to stress. For example, different protein isoforms were depicted by proteomic analyses to be specific to either life stages of the parasite [12] and a large number of PTM sites (e.g. methylation, acetylation, phosphorylation, glycosylation) has been identified during the Leishmania donovani promastigote to amastigote differentiation in vitro [16]. Moreover, proteomic analyses of affinity-enriched phosphoprotein extracts obtained from L. donovani promastigotes and axenic amastigotes revealed important differences in protein phosphorylation profiles across the two major Leishmania lifestages [19], [20], [21].
In eukaryotes, one of the best conserved stress response pathway is the phosphorylation of the alpha-subunit of eukaryotic initiation factor-2 (eIF2α) at serine 51 by stress-responsive kinases, resulting in the reduction of global translation [22], [23], [24], [25]. The phosphorylation of eIF2α prevents GDP-GTP exchange on eIF2 by the guanine nucleotide exchange factor eIF2B, thereby inhibiting recycling of the ternary complex that contains the initiator methionine Met-tRNAi [23], [24], [25]. Consequently, global translation initiation is decreased, so the cell can save energy and modulate gene expression in response to stress. There are four known eIF2α kinases in mammalian cells that phosphorylate eIF2α and are activated by different stresses. These include the double-stranded-RNA dependent protein kinase PKR, an interferon-inducible protein that is an important component in the antiviral response; the general control non-derepressible-2 kinase (GCN2), which is activated in response to amino acid starvation; the heme-regulated inhibitor (HRI) whose activity is induced by heme deficiency; and the endoplasmic reticulum (ER)-resident protein kinase PERK which is activated by the accumulation of unfolded proteins in the ER [25], [26]. Recently, we characterized the Leishmania PERK eIF2α kinase homolog and addressed its role in the parasite’s differentiation within macrophages [27]. Most importantly, we showed that lack of eIF2α phosphorylation in a PERK dominant negative mutant overexpressing a truncated PERK protein markedly delayed the Leishmania amastigote differentiation process [27].
Here, we report that Leishmania promastigotes exposed to a combined high temperature and low pH stress, a key signal triggering amastigote differentiation, exhibit a marked reduction in global translation, which coincides with eIF2α phosphorylation. Protein synthesis is also globally downregulated in axenic in vitro-generated amastigotes as compared to promastigotes while translation of known amastigote-specific genes is preferentially increased. Attenuation of global translation during amastigote differentiation may be important for allowing the parasite to adapt to the harsh environment of the phagolysosome in its mammalian host.
Results
Exposure of Leishmania to Environmental Signals Triggering Amastigote Differentiation Leads to a Decrease in Global Translation
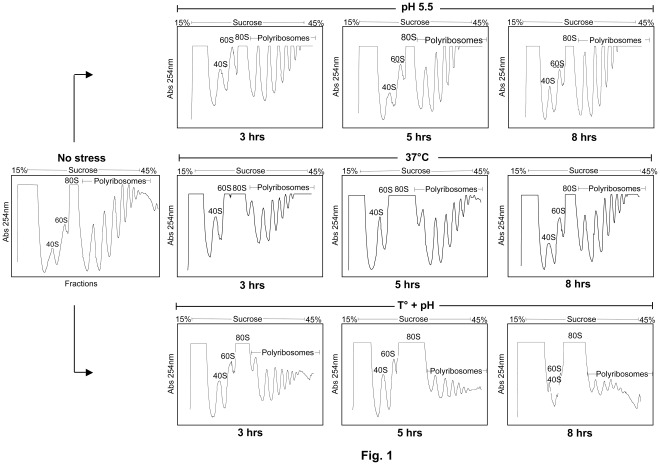
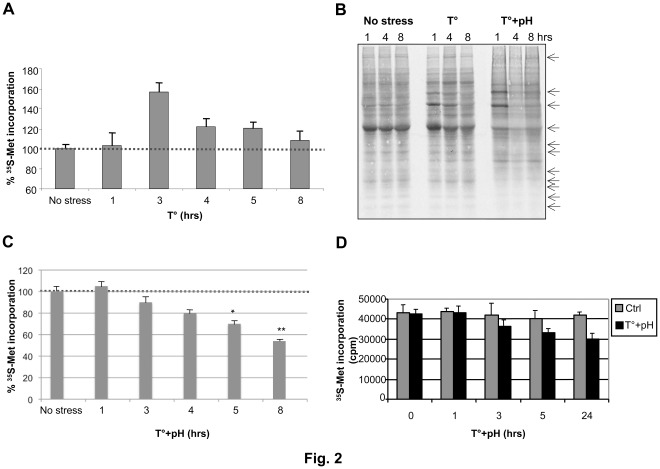
Elevated temperature and drop in pH constitute key signals triggering Leishmania promastigote to amastigote differentiation within the phagolysosome of mammalian macrophages [4], [8], [10], [28], [29]. To respond to various external stimuli and stresses, the parasite has developed several adaptive strategies allowing dynamic alterations in gene expression, mainly at the post-transcriptional level [11], [13], [14], [15], [16], [19], [30]. To better understand how Leishmania adapts and survives within the harsh phagolysosomal compartment, we first investigated the effect of elevated temperature and low pH (differentiation signals) on global translation using sucrose density gradient centrifugation to analyze ribosome profiles and metabolic cell labeling to evaluate protein synthesis rates. L. infantum promastigotes grown in RPMI medium at 25°C and pH 7.3 were exposed to either acidic or heat-shock stresses for different time points. Gradual exposure of L. infantum promastigotes for 3, 5 and 8 hours to an acidified medium or to a temperature shift from 25°C to 37°C had no significant effect on global translation, as determined by polysome profiling analysis (Figure 1, upper and middle panels). Furthermore, [35S]-methionine incorporation into Leishmania proteins in parasites grown in methionine-free medium during temperature stress corroborated the polysome profiling data (Figure 2A). Interestingly, 3 hours following temperature stress, a significant increase in [35S]-Met labeling (Figure 2A) and the accumulation of high molecular-weight polypeptides (Figure 2B) was observed. These data suggest that translation of specific proteins is increased in response to temperature stress, consistent with previous reports indicating a selective up-regulation of heat-shock proteins and of chaperones to increase cellular survival [31].
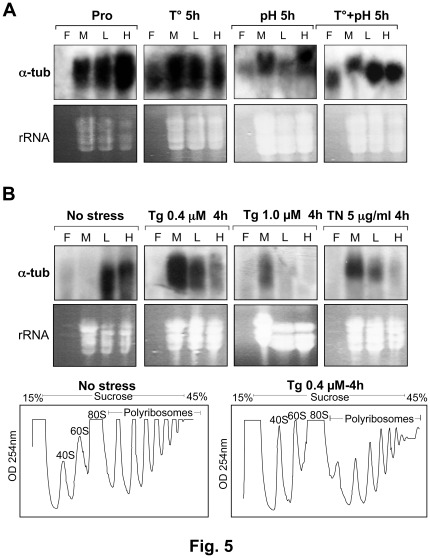
Figure 1. Effect of temperature and pH stress on general translation in Leishmania .
. Polysome profile analysis of L. infantum promastigotes (no stress; 25°C and pH 7.3) and parasites exposed for different time periods to either elevated temperature (37°C) or low pH (5.5) or to a combined temperature and acidic pH (T°+pH) stress. Both elevated temperature and low pH are major signals triggering amastigote differentiation within the phagolysosome of the host macrophage [8], [10]. Leishmania lysates were fractionated by 15% to 45% sucrose density ultracentrifugation and absorbance (Abs) at 254 nm was continuously recorded. The 40S and 60S ribosomal subunits, 80S monosome and polyribosome peaks are indicated. Data displayed here represent one of 3–5 separate experiments.
Figure 2. The effect of temperature and acidic pH stress on the de novo protein synthesis in Leishmania.
(A) Levels of [35S]-methionine incorporation (as an indicative of general translation rates) in L. infantum promastigotes subjected from 1 to 8 hours to either an elevated temperature (37°C) stress (A) or to a combined temperature and low pH (pH 5.5) stress (C). Thirty minutes before protein sample collection, 1 µCi/ml [35S]-methionine protein labeling mix was added to the culture media lacking methionine. Protein synthesis was measured as the incorporated radioactivity by a scintillation counter and expressed as cpm. Results are the mean of a minimum of six independent experiments. (B) [35S]-Met-labeled proteins resolved on 10% SDS-PAGE. Cells grown at 25°C/pH 7.3 (no stress) or exposed to elevated temperature (37°C) (T°) only or to a combined temperature and acidic pH (pH 5.5) stress for 1, 4 and 8 hours were labelled with [35S]-Met for 30 min. An autoradiograph of the SDS-PAGE analysis is shown here. Examples of upregulated or downregulated proteins under stress are indicated by arrows. (D) Pulse-chase assay to assess the stability of L. infantum proteins under stress. L. infantum promastigotes were incubated in a methionine-deprived medium supplemented with [35S]-Met for 1 hour at 25°C and then transferred to a non-radioactive methionine-containing medium at 37°C and pH 5.5 and grown for 24 hours. Aliquots were taken at 1, 3, 5 and 24 hours and the radioactivity (in cpm) was measured by a scintillation counter. A decrease in cpm numbers corresponds to protein degradation under stress. Results are the mean of three independent experiments. Significant differences between the various conditions in (C) are indicated (* p<0.05 and **p<0.01) using one-way ANOVA followed by the Tukey-Kramer test.
We next evaluated the effect of a combined temperature and acidic pH stress on L. infantum global translation using polysome profiling analysis and metabolic labeling, as described above. In contrast to the individual temperature or pH stresses, only few hours exposure of L. infantum to the combined stress resulted in a marked reduction of general translation, as illustrated by the pronounced decrease in the number and density of polysomes and the concomitant increase in free ribosomal subunits (40S and 60S) and monosomes (80S) (Figure 1, lower panels), which is consistent with reduced rates of translation initiation. A similar effect on translation upon temperature and acidic pH stress was observed with another Leishmania species, L. major (Figure S1). Moreover, [35S]-Met labeling experiments showed more than 20% decrease in de novo protein synthesis 5 hours after exposure of the parasite to combined stress and more than 40% decrease 8 hours following the same stress in comparison to the unstressed parasites (Figures 2B and 2C). This marked attenuation in translation was not related to parasite mortality as after 8 hours of exposure to high temperature and low pH, mortality rates of the parasite as assessed by propidium iodide staining were only at 8% in comparison to 2% for the unstressed control (data not shown).
To assess the levels of overall protein degradation under conditions of a combined temperature and pH stress, we carried out pulse-chase assays. Unstressed and stressed parasites were first grown in methionine-free medium to which [35S]-Met was added for 1 hour. Then, parasites were chased by replacing [35S]-Met by cold methionine in the medium while applying elevated temperature and low pH stresses for 1, 3, 5 and 24 hours. Only 16% of the radiolabeled proteins were degraded after 5 hours of combined stress as estimated by [35S]-Met incorporation analysis, but this percentage increased up to 25% following 24 hours of stress whereas less than 2% protein degradation was observed in unstressed parasites treated the same way (Figure 2D). Together, these findings indicate that combined elevated temperature and acidic pH stress represses global mRNA translation and accelerates protein degradation in Leishmania.
Leishmania Amastigotes Exhibit Generally Lower Translation Rates than the Highly Replicating Promastigote Forms
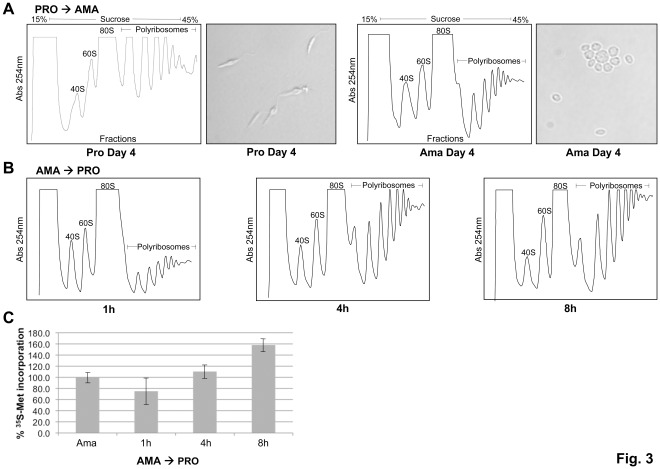
Amastigote differentiation can be induced in a host-free medium by culturing stationary promastigotes of most Leishmania species in an environment mimicking the phagolysosomal compartment of macrophages (37°C and pH 5.5 in 5% CO2) [4], [9], [32], [33], [34], [35]. Based on a number of criteria, including the parasite’s morphology, infectivity, immunochemistry, biochemical properties and gene expression patterns, axenic amastigotes share extensive similarities with macrophage-derived or lesion-derived amastigotes [4], [10], [13], [14], [15], [28], [34], [35], [36] (Figures 3A and S2). Here, we assessed the rates of global translation throughout the parasite’s life cycle. Differentiation of L. infantum promastigotes into amastigote-like forms in MAA-20 cell-free medium at 37°C and pH 5.5 for 4 days led to a significant decrease in global translation, as determined by ribosome profile analysis (Figure 3A). Interestingly, adapted axenic amastigotes grown for five consecutive passages in the MAA-20 medium demonstrated lower translation rates in comparison to the highly replicating promastigotes grown for the same number of passages (data not shown). Reconversion of axenic amastigotes back to promastigotes increased global translation (Figure 3B) to levels similar to those observed in promastigotes (Figures 1 and 3A, left panel). In line with the polysome profiling data (Figure 3B), [35S]-Met labeling experiments showed that amastigote to promastigote differentiation was associated with higher incorporation of 35S-Met into Leishmania proteins, which was proportional to the duration of growth under promastigote conditions (Figure 3C), consistent with an increase in protein synthesis under those conditions.
Figure 3. Regulation of general translation throughout the Leishmania infantum life cycle.
Promastigote to amastigote differentiation (A). Polysome profile analysis of L. infantum promastigotes (Pro) and axenic amastigotes (Ama) grown in MAA 20 medium at 37°C and pH 5.5 for 4 days in average (1st passage). L. infantum differentiates into amastigote-like forms after ∼4 days of growth under these conditions as illustrated in (A). Amastigote to promastigote differentiation (B). Polysome profile analysis of L. infantum axenic amastigotes transferred from the MAA 20 medium at 37°C and pH 5.5 to a MAA medium at 25°C and pH 7.3 for up to 8 hours. Data displayed here represent one of three separate experiments. (C) The effect of L. infantum amastigote to promastigote differentiation on global protein synthesis as estimated by the incorporation levels of [35S]-Met into the Leishmania proteins (as described in Figure 2). Results are the mean of four independent experiments.
Decrease in General Translation upon Stress and during Leishmania Amastigote Differentiation Correlates with eIF2α Phosphorylation
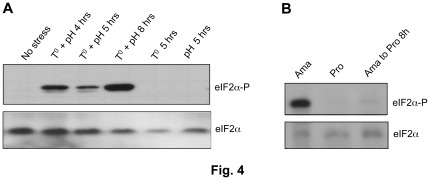
We next investigated whether attenuation of general translation in L. infantum axenic amastigotes is related to eIF2α phosphorylation, a well-documented mechanism to inhibit translation initiation in eukaryotes [25]. Phosphorylated eIF2α inhibits translation initiation, causing dissociation of polysomes and accumulation of monosomes and ribosomal subunits in stressed cells [23]. Western blot analysis using a commercially available phospho-antibody recognizing the phosphorylated form of eIF2α revealed high levels of eIF2α phosphorylation in promastigotes exposed to a combined high temperature and low pH stress for at least 4 hours but not in individually temperature-stressed or pH-stressed parasites or in unstressed promastigotes (Figure 4A). As also reported previously [27], eIF2α phosphorylation is detected in amastigotes (Figure 4B) where general translation is attenuated (Figure 3A) but not or very little in promastigotes undergoing active translation (Figures 1 and 4B). Interestingly, eIF2α phosphorylation drops down to background levels upon reconversion of amastigotes back to promastigotes (Figure 4B). EIF2α phosphorylation coincides with a marked decrease in global translation in parasites exposed to a combined temperature and acidic stress (Figure 1) or during amastigote differentiation in vitro (Figure 3A).
Figure 4. The translation initiation factor 2-alpha subunit (eIF2α) is phosphorylated upon temperature and acidic pH stress and during promastigote to amastigote differentiation.
L. infantum promastigotes (Pro) (no stress) or promastigotes exposed to elevated temperature (37°C) or to a combined temperature and low pH (pH 5.5) stress for 4, 5 and 8 hours (A) or axenic amastigotes (Ama) and or amastigotes subjected to promastigote differentiation (B) were lysed and whole-cell lysates were used in immunoblots with a rabbit polyclonal anti-eIF2α [pS51] phosphospecific antibody (eIF2α-P) to detect eIF2α phosphorylation. Protein loading was monitored using an anti-Leishmania specific eIF2α antibody described in [27].
Concomitant Exposure of Leishmania to Temperature and Acidic pH Stress Selectively Induces Translation of Developmentally Regulated Genes
Although diverse cellular stress conditions generally repress global protein synthesis, there are examples where translation of specific transcripts is selectively enhanced in response to stressful stimuli [37]. For example, amino-acid starvation enhances translation of GCN4 [38] in Saccharomyces cerevisiae and of ATF4 [39] and the cat-1 amino acid transporter [40] in mammalian cells. We therefore investigated the effect of elevated temperature, low pH and amastigote differentiation on the translational efficiency of developmentally regulated transcripts in comparison to housekeeping genes. The constitutively expressed alpha-tubulin gene and the A2 developmentally regulated gene known to be expressed specifically in L. donovani amastigotes [41] were analyzed in this study.
Under promastigote growth conditions (25°C, pH 7.3), the L. infantum alpha-tubulin transcript was predominantly associated with highly translating polysomes (Figure 5A). However, both heat stress and to a larger extent the combined temperature and low pH stress promoted a shift of the alpha-tubulin mRNA from the highly translating polysomes to the monosomes and ribosome-free fractions (Figure 5A), consistent with reduced translation under these conditions (Figure 1, bottom panels). Similarly, attenuation of general translation following endoplasmic reticulum (ER) stress induced by pharmacological agents such as thapsigargin (Figure 5B, bottom panel) or tunicamycin (data not shown) had an effect on the efficiency of alpha-tubulin mRNA translation. Indeed, increasing concentrations of thapsigargin or tunicamycin treatment resulted in gradual dissociation of the alpha-tubulin mRNA from the polyribosomes (Figure 5B, upper panels). The decrease in translation observed upon ER stress was not due to parasite mortality, as Leishmania treated with 1.0 µM thapsigargin for 4 hrs exhibited only 1% mortality in comparison to the untreated control (data not shown). Together these findings indicate that upon various stresses leading to a reduction in global translation, housekeeping transcripts such as alpha-tubulin are less efficiently translated.
Figure 5. Translation of Leishmania housekeeping genes under stress.
(A–B) Northern blot hybridization of L. infantum RNA samples isolated from sucrose gradient fractions (F: free mRNPs; M: monosomes; L: light polysomes; H: heavy polysomes) to determine the association of the constitutively expressed alpha-tubulin mRNA with translating polysomes in the absence of stress (no stress) or under temperature (T°) or acidic pH stresses (A) and or in the presence of the endoplasmic reticulum (ER)-inducing agents thapsigargin (Tg) or tunicamycin (TN) (B, upper panels). The ethidium bromide-stained gels used for northern blot analysis served as a loading control (rRNA is indicated). Polysome profiling analysis of L. infantum promastigotes untreated (no stress) or treated with 0.4 µM of thapsigargin (Tg) for 4 hours (B, lower panels). Data shown here are representative of at least four independent experiments with similar results.
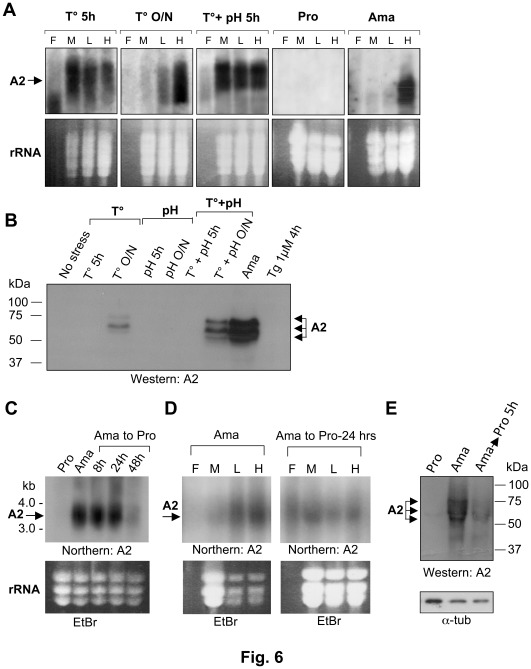
In contrast to the alpha-tubulin mRNA, translation of the stage-regulated A2 transcript markedly increased under heat stress. The A2 transcript is specifically expressed in amastigotes (Figure 6A and 6C and [41]), but elevated temperature and pH stress can also trigger its expression [10]. Northern blot hybridization revealed that 5 hours following of L. infantum exposure to elevated temperature or to a combined temperature and acidic pH stress, the A2 transcript was detected both in the monosome and polysome fractions (Figure 6A). Its partial association with the polysomes did not result however, in high levels of translated A2 protein as determined by western blot with a specific anti-A2 antibody (Figure 6B). However, after an O/N exposure of the parasite to heat stress, the A2 transcript shifted mainly to the highly translating polysome fraction, suggesting an active translation (Figure 6A) as also confirmed by western blot analysis (Figure 6B). In contrast to heat stress, acidic pH stress alone did not trigger A2 expression (Figure 6B). In addition, thapsigargin-induced ER stress, shown here to decrease global translation in L. infantum (Figure 5B, lower panel), did not induce A2 mRNA accumulation (data not shown) or protein expression (Figure 6B). In axenic amastigotes, the totality of A2 mRNA was found associated with the heavy polysomes (Figure 6A) leading to high levels of translation as confirmed by western blot analysis (Figure 6B). Interestingly, reconversion of amastigotes back to promastigote growth conditions resulted in a marked decrease in A2 mRNA accumulation (Figure 6C), a shift of the A2 mRNA association from translating polyribosomes towards the monosome and free-ribosome fraction (Figure 6D) and a significant reduction in A2 protein synthesis (Figure 6E). Although no significant decrease in the accumulation of A2 mRNA was observed during the first 24 hrs following the amastigote to promastigote switch (Figure 6C), western blot analysis revealed lower A2 protein synthesis already 5 hours after the switch (Figure 6E). Altogether, these findings suggest that A2 developmental regulation occurs mainly at the translational level.
Figure 6. Translation of L. infantum developmentally regulated transcripts under temperature and acidic pH stresses.
(A) Northern blot hybridization of L. infantum RNA isolated from sucrose gradient fractions (F, M, L and H are as in Figure 5) to assess the association of the amastigote-specific A2 transcript with the ribosomes under temperature stress or acidic pH stress or a combination of both stresses. The ethidium bromide-stained gels used for northern blot analysis served as a loading control (rRNA is indicated). (B) Western blot analysis of L. infantum protein lysates using a monoclonal anti-A2 antibody to determine expression levels of A2 protein under different stresses, including high temperature, low pH and Tg-induced ER stresses. Controls using L. infantum promastigotes (Pro) (non stress) or axenic amastigotes (Ama) were included for both northern and western blots. (C) Northern blot hybridization of L. infantum total RNA to determine A2 mRNA expression during amastigote to promastigote differentiation for up to 48 hrs. (D) Northern blot hybridization of L. infantum RNA isolated from sucrose gradient fractions to determine the association of the A2 mRNA with monosomes or translating polysomes. (E) Western blot with an anti-A2 antibody of L. infantum amastigotes transferred into MAA-20 medium at 25°C and pH 7.3 for up to 5 hours to allow its differentiation into promastigote forms. The anti-alpha-tubulin antibody was used as loading control. Data shown here are representative of three independent experiments with similar results.
Discussion
In this study, we explored the role of translational control in the context of Leishmania differentiation from free-living promastigotes to intracellular amastigote forms and vice-versa. We provide evidence that protein synthesis is generally reduced under growth conditions inducing amastigote differentiation and that reduced translation rates coincide with the phosphorylation of the alpha-subunit of eukaryotic initiation factor-2. Interestingly, we show that attenuation of global translation is not only observed in parasites transiently subjected to the amastigote differentiation signal (e.g. elevated temperature and acidic pH), but also in adapted amastigote cultures, suggesting that this life stage has to enter a slow growth state in order to adapt to the harsh environment in the phagolysosomal compartment. In addition, we show that while general translation is decreased during amastigote differentiation, translation of developmentally regulated transcripts like A2 is preferentially enhanced, in agreement with the induction of an amastigote-specific differentiation program.
During its amastigote differentiation inside macrophages, Leishmania experiences an important number of stressful conditions, including rise in temperature, a dramatic shift in extracellular pH, increased levels of oxygen and nitrogen-reactive species, a high proteolytic activity and a nutritional stress within the phagolysosome. These external stimuli induce dynamic alterations in the regulation of gene expression, mainly at the posttranscriptional level [6], [7], leading to important morphological and biochemical changes that ensure the parasite’s intracellular survival. It has been established previously that temperature shift (from 25°C to 37°C) and drop in pH (from pH 7.3 to pH 5.5) provide a key signal for promastigote to amastigote differentiation [8], [10]. Here, we show that exposure of L. infantum promastigotes to a combined elevated temperature and acidic pH stress for few hours (3–5 hrs) leads to a marked decrease in general translation. A similar decrease in polysome formation following the differentiation signal has been reported recently in L. donovani but after longer exposure times (e.g. 15–24 hrs) [8], [9], [10], [11]. Of most interest, we show that translation remains generally lower in adapted amastigotes grown for several passages in a cell-free medium. Reconversion of amastigotes back to promastigote growth conditions results in increased levels of general translation comparable to those of promastigotes, suggesting that translational control plays a key role during the Leishmania’s development. Thus, the highly replicating promastigote forms undergo more active translation than intracellular amastigotes. These conclusions are consistent with previous reports demonstrating preferential upregulation in promastigotes of genes involved in translation (e.g. ribosomal proteins, translation factors, tRNA synthetases) [13], [14].
Eukaryotic cells have evolved mechanisms to respond to various cellular stresses encountered in their environments. One of the best-characterized stress response pathways conserved from yeast to humans is the reversible phosphorylation of the alpha-subunit of eukaryotic initiation factor 2, which lowers global protein synthesis along with induced translation of selected mRNAs [23], [25]. Here, we show that attenuation of general translation upon environmental stimuli such as elevated temperature and acidic pH triggering promastigote to amastigote differentiation or during amastigote growth is associated with eIF2α phosphorylation. These data suggest that Leishmania infective promastigotes experience stress upon their entry in macrophages and that translation attenuation mediated by eIF2α phosphorylation is important to allow the necessary adaptations for the parasite’s intracellular survival in the hostile environment of the phagolysosome. EIF2α phosphorylation is linked to amastigote differentiation as reconversion of amastigotes back to promastigote forms results in a dramatic decrease of eIF2α phosphorylation levels and upregulation of translation. Recent work from our laboratory highlighted the importance of eIF2α phosphorylation for amastigote differentiation as parasites unable to phosphorylate eIF2α by the ER resident PERK eIF2α kinase were markedly delayed in their differentiation into amastigote forms within macrophages [27]. The importance of eIF2α phosphorylation in the intracellular development of parasitic protozoa has been emphasized not only in Trypanosomatidae such as Leishmania [27] and Trypanosoma cruzi [42], but also in Apicomplexa [27], [37], [43], [44]. For example, in Toxoplasma gondii, it has been shown that eIF2α phosphorylation is important for maintaining latency of the bradyzoite form in the mammalian host [44], [45]. Moreover, it has been reported that phosphorylation of the Toxoplasma eIF2α is critical for the resistance of the parasite to external stress when outside its host and that inhibition of this process significantly delays the development of acute toxoplasmosis in vivo [44]. In Plasmodium, phosphorylation of PfeIF2α by the IK2 kinase plays an important role in salivary gland sporozoite latency and transformation into liver stages [37]. While combined heat stress (37°C, a physiological temperature encountered within macrophages) and drop in pH promote a reduction in global translation and eIF2α phosphorylation in Leishmania, interestingly in the related parasite Trypanosoma brucei, it has been shown that heat-shock stress up to 41°C causes a decrease in polysomes and the formation of stress granules but independently of eIF2α phosphorylation [31]. It is possible that under extreme stress conditions such as heat-shock at 41°C, mechanisms other than eIF2α phosphorylation may operate in these parasites to reduce levels of translation.
Reduced translation during amastigote differentiation may allow Leishmania to conserve energy while reconfigurating the expression of specific sets of genes necessary for its survival in the mammalian host. Consistent with this hypothesis it has been shown previously that the effects of elevated temperature and/or acidic pH induce the expression of several individual mRNAs [13], [14], [17], [30]. Here we show that although translation of housekeeping genes like alpha-tubulin is reduced during amastigote differentiation, translation of developmentally regulated transcripts is selectively upregulated. This was the case of the A2 amastigote-specific gene [41] whose translation markedly increased upon exposure to elevated temperature or to the differentiation signal. Our findings support that developmental regulation of the A2 transcript occurs mainly at the level of translation and that is triggered by elevated temperature and not by drop in the pH. Through its higher association with polyribosomes, the A2 transcript is stabilized and is more efficiently translated. When the temperature shifts from 37°C to 25°C during amastigote to promastigote differentiation, the A2 transcript is not longer associated with translating polyribosomes and is being gradually degraded. Our data indicate that although the A2 transcript could be detected for more than 24 hrs following amastigote to promastigote differentiation in vitro, A2 translation was significantly diminished already ∼5 hrs upon differentiation, suggesting that A2 downregulation during amastigote to promastigote switch is also controlled at the level of translation. It has been shown recently that the A2 protein is induced by heat shock (40°C) and that under these conditions is complexed with the ER chaperone BiP [46], suggesting that A2 is a stress response protein that may play a role in enabling L. donovani to survive the higher temperatures associated with visceral organ infection. Despite its suggested localization in the ER following heat stress, our data indicate that A2 protein expression is not induced upon ER stress.
In this study, the findings indicate that Leishmania is capable of withstanding stressful situations during its digenetic life cycle by regulating general translation through reversible eIF2α phosphorylation. Global attenuation of translation during amastigote differentiation likely explains the slower growth rate of amastigotes inside the phagolysosome under conditions where metabolic requirements are reduced as compared to extracellular promastigotes [47]. Translational control seems also to be key in the regulation of amastigote-specific genes such as the A2 gene. Overall, these findings contribute to our better understanding of the adaptive responses of Leishmania to stress during its developmental switches and highlight the importance of translational control in promastigote to amastigote differentiation and vice-versa.
Materials and Methods
Cell Culture
The Leishmania infantum MHOM/MA/67/ITMAP-263 strain used in this study has been described elsewhere [32]. Promastigotes were cultured at 25°C and pH 7.3 in RPMI-1640 medium supplemented with 1 µg/ml d-biotin, 20 µg/ml adenosine, 5 µg/ml hemin and 10% heat-inactivated fetal calf serum (Multicell, Wisent Inc). Acidic stress was induced in RPMI medium at pH 5.5. L. infantum promastigote to amastigote differentiation in a cell-free culture and the maintenance of axenic amastigotes were carried out as described previously [9], [48]. Typically, late stationary-phase promastigotes were inoculated in MAA/20 medium supplemented with 20% serum in 25-cm2 ventilated flasks and grown at 37°C and pH 5.5 with 5% CO2 for 5 days in average.
RNA and Protein Manipulations
Total RNA was isolated with Trizol™ (Invitrogen) following the manufacturer’s instructions. Northern blot hybridizations were performed following standard procedures [49]. Leishmania lysates were clarified by centrifugation at 13,000×g for 15 min at 4o C. Protein quantification was assessed using the Bradford reagent (BioRad) and 25 µg of total protein lysates was loaded onto 10% SDS-PAGE. The gels were transferred on Immobilon-P polyvinylidene difluoride membranes (Millipore) following the manufacturer’s instructions. For the anti-alpha tubulin and anti-A2 antibodies, blocking was carried out for 60 min in phosphate-buffered saline with 5% non fat dry skim milk or 5% BSA, respectively prior to the addition of the first antibody. The A2 antibody was kindly provided by Dr Greg Matlashewski (McGill University). For the anti-eIF2alpha [pS51] phosphospecific antibody, blocking was performed O/N at 4°C in 5% BSA, prior to incubation for 2 hrs at RT° in 1% BSA. Mouse monoclonal antisera raised against L. infantum eIF2alpha [27], mouse monoclonal anti-alpha tubulin antibody (Sigma) and rabbit polyclonal anti-eIF2alpha (human) [pS51] phosphospecific antibody (BioSource™) were used at 1∶1000 dilutions. Phosphate-buffered saline with 0.1% Tween 20 was used to wash the membranes after the blocking step. Anti-mouse or anti-rabbit antibodies that had been conjugated to horseradish peroxidase (Santa Cruz Biotechnology) were diluted at 1∶10,000 in PBS-Tween 0.1% and 5% non fat dry skim milk and added to the membrane for 60 min. Three X 10 min washes (PBS with 0.1% Tween 20) were performed before visualizing the signal on the membrane by chemiluminescence using the Amersham Hyperfilm™/ECL™ kit (GE HealthCare). After exposure of the film to the membrane, protein amounts were quantified by densitometric analyses using a PhosphorImager with ImageQuaNT 3.1 software.
Polysome Profiling Analysis
A total of 3×109 L. infantum late-log/stationary-phase promastigotes treated with 0.4 µM or 1 µM thapsigargin or 5 µg/ml tunicamycin (Sigma) or subjected to heat-shock or acidic stress or to amastigote differentiation in culture were incubated with 100 µg/ml cycloheximide (Sigma) for 10 min, washed with cycloheximide-containing PBS buffer and lysed with a Dounce homogenizer in lysis buffer [10 mM Tris-HCl pH 7.4, 150 mM NaCl, 10 mM MgCl2, 1 mM DTT, 0.5% IGEPAL, 100 µg/ml cycloheximide, 100 U/ml RNAseOUT (Amersham), 1 mM PMSF, 15 µl/ml of protease inhibitor cocktail (Sigma)]. Leishmania lysates were pelleted by centrifugation and the supernatant (40 OD260 nm units) was layered on top of a 15% to 45% linear sucrose gradient (10 ml) in gradient buffer (50 mM Tris-HCl pH 7.4, 50 mM KCl, 10 mM MgCl2, 1 mM DTT, 100 U/ml RNaseOUT) as described previously [27], [50]. Ribosomal subunits (40S and 60S), monosomes (80S) and polyribosomes were sedimented by centrifugation in a Beckman SW40 Ti rotor at 35,000 rpm for 2.15 hours at 4°C and fractions were collected using an ISCO Density Gradient Fractionation System under constant monitoring of the absorbance at 254 nm. RNA was extracted from the free subunit (F), monosome (M), light polysome (L) and heavy polysome (H) fractions by phenol-chloroform followed by ethanol precipitation and analyzed by northern blot hybridization.
Metabolic Labeling
Approximately 5×107 L. infantum promastigotes grown in RPMI-1640 medium were subjected to either heat-shock from 25°C to 37°C or to low pH (from pH 7. 3 to pH 5.5) or to both conditions for different time periods. Thirty minutes before each time point, 0.5 ml of the culture was transferred to a methionine-free medium (Gibco) supplemented with 1 µCi/ml [35S]-methionine (GE Healthcare) and re-incubated under the same conditions for the rest of the stress period. Cells were pelleted, washed twice with ice-cold phosphate-buffer saline (PBS) and lysed in SDS-PAGE sample buffer. [35S]-methionine incorporation was measured with a scintillation counter (Beckman LS 6000TA). Samples were analyzed by SDS-PAGE and autoradiography.
Pulse Chase Assay
L. infantum promastigotes were washed twice in PBS and incubated in a methionine-free medium supplemented with 1 µCi/ml [35S]-methionine for 1 hour at 25°C. Following two washes in PBS, stress was induced in a cold methionine-containing medium (Gibco) at 37°C and pH 5.5. After different time points, 0.5 ml of culture were washed twice in PBS and the cells were lysed in Laemmli buffer. Non stressed cells were grown for the same period as the stressed cells and treated the same way. Values were normalized with the non-stressed control. [35S]-methionine incorporation was measured with a scintillation counter (Beckman LS 6000TA).
Supporting Information
Global translation is reduced in L. major promastigotes subjected to temperature and acidic pH stress. Polysome profile analysis of L. major LV39 promastigotes (no stress) and parasites exposed to a combined stress of elevated temperature (37°C) and acidic pH (5.5) for 4 hours. Cell lysates were sedimented on 15% to 45% sucrose gradients. Gradients were fractionated and absorbance (Abs) at 254 nm was continuously recorded. The 40S and 60S subunits, 80S monosome and polysome peaks are indicated. Data displayed represent one of two separate experiments.
(TIF)
Morphological changes of L. infantum during axenic amastigote differentiation. Morphological analysis of L. infantum axenic differentiation from elongated flagellated promastigotes into round aflagellated amastigote-like forms using a phase-contrast microscope. L. infantum promastigotes (Pro) exposed to either elevated temperature (37°C) or a combination of high temperature and low pH (pH 5.5) for 4 and 8 hours, respectively are shown here.
(TIF)
Acknowledgments
We thank Dr Greg Matlashewski (McGill University) for kindly providing us with the A2 antibody.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by an operating grant from the Canadian Institutes of Health Research (CIHR) (MOP-12182) awarded to BP. SC was awarded by a CIHR Research Doctoral Fellowship and by a doctoral research bridging award from the Centre for Host-Parasite Interactions of the « Fonds Québecois de la Recherche sur la Nature et les Technologies ». CC was awarded by a postdoctoral fellowship from the CIHR STP-53924 Strategic Training Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Turco SJ, Sacks DL. Expression of a stage-specific lipophosphoglycan in Leishmania major amastigotes. Mol Biochem Parasitol. 1991;45:91–99. doi: 10.1016/0166-6851(91)90030-a. [DOI] [PubMed] [Google Scholar]
- 3.McConville MJ, Blackwell JM. Developmental changes in the glycosylated phosphatidylinositols of Leishmania donovani. Characterization of the promastigote and amastigote glycolipids. J Biol Chem. 1991;266:15170–15179. [PubMed] [Google Scholar]
- 4.Saar Y, Ransford A, Waldman E, Mazareb S, Amin-Spector S, et al. Characterization of developmentally-regulated activities in axenic amastigotes of Leishmania donovani. Mol Biochem Parasitol. 1998;95:9–20. doi: 10.1016/s0166-6851(98)00062-0. [DOI] [PubMed] [Google Scholar]
- 5.Goyard S, Segawa H, Gordon J, Showalter M, Duncan R, et al. An in vitro system for developmental and genetic studies of Leishmania donovani phosphoglycans. Mol Biochem Parasitol. 2003;130:31–42. doi: 10.1016/s0166-6851(03)00142-7. [DOI] [PubMed] [Google Scholar]
- 6.Clayton C, Shapira M. Post-transcriptional regulation of gene expression in trypanosomes and leishmanias. Mol Biochem Parasitol. 2007;156:93–101. doi: 10.1016/j.molbiopara.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Haile S, Papadopoulou B. Developmental regulation of gene expression in trypanosomatid parasitic protozoa. Curr Opin Microbiol. 2007;10:569–577. doi: 10.1016/j.mib.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Zilberstein D, Shapira M. The role of pH and temperature in the development of Leishmania parasites. Annu Rev Microbiol. 1994;48:449–470. doi: 10.1146/annurev.mi.48.100194.002313. [DOI] [PubMed] [Google Scholar]
- 9.Sereno D, Lemesre JL. Axenically cultured amastigote forms as an in vitro model for investigation of antileishmanial agents. Antimicrob Agents Chemother. 1997;41:972–976. doi: 10.1128/aac.41.5.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barak E, Amin-Spector S, Gerliak E, Goyard S, Holland N, et al. Differentiation of Leishmania donovani in host-free system: analysis of signal perception and response. Mol Biochem Parasitol. 2005;141:99–108. doi: 10.1016/j.molbiopara.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Lahav T, Sivam D, Volpin H, Ronen M, Tsigankov P, et al. Multiple levels of gene regulation mediate differentiation of the intracellular pathogen Leishmania. Faseb J. 2011;25:515–525. doi: 10.1096/fj.10-157529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNicoll F, Drummelsmith J, Muller M, Madore E, Boilard N, et al. A combined proteomic and transcriptomic approach to the study of stage differentiation in Leishmania infantum. Proteomics. 2006;6:3567–3581. doi: 10.1002/pmic.200500853. [DOI] [PubMed] [Google Scholar]
- 13.Saxena A, Lahav T, Holland N, Aggarwal G, Anupama A, et al. Analysis of the Leishmania donovani transcriptome reveals an ordered progression of transient and permanent changes in gene expression during differentiation. Mol Biochem Parasitol. 2007;152:53–65. doi: 10.1016/j.molbiopara.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rochette A, Raymond F, Corbeil J, Ouellette M, Papadopoulou B. Whole-genome comparative RNA expression profiling of axenic and intracellular amastigote forms of Leishmania infantum. Mol Biochem Parasitol. 2009;165:32–47. doi: 10.1016/j.molbiopara.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Rochette A, Raymond F, Ubeda JM, Smith M, Messier N, et al. Genome-wide gene expression profiling analysis of Leishmania major and Leishmania infantum developmental stages reveals substantial differences between the two species. BMC Genomics. 2008;9:255. doi: 10.1186/1471-2164-9-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenzweig D, Smith D, Opperdoes F, Stern S, Olafson RW, et al. Retooling Leishmania metabolism: from sand fly gut to human macrophage. Faseb J. 2008;22:590–602. doi: 10.1096/fj.07-9254com. [DOI] [PubMed] [Google Scholar]
- 17.Cohen-Freue G, Holzer TR, Forney JD, McMaster WR. Global gene expression in Leishmania. Int J Parasitol. 2007;37:1077–1086. doi: 10.1016/j.ijpara.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Paape D, Lippuner C, Schmid M, Ackermann R, Barrios-Llerena ME, et al. Transgenic, fluorescent Leishmania mexicana allow direct analysis of the proteome of intracellular amastigotes. Mol Cell Proteomics. 2008;7:1688–1701. doi: 10.1074/mcp.M700343-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morales MA, Watanabe R, Laurent C, Lenormand P, Rousselle JC, et al. Phosphoproteomic analysis of Leishmania donovani pro- and amastigote stages. Proteomics. 2008;8:350–363. doi: 10.1002/pmic.200700697. [DOI] [PubMed] [Google Scholar]
- 20.Morales MA, Watanabe R, Dacher M, Chafey P, Osorio y Fortea J, et al. Phosphoproteome dynamics reveal heat-shock protein complexes specific to the Leishmania donovani infectious stage. Proc Natl Acad Sci U S A. 2010;107:8381–8386. doi: 10.1073/pnas.0914768107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hem S, Gherardini PF, Osorio y Fortea J, Hourdel V, Morales MA, et al. Identification of Leishmania-specific protein phosphorylation sites by LC-ESI-MS/MS and comparative genomics analyses. Proteomics. 2010;10:3868–3883. doi: 10.1002/pmic.201000305. [DOI] [PubMed] [Google Scholar]
- 22.Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nat Rev Mol Cell Biol. 2005;6:318–327. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]
- 23.Dever TE. Gene-specific regulation by general translation factors. Cell. 2002;108:545–556. doi: 10.1016/s0092-8674(02)00642-6. [DOI] [PubMed] [Google Scholar]
- 24.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 26.Ron D, Harding HP. eIF2α phosphorylation in celluar stress responses and disease. In: Mathews M, Sonenberg N, Hershey JWB, editors. Translational Control in Biology and Medicine. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2007. pp. 345–368. [Google Scholar]
- 27.Chow C, Cloutier S, Dumas C, Chou MN, Papadopoulou B. Promastigote to amastigote differentiation of Leishmania is markedly delayed in the absence of PERK eIF2alpha kinase-dependent eIF2alpha phosphorylation. Cell Microbiol. 2011;13:1059–1077. doi: 10.1111/j.1462-5822.2011.01602.x. [DOI] [PubMed] [Google Scholar]
- 28.Debrabant A, Joshi MB, Pimenta PF, Dwyer DM. Generation of Leishmania donovani axenic amastigotes: their growth and biological characteristics. Int J Parasitol. 2004;34:205–217. doi: 10.1016/j.ijpara.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Bates PA. The developmental biology of Leishmania promastigotes. Exp Parasitol. 1994;79:215–218. doi: 10.1006/expr.1994.1084. [DOI] [PubMed] [Google Scholar]
- 30.Alcolea PJ, Alonso A, Gomez MJ, Sanchez-Gorostiaga A, Moreno-Paz M, et al. Temperature increase prevails over acidification in gene expression modulation of amastigote differentiation in Leishmania infantum. BMC Genomics. 2010;11:31. doi: 10.1186/1471-2164-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kramer S, Queiroz R, Ellis L, Webb H, Hoheisel JD, et al. Heat shock causes a decrease in polysomes and the appearance of stress granules in trypanosomes independently of eIF2(alpha) phosphorylation at Thr169. J Cell Sci. 2008;121:3002–3014. doi: 10.1242/jcs.031823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sereno D, Cavaleyra M, Zemzoumi K, Maquaire S, Ouaissi A, et al. Axenically grown amastigotes of Leishmania infantum used as an in vitro model to investigate the pentavalent antimony mode of action. Antimicrob Agents Chemother. 1998;42:3097–3102. doi: 10.1128/aac.42.12.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Somanna A, Mundodi V, Gedamu L. In vitro cultivation and characterization of Leishmania chagasi amastigote-like forms. Acta Trop. 2002;83:37–42. doi: 10.1016/s0001-706x(02)00054-2. [DOI] [PubMed] [Google Scholar]
- 34.Bates PA, Robertson CD, Tetley L, Coombs GH. Axenic cultivation and characterization of Leishmania mexicana amastigote-like forms. Parasitology 105 (Pt. 1992;2):193–202. doi: 10.1017/s0031182000074102. [DOI] [PubMed] [Google Scholar]
- 35.Rainey PM, Spithill TW, McMahon-Pratt D, Pan AA. Biochemical and molecular characterization of Leishmania pifanoi amastigotes in continuous axenic culture. Mol Biochem Parasitol. 1991;49:111–118. doi: 10.1016/0166-6851(91)90134-r. [DOI] [PubMed] [Google Scholar]
- 36.Gupta N, Goyal N, Rastogi AK. In vitro cultivation and characterization of axenic amastigotes of Leishmania. Trends Parasitol. 2001;17:150–153. doi: 10.1016/s1471-4922(00)01811-0. [DOI] [PubMed] [Google Scholar]
- 37.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 38.Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol. 2005;59:407–450. doi: 10.1146/annurev.micro.59.031805.133833. [DOI] [PubMed] [Google Scholar]
- 39.Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A. 2004;101:11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yaman I, Fernandez J, Liu H, Caprara M, Komar AA, et al. The zipper model of translational control: a small upstream ORF is the switch that controls structural remodeling of an mRNA leader. Cell. 2003;113:519–531. doi: 10.1016/s0092-8674(03)00345-3. [DOI] [PubMed] [Google Scholar]
- 41.Charest H, Matlashewski G. Developmental gene expression in Leishmania donovani: differential cloning and analysis of an amastigote-stage-specific gene. Mol Cell Biol. 1994;14:2975–2984. doi: 10.1128/mcb.14.5.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tonelli RR, Augusto Lda S, Castilho BA, Schenkman S. Protein Synthesis Attenuation by Phosphorylation of eIF2alpha Is Required for the Differentiation of Trypanosoma cruzi into Infective Forms. PLoS One. 2011;6:e27904. doi: 10.1371/journal.pone.0027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moraes MC, Jesus TC, Hashimoto NN, Dey M, Schwartz KJ, et al. Novel membrane-bound eIF2alpha kinase in the flagellar pocket of Trypanosoma brucei. Eukaryot Cell. 2007;6:1979–1991. doi: 10.1128/EC.00249-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joyce BR, Queener SF, Wek RC, Sullivan WJ., Jr Phosphorylation of eukaryotic initiation factor-2{alpha} promotes the extracellular survival of obligate intracellular parasite Toxoplasma gondii. Proc Natl Acad Sci U S A. 2010;107:17200–17205. doi: 10.1073/pnas.1007610107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Narasimhan J, Joyce BR, Naguleswaran A, Smith AT, Livingston MR, et al. Translation regulation by eukaryotic initiation factor-2 kinases in the development of latent cysts in Toxoplasma gondii. J Biol Chem. 2008;283:16591–16601. doi: 10.1074/jbc.M800681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCall LI, Matlashewski G. Localization and induction of the A2 virulence factor in Leishmania: evidence that A2 is a stress response protein. Mol Microbiol. 2010;77:518–530. doi: 10.1111/j.1365-2958.2010.07229.x. [DOI] [PubMed] [Google Scholar]
- 47.McConville MJ, Naderer T. Annu Rev Microbiol; 2011. Metabolic Pathways Required for the Intracellular Survival of Leishmania. [DOI] [PubMed] [Google Scholar]
- 48.Wu Y, El Fakhry Y, Sereno D, Tamar S, Papadopoulou B. A new developmentally regulated gene family in Leishmania amastigotes encoding a homolog of amastin surface proteins. Mol Biochem Parasitol. 2000;110:345–357. doi: 10.1016/s0166-6851(00)00290-5. [DOI] [PubMed] [Google Scholar]
- 49.Sambrook J, Fritsch EF, Maniatis T. New York: Cold Spring Harbor Laboratory Press; 1989. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- 50.McNicoll F, Muller M, Cloutier S, Boilard N, Rochette A, et al. Distinct 3'-untranslated region elements regulate stage-specific mRNA accumulation and translation in Leishmania. J Biol Chem. 2005;280:35238–35246. doi: 10.1074/jbc.M507511200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Global translation is reduced in L. major promastigotes subjected to temperature and acidic pH stress. Polysome profile analysis of L. major LV39 promastigotes (no stress) and parasites exposed to a combined stress of elevated temperature (37°C) and acidic pH (5.5) for 4 hours. Cell lysates were sedimented on 15% to 45% sucrose gradients. Gradients were fractionated and absorbance (Abs) at 254 nm was continuously recorded. The 40S and 60S subunits, 80S monosome and polysome peaks are indicated. Data displayed represent one of two separate experiments.
(TIF)
Morphological changes of L. infantum during axenic amastigote differentiation. Morphological analysis of L. infantum axenic differentiation from elongated flagellated promastigotes into round aflagellated amastigote-like forms using a phase-contrast microscope. L. infantum promastigotes (Pro) exposed to either elevated temperature (37°C) or a combination of high temperature and low pH (pH 5.5) for 4 and 8 hours, respectively are shown here.
(TIF)