Abstract
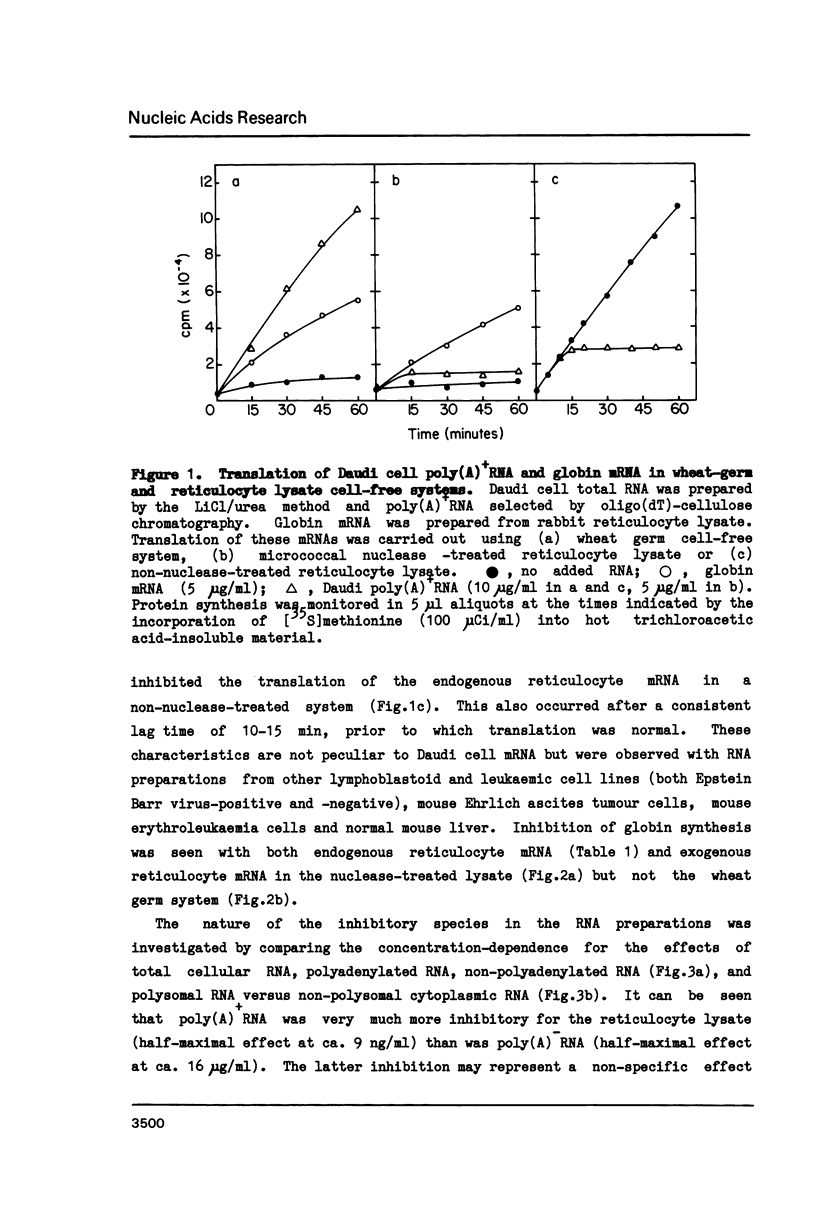
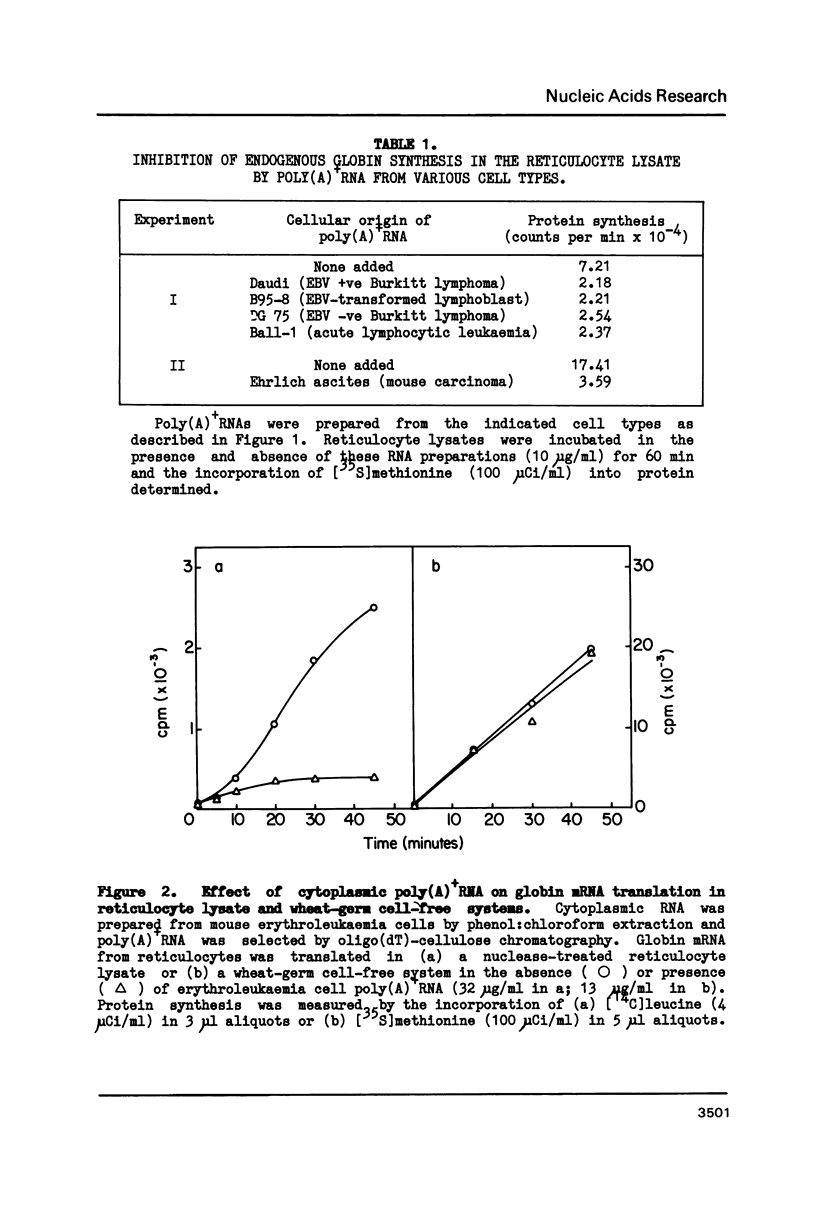
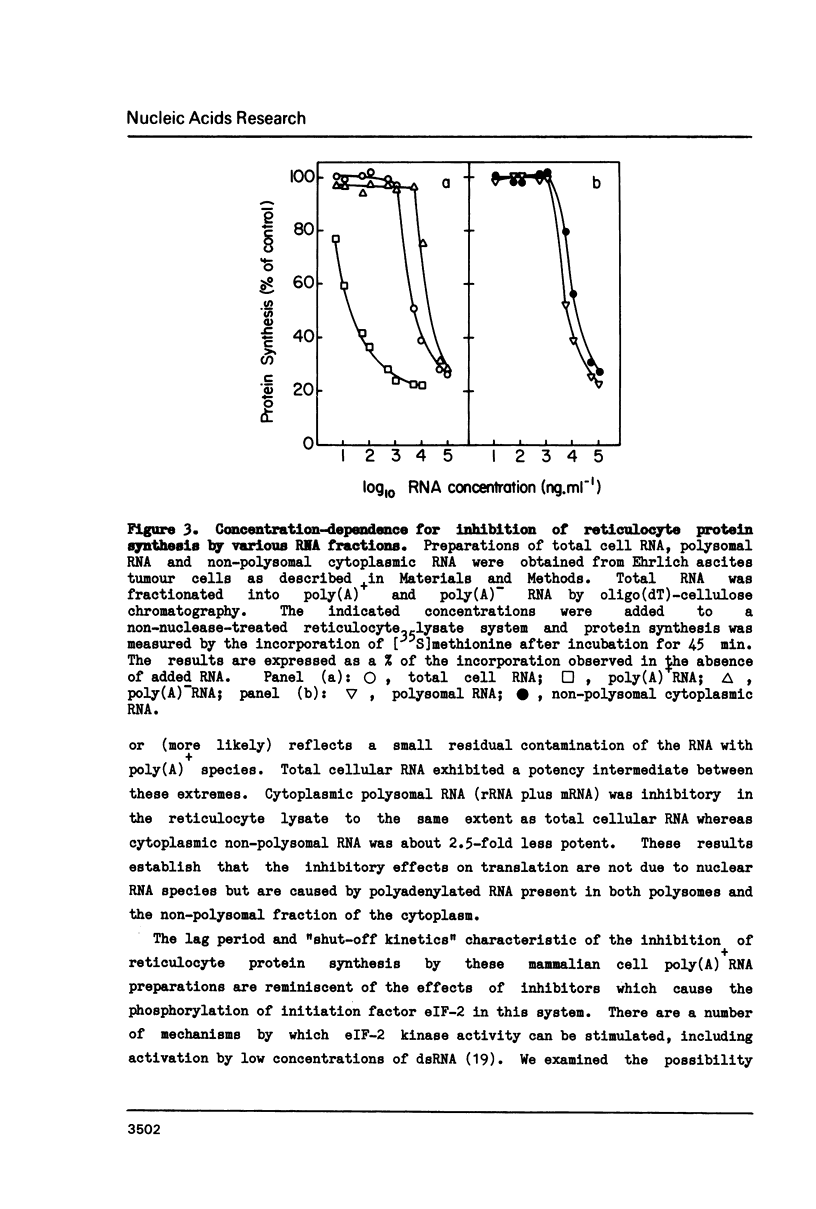
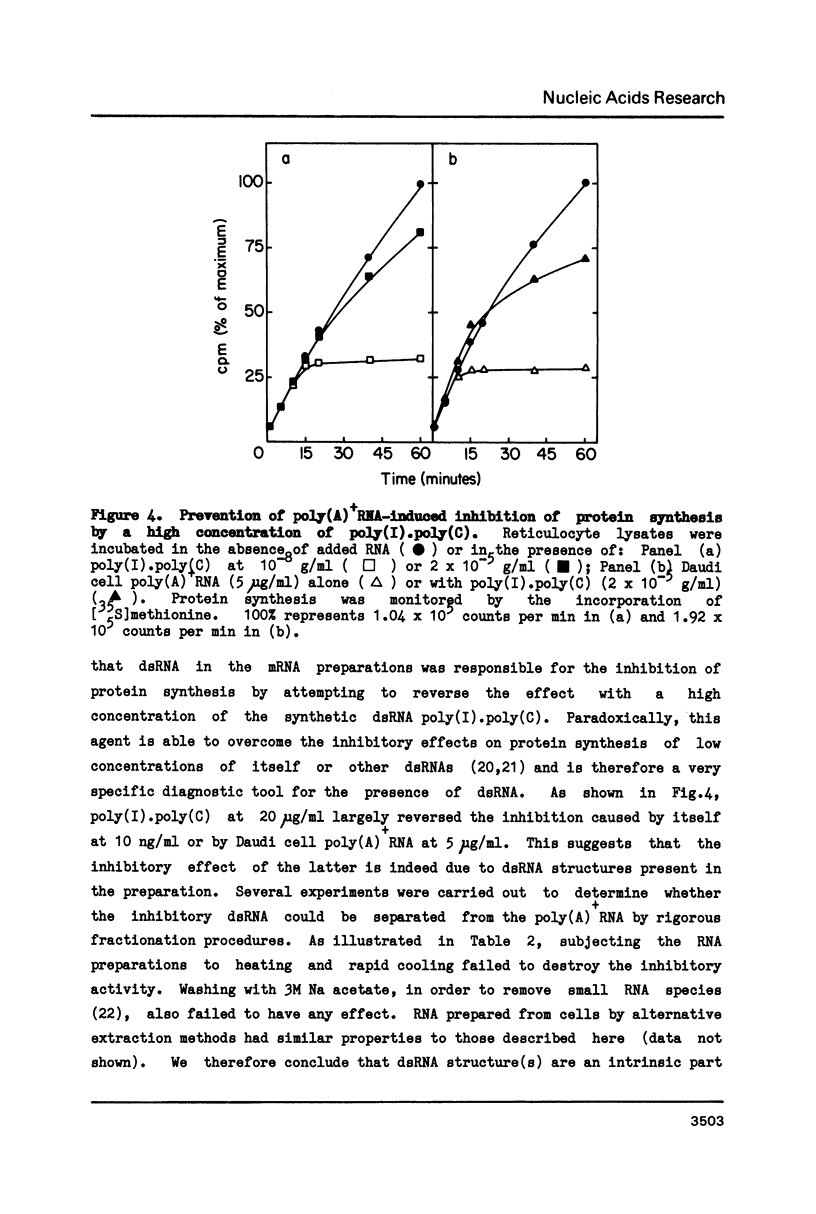
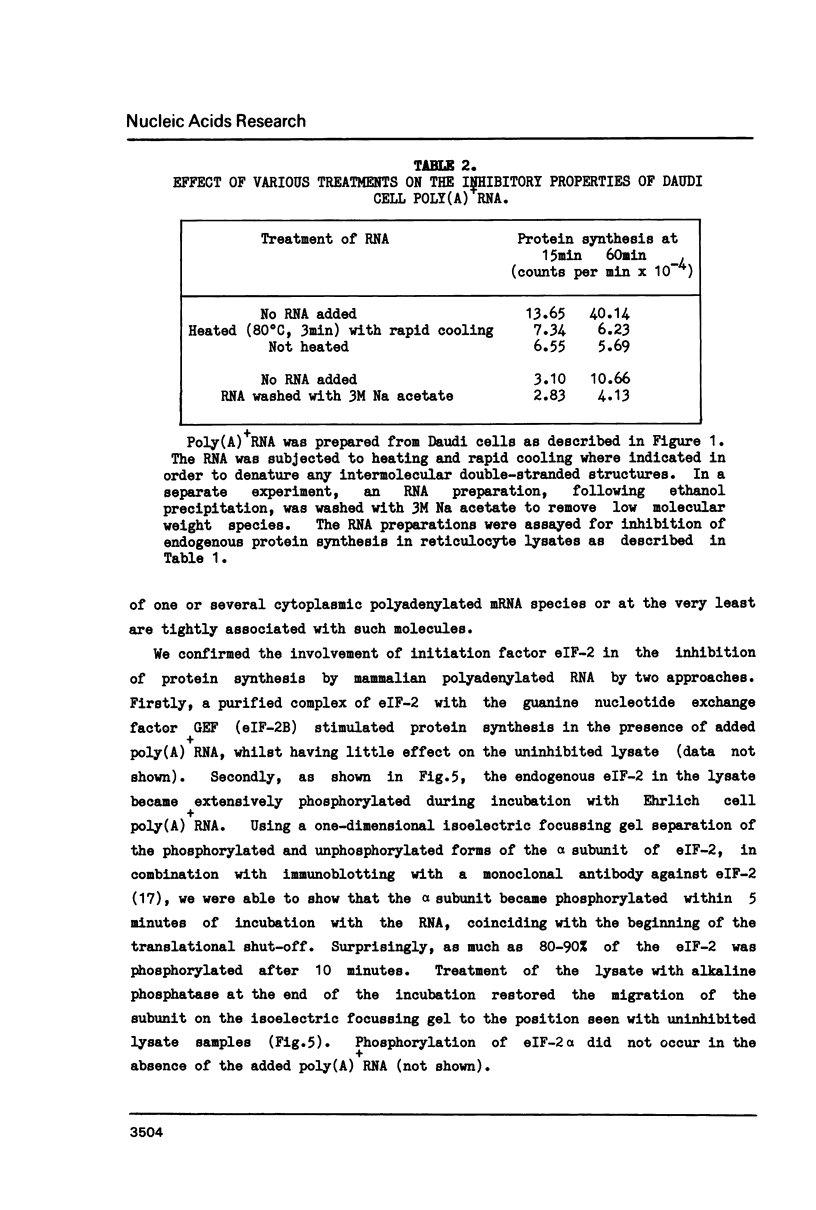
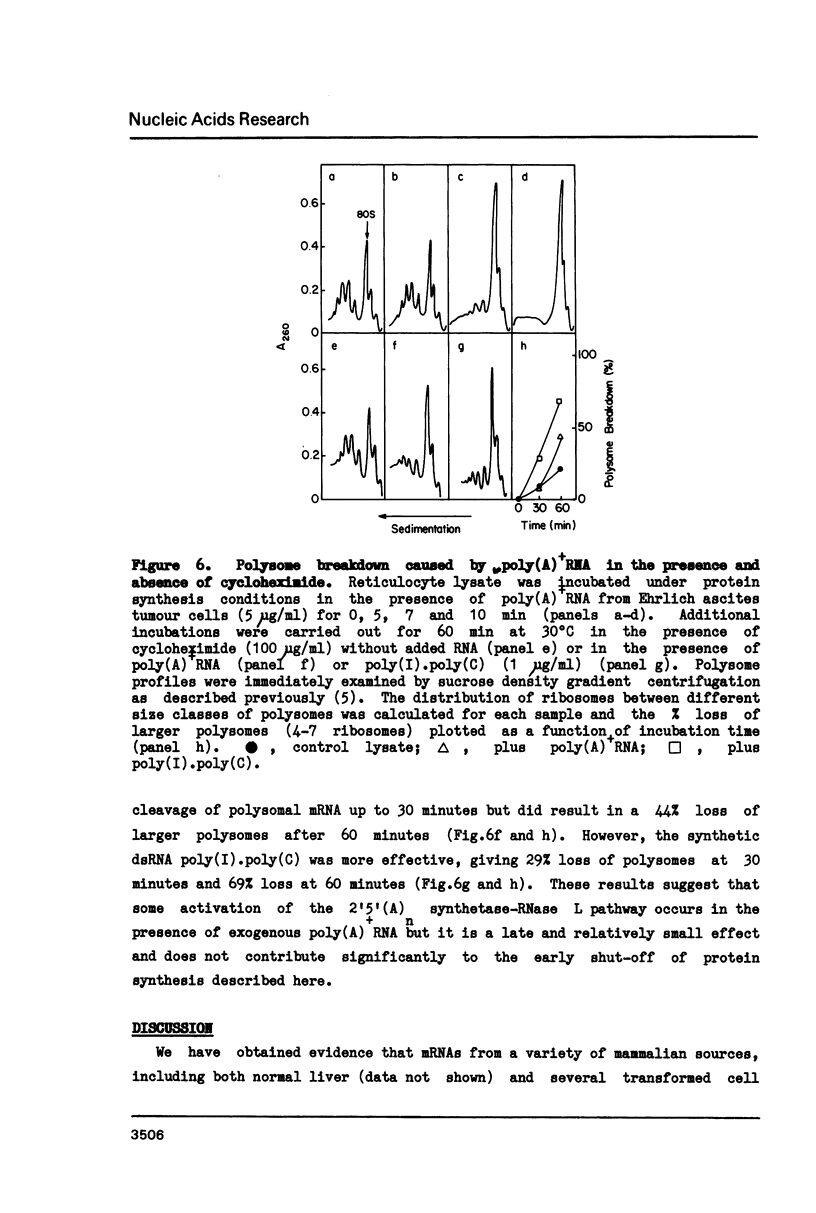
Polyadenylated mRNA has been purified from a variety of human and mouse cell sources. These preparations are actively translated in the wheat germ cell-free system but have only poor ability to stimulate the nuclease-treated reticulocyte lysate. The translation of endogenous and exogenous globin mRNA is strongly inhibited by the poly(A)+ RNA preparations in reticulocyte lysates. Both polysomal and non-polysomal RNA have similar effects but poly(A)+ RNA is almost 2000-fold more inhibitory than poly(A)-RNA on a weight basis. The inhibition is abolished in the presence a high concentration of poly(I).poly(C). Analysis of endogenous eIF-2 in the lysate reveals that the subunit becomes extensively phosphorylated in the presence of the inhibitory poly(A)+ RNA. Prolonged incubation of lysate with poly(A)+ RNA also causes some nucleolytic degradation of polysomal globin mRNA. These characteristics suggest that some eukaryotic cell mRNAs contain regions of double-stranded structure which are sufficiently extensive to activate translational control mechanisms in the reticulocyte lysate.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bablanian R., Banerjee A. K. Poly(riboadenylic acid) preferentially inhibits in vitro translation of cellular mRNAs compared with vaccinia virus mRNAs: possible role in vaccinia virus cytopathology. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1290–1294. doi: 10.1073/pnas.83.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bases R., Kaplan B. H. Double-stranded RNA from HeLa cell nuclei inhibits initiation of protein synthesis. Biochim Biophys Acta. 1973 Jul 13;312(3):574–580. doi: 10.1016/0005-2787(73)90455-3. [DOI] [PubMed] [Google Scholar]
- Baum E. Z., Ernst V. G. Inhibition of protein synthesis in reticulocyte lysates by a double-stranded RNA component in HeLa mRNA. Biochem Biophys Res Commun. 1983 Jul 18;114(1):41–49. doi: 10.1016/0006-291x(83)91591-7. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., McNurlan M. A. Regulation of cell proliferation and differentiation by interferons. Biochem J. 1985 Mar 1;226(2):345–360. doi: 10.1042/bj2260345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens M. J., Safer B., Merrick W. C., Anderson W. F., London I. M. Inhibition of protein synthesis in rabbit reticulocyte lysates by double-stranded RNA and oxidized glutathione: indirect mode of action on polypeptide chain initiation. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1286–1290. doi: 10.1073/pnas.72.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens M. J., Williams B. R. Inhibition of cell-free protein synthesis by pppA2'p5'A2'p5'A: a novel oligonucleotide synthesized by interferon-treated L cell extracts. Cell. 1978 Mar;13(3):565–572. doi: 10.1016/0092-8674(78)90329-x. [DOI] [PubMed] [Google Scholar]
- Colby W. W., Chen E. Y., Smith D. H., Levinson A. D. Identification and nucleotide sequence of a human locus homologous to the v-myc oncogene of avian myelocytomatosis virus MC29. Nature. 1983 Feb 24;301(5902):722–725. doi: 10.1038/301722a0. [DOI] [PubMed] [Google Scholar]
- Content J., Lebleu B., De Clercq E. Differential effects of various double-stranded RNAs on protein synthesis in rabbit reticulocyte lysates. Biochemistry. 1978 Jan 10;17(1):88–94. doi: 10.1021/bi00594a012. [DOI] [PubMed] [Google Scholar]
- De Benedetti A., Baglioni C. Inhibition of mRNA binding to ribosomes by localized activation of dsRNA-dependent protein kinase. Nature. 1984 Sep 6;311(5981):79–81. doi: 10.1038/311079a0. [DOI] [PubMed] [Google Scholar]
- Dionne C. A., Stearns G. B., Kramer G., Hardesty B. Inhibition of peptide initiation by a low molecular weight RNA from rabbit reticulocytes. J Biol Chem. 1982 Oct 25;257(20):12373–12379. [PubMed] [Google Scholar]
- Ehrenfeld E., Hunt T. Double-stranded poliovirus RNA inhibits initiation of protein synthesis by reticulocyte lysates. Proc Natl Acad Sci U S A. 1971 May;68(5):1075–1078. doi: 10.1073/pnas.68.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Sen G. C., Dubois M. F., Ratner L., Slattery E., Lengyel P. Interferon action: two distinct pathways for inhibition of protein synthesis by double-stranded RNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5893–5897. doi: 10.1073/pnas.75.12.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewert D. R., Moore G., Clemens M. J. Inhibition of cell division by interferons. The relationship between changes in utilization of thymidine for DNA synthesis and control of proliferation in Daudi cells. Biochem J. 1983 Sep 15;214(3):983–990. doi: 10.1042/bj2140983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Kerr I. M. Synthesis of an oligonucleotide inhibitor of protein synthesis in rabbit reticulocyte lysates analogous to that formed in extracts from interferon-treated cells. Eur J Biochem. 1978 Mar;84(1):149–159. doi: 10.1111/j.1432-1033.1978.tb12151.x. [DOI] [PubMed] [Google Scholar]
- Hunter T., Hunt T., Jackson R. J., Robertson H. D. The characteristics of inhibition of protein synthesis by double-stranded ribonucleic acid in reticulocyte lysates. J Biol Chem. 1975 Jan 25;250(2):409–417. [PubMed] [Google Scholar]
- Jacobson A., Favreau M. Possible involvement of poly(A) in protein synthesis. Nucleic Acids Res. 1983 Sep 24;11(18):6353–6368. doi: 10.1093/nar/11.18.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkosky J. W., Wood W. M., Edmonds M. Location of oligo(uridylic acid) sequences within messenger ribonucleic acid molecules of HeLa cells. Biochemistry. 1985 Jul 2;24(14):3678–3686. doi: 10.1021/bi00335a042. [DOI] [PubMed] [Google Scholar]
- Lemay G., Millward S. Inhibition of translation in L-cell lysates by free polyadenylic acid: differences in sensitivity among different mRNAs and possible involvement of an initiation factor. Arch Biochem Biophys. 1986 Aug 15;249(1):191–198. doi: 10.1016/0003-9861(86)90574-6. [DOI] [PubMed] [Google Scholar]
- Levin D. H., Petryshyn R., London I. M. Characterization of double-stranded-RNA-activated kinase that phosphorylates alpha subunit of eukaryotic initiation factor 2 (eIF-2 alpha) in reticulocyte lysates. Proc Natl Acad Sci U S A. 1980 Feb;77(2):832–836. doi: 10.1073/pnas.77.2.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J. L., Pryhitka B. Structural and functional aspects of oligo(uridylic acid)-containing and poly(adenylic acid)-containing ribonucleic acids from rat liver. Biochem Biophys Res Commun. 1982 Aug;107(3):989–997. doi: 10.1016/0006-291x(82)90620-9. [DOI] [PubMed] [Google Scholar]
- Nielsen P. J., McMaster G. K., Trachsel H. Cloning of eukaryotic protein synthesis initiation factor genes: isolation and characterization of cDNA clones encoding factor eIF-4A. Nucleic Acids Res. 1985 Oct 11;13(19):6867–6880. doi: 10.1093/nar/13.19.6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain V. M. Initiation of protein synthesis in mammalian cells. Biochem J. 1986 May 1;235(3):625–637. doi: 10.1042/bj2350625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryshyn R., Chen J. J., London I. M. Growth-related expression of a double-stranded RNA-dependent protein kinase in 3T3 cells. J Biol Chem. 1984 Dec 10;259(23):14736–14742. [PubMed] [Google Scholar]
- Ponte P., Ng S. Y., Engel J., Gunning P., Kedes L. Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucleic Acids Res. 1984 Feb 10;12(3):1687–1696. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. D., Hunter T. Sensitive methods for the detection and characterization of double helical ribonucleic acid. J Biol Chem. 1975 Jan 25;250(2):418–425. [PubMed] [Google Scholar]
- Rychlik W., Domier L. L., Gardner P. R., Hellmann G. M., Rhoads R. E. Amino acid sequence of the mRNA cap-binding protein from human tissues. Proc Natl Acad Sci U S A. 1987 Feb;84(4):945–949. doi: 10.1073/pnas.84.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel C. E., Duncan R., Knutson G. S., Hershey J. W. Mechanism of interferon action. Increased phosphorylation of protein synthesis initiation factor eIF-2 alpha in interferon-treated, reovirus-infected mouse L929 fibroblasts in vitro and in vivo. J Biol Chem. 1984 Nov 10;259(21):13451–13457. [PubMed] [Google Scholar]
- Sarkar S., Mukherjee A. K., Guha C. A ribonuclease-resistant cytoplasmic 10 S ribonucleoprotein of chick embryonic muscle. A potent inhibitor of cell-free protein synthesis. J Biol Chem. 1981 May 25;256(10):5077–5086. [PubMed] [Google Scholar]
- Sarma M. H., Beach T. A., Chatterjee N. K. Inhibition of exogenous RNA-dependent protein synthesis by a low-molecular-weight RNA from nuclear ribonucleoprotein particles of adenovirus-infected HeLa cells. Biochem Biophys Res Commun. 1978 May 15;82(1):384–391. doi: 10.1016/0006-291x(78)90621-6. [DOI] [PubMed] [Google Scholar]
- Scorsone K. A., Panniers R., Rowlands A. G., Henshaw E. C. Phosphorylation of eukaryotic initiation factor 2 during physiological stresses which affect protein synthesis. J Biol Chem. 1987 Oct 25;262(30):14538–14543. [PubMed] [Google Scholar]
- Shore G. C., Tata J. R. Two fractions of rough endoplasmic reticulum from rat liver. II. Cytoplasmic messenger RNA's which code for albumin and mitochondrial proteins are distributed differently between the two fractions. J Cell Biol. 1977 Mar;72(3):726–743. doi: 10.1083/jcb.72.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery E., Ghosh N., Samanta H., Lengyel P. Interferon, double-stranded RNA, and RNA degradation: activation of an endonuclease by (2'-5')An. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4778–4782. doi: 10.1073/pnas.76.10.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torelli U., Cadossi R., Torelli G., Ferrari S., Ferrari S. Inhibition of protein synthesis in reticulocyte lysates by double-stranded ribonucleic acid extracted from nuclei of leukemic cells. FEBS Lett. 1975 Aug 1;56(1):96–100. doi: 10.1016/0014-5793(75)80119-0. [DOI] [PubMed] [Google Scholar]
- Van Ventrooij W. J., Henshaw E. C., Hirsch C. A. Nutritional effects on the polyribosome distribution and rate of protein synthesis in Ehrlich ascites tumor cells in culture. J Biol Chem. 1970 Nov 25;245(22):5947–5953. [PubMed] [Google Scholar]
- Williams B. R., Gilbert C. S., Kerr I. M. The respective roles of the protein kinase and pppA2' p5' A2' p5 A-activated endonuclease in the inhibition of protein synthesis by double stranded RNA in rabbit reticulocyte lysates. Nucleic Acids Res. 1979 Apr;6(4):1335–1350. doi: 10.1093/nar/6.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. R., Golgher R. R., Brown R. E., Gilbert C. S., Kerr I. M. Natural occurrence of 2-5A in interferon-treated EMC virus-infected L cells. Nature. 1979 Dec 6;282(5739):582–586. doi: 10.1038/282582a0. [DOI] [PubMed] [Google Scholar]
- Wong S. T., Mastropaolo W., Henshaw E. C. Differential phosphorylation of soluble versus ribosome-bound eukaryotic initiation factor 2 in the Ehrlich ascites tumor cell. J Biol Chem. 1982 May 10;257(9):5231–5238. [PubMed] [Google Scholar]
- Wood W. M., Wallace J. C., Edmonds M. Sequence content of oligo(uridylic acid)-containing messenger ribonucleic acid from HeLa cells. Biochemistry. 1985 Jul 2;24(14):3686–3693. doi: 10.1021/bi00335a043. [DOI] [PubMed] [Google Scholar]
- Wreschner D. H., James T. C., Silverman R. H., Kerr I. M. Ribosomal RNA cleavage, nuclease activation and 2-5A(ppp(A2'p)nA) in interferon-treated cells. Nucleic Acids Res. 1981 Apr 10;9(7):1571–1581. doi: 10.1093/nar/9.7.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]




