Abstract
Study Objectives:
To evaluate functional outcomes in adults with REM-related obstructive sleep apnea (OSA) treated with positive airway pressure (PAP) therapy.
Design:
Retrospective observational study.
Setting:
Outpatient sleep clinic.
Patients:
330 adults (171 males) with OSA receiving PAP therapy, including 130 with REM OSA and 200 with OSA not restricted to REM.
Measurements and Results:
REM OSA was defined as a REM apnea-hypopnea index (AHI) / NREM AHI > 2 and NREM AHI < 15. Patients had baseline and post-PAP functional outcomes, including Epworth Sleepiness Scale (ESS), Fatigue Severity Scale (FSS), Patient Health Questionnaire-9 (PHQ-9), and Functional Outcomes Sleep Questionnaire (FOSQ) scores. We compared functional outcomes, demographic, clinical and polysomnographic features, and PAP adherence in patients with REM OSA and OSA not restricted to REM. Female gender was significantly more common in REM OSA. Age, BMI, neck girth, and baseline ESS, FSS, PHQ-9, and FOSQ were similar between groups. Smoking history and comorbid disorders were also similar except for a higher prevalence of depression and cardiovascular disease in OSA not restricted to REM. All functional outcomes improved significantly after PAP therapy in both groups. Change from baseline to post treatment was similar for all functional outcomes between groups.
Conclusions:
The study is the first addressing clinical outcomes in REM OSA using validated measures. Functional outcomes in patients with REM OSA improve after treatment with PAP therapy comparable to that observed in patients with OSA not restricted to REM.
Commentary:
A commentary on this article appears in this issue on page 249.
Citation:
Su CS; Liu KT; Panjapornpon K; Andrews N; Foldvary-Schaefer N. Functional outcomes in patients with rem-related obstructive sleep apnea treated with positive airway pressure therapy. J Clin Sleep Med 2012;8(3):243-247.
Keywords: REM OSA, obstructive sleep apnea, positive airway pressure, Epworth Sleepiness Scale, Fatigue Severity Scale, Patient Health Questionnaire-9, Functional Outcomes Sleep Questionnaire
Obstructive sleep apnea (OSA) is a common health problem, affecting 5% to 24% of men and 1% to 9% of women,1–3 and increasing with advancing age.4 The most common presenting symptoms of OSA are daytime sleepiness or fatigue, snoring, and witnessed apneas.5,6 Associations between OSA and a variety of adverse health and social outcomes including hypertension, cardiovascular disease, stroke, glucose intolerance, and traffic or occupational accidents have been reported.7–11 Positive airway pressure (PAP) therapy is the gold standard treatment of moderate to severe OSA.12,13 Patients treated with PAP therapy experience improvement in sleepiness, mood, cognitive impairment, and quality of life, as well as reductions in blood pressure, serum lipids, insulin sensitivity, risk of cardiovascular death, acute coronary syndrome, and atherogenic plaques.7,–15
REM OSA, in which respiratory events occur exclusively or predominately in REM, is estimated to constitute 14% to 36% of all OSA cases.16–19 Previous investigators have found REM OSA to be more common in females, particularly in those presenting with depressive symptoms.16,18,19 REM sleep is characterized by high-frequency irregular respirations, decreased tidal volume and minute ventilation, and reduced responsiveness to respiratory modulation.12 Respiratory events in patients with OSA are often more frequent and longer in REM sleep with more profound oxygen desaturation than in any other sleep stage, yet more resistant to eliciting arousal than respiratory events in NREM sleep. However, the clinical manifestations and response to treatment of REM OSA are poorly elucidated. Specifically, it remains unproven as to whether REM OSA accounts for the same degree of daytime functional impairment as general OSA; treatment outcomes have not been reported.
BRIEF SUMMARY
Current Knowledge/Study Rationale: The clinical manifestations of REM OSA are incompletely elucidated. It is unclear whether REM OSA accounts for the same degree of daytime functional impairment as general OSA. This study was conducted to characterize clinical characteristics and functional outcomes in patients with REM OSA treated with PAP therapy compared to a group of OSA patients without REM-predominant respiratory events.
Study Impact: Functional outcomes in patients with REM OSA improved after treatment with PAP therapy comparable to those observed in patients with OSA not restricted to REM. While the study's limitations preclude using these findings to alter patient care strategies, we believe these observations are important and may prompt prospective studies addressing treatment outcomes for REM OSA.
Since 2007, all patients presenting to the Cleveland Clinic Sleep Disorders Clinic have completed standardized assessments of general and sleep functional outcomes, called the Knowledge Program (KP), at each outpatient clinic appointment. This study was conducted using KP data to characterize clinical characteristics and functional outcomes in patients with REM OSA treated with PAP therapy compared to a group of OSA patients without REM-predominant respiratory events.
METHODS
This was a retrospective study approved by the Institutional Review Board of the Cleveland Clinic.
Patient Selection
Consecutive patients with a diagnostic polysomnogram (PSG) performed at the Cleveland Clinic Sleep Disorders Center between January 1, 2008, and December 31, 2009, were identified. Inclusion criteria were as follows: (1) age ≥ 18 years; (2) overall apnea-hypopnea index (AHI) ≥ 5/h; (3) total sleep time ≥ 150 min and total time spent in REM sleep ≥ 10 min; (4) treated with PAP therapy; and (5) baseline and at least one post-PAP (30-90 days) clinic visit with completed functional assessments. REM OSA was defined as REM AHI /NREM AHI > 2 and NREM AHI < 15. Patients with an AHI not meeting the criteria for REM OSA constituted the OSA group.
A review of the electronic medical record (EMR) was performed for demographic data, smoking status, comorbid systemic diseases, and comorbid sleep disorders. Comorbid systemic disorders assessed included hypertension, diabetes mellitus, hyperlipidemia, cardiovascular disease (coronary artery disease, myocardial ischemia/infarction, and stroke), heart failure, pulmonary disease, hypothyroidism, and depression. Comorbid conditions were identified through the Past Medical History tab, typically completed by PCPs, and office notes from PCPs and specialists in the EMR.
Polysomnography
Polysomnograms were performed using standard guidelines.20 Hypopnea was defined as a reduction in airflow ≥ 50% in the nasal pressure channel for ≥ 10 sec resulting in an arousal or ≥ 3% oxygen desaturation.
PAP titration studies were performed following laboratory protocol beginning at a pressure of 5 cm H2O and increasing by 2 cm H2O for respiratory events and 1 cm H2O for arousals, cyclical desaturations, and snoring, aiming to record REM and supine sleep. PAP titration studies were categorized as optimal, good, adequate, or inadequate based on the quality of the study. An optimal titration normalized the AHI for ≥ 15 min and included supine REM sleep at the selected pressure. A good titration reduced the AHI to < 10 or by 50% if the baseline AHI was < 15 and included supine REM sleep at the selected pressure. An adequate titration did not reduce the AHI to < 10 but reduced it by 75% from baseline or did not record supine REM sleep at the selected pressure. Titration studies not meeting any of the above criteria (including pressure or interface intolerance) and patients who did not undergo laboratory titration excluding autoPAP cases were deemed inadequate.
PAP Adherence
Adherence to therapy, defined as average number of hours per night of PAP use and ≥ 4 h use on average, was determined by subject self-report at the post-PAP visit and objectively during the first 90 days of treatment, when available.21
Functional Outcomes
The following outcomes were measured at baseline and post-PAP.
Epworth Sleepiness Scale (ESS)
The ESS22 is the most widely used questionnaire to assess subjective daytime sleepiness in sleep disorder subjects. Composed of 8 items of daily living, subjects rate their chance of dozing on a scale ranging from never (0) to high chance of dozing (3). Responses to the 8 items are tallied, producing a total score from 0 to 24. An ESS score > 10 is considered indicative of EDS.
Fatigue Severity Scale (FSS)
The FSS23 is a 9-item self-report questionnaire to evaluate the severity of fatigue in different situations. Each item is graded on a scale from 1 (disagree) to 7 (agree). The total score range is 9-63, and a score ≥ 36 is abnormal.
Patient Health Questionnaire-9 (PHQ-9)
The PHQ-924 is a self-administered questionnaire based on 9 DSM-IV criteria of depression. Each item is graded as 0 (not at all) to 3 (nearly every day). A score ≥ 10 is abnormal, having a sensitivity of 88% and specificity of 88% for depression.
Functional Outcomes Sleep Questionnaire (FOSQ)
The FOSQ25 is a self-reported assessment measuring functional measures in OSA. It includes 30 items categorized in each of 5 subscales including activity level, vigilance, intimacy and sexual relationships, general productivity, and social outcome. Responses are graded from no (4) to extreme (1) difficulty. The total score is the sum of the subscale scores. Lower scores indicate greater functional impairment.
Statistical Analysis
Analyses were performed using SPSS. Continuous variables are presented as mean ± SD and categorical variables as n (%). T-tests and χ2 or Fisher exact tests were used to compare differences between groups on continuous and categorical variables, respectively. Paired t-tests were used to compare baseline and post-PAP functional outcomes between groups. Probability values ≤ 0.05 were considered significant.
RESULTS
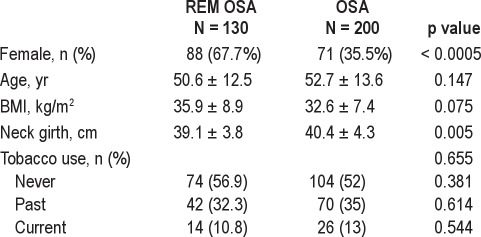
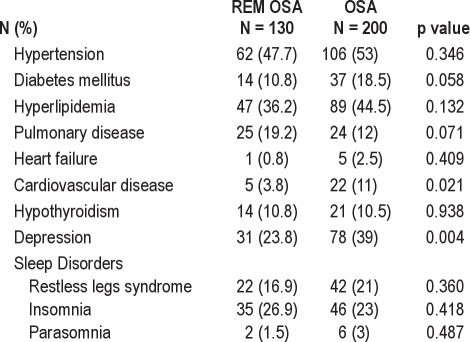
Subject and PSG characteristics are summarized in Tables 1, 2, and 3. Overall, 330 patients (171 males) were included. One hundred thirty subjects met criteria for REM OSA, and 200 subjects constituted the OSA group in which events were not restricted to REM. Female gender and smaller neck girth were significantly more common in REM OSA. No differences were found between groups for age, BMI, or smoking status, although male subjects with REM OSA were significantly younger than males in the OSA group (REM: 48.9 ± 14.4 years; OSA: 53.9 ± 13.7 years, p = 0.05). The prevalence of depression and cardiovascular disease (including coronary artery disease, myocardial ischemia/infarction, and stroke) was higher in subjects with OSA not restricted to REM.
Table 1.
Sample characteristics
Table 2.
Comorbid disorders by OSA group
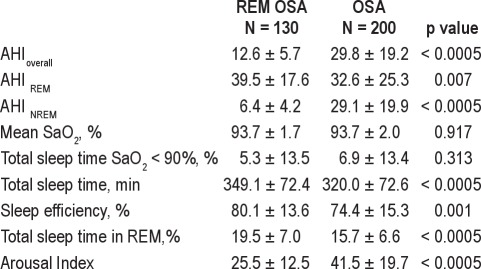
Table 3.
Baseline polysomnographic variables by OSA group
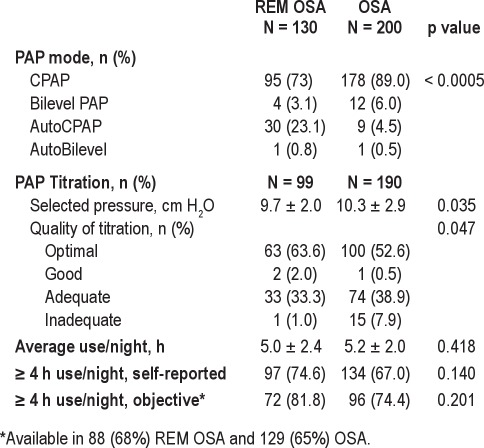
Table 4 summarizes the results of PAP titration studies, modes of therapy, and PAP adherence. Thirty-one subjects (23.9%) with REM OSA and 10 (5%) with OSA used AutoPAP (p < 0.0005). Subjects with REM OSA had fewer inadequate titrations (p = 0.047) and lower optimal pressure (p = 0.035) than the OSA group. Adherence was not different between groups.
Table 4.
PAP therapy and adherence by OSA group
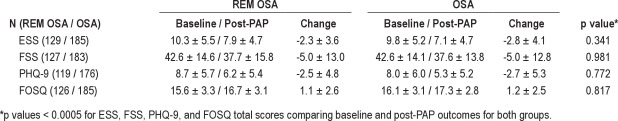
Baseline and post-PAP functional outcomes are shown in Table 5 excluding subjects with inadequate titration studies. There was no difference in baseline scores between groups. Significant and comparable improvement was observed in the ESS, FSS, PHQ-9, and FOSQ total scores for both groups.
Table 5.
Functional outcomes by OSA group
DISCUSSION
This is the first study to investigate PAP therapy functional outcomes in patients with REM OSA. Our primary finding is that patients with REM OSA achieve measurable improvement in functional outcomes with PAP therapy. These changes were comparable to those observed in patients with more severe OSA not restricted to REM sleep. Our study extends these observations by demonstrating improvements in daytime sleepiness, fatigue, mood, and overall functional status with PAP therapy.
Early investigations of REM OSA were largely limited to clinical characteristics, demonstrating a female predominance of REM OSA, particularly in younger patients.16,17,19 Subsequent reports examined daytime sleepiness in patients with REM OSA, some correlating subjective and objective measures.,–29 Results of these studies are conflicting. Consequently, CPAP therapy may be considered for patients with REM OSA since a subset of affected individuals appear to respond to therapy.30
Yet the clinical presentation of REM OSA remains incompletely elucidated and would be expected to differ from that of OSA not restricted to REM due to differences in arousal responses to respiratory stimuli, with greater intensities required to produce an arousal in REM than NREM sleep.31 In a cross-sectional study of over 1800 subjects with OSA with mean AHI of 38, the NREM AHI and not the REM AHI correlated with scores on the MSLT.27 REM OSA was defined by the lowest quartile of NREM AHI being < 8.3, in contrast to the criteria applied to our sample. The authors proposed an alternative method of disease classification based on state dependent AHI, more meaningful than the overall AHI that can be discrepant with clinical symptoms in patients with respiratory events that are strikingly state dependent. In another cross-sectional analysis involving over 5000 Sleep Heart Health Study participants, in the absence of NREM OSA, REM OSA was not independently associated with daytime sleepiness measured by the ESS, impaired health-related QOL, or self-reported sleep disruption.29 Studies correlating the ESS and MSLT have been conflicting.32 However, a recent report found a clear association between the ESS and the average sleep latency on the MSLT using a survival analysis.33 While we found improvements in the ESS with PAP therapy in REM OSA comparable to OSA not restricted to REM, it is possible that a variety of factors not measurable, such as subjects' desire for treatment and mood, may have contributed to this finding not observed in prior studies.
In our series, REM OSA constituted 39.4% of all patients with an overall AHI ≥ 5 meeting inclusion criteria. Several definitions of REM OSA have been proposed. We used a previously published definition of REM AHI / NREM AHI > 2 and NREM AHI < 15.18 Prior studies using the same definition also found a higher prevalence of REM OSA in female subjects.16,18,19 The proposed explanation for this finding is a protective airway effect by progesterone in NREM sleep and smaller upper airway dimension rendering it more vulnerable to collapse in REM sleep.16,19 Female subjects with REM OSA were more obese than males.16 In the current study, females were more obese in both OSA groups, consistent with prior reports of gender differences in OSA.34–36 Since women with OSA more often present with nonspecific symptoms37,38 and REM OSA, by definition, is associated with a lower overall AHI,17 treatment guidelines are not standardized. Our findings suggest that patients with REM OSA defined in a specific manner benefit from PAP therapy in a variety of functional domains beyond daytime sleepiness and are adherent to PAP therapy comparable to more severe OSA patients.
The retrospective study design of this research introduces selection bias that limits the validity and generalizability of our results. Our sample was restricted to patients referred to the Cleveland Clinic Sleep Disorders Center main campus outpatient clinic with newly diagnosed OSA who had diagnostic PSG in one of our laboratories, received PAP therapy, and had pre- and 30-90 day post-PAP visits during which functional assessments were completed. This represented only a portion of patients seen at our institution since our outcomes program was in its infancy and not yet launched in locations outside of the main campus facility. Sleep disorder patients were seen by sleep physicians at several additional locations in the community during the time frame under consideration and were not included for this reason.
Our study design also excluded subjects who underwent split-night studies because of concerns that limited REM sleep time would result in incorrect categorization of OSA group. Over the time frame under consideration, 53% of split-night PSGs performed in our laboratories had no REM recorded during the diagnostic portion of the study, and the median number of minutes of REM sleep in the diagnostic portion of split-night studies was 9. Given this, the number of subjects excluded who otherwise would have met the criteria for REM OSA is low, and these cases would have been more likely to be miscategorized given the shorter recording time.
In addition, the current study excluded subjects with REM OSA not offered PAP therapy. The decision to initiate PAP therapy was made on a case-by-case basis by the treating physician and was likely affected by a variety of factors not accounted for in this study, such as physician preference, severity of daytime impairment, and patient desire to be treated. Despite these sources of selection bias discussed, the characteristics of our sample are quite similar to previous reports of REM OSA (although one prior report was from our institution several years prior to the current investigation).16,19
One final study limitation is the reliance on self-reported data for PAP adherence. Our outcomes database was limited to subjective adherence data during the time frame under consideration. Objective adherence data, available in approximately two-thirds of cases, confirmed acceptable PAP adherence in 74% of OSA and 82% of REM OSA subjects, with no significant difference between groups. Therefore, we believe our findings are not likely to be substantially influenced by variations in PAP adherence between groups.
Despite the aforementioned limitations, our study's strengths include the use of a standardized outcomes assessment program and specifically defined criteria for REM OSA. Our study is the first addressing clinical outcomes in subjects with REM OSA using validated measures. Functional outcomes in patients with REM OSA improved after treatment with PAP therapy comparable to those observed in patients with OSA not restricted to REM. While the study's limitations preclude using these findings to alter patient care strategies, we believe these observations are important nonetheless and may prompt prospective studies addressing treatment outcomes for REM OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Foldvary-Schaefer has received research support from UCB Pharma, CleveMed, and the American Sleep Foundation. She has participated in the speakers bureaus for Jazz Pharmaceuticals and UCB Pharma. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the Knowledge Program Data Registry of the Cleveland Clinic, Cleveland, OH and Nengah Hariadi for providing the data used in these analyses. The study was performed at the Cleveland Clinic, Cleveland, OH.
REFERENCES
- 1.Olson LG, King MT, Hensley MJ, Saunders NA. A community study of snoring and sleep-disordered breathing. Prevalence. Am J Respir Crit Care Med. 1995;152:711–6. doi: 10.1164/ajrccm.152.2.7633731. [DOI] [PubMed] [Google Scholar]
- 2.Hoch CC, Reynolds CF, 3rd, Monk TH, et al. Comparison of sleep-disordered breathing among healthy elderly in the seventh, eighth, and ninth decades of life. Sleep. 1990;13:502–11. doi: 10.1093/sleep/13.6.502. [DOI] [PubMed] [Google Scholar]
- 3.Kripke DF, Ancoli-Israel S, Klauber MR, Wingard DL, Mason WJ, Mullaney DJ. Prevalence of sleep-disordered breathing in ages 40-64 years: a population-based survey. Sleep. 1997;20:65–76. doi: 10.1093/sleep/20.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 5.Partinen M, Telakivi T. Epidemiology of obstructive sleep apnea syndrome. Sleep. 1992;15(6 Suppl):S1–4. doi: 10.1093/sleep/15.suppl_6.s1. [DOI] [PubMed] [Google Scholar]
- 6.Ward Flemons W, McNicholas WT. Clinical prediction of the sleep apnea syndrome. Sleep Med Rev. 1997;1:19–32. doi: 10.1016/s1087-0792(97)90003-4. [DOI] [PubMed] [Google Scholar]
- 7.Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med. 2009;122:1122–7. doi: 10.1016/j.amjmed.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5:573–81. [PMC free article] [PubMed] [Google Scholar]
- 9.Dyken ME, Im KB. Obstructive sleep apnea and stroke. Chest. 2009;136:1668–77. doi: 10.1378/chest.08-1512. [DOI] [PubMed] [Google Scholar]
- 10.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 11.Rodenstein D. Sleep apnea: traffic and occupational accidents--individual risks, socioeconomic and legal implications. Respiration. 2009;78:241–8. doi: 10.1159/000222811. [DOI] [PubMed] [Google Scholar]
- 12.Kryger MH, Roth T, Dement WC. Principles and practice of sleep medicine. 4th edition. Elsevier; 2005. [Google Scholar]
- 13.McDaid C, Duree KH, Griffin SC, et al. A systematic review of continuous positive airway pressure for obstructive sleep apnoea-hypopnoea syndrome. Sleep Med Rev. 2009;13:427–36. doi: 10.1016/j.smrv.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Keles T, Durmaz T, Bayram NA, et al. Effect of continuous positive airway pressure therapy on aortic stiffness in patients with obstructive sleep apnea syndrome. Echocardiography. 2009;26:1217–24. doi: 10.1111/j.1540-8175.2009.00957.x. [DOI] [PubMed] [Google Scholar]
- 15.Milleron O, Pilliere R, Foucher A, et al. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur Heart J. 2004;25:728–34. doi: 10.1016/j.ehj.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Koo BB, Dostal J, Ioachimescu O, Budur K. The effects of gender and age on REM-related sleep-disordered breathing. Sleep Breath. 2008;12:259–64. doi: 10.1007/s11325-007-0161-7. [DOI] [PubMed] [Google Scholar]
- 17.Haba-Rubio J, Janssens JP, Rochat T, Sforza E. Rapid eye movement-related disordered breathing: clinical and polysomnographic features. Chest. 2005;128:3350–7. doi: 10.1378/chest.128.5.3350. [DOI] [PubMed] [Google Scholar]
- 18.O'Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1465–72. doi: 10.1164/ajrccm.161.5.9904121. [DOI] [PubMed] [Google Scholar]
- 19.Koo BB, Patel SR, Strohl K, Hoffstein V. Rapid eye movement-related sleep-disordered breathing: influence of age and gender. Chest. 2008;134:1156–61. doi: 10.1378/chest.08-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF. AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 21.Lloberes P, Marti S, Sampol G, et al. Predictive factors of quality-of-life improvement and continuous positive airway pressure use in patients with sleep apnea-hypopnea syndrome: study at 1 year. Chest. 2004;126:1241–7. doi: 10.1378/chest.126.4.1241. [DOI] [PubMed] [Google Scholar]
- 22.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 23.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 24.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 26.Kass JE, Akers SM, Bartter TC, Pratter MR. Rapid-eye-movement-specific sleep-disordered breathing: a possible cause of excessive daytime sleepiness. Am J Respir Crit Care Med. 1996;154:167–9. doi: 10.1164/ajrccm.154.1.8680674. [DOI] [PubMed] [Google Scholar]
- 27.Punjabi NM, Bandeen-Roche K, Marx JJ, Neubauer DN, Smith PL, Schwartz AR. The association between daytime sleepiness and sleep-disordered breathing in NREM and REM sleep. Sleep. 2002;25:307–14. [PubMed] [Google Scholar]
- 28.Chervin RD, Aldrich MS. The relation between multiple sleep latency test findings and the frequency of apneic events in REM and non-REM sleep. Chest. 1998;113:980–4. doi: 10.1378/chest.113.4.980. [DOI] [PubMed] [Google Scholar]
- 29.Chami HA, Baldwin CM, Silverman A, et al. Sleepiness, quality of life, and sleep maintenance in REM versus non-REM sleep-disordered breathing. Am J Respir Crit Care Med. 2010;181:997–1002. doi: 10.1164/rccm.200908-1304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patil SP, Schneider H, Schwartz AR, Smith PL. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest. 2007;132:325–37. doi: 10.1378/chest.07-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillipson E, Bowes G. Control of breathing during sleep. In: Cherniack N, Widdicombe JG, editors. Handbook of physiology. 2nd ed. Bethesda, MD: American Physiologic Society; 1986. pp. 649–89. [Google Scholar]
- 32.Sullivan SS, Kushida CA. Multiple sleep latency test and maintenance of wakefulness test. Chest. 2008;134:854–61. doi: 10.1378/chest.08-0822. [DOI] [PubMed] [Google Scholar]
- 33.Aurora RN, Caffo B, Crainiceanu C, Punjabi NM. Correlating subjective and objective sleepiness: revisiting the association using survival analysis. Sleep. 2011;34:1707–14. doi: 10.5665/sleep.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med. 1994;149(3 Pt 1):722–6. doi: 10.1164/ajrccm.149.3.8118642. [DOI] [PubMed] [Google Scholar]
- 35.Guilleminault C, Quera-Salva MA, Partinen M, Jamieson A. Women and the obstructive sleep apnea syndrome. Chest. 1988;93:104–9. doi: 10.1378/chest.93.1.104. [DOI] [PubMed] [Google Scholar]
- 36.Young T, Hutton R, Finn L, Badr S, Palta M. The gender bias in sleep apnea diagnosis. Are women missed because they have different symptoms? Arch Intern Med. 1996;156:2445–51. [PubMed] [Google Scholar]
- 37.Quintana-Gallego E, Carmona-Bernal C, Capote F, et al. Gender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patients. Respir Med. 2004;98:984–9. doi: 10.1016/j.rmed.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Wahner-Roedler DL, Olson EJ, Narayanan S, et al. Gender-specific differences in a patient population with obstructive sleep apnea-hypopnea syndrome. Gend Med. 2007;4:329–38. doi: 10.1016/s1550-8579(07)80062-3. [DOI] [PubMed] [Google Scholar]