Abstract
Study Objectives:
The purpose of this study was to determine if subjects with moderate-to-severe obstructive sleep apnea would experience increasing treatment effect when a tongue retention component was added to a mandibular repositioning appliance.
Design:
Cohort study.
Setting:
Sleep clinic.
Patients:
Forty-four sequentially recruited patients with moderate-to-severe obstructive sleep apnea.
Interventions:
Subjects were sleep tested at 4 treatment stages of oral appliance therapy. The 4 stages were: 6-mm mandibular protrusion, 8-mm protrusion, 6-mm protrusion with a tongue retention bulb, and 8-mm protrusion with a tongue retention bulb.
Measurements and Results:
Forty-one of 44 subjects completed the protocol. There was a decrease in mean respiratory disturbance index from 33.5 events/h at baseline to 18.1 events/h at stage 4 (p = 0.001). Mean Epworth Sleepiness Scale (ESS) decreased from 12.3 at baseline to 9.0 at stage 4 (p = 0.0001.
Conclusions:
A combined approach utilizing both mandibular protrusion and tongue retention can provide effective treatment for moderate-to-severe obstructive sleep apnea. The addition of a tongue bulb may provide further treatment effect when mandibular protrusion is limited. Appliance designs that allow for convenient combination therapy need to be developed for this purpose.
Citation:
Dort L; Remmers J. A combination appliance for obstructive sleep apnea: the effectiveness of mandibular advancement and tongue retention. J Clin Sleep Med 2012;8(3):265-269.
Keywords: Obstructive sleep apnea, therapy, oral appliances, mandibular advancement, tongue retention
Obstructive sleep apnea (OSA) is a common condition characterized by repetitive collapse of the pharynx during sleep.1,2 The resultant hypoxemia, bursts of sympathetic activity and sleep disruption are associated with significant health consequences including hypertension, stroke, and early mortality.3–5 Accompanying the pathophysiological consequences of OSA are the necessary health resource costs that consume an ever increasing portion of health care resources.6
Oral appliances (OA) are an indicated primary treatment for those with mild-to-moderate OSA and an alternative in patients with severe OSA who fail CPAP treatment.7 The most extensively studied OAs are custom-made, adjustable mandibular repositioning appliances (MRAs).8,9 The fabrication process makes custom-made appliances impractical for trial or temporary use. Non-customized, prefabricated, “boil and bite” OAs have been suggested as trial appliances. Unfortunately, the types of these devices developed to date are much less effective or comfortable, and the results do not indicate whether custom devices will be effective.10 Tongue retaining devices (TRDs) that open the airway utilizing suction to hold the tip of the tongue forward are an alternate device but have been the subject of far fewer investigations than MRAs.11,12 Several recent publications may demonstrate a renewed interest in the use of TRDs.13–17
Identifying those who will experience therapeutic success, even with custom MRAs, is challenging. Patient characteristics including mild-to-moderate OSA severity, female gender, position-dependent OSA, and body mass index (BMI) are associated with but not strongly predictive of therapeutic success.18–21 Mandibular advancement during polysomnography may be helpful, but appropriate devices are not yet commercially available.22–25 Recently, spirometry, nasopharyngosgopy, and therapeutic CPAP pressures have been investigated as predictors of success with OAs, but these need confirmation of clinically useful protocols.26–29 There is a need to develop practical, clinically relevant protocol for maximizing response to OA treatment.
BRIEF SUMMARY
Current Knowledge/Study Rationale: The study was done to investigate the practicality and effectiveness of combining tongue retention devices with mandibular advancement appliances in the treatment of OSA. The study was also conducted to evaluate the effectiveness of appliance therapy in moderate to severe OSA as most previous studies have enrolled subjects with mild to moderate OSA.
Study Impact: The results suggest that development of a combination appliance may provide increased therapeutic effect when patients have limited protrusive capabilities. The study suggests that those with moderate to severe OSA may experience considerable benefit from even modest mandible and tongue repositioning with an appliance.
The purpose of this study was to determine, using a clinically practical protocol in a pragmatic trial, if significantly more patients would experience a complete or partial response when a tongue retention component was added to a mandibular repositioning appliance than with the mandibular repositioning appliance alone.
METHODS
Subjects were recruited from the Alberta Lung Association Sleep Center, the major referral center for southern Alberta, with a population of over 1.5 million. Referral sources for the center include internists, family physicians, otolaryngologists, dentists, and psychiatrists. Patients diagnosed with mild-to-moderate OSA and those with severe OSA who have failed or refused CPAP are routinely given the opportunity to have a consultation regarding oral appliance therapy. Patients referred for oral appliance consultation by a physician during the recruiting period were eligible for inclusion. Inclusion criteria were moderate-to-severe OSA (RDI ≥ 15 events/h)30 and ≥ 10 teeth per arch. The study was approved by the Conjoint Ethics Committee of the University of Calgary and written informed consent was obtained from all participants.
Oral Appliances
An MRA (EMA)31 was custom-made for each patient. The MRA was made from thermoplastic polymer pressure-formed to dental study models. Protrusion of the mandible was achieved through elastic straps of varying lengths and stiffness (durometer 60-90), attached bilaterally.
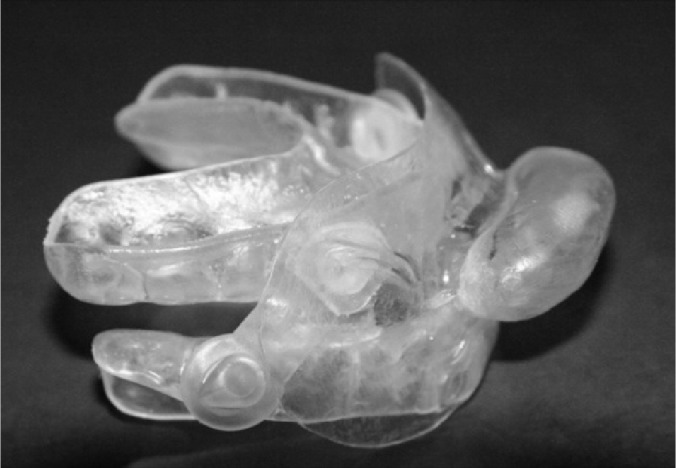
The tongue devices (TD) added to the MRAs were fabricated by vacuum-molding a single piece of ethyl vinyl acetate, a dental mouth guard material. These devices have been previously found to reduce the RDI by ≥ 50% in 34% of subjects and to control snoring in 73% of subjects13 (Figure 1). Subjects progressed through 4 stages: 6-mm advancement, 8-mm advancement, 6-mm advancement with TD, and 8-mm advancement with TD.
Figure 1. MRA with TD attached.
Sleep tests were planned for each subject at each of the 4 study conditions. The timing of the tests was dependent on the individual's ability to acclimate to a particular test condition.
Portable Monitoring
The RDI and oxygen parameters were assessed at baseline and each of the 4 study conditions using a portable monitor (Sagatech, Calgary, Canada) that digitally records oxygen saturation, heart rate with a pulse oximeter, snoring sounds with a microphone, and body position with a gyroscope. An off-line algorithm analyzes the nocturnal oxygen saturation signal to determine the RDI. The monitor-derived RDI provides a close estimate of polysomnography-derived apnea hypopnea index (AHI).32,33 Using a diagnostic criterion value ≥ 15 events/h, the analysis algorithm has a sensitivity and specificity of 98% and 88%, respectively.32 The portable monitor used in the study was the same monitor used by the sleep center for primary diagnosis. Subjects were instructed to use the sleep monitors only while the appliances were in use and to turn off the monitors when they removed the appliance.
Study Design
This study employed a prospective repeated measures design, in which each subject acted as his own control. Patients who agreed to participate underwent clinical evaluation including impressions for dental study models. Subjects were seen in the dental clinic as they progressed through the 4 study stages: 6-mm protrusion, 8-mm protrusion, 6-mm protrusion with tongue device, and 8-mm protrusion with tongue device. Portable monitoring was performed at baseline and at each stage when a subject was able to tolerate the MRA ≥ 4 h/night. A subject's final outcome was the study stage furthest along the sequence they were able to tolerate comfortably during nightly use.
Outcome Measures
The primary outcome measure was the reduction in RDI as subjects progressed through the study stages. Complete response was defined as a reduction of RDI to < 10 events/h. Partial response was defined as a reduction of RDI ≥ 50% and to < 20 events/h. Secondary outcome was the Epworth Sleepiness Scale (ESS). The ESS is a questionnaire commonly used to assess daytime sleepiness.34
Statistical Analysis
Mixed regression modeling was used to examine mean RDI under the various study conditions. Mixed regression modeling for repeated measures was chosen as appropriate as the sleep tests at each stage were carried out at different times for the individual subjects and not all individuals have observations at all stages. Mixed regression is an alternative to repeated measures ANOVA under these conditions.35 Differences were considered significant at the 5% (p < 0.05) level. STATA 10.0 (Statacorp) was used for all statistical analyses. A power calculation indicated that a sample size of 35 was require to give a 90% power to detect a 5 event/h decrease in RDI at each stage (p = 0.05).
RESULTS
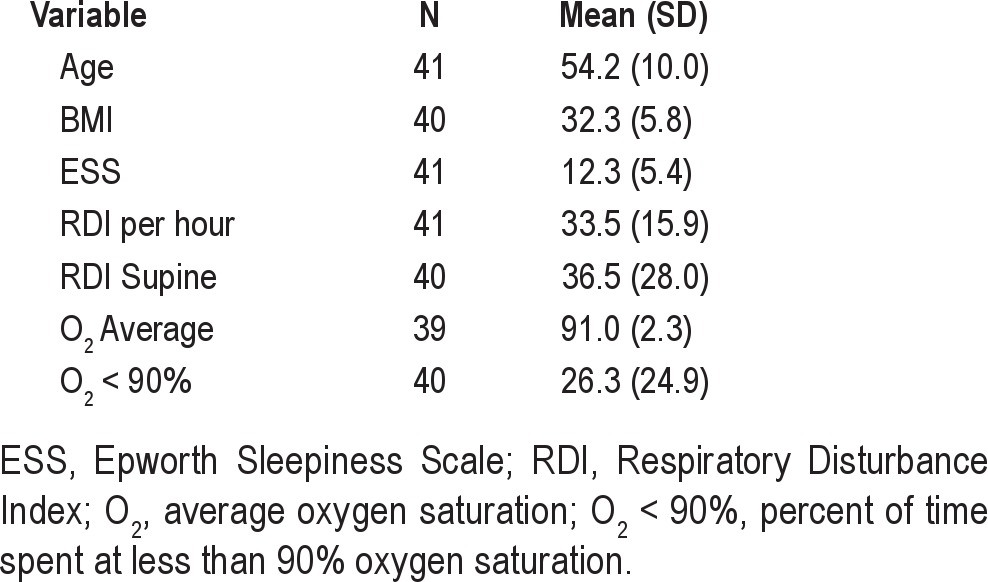
The study population consisted of 44 consecutively recruited subjects from 183 referred during the recruitment period (Figure 2). Three subjects were unable to schedule follow-up tests and were excluded from the analysis. These subjects reported that they were “too busy” or it was “too inconvenient to come in for follow-up.” There were no significant differences between those who had at least one post-treatment test and those who refused to return for follow-up testing. Forty-one subjects (12 female, 29 male) had at least one post-treatment sleep test (Table 1).
Figure 2. Study flow diagram.
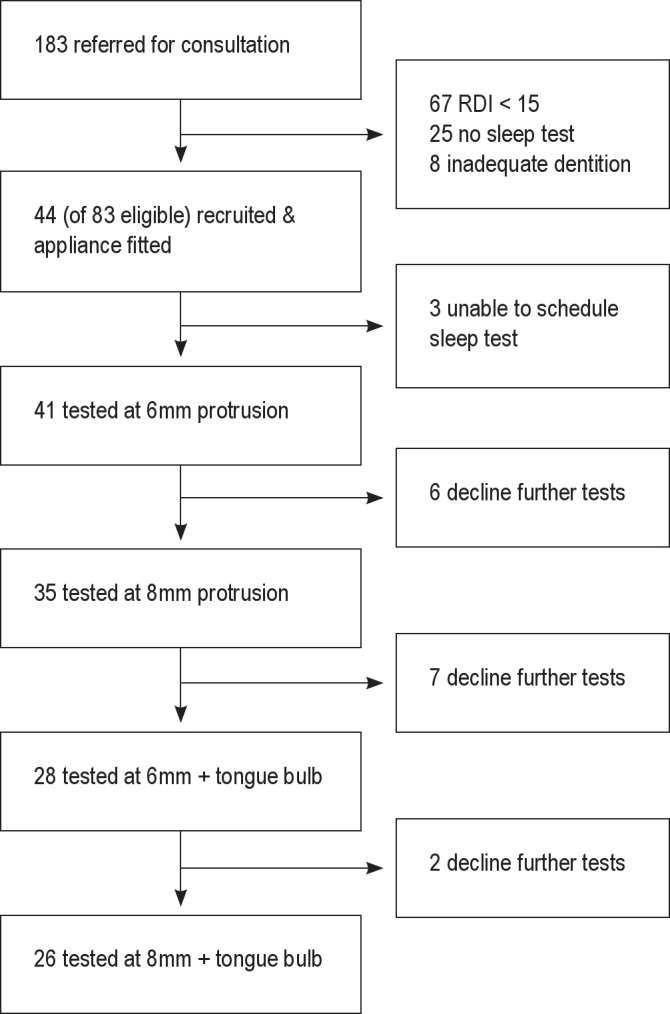
Table 1.
Characteristics of the study population
The mean RDI decreased from 33.5 (15.9) events/h at baseline to 18.1 (2.6) events/h at stage 4, p = 0.001 (Table 2). Regression modeling confirmed stage as a significant factor and estimated a decrease of 4.18 (p < 0.001, 95% CI: 5.28-3.08) events/h per stage. Sixty-one percent of subjects were either complete or partial responders. Complete response (RDI < 10) was achieved in 34% (14/41) of subjects, and partial response (RDI < 20 and reduced ≥ 50%) in an additional 27% (11/41) of subjects (Figure 3).
Table 2.
Primary outcome comparisons
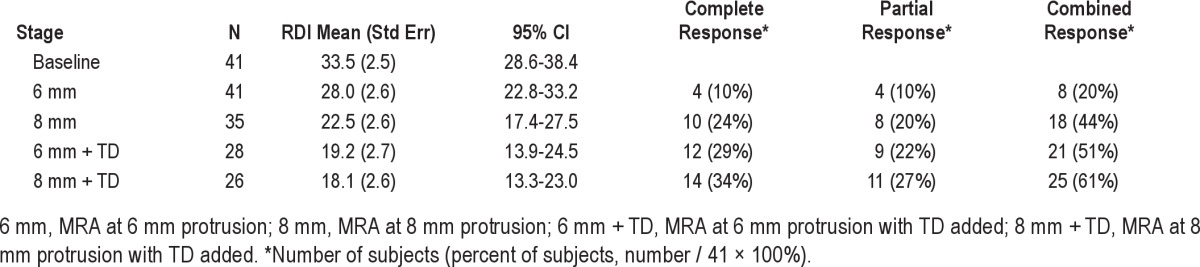
Figure 3. Percent response by stage.
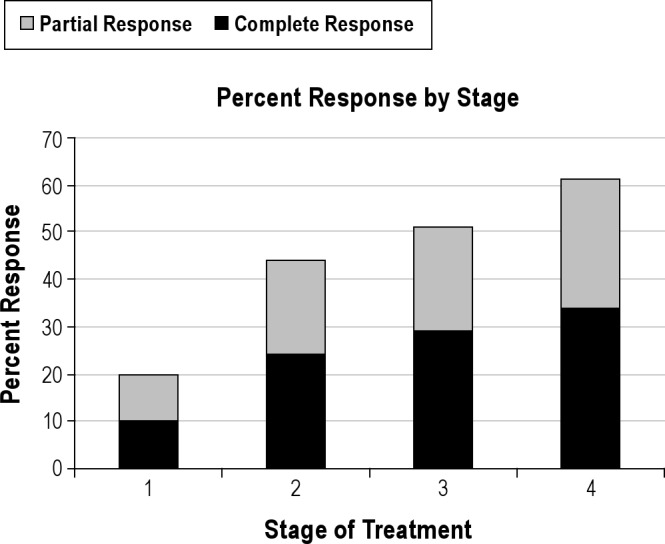
Stage 1, 6mm protrusion; Stage 2, 8mm protrusion; Stage 3, 6mm plus tongue bulb; Stage 4, 8mm plus tongue bulb.
Sixteen subjects (39%) were non-responders according to predetermined response criteria. There were 7 severe subjects and 9 moderate subjects in the non-responder group.
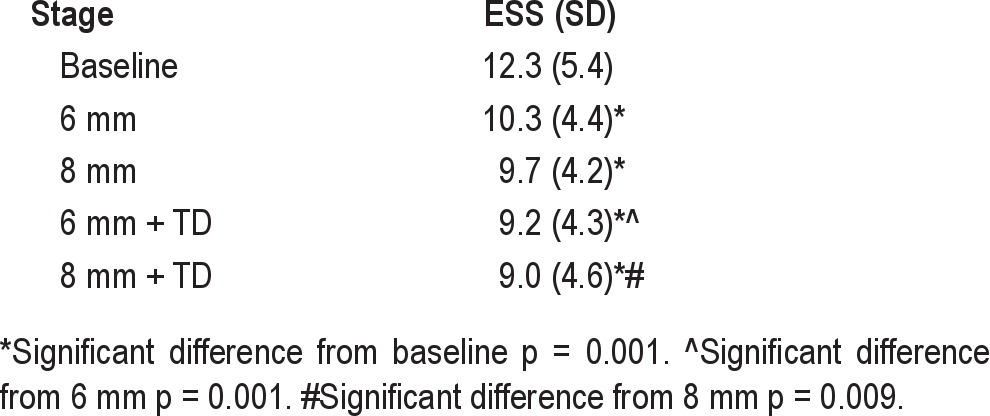
Sleepiness as measured by the ESS showed a continuous improvement from 12.3 at baseline to 9.0 at the final stage (Table 3). A proxy for compliance was the number of subjects tested at each stage (Table 2). Sixty-three percent (26/41) of subjects were able to adapt to wearing the appliance ≥ 4 h at night at all stages, although not all had complete or partial response.
Table 3.
Secondary outcome comparisons: ESS
DISCUSSION
Oral appliances are being used increasingly as therapy for obstructive sleep apnea. They are recommended first-line therapy for mild-to-moderate OSA and second-line therapy for severe OSA.7 The majority of studies have investigated effectiveness in mild-to-moderate OSA and have reported complete or partial response to MRAs in the range of 65% for mild-to-moderate OSA.8 Few reports exist of effectiveness of either MRAs or TDs in the moderate-to-severe OSA population.The present study examined the effectiveness of a staged approach to oral appliance therapy for OSA, using a combination of MRA and TD. Forty-one subjects with moderate-to-severe OSA were studied at baseline and after each of 4 treatment stages. We found a statistically significant effect of treatment. Treatment stage predicted 66% of the variability in RDI.
The decrease in RDI was statistically significant and represented a meaningful change in RDI from baseline to final stage. At the start of treatment, the mean RDI indicated severe OSA (> 30 events/h); by the final stage the mean RDI was moderate (RDI 15-30) events per hour. The ESS decreased from 12.3 at baseline to 9.0 (p = 0.001) at the final stage.
Sixty-one percent of subjects were either complete or partial responders. Using the same response criteria, Deane16 reported complete or partial response in 71%, Mehta in 71%,36 and Gindre in 73%.37 The response in our study may have been limited by the design of the combination device. The titration protocol may also have limited the response. Gradual titration of MRAs (stepwise increase) is standard recommended clinical protocol and is meant to allow patients to increase tolerance of the device in new positions over time. Numerous studies have reported increased response with increased protrusion.23,24,37 Previous studies have allowed patients to advance (titrate) their appliances to maximum tolerable protrusion or symptom relief. Our subjects were only allowed a limited number of protrusion settings
Mehta reports a mean acclimatization period of more than 19 weeks.36 While our subjects were not tested until they could tolerate the device for at least four hours of sleep at any particular stage, our entire study was conducted in less than 20 weeks. If subjects had a longer time to acclimate to the devices, they may have had a greater response.
Patient preference was not predetermined as an outcome measure, but it is suggested by the number tested at each stage of the study. Deane reported that subjects preferred the MRA to the TD and that more subjects removed the TD during sleep compared to the MRA (86% vs 9%).16 The design used in this study made the TD impossible to remove separately. While the addition of a TD may be of benefit some patients with limited protrusion, some of our subjects may have had a better response with further protrusion rather than the addition of the relatively uncomfortable TD. A further limitation of TDs is that the amount of tissue in the bulb and the strength of suction is determined by the patient and cannot be controlled.
A recent study by Sutherland was the first to examine airway structure and volumetric changes produced by a TD compared to MRA using magnetic resonance imaging (MRI).17 They report differences in the location and degree of airway change produced by TD compared to MRA. The TD increased the velopharyngeal lateral diameter to a greater extent than the MRA. The TD also increased antero-posterior diameter with anterior displacement of the tongue. The MRA produced significant anterior displacement of the tongue base muscles. They did not examine the association between volume of tongue protruded and response. If explored in future studies, the relationships between tongue volume in the TD and response could inform appliance design.
Based on the findings of Sutherland, the response of the combination device can be considered in terms of the different expected effect on airway caliber produced by the individual components. The difference between response at 6-mm protrusion (20%) and 8-mm (44%) is consistent with improved response with titration found in previous studies.23,24,37 This difference could reflect the increase in response due to anterior displacement of the tongue base muscles. The difference in response between 6 mm (20%) and 6 mm plus TD (51%) as well as between 8 mm (44%) and 8 mm plus TD (61%) possibly shows the effect of increased airway caliber unique to the action of a TD. The TD may increase response more at less mandibular protrusion than at greater mandibular protrusion. If so, it could be of clinical significance to increasing response in those with limited mandibular protrusion.
Our results may have been influenced by selection bias, as the study design was not randomized. Other limitations arise from the bulky nature of the combination device. Our trial involved putting together two separate devices, MRA and TD, which were not designed to go together. A device designed for combination would likely be more comfortable. As the protrusion in the MRA used is obtained through the use of flexible and stretchable straps, the measures of 6 mm and 8 mm are likely overestimates of the actual protrusion. The use of portable monitors is potentially limiting. Portable monitors may produce an underestimation of the RDI, as electroencephalography is not used and the monitor is not able to record respiratory effort related arousals (RERAs). Study time rather than sleep time is the denominator when calculating the events per hour. The limitations of portable monitors was partially controlled, as the same monitor was used for diagnosis by the referring sleep physicians and that the portable monitor protocol followed the AASM and the Canadian Thoracic Society guidelines.38,39
Further studies are needed, using devices designed for combination, to explore the effectiveness of combining mandibular protrusion and tongue retention in the treatment of OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–8. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 3.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906–14. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 4.Nieto FJ, Peppard PE, Young TB. Sleep disordered breathing and metabolic syndrome. WMJ. 2009;108:263–65. [PMC free article] [PubMed] [Google Scholar]
- 5.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 6.Reuveni H, Greenberg-Dotan S, Simon-Tuval T, Oksenberg A, Tarasiuk A. Elevated healthcare utilisation in young adult males with obstructive sleep apnoea. Eur Respir J. 2008;31:273–79. doi: 10.1183/09031936.00097907. [DOI] [PubMed] [Google Scholar]
- 7.Kushida CA, Morgenthaler TI, Littner MR, et al. Practice parameters for the treatment of snoring and Obstructive Sleep Apnea with oral appliances: an update for 2005. Sleep. 2006;29:240–3. doi: 10.1093/sleep/29.2.240. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29:244–62. doi: 10.1093/sleep/29.2.244. [DOI] [PubMed] [Google Scholar]
- 9.Lim J, Lasserson TJ, Fleetham J, Wright J. Oral appliances for obstructive sleep apnoea. Cochrane Database Syst Rev. 2006:CD004435. doi: 10.1002/14651858.CD004435. [DOI] [PubMed] [Google Scholar]
- 10.Vanderveken OM, Devolder A, Marklund M, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med. 2008;178:197–202. doi: 10.1164/rccm.200701-114OC. [DOI] [PubMed] [Google Scholar]
- 11.Cartwright R. Return of the TRD. J Clin Sleep Med. 2009;5:439–40. [PMC free article] [PubMed] [Google Scholar]
- 12.Chan AS, Lee RW, Cistulli PA. Non-positive airway pressure modalities: mandibular advancement devices/positional therapy. Proc Am Thorac Soc. 2008;5:179–84. doi: 10.1513/pats.200707-104MG. [DOI] [PubMed] [Google Scholar]
- 13.Dort L, Brant R. A randomized, controlled, crossover study of a noncustomized tongue retaining device for sleep disordered breathing. Sleep Breath. 2008;12:369–73. doi: 10.1007/s11325-008-0187-5. [DOI] [PubMed] [Google Scholar]
- 14.Lazard DS, Blumen M, Levy P, et al. The tongue-retaining device: efficacy and side effects in obstructive sleep apnea syndrome. J Clin Sleep Med. 2009;5:431–38. [PMC free article] [PubMed] [Google Scholar]
- 15.Kurtulmus H, Cotert HS. Management of obstructive sleep apnea with a mandibular and tongue advancement splint (MTAS) in a completely edentulous patient. a clinical report. J Prosthodont. 2009;18:348–52. doi: 10.1111/j.1532-849X.2008.00432.x. [DOI] [PubMed] [Google Scholar]
- 16.Deane SA, Cistulli PA, Ng AT, Zeng B, Petocz P, Darendeliler MA. Comparison of mandibular advancement splint and tongue stabilizing device in obstructive sleep apnea: a randomized controlled trial. Sleep. 2009;32:648–53. doi: 10.1093/sleep/32.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutherland K, Deane SA, Chan AS, et al. Comparative effects of two oral appliances on upper airway structure in obstructive sleep apnea. Sleep. 2011;34:469–77. doi: 10.1093/sleep/34.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marklund M, Stenlund H, Franklin KA. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success. Chest. 2004;125:1270–8. doi: 10.1378/chest.125.4.1270. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Lowe AA. Factors related to the efficacy of an adjustable oral appliance for the treatment of obstructive sleep apnea. Chin J Dent Res. 2000;3:15–23. [PubMed] [Google Scholar]
- 20.Dahlqvist J, Dahlqvist A, Marklund M, Berggren D, Stenlund H, Franklin KA. Physical findings in the upper airways related to obstructive sleep apnea in men and women. Acta Otolaryngol. 2007;127:623–30. doi: 10.1080/00016480600987842. [DOI] [PubMed] [Google Scholar]
- 21.Hoekema A, Doff MH, de Bont LG, et al. Predictors of obstructive sleep apnea-hypopnea treatment outcome. J Dent Res. 2007;86:1181–6. doi: 10.1177/154405910708601208. [DOI] [PubMed] [Google Scholar]
- 22.Dort LC, Hadjuk E, Remmers JE. Mandibular advancement and obstructive sleep apnoea: a method for determining effective mandibular protrusion. Eur Respir J. 2006;27:1003–9. doi: 10.1183/09031936.06.00077804. [DOI] [PubMed] [Google Scholar]
- 23.Tsai WH, Vazquez JC, Oshima T, et al. Remotely controlled mandibular positioner predicts efficacy of oral appliances in sleep apnea. Am J Respir Crit Care Med. 2004;170:366–70. doi: 10.1164/rccm.200310-1446OC. [DOI] [PubMed] [Google Scholar]
- 24.Almeida FR, Parker JA, Hodges JS, Lowe AA, Ferguson KA. Effect of a titration polysomnogram on treatment success with a mandibular repositioning appliance. J Clin Sleep Med. 2009;5:198–204. [PMC free article] [PubMed] [Google Scholar]
- 25.Fleury B, Rakotonanahary D, Petelle B, et al. Mandibular advancement titration for obstructive sleep apnea: optimization of the procedure by combining clinical and oximetric parameters. Chest. 2004;125:1761–67. doi: 10.1378/chest.125.5.1761. [DOI] [PubMed] [Google Scholar]
- 26.Zeng B, Ng AT, Darendeliler MA, Petocz P, Cistulli PA. Use of flow-volume curves to predict oral appliance treatment outcome in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;175:726–30. doi: 10.1164/rccm.200608-1205OC. [DOI] [PubMed] [Google Scholar]
- 27.Zeng B, Ng AT, Qian J, Petocz P, Darendeliler MA, Cistulli PA. Influence of nasal resistance on oral appliance treatment outcome in obstructive sleep apnea. Sleep. 2008;31:543–47. doi: 10.1093/sleep/31.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan AS, Lee RW, Srinivasan VK, Darendeliler MA, Grunstein RR, Cistulli PA. Nasopharyngoscopic evaluation of oral appliance therapy for obstructive sleep apnoea. Eur Respir J. 2010;35:836–42. doi: 10.1183/09031936.00077409. [DOI] [PubMed] [Google Scholar]
- 29.Tsuiki S, Kobayashi M, Namba K, et al. Optimal positive airway pressure predicts oral appliance response to sleep apnoea. Eur Respir J. 2010;35:1098–105. doi: 10.1183/09031936.00121608. [DOI] [PubMed] [Google Scholar]
- 30.Epstein LJ, Kristo D, Strollo PJ, Jr., et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 31.Henke KG, Frantz DE, Kuna ST. An oral elastic mandibular advancement device for obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:420–5. doi: 10.1164/ajrccm.161.2.9903079. [DOI] [PubMed] [Google Scholar]
- 32.Vazquez JC, Tsai WH, Flemons WW, et al. Automated analysis of digital oximetry in the diagnosis of obstructive sleep apnoea. Thorax. 2000;55:302–7. doi: 10.1136/thorax.55.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Issa FG, Morrison D, Hadjuk E, Iyer A, Feroah T, Remmers JE. Digital monitoring of sleep-disordered breathing using snoring sound and arterial oxygen saturation. Am Rev Respir Dis. 1993;148:1023–9. doi: 10.1164/ajrccm/148.4_Pt_1.1023. [DOI] [PubMed] [Google Scholar]
- 34.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 35.Rabe-Hesketh S, Skrondal A. 2nd ed. College Station, TX: Stata Press; 2008. Multilevel and longitudinal modeling using Stata. [Google Scholar]
- 36.Mehta A, Qian J, Petocz P, Darendeliler MA, Cistulli PA. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. [see comment] Am J Respir Crit Care Med. 2001;163:1457–61. doi: 10.1164/ajrccm.163.6.2004213. [DOI] [PubMed] [Google Scholar]
- 37.Gindre L, Gagnadoux F, Meslier N, Gustin JM, Racineux JL. Mandibular advancement for obstructive sleep apnea: dose effect on apnea, long-term use and tolerance. Respiration. 2008;76:386–92. doi: 10.1159/000156861. [DOI] [PubMed] [Google Scholar]
- 38.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]
- 39.Blackman A, McGregor C, Dales R, et al. Canadian Sleep Society/Canadian Thoracic Society position paper on the use of portable monitoring for the diagnosis of obstructive sleep apnea/hypopnea in adults. Can Respir J. 2010;17:229–32. doi: 10.1155/2010/923718. [DOI] [PMC free article] [PubMed] [Google Scholar]


