Here comes the sun, Here comes the sun, and I say, “It’s alright.” —George Harrison
Skin cancer is the most common neoplasm in Caucasians in the United States with a lifetime risk nearly equal to that of all other cancers combined (1). More than 800,000 people are expected to develop nonmelanoma skin cancer [basal cell carcinoma (BCC) or squamous cell carcinoma (SCC)] this year in the United States (2). Sun exposure is the major environmental agent implicated in induction of nonmelanoma skin cancer (3). While sun exposure begins early in life, the average patient with nonmelanoma skin cancer is about 60 years old (1) (Fig. 1). The article by Jonason et al. (4) in a previous issue of the Proceedings provides a new insight into the link between sun exposure and nonmelanoma skin cancer and furnishes information about events occurring between the time of initial sun exposure and subsequent skin cancer years later.
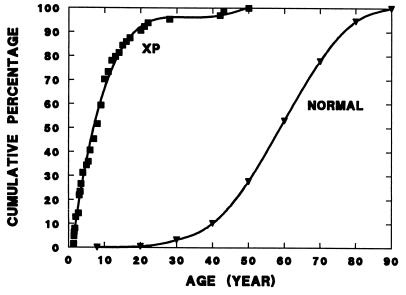
Figure 1.
Age of onset of skin cancers in normal and xeroderma pigmentosum (XP) skin cancer patients. The cumulative percentage of patients with BCCs or SCCs of the skin is plotted versus the age at diagnosis. The curve for the normal population is based on 29,757 skin cancers surveyed by the National Cancer Institute (1). The curve for the xeroderma pigmentosum patients is based on 63 skin cancers reported to the Xeroderma Pigmentosum Registry (unpublished data).
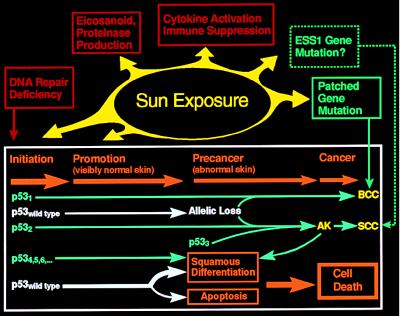
The multistage theory of carcinogenesis is based on experimental studies in rodents and has been proposed as a general model for environmental carcinogenesis (5) (Fig. 2). In the first stage—initiation—a carcinogen mutates a target gene. Initiation is followed by promotion, a process in visibly normal skin in which the single damaged cell expands to form a clone of damaged cells. These changes progress, leading to precancerous clinically abnormal skin and then to cancer. Many experimental studies have been designed to dissect the cellular and molecular mechanisms involved in this process. These studies involve investigations of DNA repair, eicosanoid and proteinase production, cytokine activation and immune suppression, and specific tumor-suppressor genes including patched and p53 (Fig. 2).
Figure 2.
Effect of sun exposure on the tumor suppressor gene, p53, and on other cellular processes involved in induction of nonmelanoma skin cancer. Sun exposure causes mutations of the tumor suppressor gene, p53 (p531, p532, p533, p534, … ), which results in initiated cells, some of which are resistant to apoptosis. Additional sun exposure acts as a promotor permitting these apoptotic-resistant cells to continue to proliferate following UV exposures that inhibit neighboring cells. In a small fraction of cells allelic loss or acquisition of a second p53 mutation on the other allele is associated with a precancerous state, such as an actinic keratosis (AK). A small fraction of these cells eventually become cancerous. This entire process is modified by additional cellular processes such as DNA repair and by processes that are altered by sun exposure including eicosanoid and proteinase production, cytokine activation and immune suppression, and sunlight-induced mutations of other tumor suppressor genes including patched (PTCH) for BCCs and possibly the ESS1 gene [for the multiple self-healing squamous cell epithelioma disorder described by Ferguson-Smith (52, 53)] for SCCs. Additional details are provided in the text.
DNA Repair
Patients with the rare inherited disorder, xeroderma pigmentosum (XP), are very sensitive to sun exposure and have a risk of developing skin cancer about 1000 times that of the general population (6, 7). The age of onset of nonmelanoma skin cancer is reduced by about 50 years in XP patients in comparison to that of the general population (Fig. 1). Cells from XP patients are hypersensitive to killing by UV and to induction of mutations in their DNA by UV exposure (8). These abnormalities are caused by a defect in DNA nucleotide excision repair (9). Work by laboratories throughout the world in the past few years has resulted in cloning of seven different DNA repair genes (XP-A to XP-G) involved in XP (10–12). Mice in which the murine homologue of human XP-A and XP-C genes have been inactivated were shown to have a markedly increased susceptibility to UV induction of skin cancer (13–15). These studies of XP strongly implicate DNA repair in protection against sunlight-induced skin cancer. Recent reports suggest that DNA repair may also be defective in apparently normal individuals with early onset of basal cell carcinoma (16) and in normal people as they age (17).
Eicosanoid and Proteinase Production
Eicosanoids such as arachidonic acid and its metabolites including prostaglandins and leucotrienes are major mediators of the inflammatory response generated by UV exposure (18). These are also produced in response to skin application of chemical tumor promoters such as the phorbol ester phorbol 12-myristate 13-acetate (PMA), which binds to protein kinase C. Inhibitor studies suggest that eicosanoids are essential components of the skin tumor promotion process.
Small doses of UVB to human volunteers were shown to activate cutaneous proteinases (19). This appears to be activated through the AP1 transcription factor and inhibited with retinoids. These proteinases may contribute to tumor cell spreading.
Cytokine Activation and Immune Suppression
People with kidney transplants who are receiving immunosuppressive medications have a very high frequency of developing squamous cell carcinomas on sun exposed skin (20). Studies of the effect of UV on mice with highly antigenic transplanted tumors have indicated that sunlight interferes with host immunity against these cancers (21, 22). UV treatment of the mouse skin resulted in systemic immunosuppression that could be transferred to untreated mice by transfusion of T-lymphocytes (suppressor T cells) and also in keratinocyte production of soluble cytokines such as interleukin 10. In addition to this tumor-specific systemic effect, UV treatment produces a nonspecific local impairment of resistance to tumor growth. These complex mechanisms are mediated through several different factors including alteration of antigen-presenting activity of Langerhans cells, local production of immunomodulatory cytokines such as tumor necrosis factor α, UV isomerization of urocanic acid in the skin and infiltration of the skin by new antigen presenting macrophages.
Studies utilizing liposome encapsulated DNA repair enzymes indicate that DNA photoproducts are involved in induction of immunosuppression (21). Recently, mice that have the XP-A DNA repair gene knocked out were found to have defective post-UV immunity with marked sensitivity to UV-induced depletion of Langerhans cells and greatly enhanced UVB induction of local and systemic immunosuppression (23).
p53 Tumor Suppressor Gene
The p53 tumor suppressor gene is involved in many cellular functions including cell cycle inhibition, regulation of differentiation, transcription, DNA repair, and apoptosis of cells sustaining DNA damage (24). The retinoblastoma gene in familial retinoblastoma is the paradigm of the tumor suppressor gene (25). Affected patients inherit one defective allele and the second allele is subsequently lost or inactivated resulting in a tumor. Patients with the Li–Fraumeni syndrome inherit a mutated form of the p53 gene (26). They have a high frequency of many cancers at an early age. These cancers include rhabdomyosarcoma, soft tissue sarcomas, breast cancer, brain tumors, osteosarcoma, leukemia, adrenocortical carcinoma, lung adenocarcinoma, and melanoma of the skin. These tumors have additional p53 gene mutations.
p53 and Molecular Fingerprints
Mutations in the p53 gene have also been found in about half of all sporadic human cancer cases examined in the general population (27). These p53 mutations have been used in studies of molecular epidemiology because many mutations can inactivate its function and some carcinogens, like UV, leave characteristic fingerprints (24).
UV radiation causes damage to DNA primarily at sites of adjacent pyrimidines (9). The most frequent photoproducts are cyclobutane dimers that are formed at adjacent thymidines (TT), with thymine-cytosine (TC) and cytosine-cytosine (CC) dimers occurring less frequently. Collectively, cyclobutane dimers represent about three-quarters of the photoproducts. The remaining nondimer photoproducts consist mostly of 6–4 pyrimidine–pyrimidone lesions at TC, CC, or TT bases on the same DNA strand. All these photoproducts are removed in normal cells by DNA excision repair (see above) (8–12).
Unrepaired photoproducts may result in a block to replication resulting in cell death. Alternatively, bypass of unrepaired lesions during replication may result in incorporation of the incorrect base opposite the photoproduct resulting in a mutation. When a shuttle vector plasmid was treated with UV in vitro and then passed through human cells, the major mutation introduced into the replicated plasmid by the human cells was the G:C to A:T transition (28). This C to T transition was seen in 75% of the mutant plasmids recovered from repair proficient cells and in 96% recovered from repair deficient XP cells (28). All of the five other types of base substitution mutations were also seen, but at a much lower frequency. Most of the C to T mutations occurred at 5′TC sites. In addition to single base substitutions, tandem 5′CC to TT mutations were found. These UV-induced mutations are sufficiently different from those induced by other carcinogens as to form a characteristic fingerprint pattern and have been used to determine the etiologic agent in cases of environmentally induced cancers.
The p53 tumor suppressor gene has been found to be mutated in more than 90% of human cutaneous SCCs and about 50% of human BCCs. Combined analysis of skin cancer mutations from several laboratories (29–35) showed that 69% of the mutations were G:C to A:T with normal patients, and that 90% of the mutations were G:C to A:T with XP patients (35–37). The types of mutations and differences between cancers from repair-proficient and repair-deficient patients were similar to those seen with the UV-treated shuttle vector plasmids strongly supporting the UV origin of these mutations in p53.
p53 Mutations in Skin
While these changes in p53 are seen in frank malignancies, when do they first occur? Do they actually contribute to the neoplastic process or are they merely markers of sun exposure (Fig. 2)? Epidemiologic evidence points to a close linkage between cutaneous SCC and sun exposure. The actinic keratosis (AK) is a premalignant lesion that infrequently (on the order of 1:1000) progresses to SCC (38). AK have a high frequency of loss of portions of several chromosomes resulting in loss of alleles of many genes including p53 (39). Studies of AK have revealed a high frequency of p53 mutations of the type seen following UV exposure (40, 41).
The location of mutations in the p53 gene is not random. Hotspots of C to T and CC to TT mutations are found in skin cancers at certain codons that are different from those seen in internal tumors such as liver cancers induced by aflatoxin or lung tumors induced by cigarette smoking (22). The paper by Jonason et al. (4) examined normal appearing skin in patients who did not have skin cancer. They found that sun exposed skin contains clones of cells with characteristic UV-type p53 mutations and a few of these are also in the same sites as seen in AK and SCC.
The protein product of the p53 gene is normally unstable and does not persist within cells. Antibodies to p53 stain cells that contain stable p53 protein. Sequencing of the p53 gene from such cells often, but not always, shows mutations. Nonmelanoma skin cancers and precancers in humans and mice often stain positive for p53. Berg et al. (42) demonstrated that 30 daily doses of UVB to hairless mice (which would produce skin tumors at 30 weeks) result in clusters of p53 positive cells in exposed skin. These clusters were observed in 93% of biopsies 1 week after discontinuation of UVB and in 47% of biopsies at 2 weeks. Staining with an antibody for a mutant p53 was positive in 64% and 37% of the biopsies, respectively, indicating an early onset of mutation in histologically normal appearing mouse skin. The paper by Jonason et al. (4) examined p53 staining in sun exposed and sun shielded skin from cancer-free normal human donors. They separated the epidermis from the dermis and stained the resulting horizontal sheets of epidermis. Remarkably, they found numerous compact areas of staining in sun exposed normal skin. The frequency and size of staining areas was roughly correlated with the extent of sun exposure and involved up to 4% of the skin cells in sun-exposed areas.
The cells of the human epidermis are constantly turning over (43). Cells in the basal layer divide and daughter cells migrate upward and differentiate into squamous cells, producing keratin and other proteins. Continued squamous differentiation results in flattening of the cell with loss of nuclei and, ultimately, sloughing off of the dead squamous cells (Fig. 2). This process takes about 1 month in normal epidermis of 0.1-mm thickness (43). In order for the skin to remain viable, some cells must remain in the basal layer. These putative stem cells divide infrequently but produce other rapidly dividing cells that then differentiate forming an “epidermal proliferative unit” (43). Normal stem cells have not been unequivocally identified in human skin. Jonason et al. (4) used a scanning confocal microscope to reconstruct a three-dimensional immunofluorescent cone of p53 mutated keratinocytes from an epidermal whole mount of sun exposed skin. The apex of the cone was found to be at the dermal–epidermal junction pointing toward the location of an initiating stem cell. The p53 mutation thus serves as a marker for the progeny of a single stem cell. This is perhaps the best evidence to date of the existence of a stem cell in human skin. However, because these cells are mutated, behavior of clones from nonmutated stem cells may be different.
p53, Sun Exposure, and Skin Cancer
A picture emerges from these studies of the role of p53 in sunlight-induced skin cancer (Fig. 2). Sun exposure of normal skin results in many p53 mutations that serve as an initiation process that start the cells on a path toward cancer (40, 44). Sequence analysis of 7 mutations by Jonason et al. (4) combined with two more by Ren et al. (40) indicates that all nine UV type C to T p53 mutations found in normal skin result in a change an amino acid of the p53 protein. Are these mutations only passive indicators of sun exposure or do they offer a selective growth advantage? Passive dosimeters should involve all possible positions in a codon with some C to T mutations resulting in silent mutations that do not change an amino acid. Because the knowledge of all inactivating p53 mutations is incomplete, an approximation of what would be expected can be based on the genetic code: of the 48 possible C to T mutations in all triplet codons, 18 will not result in change of an amino acid. Thus finding no silent mutations out of nine sequenced is highly unlikely to be due to chance alone (P < 0.02). This is evidence that these p53 mutations do indeed offer some selective growth advantage to the initiated cells.
Sun exposure also serves as a tumor promoter. Normally functioning p53 serves as a type of monitor. Cell damage results in increased stabilization of p53 protein that slows down the cell cycle to permit repair of DNA damage and turns cells sustaining unrepaired DNA damage toward apoptosis (programmed cell death) rather than to normal squamous differentiation (Fig. 2). This is a protective mechanism that rids the skin of severely damaged cells. Cells with defective DNA repair of transcribed genes, such as those from patients with xeroderma pigmentosum complementation group A or Cockayne syndrome induce nuclear accumulation of p53 and apoptosis at much lower UV doses than normal (45, 46).
In skin, cells appearing after UV exposure that have a histologic appearance that has been called “sunburn cells” were demonstrated to be apoptotic cells (47). These sunburn cells are virtually absent in UV-exposed skin of mice with a homozygous knockout of the p53 gene (p53−/−), while heterozygous p53 knockout mice (p53+/−) have a partially reduced response in comparison to normal (p53+/+) mice (47). Thus cells with a single p53 mutation are more susceptible to the tumor promoting effects of sun exposure: they have a diminished p53-mediated apoptotic cell death protective mechanism thereby permitting these cells to continue to survive in an area of skin in which surrounding cells with wild-type p53 are killed by apoptosis. Quantitatively, however, because up to 4% of the normal appearing sun-exposed skin cells have p53 mutations and very few develop into actinic keratoses or cancer, most must eventually undergo squamous differentiation or apoptosis and cell death (Fig. 2).
Eventually, repeated UV insults to cells containing a single p53 gene mutation may lead to a second mutation in the other allele or to loss of a portion of the normal chromosome. This allelic loss is commonly seen in actinic keratoses and is correlated with the appearance of clinically and histologically abnormal skin (39). However, only about 1 in 1000 actinic keratoses develop into squamous cell carcinomas (38). Other factors such as mutations in tumor-specific genes may determine the outcome.
Patched Gene
Patients with the dominant disorder, basal cell nevus syndrome (BCNS), develop multiple cutaneous BCCs, but not SCCs, especially on sun exposed skin (48). In addition, they have a high frequency of developmental abnormalities including jaw cysts, cleft palate, abnormal vertebrae and ribs, and an elevated risk of developing benign and malignant internal tumors such as ovarian fibromas and carcinomas, medulloblastoma of the brain, and cardiac fibromas (48). Therapeutic x-radiation of the skin of BCNS patients results in the appearance of numerous cutaneous BCC. PTCH, the human homologue of the Drosophila patched gene on chromosome 9 q22.3, was recently found to be defective in patients with BCNS (49, 50). This gene encodes a transmembrane protein that represses transcription in specific genes encoding members of the transforming growth factor β family and is involved in Drosophila development.
The PTCH gene was also reported to contain mutations in about one-third of sporadic basal cell carcinomas (51). The types of mutations found, predominately C to T, are characteristic of UV mutagenesis. Thus the PTCH gene may play an essential role in sunlight induction of BCC.
A SCC Gene?
A patient with multiple self-healing squamous epithelioma (multiple keratoacanthomas) with the histologic appearance of squamous cell carcinoma was described by Ferguson-Smith (52, 53). Studies of 13 affected families with this squamous cell-specific disorder indicated dominant inheritance and located a gene (called ESS1) at 9q31 by linkage analysis (54). The pattern of allelic loss on chromosome 9 is different in SCC from that in BCC (55, 56). The ESS1 gene has not yet been identified, but by analogy to the patched gene in BCNS and BCCs, the ESS1 gene may play a role in squamous cell carcinomas.
Conclusion
The Jonason et al. (4) paper shows that p53 mutations occur very early in the process of skin carcinogenesis and are seen in many relatively large areas of normal appearing, sun exposed skin. Most of these initiated cells containing p53 mutations eventually are lost through normal differentiation or cell death. However, these mutations appear to confer a survival advantage following repeated sun exposure (promotion) leading to abnormal appearing precancerous cells with a second p53 mutation or with loss of a portion of the normal chromosome. Most of these cells eventually die, but some go on to form skin cancer. It appears that this is only a part of the story. Patients with germ line p53 mutations (the Li–Fraumeni syndrome) do not have a high frequency of nonmelanoma skin cancers (or indeed, of colon or liver cancers that also usually have p53 mutations). Additional factors are probably essential for UV carcinogenesis such as diminished DNA repair, or sunlight-induced production of eicosanoids, proteinases, and cytokines resulting in immune suppression as well as mutations of tumor-specific genes.
References
- 1.Scotto J, Fears T R, Fraumeni J F. Incidence of Non-Melanoma Skin Cancer in the United States. Bethesda, MD: U.S. Department of Health and Human Services; 1982. [Google Scholar]
- 2.Parker S L, Tong T, Bolden S, Wingo P A. Ca Cancer J Clin. 1996;46:5–27. doi: 10.3322/canjclin.46.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Kripke M L. In: Dermatology in General Medicine. 4th Ed. Fitzpatrick T B, Eisen A Z, Wolff K, Freedberg I M, Austen K F, editors. New York: McGraw–Hill; 1993. pp. 797–804. [Google Scholar]
- 4.Jonason A S, Kunala S, Price G J, Restifo R J, Spinelli H M, Persing J A, Leffell D, J, Tarone R E, Brash D E. Proc Natl Acad Sci USA. 1966;93:14025–14029. doi: 10.1073/pnas.93.24.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuspa S H, Dlugosz A A, Cheng C K, Denning M F, Tennenbaum T, Glick A B, Weinberg W C. J Invest Dermatol. 1994;103:90S–95S. doi: 10.1111/1523-1747.ep12399255. [DOI] [PubMed] [Google Scholar]
- 6.Kraemer K H, Lee M M, Scotto J. Carcinogenesis. 1984;5:511–514. doi: 10.1093/carcin/5.4.511. [DOI] [PubMed] [Google Scholar]
- 7.Kraemer K H, Lee M-M, Andrews A D, Lambert W C. Arch Dermatol. 1994;130:1018–1021. [PubMed] [Google Scholar]
- 8.Cleaver J E, Kraemer K H. In: The Metabolic and Molecular Bases of Inherited Disease. 7th Ed. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 1995. pp. 4393–4419. [Google Scholar]
- 9.Friedberg E C, Walker G C, Siede W. DNA Repair and Mutagenesis. Washington, DC: Am. Soc. for Microbiol.; 1995. [Google Scholar]
- 10.Bootsma D, Weeda G, Vermeulen W, Van Vuuren H, Troelstra C, Van der Spek P, Hoeijmakers J. Philos Trans R Soc London B. 1995;347:75–81. doi: 10.1098/rstb.1995.0012. [DOI] [PubMed] [Google Scholar]
- 11.Sancar A. Annu Rev Genet. 1995;29:69–105. doi: 10.1146/annurev.ge.29.120195.000441. [DOI] [PubMed] [Google Scholar]
- 12.Aboussekhra A, Biggerstaff M, Shivji M K K, Vilpo J A, Moncollin V, Podust V N, Protic M, Hübscher U, Egly J-M, Wood R D. Cell. 1995;80:859–868. doi: 10.1016/0092-8674(95)90289-9. [DOI] [PubMed] [Google Scholar]
- 13.Nakane H, Takeuchi S, Yuba S, Saijo M, Nakatsu Y, et al. Nature (London) 1995;377:165–168. doi: 10.1038/377165a0. [DOI] [PubMed] [Google Scholar]
- 14.De Vries A, Van Oostrom C T M, Hofhuis F M A, Dortant P M, Berg R J W, De Gruijl F R, Wester P W, Van Kreijl C F, Capel P J A, Van Steeg H, Verbeek S J. Nature (London) 1995;377:169–173. doi: 10.1038/377169a0. [DOI] [PubMed] [Google Scholar]
- 15.Sands A T, Abuin A, Sanchez A, Conti C J, Bradley A. Nature (London) 1995;377:162–165. doi: 10.1038/377162a0. [DOI] [PubMed] [Google Scholar]
- 16.Wei Q, Matanoski G M, Farmer E R, Hedayati M A, Grossman L. Proc Natl Acad Sci USA. 1993;90:1614–1618. doi: 10.1073/pnas.90.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moriwaki S-I, Ray S, Tarone R E, Kraemer K H, Grossman L. Mutat Res DNA Repair. 1996;364:117–123. doi: 10.1016/0921-8777(96)00029-8. [DOI] [PubMed] [Google Scholar]
- 18.Mukhtar H. Skin Cancer. Boca Raton, FL: CRC; 1995. [Google Scholar]
- 19.Fisher G J, Datta S C, Talwar H S, Wang Z Q, Varani J, Kang S, Voorhees J J. Nature (London) 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 20.Glover M T, Niranjan N, Kwan J T C, Leigh I M. Br J Plast Surg. 1994;47:86–89. doi: 10.1016/0007-1226(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 21.Yarosh D B, Kripke M L. Mutat Res. 1996;350:255–260. doi: 10.1016/0027-5107(95)00108-5. [DOI] [PubMed] [Google Scholar]
- 22.Kripke M L. J Dermatol. 1991;18:429–433. doi: 10.1111/j.1346-8138.1991.tb03111.x. [DOI] [PubMed] [Google Scholar]
- 23.Miyauchi-Hashimoto H, Tanaka K, Horio T. J Invest Dermatol. 1996;107:343–348. doi: 10.1111/1523-1747.ep12363295. [DOI] [PubMed] [Google Scholar]
- 24.Harris C C. Environ Health Perspect. 1996;104:435–439. doi: 10.1289/ehp.96104s3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knudson A G. Proc Natl Acad Sci USA. 1993;90:10914–10921. doi: 10.1073/pnas.90.23.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frebourg T, Barbier N, Yan Y, Garber J E, Dreyfus M, Fraumeni J, Jr, Li F P, Friend S H. Am J Hum Genet. 1995;56:608–615. [PMC free article] [PubMed] [Google Scholar]
- 27.Béroud C, Verdier F, Soussi T. Nucleic Acids Res. 1996;24:147–150. doi: 10.1093/nar/24.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy D D, Saijo M, Tanaka K, Kraemer K H. Carcinogenesis. 1995;16:1557–1564. doi: 10.1093/carcin/16.7.1557. [DOI] [PubMed] [Google Scholar]
- 29.Brash D E, Rudolph J A, Simon J A, Lin A, McKenna G J, Baden H P, Halperin A J, Pontën J. Proc Natl Acad Sci USA. 1991;88:10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rady P, Scinicariello F, Wagner R F, Jr, Tyring S K. Cancer Res. 1992;52:3804–3806. [PubMed] [Google Scholar]
- 31.Ziegler A, Leffell D J, Kunala S, Sharma H W, Gailani M, Simon J A, Halperin A J, Baden H P, Shapiro P E, Bale A E, Brash D E. Proc Natl Acad Sci USA. 1993;90:4216–4220. doi: 10.1073/pnas.90.9.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell C, Quinn A G, Ro Y S, Angus B, Rees J L. J Invest Dermatol. 1993;100:746–748. doi: 10.1111/1523-1747.ep12475717. [DOI] [PubMed] [Google Scholar]
- 33.Molës J P, Moyret C, Guillot B, Jeanteur P, Guilhou J J, Theillet C, Basset-Sëguin N. Oncogene. 1993;8:583–588. [PubMed] [Google Scholar]
- 34.Van der Riet P, Karp D, Farmer E, Wei Q, Grossman L, Tokino K, Ruppert J M, Sidransky D. Cancer Res. 1994;54:25–27. [PubMed] [Google Scholar]
- 35.Kubo Y, Urano Y, Yoshimoto K, Iwahana H, Fukuhara K, Arase S, Itakura M. J Invest Dermatol. 1994;102:440–444. doi: 10.1111/1523-1747.ep12373002. [DOI] [PubMed] [Google Scholar]
- 36.Dumaz N, Drougard C, Sarasin A, Daya-Grosjean L. Proc Natl Acad Sci USA. 1993;90:10529–10533. doi: 10.1073/pnas.90.22.10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato M, Nishigori C, Zghal M, Yagi T, Takebe H. Cancer Res. 1993;53:2944–2946. [PubMed] [Google Scholar]
- 38.Marks R, Rennie G, Selwood T S. Lancet. 1988;i:795–797. doi: 10.1016/s0140-6736(88)91658-3. [DOI] [PubMed] [Google Scholar]
- 39.Rehman I, Takata M, Wu Y Y, Rees J L. Oncogene. 1996;12:2483–2490. [PubMed] [Google Scholar]
- 40.Ren Z P, Hedrum A, Pontén F, Nistér M, Ahmadian A, Lundeberg J, Uhlén M, Pontén J. Oncogene. 1996;12:765–773. [PubMed] [Google Scholar]
- 41.Brash D E, Ziegler A, Jonason A S, Simon J A, Kunala S, Leffell D J. J Invest Dermatol Symp Proc. 1996;1:136–142. [PubMed] [Google Scholar]
- 42.Berg R J W, Van Kranen H J, Rebel H G, De Vries A, Van Vloten W A, Van Kreijl C F, Van der Leun J C, De Gruijl F R. Proc Natl Acad Sci USA. 1996;93:274–278. doi: 10.1073/pnas.93.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dover R, Wright N A. In: Dermatology in General Medicine. 4th Ed. Fitzpatrick T B, Eisen A Z, Wolff K, Freedberg I M, Austen K F, editors. New York: McGraw–Hill; 1993. pp. 159–171. [Google Scholar]
- 44.Nakazawa H, English D, Randell P L, Nakazawa K, Martel N, Armstrong B K, Yamasaki H. Proc Natl Acad Sci USA. 1994;91:360–364. doi: 10.1073/pnas.91.1.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ljungman M, Zhang F. Oncogene. 1996;13:823–831. [PubMed] [Google Scholar]
- 46.Yamaizumi M, Sugano T. Oncogene. 1994;9:2775–2784. [PubMed] [Google Scholar]
- 47.Ziegler A, Jonason A S, Leffell D J, Simon J A, Sharma H W, Kimmelman J, Remington L, Jacks T, Brash D E. Nature (London) 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 48.Gorlin R J. Medicine (Baltimore) 1987;66:98–113. doi: 10.1097/00005792-198703000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Hahn H, Wicking C, Zaphiropoulos P G, Gailani M R, Shanley S, et al. Cell. 1996;85:841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 50.Johnson R L, Rothman A L, Xie J W, Goodrich L V, Bare J W, Bonifas J M, Quinn A G, Myers R M, Cox D R, Epstein E H, Jr, Scott M P. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 51.Gailani M R, Stãhle-Backdahl M, Leffell D J, Glynn M, Zaphiropoulos P G, Pressman C, Unden A B, Dean M, Brash D E, Bale A E, Toftgãrd R. Nat Genet. 1996;14:78–81. doi: 10.1038/ng0996-78. [DOI] [PubMed] [Google Scholar]
- 52.Ferguson-Smith J. Br J Dermatol. 1934;46:267–272. [Google Scholar]
- 53.Ferguson-Smith J. Br J Dermatol. 1948;60:315–319. [Google Scholar]
- 54.Goudie D R, Yuille M A, Leversha M A, Furlong R A, Carter N P, Lush M J, Affara N A, Ferguson-Smith M A. Nat Genet. 1993;3:165–169. doi: 10.1038/ng0293-165. [DOI] [PubMed] [Google Scholar]
- 55.Quinn A G, Sikkink S, Rees J L. Genes Chromosomes Cancer. 1994;11:222–225. doi: 10.1002/gcc.2870110404. [DOI] [PubMed] [Google Scholar]
- 56.Holmberg E, Rozell B L, Toftgard R. Br J Cancer. 1996;74:246–250. doi: 10.1038/bjc.1996.345. [DOI] [PMC free article] [PubMed] [Google Scholar]