Abstract
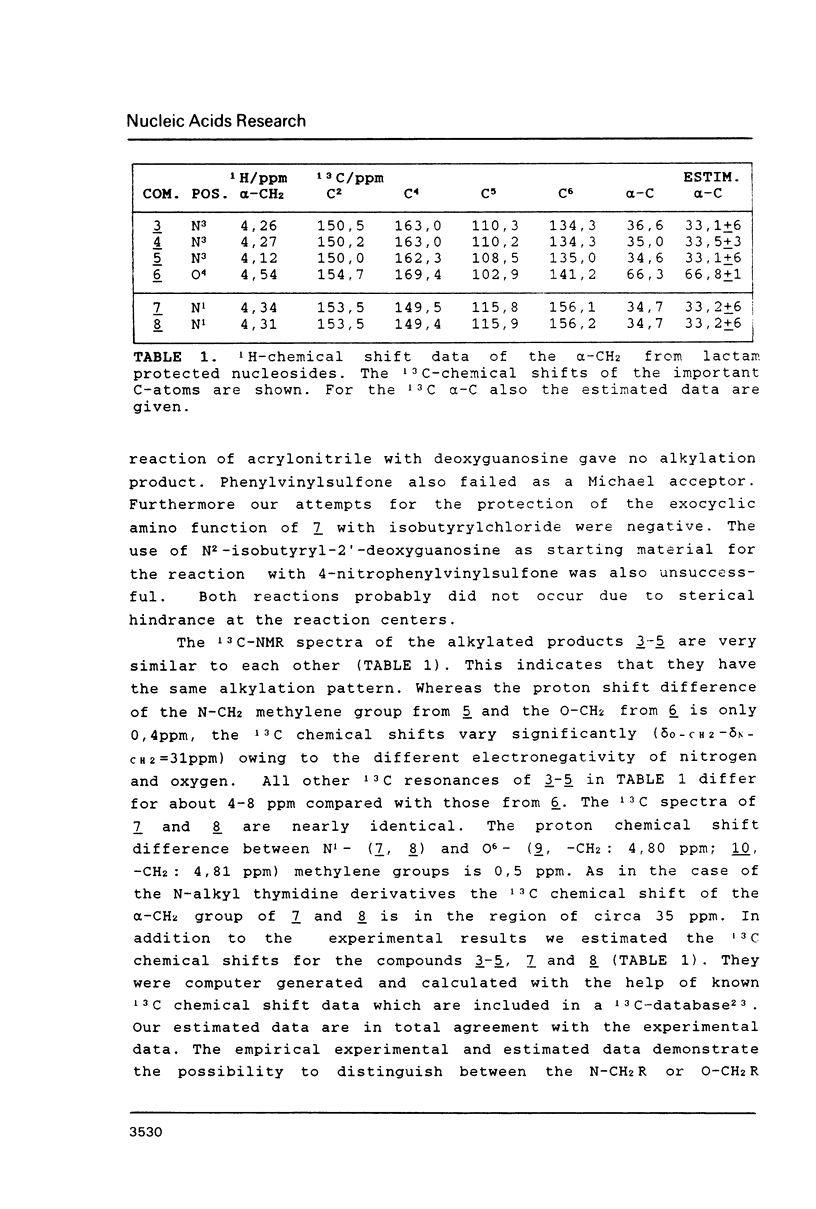
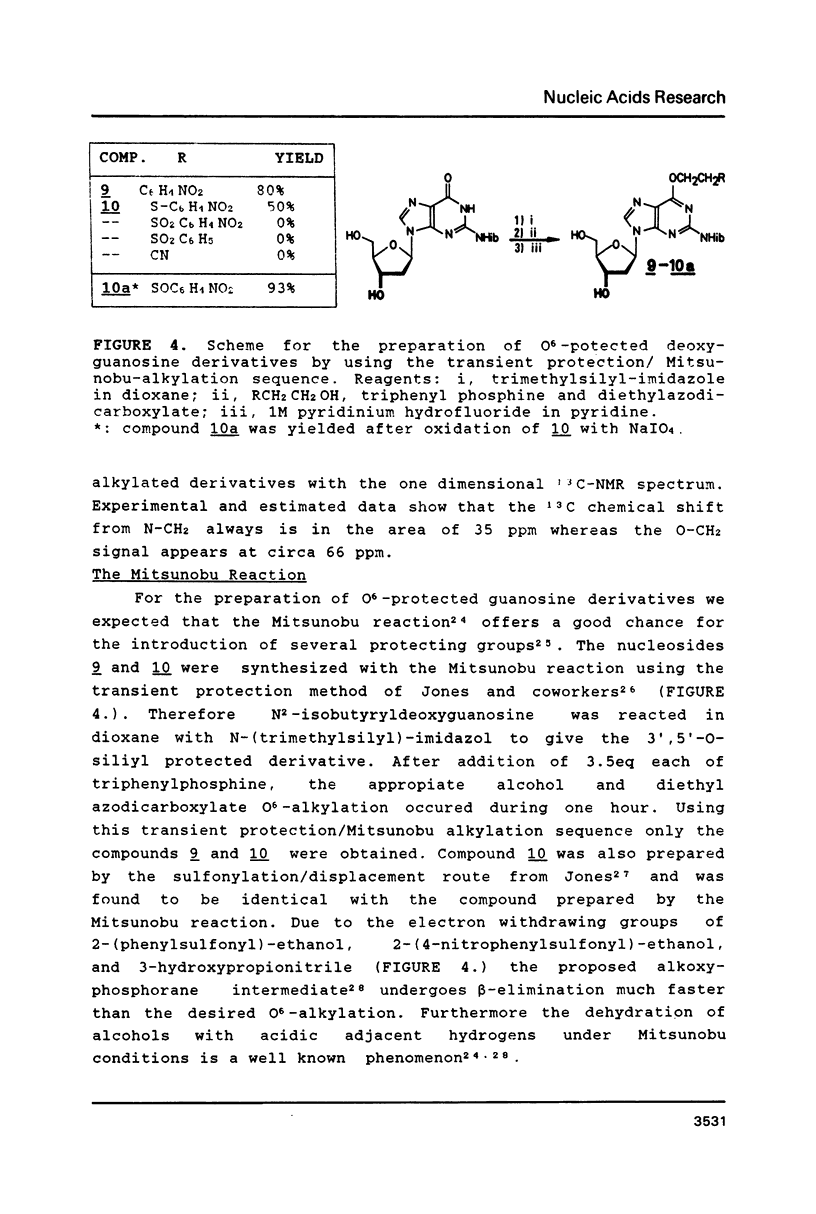
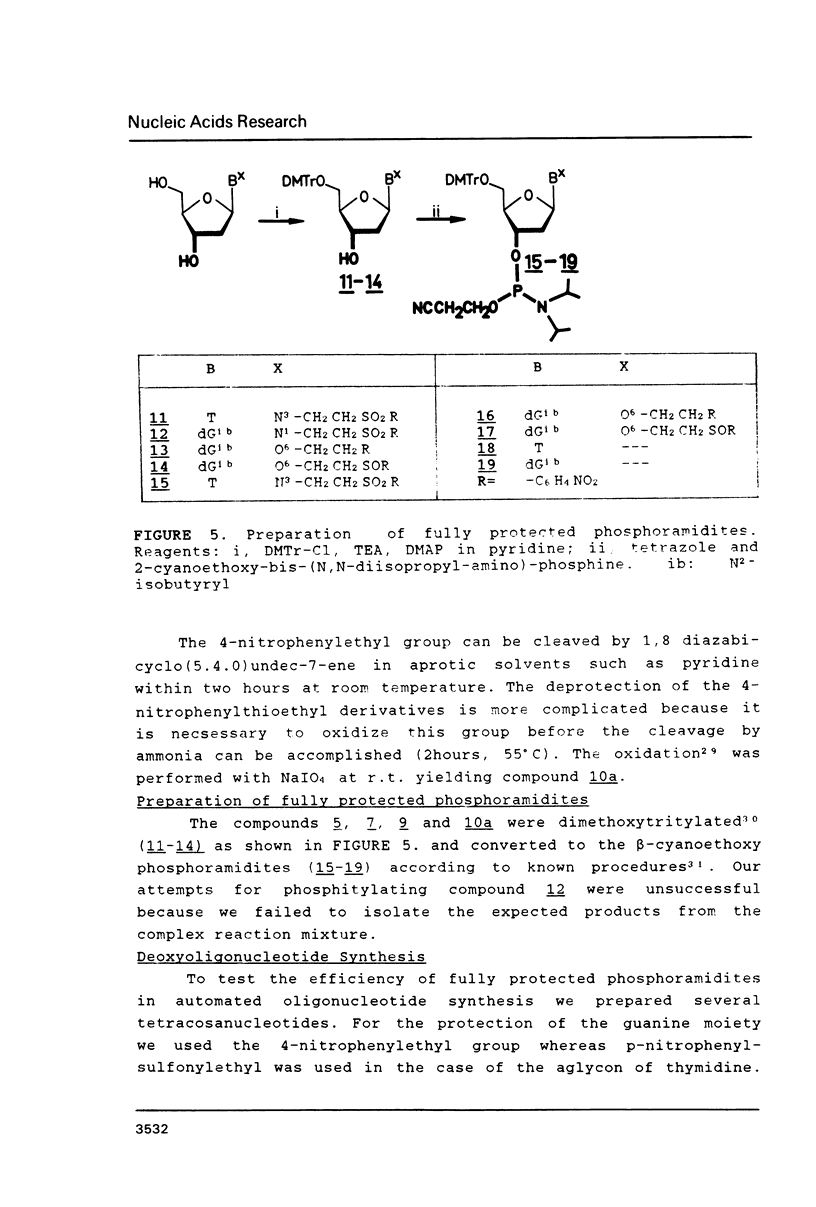
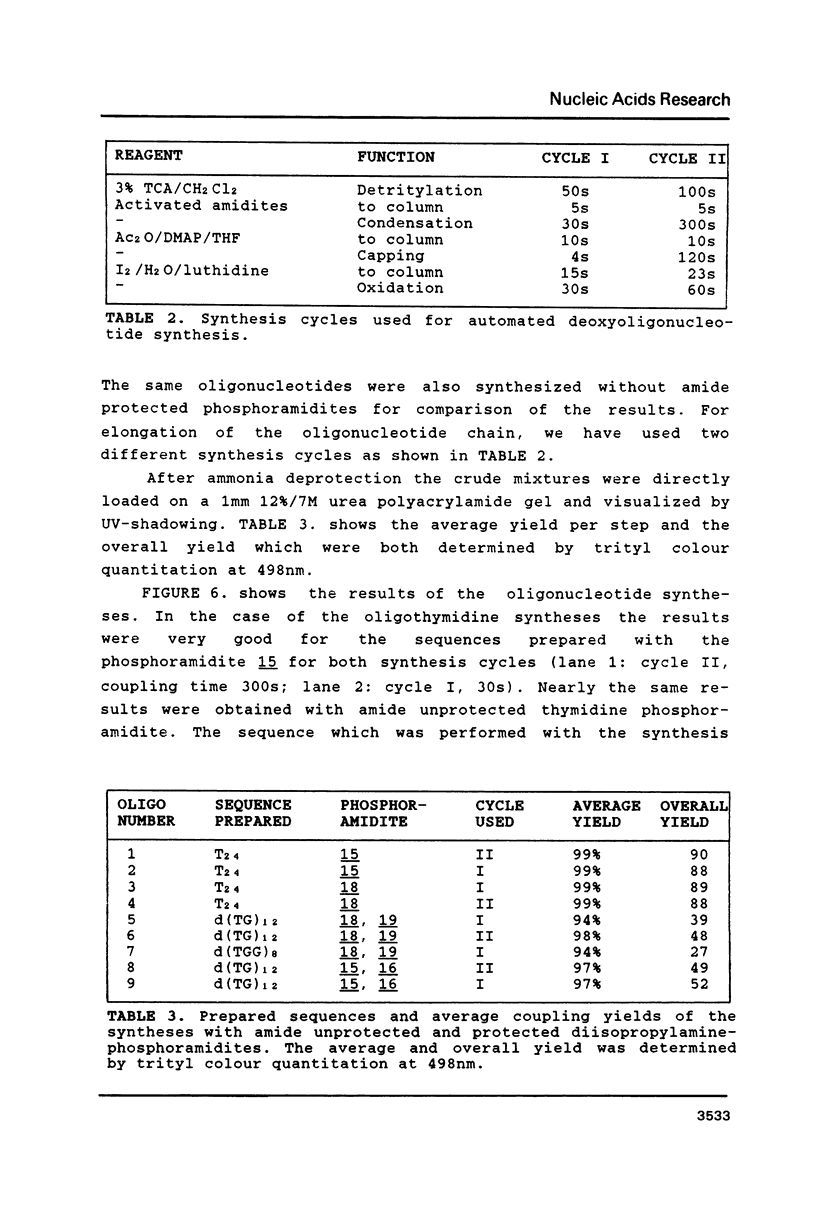
The syntheses of several amide protected deoxyguanosine- as well as thymidine nucleosides are described. These compounds were synthesized according to the Mitsunobu reaction and Michael addition. In contradiction to previous studies we have discovered that the Michael addition gives only products derived from N-alkylation. The occurrence of N- or O-alkylation was assigned by means of two dimensional 1H, 1 3 C-COLOC-NMR spectroscopy. Further, we have found that the Mitsunobu reaction used for the protection of the amide function of dG is limited to alcohols without acidic hydrogen atoms. Amide protected phosphormidites (15, 16) were used for the preparation of deoxyoligonucleotides with a large number of guanine and thymine bases using two different coupling times. We have shown that there is no experimentally detectable difference in the quality of the products if the starting monomer is amide protected or not.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barone A. D., Tang J. Y., Caruthers M. H. In situ activation of bis-dialkylaminophosphines--a new method for synthesizing deoxyoligonucleotides on polymer supports. Nucleic Acids Res. 1984 May 25;12(10):4051–4061. doi: 10.1093/nar/12.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Gaffney B. L., Senior M., Riddle R. R., Jones R. A. Methylation of thymine residues during oligonucleotide synthesis. Nucleic Acids Res. 1985 Jan 25;13(2):573–584. doi: 10.1093/nar/13.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J., Taagaard M., Marugg J. E., van Boom J. H., Dahl O. Application of 2-cyanoethyl N,N,N',N'-tetraisopropylphosphorodiamidite for in situ preparation of deoxyribonucleoside phosphoramidites and their use in polymer-supported synthesis of oligodeoxyribonucleotides. Nucleic Acids Res. 1986 Sep 25;14(18):7391–7403. doi: 10.1093/nar/14.18.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pon R. T., Damha M. J., Ogilvie K. K. Modification of guanine bases by nucleoside phosphoramidite reagents during the solid phase synthesis of oligonucleotides. Nucleic Acids Res. 1985 Sep 25;13(18):6447–6465. doi: 10.1093/nar/13.18.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pon R. T., Usman N., Damha M. J., Ogilvie K. K. Prevention of guanine modification and chain cleavage during the solid phase synthesis of oligonucleotides using phosphoramidite derivatives. Nucleic Acids Res. 1986 Aug 26;14(16):6453–6470. doi: 10.1093/nar/14.16.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]