Abstract
Despite recent advances in the development of new cancer therapies, the treatment options for glioma remain limited, and the survival rate of patients has changed little over the past three decades. Here, we show that 2-hydroxyoleic acid (2OHOA) induces differentiation and autophagy of human glioma cells. Compared to the current reference drug for this condition, temozolomide (TMZ), 2OHOA combated glioma more efficiently and, unlike TMZ, tumor relapse was not observed following 2OHOA treatment. The novel mechanism of action of 2OHOA is associated with important changes in membrane-lipid composition, primarily a recovery of sphingomyelin (SM) levels, which is markedly low in glioma cells before treatment. Parallel to membrane-lipid regulation, treatment with 2OHOA induced a dramatic translocation of Ras from the membrane to the cytoplasm, which inhibited the MAP kinase pathway, reduced activity of the PI3K/Akt pathway, and downregulated Cyclin D-CDK4/6 proteins followed by hypophosphorylation of the retinoblastoma protein (RB). These regulatory effects were associated with induction of glioma cell differentiation into mature glial cells followed by autophagic cell death. Given its high efficacy, low toxicity, ease of oral administration, and good distribution to the brain, 2OHOA constitutes a new and potentially valuable therapeutic tool for glioma patients.
Keywords: fatty acids, sphingomyelin synthase, cancer drug target, glioma biomarker
Cancer cells of undifferentiated phenotype (e.g., glioma) have a poor prognosis and limited treatment options. Primary brain tumors, of which glioma is the most common, are generally associated with very high rates of mortality (ca. 90%), being the median survival of patients about 1 y (1, 2). Chemotherapy provides only modest benefits to radiotherapy and surgery being the alkylating agent temozolomide (TMZ) the reference drug; however, tumor relapse is usually observed, and TMZ only increases the patients’ life expectancy about 2.5 m (from 12.1 to 14.6 m: ref. 3). The present study was designed to investigate the efficacy of 2OHOA against glioma and its molecular mechanisms of action. 2OHOA exhibited a greater efficacy than TMZ in the treatment of glioma, and there was no relapse after long-term treatment with 2OHOA. This efficacy and lack of toxicity at therapeutic doses has been acknowledged recently by the European Medicines Agency (EMA) to designate 2OHOA orphan drug for the treatment of glioma. In previous studies, we showed that this compound induces cell cycle arrest of lung cancer cells (4–6). Here, we showed that 2OHOA reversed the altered lipid profile of glioma cells and how this modification regulated cell signaling to induce autophagy specifically in glioma but not normal cells.
Moreover, in the present study we demonstrated that the changes induced by 2OHOA were specific to cancer cells with no significant effects observed in normal cells and no adverse effects in treated animals, features not shared by most anticancer drugs. The efficacy of this compound in the absence of any relevant toxicity indicates that 2OHOA may be a useful and innovative therapeutic tool to treat glioma.
Results
Efficacy of 2OHOA and TMZ Against Glioma.
The efficacy of 2OHOA against glioma was tested in the human glioma cell lines SF767, U118, A172, and T98G. In these lines, 2OHOA induced a time and concentration dependent inhibition of cell growth (Fig. 1A and Fig. S1A). Likewise, TMZ induced inhibition of human SF767 glioma cell growth, but it failed to kill all the cancer cells in culture exhibiting a lower efficacy in this model of human glioma than 2OHOA.
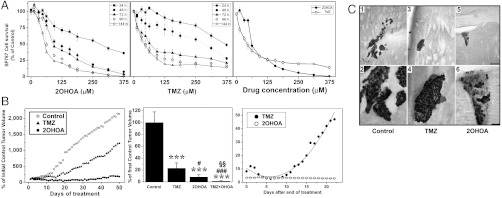
Fig. 1.
Efficacy of 2OHOA against human glioma (SF767) cells and tumors. (A) Time and concentration dependent inhibition of human glioma (SF767) cell growth by 2OHOA (left), TMZ (center), and their compared efficacy at 96 h (right, N = 6–8). (B) Effects of vehicle (control), 2OHOA, TMZ or both against SF767-derived tumor growth in mice during 50 d treatments (left, N = 20), 60-d treatments (center, N = 15), and tumor volumes during 3 wk following 60 d treatments (right). ***P < 0.001 with respect to control; #, P < 0.05, ###, P < 0.001 with respect to TMZ alone; §§, P < 0.01 with respect to 2OHOA or TMZ alone. (C) Effects of vehicle (1, 2), TMZ (3, 4), and 2OHOA (5, 6) on orthotopic human glioma growth (SF767 cells) in the brain of nude mice after 42 d treatments (N = 5). Additional pictures are shown in Fig. S1. In all cases, the doses were 600 mg/kg for 2OHOA and 80 mg/kg for TMZ (p.o., daily). Scale bars = 200 μm (1, 3, 5) and 50 μm (2, 4, 6).
In a xenograft model of human glioma (SF767 cells), 2OHOA was also more potent than TMZ (Fig. 1B). This effect was dose dependent being the sodium salt more potent than other forms of 2OHOA (Fig. S1B); therefore, it was used throughout this work. In this context, the combined treatment with both was more efficient than either alone possibly because their different modes of action (600 mg/kg 2OHOA and/or 80 mg/kg TMZ, p.o., daily, 50 d: Fig. 1B). To determine possible tumor relapse after treatment, both compounds were assessed for a further 21 d after 60 d treatments. Following TMZ treatment, the tumors derived from human glioma SF767 cells again grew in an aggressive manner (Fig. 1B). Similar results have been reported in patients with glioma in whom TMZ treatment only increases median survival by 10 wk (3, 7, 8). In contrast, tumor relapse was not observed after 2OHOA treatment (Fig. 1B). Using an orthotopic model of human glioma, oral administration of 2OHOA completely eliminated glioma cell tumors in the brain of three mice and, in the other two, only a few SF767 cells remained (Fig. 1C and Fig. S1C). In this context, HuNu+ (i.e., human glioma) cells were in the vicinity of the ventricle with some labeling on the choroid plexus. In animals treated with TMZ, a reduction of the tumor size was also observed; though, the size of tumors and their immunoreactivity were greater than in animals treated with 2OHOA (Fig. 1 and Fig. S1).
2OHOA Regulates Glioma Membrane-Lipid Composition and Structure.
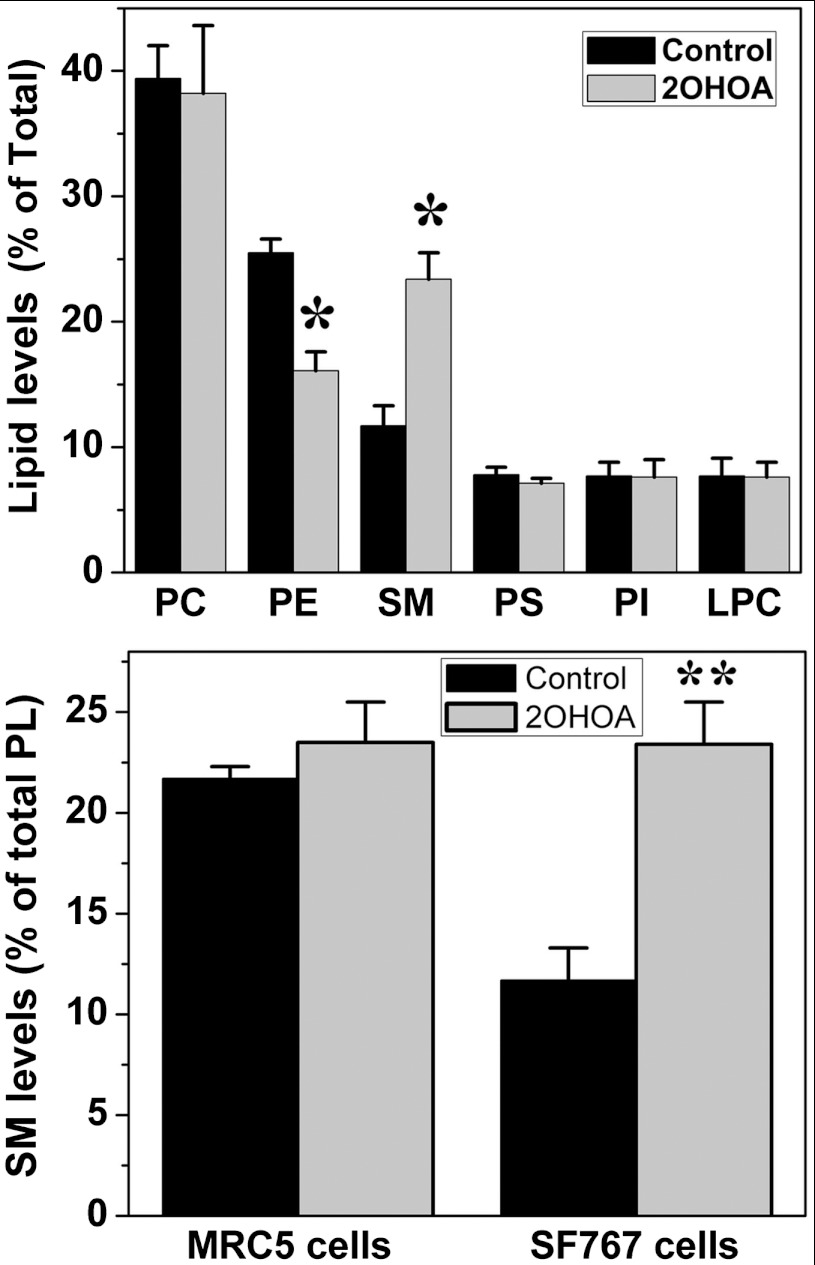
2OHOA is a synthetic fatty acid that readily binds to the plasma membrane and regulates its lipid structure (4, 9). It induces important changes in the membrane structure that favor the binding of certain proteins like protein kinase Cα (PKCα: 4, 9). 2OHOA was detected in SF767 cell membranes as a free fatty acid (ca. 7%) or incorporated in phospholipids (ca. 30%), constituting a major membrane fatty acid upon treatment. In addition, significant alterations in the levels of various membrane lipids were observed after 2OHOA treatment including marked increases in SM (ca. 2-fold: Fig. 2) and 1,2-diacylglycerol (DAG, from 2.05 ± 0.17 in untreated cells to 3.02 ± 0.11 nmole/mg protein in 2OHOA-treated cells) and marked decreases in phosphatidylethanolamine (PE) mass (Fig. 2). No such changes were observed following 2OHOA treatment of normal (MRC-5) cells (Fig. 2 and Fig. S2) that already exhibit high basal levels of SM. The increase in DAG and the presence of 2OHOA itself favors the cytosol to membrane translocation and activation of PKCα (4, 10) that is associated with knockdown of E2F-1 and DHFR (5, 6). The increase in membrane 2OHOA was likely associated with short-term (10 min) Ras release from the membrane and the subsequent inhibition of the ERK pathway, and changes in SM and PE could be related the long-term Ras translocation (Fig. 3 and Fig. S3).
Fig. 2.
2OHOA (72 h, 200 μM) induces changes in SF767 membrane-lipid composition. Upper panel, Levels of the major phospholipid classes before (black bars) and after (gray bars) 2OHOA treatment: PC, phosphatidylcholine; PE, phosphatidylethanolamine; SM, sphingomyelin; PS, phosphatidylserine; PI, phosphatidylinositol; LPC, lyso-phosphatidylcholine. Lower panel, effects of 2OHOA (72 h, 200 μM) on SM levels in normal (MRC5) and glioma (SF767) cells before (black) and after (gray) treatment (N = 6–8).
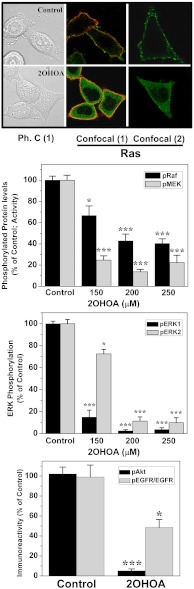
Fig. 3.
Effect of 2OHOA on the MAPK (ERK) and PI3K/Akt pathways in SF767 cells. Top: 2OHOA (150 μM) induces Ras (green fluorescence) translocation from the membrane to the cytoplasm after incubations of 10 min (1) or 24 h (2). Ph. C.: phase contrast micrography. In confocal micrographies, the red fluorescence corresponds to the membrane labeling as detected with the raft marker Vybrant Alexa Fluor 594 (Molecular Probes). Effects of 2OHOA (0, 150, 200, and 250 μM) on the phosphorylation (i.e., activity) of Raf (second panel), MEK and ERK1/2 (third panel), and EGF-stimulated Akt and EGFR phosphorylation (150 μM, 12 h after a 15 min stimulation with 35 ng/ml EGF; bottom panel). N = 6–8 in all cases.
2OHOA Inhibits the EGFR/Ras/MAP Kinase and PI3K/Akt Pathways in Glioma cells.
The EGFR/Ras/MAP kinase and PI3K/Akt pathways are usually hyperactive and cause proliferation and loss of differentiation in glial cells (11–14). Treatment with 2OHOA, which induced changes in the membrane-lipid composition of glioma cells, caused translocation of Ras from the plasma membrane to the cytoplasm (the perimembranal content of Ras determined by confocal microscopy changed from 87 ± 6% to 6 ± 3% in the absence or presence of 2OHOA); but, it did not significantly change the total cellular Ras content (98.6 ± 4.4% and 94.7 ± 6.2% in control and 2OHOA-treated cells, as determined by immunoblotting: Fig. S4). Ras propagates incoming messages from membrane growth factor receptors (e.g., EGFR) to downstream proteins such as Raf at defined ld lipid microdomains in the plasma membrane (15, 16). As such, its translocation to the cytoplasm greatly affected the proliferative Ras/MAPK signaling, which is frequently overactive in cancer cells and responsible for their loss of differentiation (17, 18). Indeed, a significant reduction in the levels of phospho-EGFR and phosphorylated (i.e., active) cRaf, MEK and MAP kinases (ERK1 and ERK2) was observed in vitro (SF767 cells) and in vivo (tumors) after 2OHOA treatments (Fig. 3, and Figs. S3 and S4). No significant changes in the levels of total Raf, MEK, or ERK were observed (Fig. S4). The PI3K/Akt signaling pathway is involved in cell survival and growth in cooperation with the Ras/MAPK pathway in cancer cells (17). Following 2OHOA treatment, we observed a significant reduction in Akt phosphorylation indicating that this protein is preferentially inactive (Fig. 3).
2OHOA Induces Cell Cycle Arrest in Human Glioma Cells.
Cell cycle arrest has been shown to occur in response to PKC activation in various cancer cell types (4–6, 19, 20). In human glioma cells, we have seen that 2OHOA induced a rapid (minutes) and sustained (over 24 h) activation (translocation to membrane) of PKC that caused overexpression of p21Cip1 and a simultaneous increase of p27Kip1 (Fig. S5). These potent CDK inhibitors (CDKIs, ref. 21) are frequently downregulated in gliomas, and their rise induced decreases of Cyclin D1, Cyclin D3, and CDK4 and CDK6 that caused RB hypophosphorylation, E2F-1 downregulaiton and DHFR knockdown (Fig. S5) followed by cell cycle arrest in G0/1 of glioma cells. No significant changes in the levels of these proteins were seen in MRC-5 cells (immunoreactivities for these proteins were 91–112% for all these proteins being P > 0.05 always).
2OHOA Induces Human Glioma Cell Differentiation.
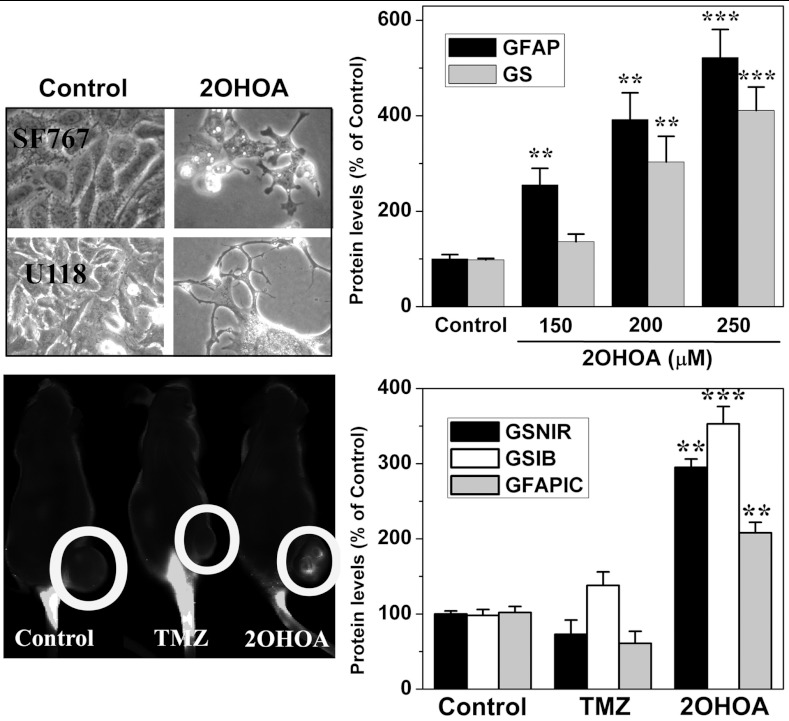
In addition to their increased proliferation, glioma cells lose many molecular and morphological features of differentiated glial cells such as the typical stellar shape and the expression of glutamine synthetase (GS) and glial fibrillary acidic protein (GFAP; 22, 23). It has been proposed that inducing differentiation may be a suitable approach to treat cancer given its potential specificity and low level of toxicity (24). In this context, treatment with 2OHOA induced a marked differentiation of glioma cells resulting in the recovery of the stellar morphology of mature astrocytes and the expression (in vitro and in vivo) of the glial differentiation markers GFAP and GS, in SF767 cells (Fig. 4). Because the final effect of 2OHOA was induction of autophagy, it could be feasible that redifferentiation may lead to the initiation of death programs in cancer cells, though this point requires further studies.
Fig. 4.
2OHOA induces glioma cell differentiation in vitro and in vivo. Top, phase contrast micrographs of SF767 and U118 (human glioma) cells maintained in the presence or absence (control) of 2OHOA for 72 h (Left, 200 μM, 200x magnification). 2OHOA induced the expression of GFAP and GS (right) in SF767 cells as determined in immunoblots. Bottom, In vivo near infrared detection of a fluorolabeled anti-GS antibody in nude mice with SF767-derived tumors following treatment with the vehicle lone (control), 2OHOA and TMZ (Left, N = 10 per group) and the corresponding quantification (Right, GSNIR, mean ± SEM). GS was also determined ex vivo in these animals using immunoblotting (GSIB). GFAP was also quantified by immunocytochemistry (GFAPIC, Right).
2OHOA Induces Glioma Cell Autophagy.
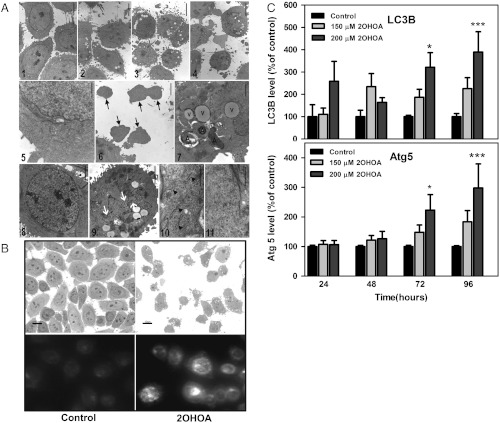
2OHOA treatment resulted in the death of the majority of cells in tumors derived from SF767 cells (Fig. 5 and Fig. S6). Similarly, in cultured SF767 cells, long-term (120 h) incubation with 2OHOA induced important morphological alterations that were observed in semithin sections (1.5 μm) by optical microscopy and thin (0.06–0.09 μm) sections by electron microscopy (Fig. 5). Thus, optical microscopy images revealed that untreated cells were oval in shape, with nuclei containing multiple nucleoli distributed along the nuclear matrix and few invaginations. In contrast, 2OHOA-treated cells (120 h, 300 μM) exhibited irregular morphology and were markedly smaller. Their nuclei were also smaller and contained deep invaginations whose depth was concentration dependent (Fig. 5). Notably, 2OHOA induced the appearance of lipid droplets and dense bodies, the latter scattered throughout the cytoplasm and some exhibiting morphological characteristics of autophagosomes (Fig. 5). At low and high 2OHOA concentrations, a loss of ER cisternae was observed in the cytoplasm consistent with the autophagic process (Fig. 5).
Fig. 5.
(A) Electron microscopy of SF767 cells maintained for 120 h in the presence or absence (control, panels 1, 5, 8, and 10) of 2OHOA (100 μM: panel 2; 200 μM: panels 3, 6, and 7; 300 μM: panels 4, 9 and 11). Arrows in panel 6 correspond to SF767 fragments. In panel 7, V corresponds to lipid vesicles and A accounts for autophagosomes. The nuclei of SF767 cells cultured in the presence (panel 9) or absence (panel 8) of 2OHOA are shown, and the white arrows correspond to nuclear invaginations (bar = 2.5μm). 2OHOA treatment caused loss of rough endoplasmic reticulum cisternae in 2OHOA-treated cells (panel 11) compared with control cells (panel 10, arrows show cisternae; bar = 0.5 μm). N = 300 for each concentration and time used. (B) Upper panels, optical microscope photographs (1.5 μm semithin sections) showing SF767 cells treated for 120 h with vehicle (control) or 2OHOA (300 μM). Bottom panels, Fluorescence of lysosome/autophagosome labeled with Lysosensor in SF767 cells in the presence or absence (left) of 2OHOA (150 μM for 48 h, right). N = 400 cells from five independent experiments. The relative fluorescence was quantified using the Image J 1.38x software, and data are indicated in the Results section. (C) Effect of 2OHOA (150 μM and 200 μM) at different times (24–96 h) on the markers of autophagy, LC3B (upper panel) and Atg5 (lower panel).
In turn, the unspecific ER stress inducer palmitic acid caused similar autophagosome synthesis induction in SF767 and MRC-5 cells (Fig. S6B). Finally, the expression of the autophagosome proteins LC3B and ATG5 increased significantly in a time and concentration dependent manner in SF767 cells (Fig. 5).
Discussion
In this study, the efficacy of the synthetic fatty acid 2OHOA in the treatment of glioma was demonstrated, and its mode of action described, driving glioma-to-glial cell differentiation that was followed by autophagy through the specific inhibition of the Ras-MAPK, Cyclin/CDK-DHFR, and PI3K-Akt pathways. 2OHOA is more efficient drug against human glioma than TMZ, the current reference drug to treat this condition. Unlike TMZ, no tumor relapse was observed following 2OHOA treatment (Fig. 1). Its low toxicity (IC50 > 5,000 μM, in normal MRC5 cells, and minimum lethal dose > 3,000 mg/kg in rats) is unusual for anticancer drugs further supporting the specificity of 2OHOA and its use as differentiation therapy agent to treat cancer (24). Based on these facts, the EMA has acknowledged the potential significant benefit of 2OHOA and has recently designated this molecule an orphan drug for the treatment of glioma.
The plasma membrane contains thousands of different lipids that form various types of membrane microdomains that can be differentially and specifically regulated by drugs targeting the lipid bilayer. Thus, membrane-lipid therapy aims at the specific regulation of certain membrane-lipid structures to treat cancer and other human pathologies (25). In this context, very low levels of SM were found in human glioma (SF767) cells when compared with normal (MRC-5) cells, a characteristic common to all the cancer cell lines that we have studied to date (leukemia, lung cancer, and other glioma cells; ref. 26). In SF767 cells, 2OHOA treatments induced restoration of SM to levels similar to those observed in nontumor cells (Fig. 2). This observation (along with other results shown here) suggests that lower SM levels in cancer cells could facilitate high Ras-MAPK activity to express the malignant phenotype. The bulky isoprenyl moiety of Ras proteins can be anchored in membrane domains with high content of PE whereas it is excluded from SM-rich domains where the dense surface membrane packing prevents isoprenyl binding. Thus, Ras translocation to the cytoplasm (Fig. 3) was probably caused by changes in membrane lipids induced by 2OHOA, which could impair productive interactions between EGFR and Ras and Ras and Raf at the plasma membrane, and finally inactivate the MAPK cascade (protein expression was not altered). In fact, tipifarnib and other farnesyl transferase inhibitors exert their anticancer effects impairing Ras binding to membranes by blocking Ras isoprenylation (27). Therefore, the presence of 2OHOA first and the normalization of SM and PE levels, later induced changes in the membrane-lipid structure that caused recovery of the localization and activity of relevant signaling proteins (Fig. 2, and Figs. S2 and S3), which constitutes an alternative approach for the treatment of cancer (28). In this context, the regulation of SM levels by 2OHOA (via activation of sphingomyelin synthetase, ref. 26) is crucial in its mechanism of action against glioma. In line with these results, the addition of SM to culture medium enhances gemcitabine-mediated pancreatic cancer cell death (29) further indicating the relevance of this lipid in cancer cell survival. On the other hand, 2OHOA treatments induced specific changes in the levels of other membrane lipids (DAG, PE, and 2OHOA) that contributed to remodel membrane microdomains and regulated glioma cell signaling (30–32). These changes also facilitated the membrane binding and activation of PKCα (4, 10, and Fig. S5) that, itself, triggers inhibitory effects against cancer cell growth (4, 19, 20, and Fig. 5).
The canonical signaling cassette made up of EGFR, Ras, Raf, MEK, and ERK and/or the PI3K/Akt signaling pathway are overactivated in most human gliomas as well as in other types of cancer, and they often cooperate to induce malignant transformation of cancer cells (15, 17, 18, 33–36). In the present study, we demonstrated that 2OHOA mediates the translocation of Ras from the plasma membrane to the cytoplasm as well as the subsequent inhibition of the MAP kinase (ERK, in vitro and in vivo) PI3K/Akt and Cyclin/CDK pathways (Fig. 3 and Fig. S4). In glioma cells, activation of the EGFR/Ras/Raf/ERK pathway blocks differentiation and induces the dedifferentiation of glial cells (11). Thus, inhibition of this signaling cascade constitutes a central event in the glioma-to-glial cell differentiation induced by 2OHOA, and it is most likely involved in 2OHOA-mediated cell cycle arrest and induction of autophagy.
2OHOA-induced PKC translocation to the membrane (and its concomitant activation) is associated with overexpression of the CDKI p21Cip1 (4) and possibly of p27Kip1 (Fig. S5), and with β-catenin downregulation (4, 19, 20). 2OHOA-induced overexpression of CDKIs and inactivation of cyclin D-CDK4/6 complexes (Fig. S5) is also associated with decreased Akt levels and RB phosphorylation (4, 37) and, therefore, with lower cell proliferation and reduced survival. Hypophosphorylation of RB prevents its dissociation from E2F-1 inhibiting the expression and activation of E2F-1, a pivotal transcription factor in cell cycle progression. These multiple regulatory effects probably contributed to glioma cell differentiation via inhibition of the MAPK-pathway as determined by the morphological (astroglial shape recovery) and molecular (increased expression of GS and GFAP) changes caused by 2OHOA, in vitro and in vivo.
We have shown in the present and previous studies (26) that the plasma membrane of glioma and all other cancer cells studied exhibit markedly low SM levels that appear to constitute a basic requirement to express the malignant phenotype. In the present study, we showed that 2OHOA induced recovery of SM levels and it was associated with potent effects against glioma. This fact suggests that remodeling of the membrane structure and composition would be upstream to the oncogenic action of Ras in cancer cells. Furthermore, this anticancer effect was associated with a dual-mode mechanism of action. On the one hand, the presence of 2OHOA in membranes and the increase in DAG would induce PKC translocation to membranes followed by CDKI overexpression and pRb hypophosphorylation (this work and refs. 4–6). On the other hand, Ras translocation to the cytosol would cause MAPK and Akt inactivation. These two pathways have been consistently seen to be involved in the loss of differentiation, increased proliferation, and survival of cancer cells so that their regulation by 2OHOA is most likely responsible for the induction of differentiation and autophagy observed upon treatment. In any case, additional molecular events/mechanisms should not be discarded. Indeed, we have recently seen that 2OHOA also induces marked increases in the levels of nuclear SM (26). In this context, nuclear phospholipids have been shown to participate in nuclear signaling and could account for some of the cellular effects induced by 2OHOA including glioma cell proliferation, differentiation and death (38).
Autophagy is an alternative program of cell death that may overcome the resistance of many cancers (e.g., glioma) to enter the apoptotic program (39). In this context, we detected that 2OHOA-induced autophagy in SF767 cells (Fig. 5). Thus, there were observed marked time and concentration dependent increases in the levels of ATG5 and LC3B, both proteins fundamental for the formation of autophagosomes (40). The induction of autophagy in glioma cells is triggered by RB hypophosphorylation and p27kip1-mediated Akt inhibition (41) events induced by 2OHOA on these cells. In this context, the modifications in glioma cells caused by 2OHOA resulted in profound morphological changes, cell fragmentation, the release of cytosolic bodies containing ER cisternae, and an increase in lysosomes/autophagosomes, which is further evidence that autophagy is initiated. The high extent of autophagy in glioma cells upon treatment with 2OHOA specifically kills cancer but not nontumor cells and supports a recent hypothesis suggesting that autophagy might be used as a potential cancer therapy (42). The fact that autophagy occurred after induction of differentiation in SF767 cells treated with 2OHOA suggests that recovery of the features of mature cells may cause a molecular conflict to cancer cells. In contrast, neither autophagy nor the other molecular events here described were induced by 2OHOA in normal cells. This mechanism of action explains the extensive tumor cell death and lack of relapse in animals treated with 2OHOA. Thus, 2OHOA is a first-in-class nontoxic membrane-lipid anticancer drug that activates sphingomyelin synthase and subsequently inhibits the MAPK and related oncogenic pathways.
Methods
Cells were incubated in DMEM (SF767) or RPMI 1640 (U118, A172 and T98G) in the presence of 10% FBS and antibiotics and in the presence or absence of 2OHOA or TMZ. Xenograft gliomas (subcutaneous and orthotopic) were developed in immunosuppressed mice by inoculation of SF767 cells. The data are expressed as the mean ± SEM values from 6–8 independent experiments or the number of animals indicated. Statistical significance was indicated: *, P < 0.05, **, P < 0.01, and ***, P < 0.001. Further details of the experiments are provided in the supporting information section.
Supplementary Material
Acknowledgments.
We are indebted to Prof. John E. Halver for his valuable ideas and suggestions. This work was supported by Grants BIO2010-21132, IPT-010000-2010-016 (Ministerio de Ciencia e Innovación, Spain), PROMETEO (Generalitat Comunitat Valenciana), and by the Marathon Foundation. S.T. and G.B.-C. were supported by Torres-Quevedo Research Contracts. M.L.M. and M.A.N.-S. were supported by fellowships from the Govern de les Illes Balears.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118349109/-/DCSupplemental.
References
- 1.Brenner H, et al. Long-term survival expectations of cancer patients in Europe in 2000–2002. Eur J Cancer. 2009;45:1028–1041. doi: 10.1016/j.ejca.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN. Molecular pathology of malignant gliomas. Annu Rev Pathol. 2006;1:97–117. doi: 10.1146/annurev.pathol.1.110304.100043. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Martínez J, et al. Membrane structure modulation, protein kinase Cα activation, and anticancer activity of Minerval. Mol Pharmacol. 2005;67:531–540. doi: 10.1124/mol.104.000778. [DOI] [PubMed] [Google Scholar]
- 5.Martínez J, et al. The repression of E2F-1 is critical for the activity of Minerval against cancer. J Pharmacol Exp Ther. 2005;315:466–474. doi: 10.1124/jpet.105.088716. [DOI] [PubMed] [Google Scholar]
- 6.Lladó V, et al. Pivotal role of dihydrofolate reductase knockdown in the anticancer activity of 2-hydroxyoleic acid. Proc Natl Acad Sci USA. 2009;106:13754–13758. doi: 10.1073/pnas.0907300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yung WK, et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse Temodal Brain Tumor Group. J Clin Oncol. 1999;17:2762–2771. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]
- 8.Omar AI, Mason WP. Temozolomide: The evidence for its therapeutic efficacy in malignant astrocytomas. Core Evid. 1999;4:93–111. doi: 10.2147/ce.s6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barceló F, et al. The hypotensive drug 2-hydroxyoleic acid modifies the structural properties of model membranes. Mol Membr Biol. 2004;21:261–268. doi: 10.1080/09687680410001716835. [DOI] [PubMed] [Google Scholar]
- 10.Goñi FM, Alonso A. Structure and functional properties of diacylglycerols in membranes. Prog Lipid Res. 1999;38:1–48. doi: 10.1016/s0163-7827(98)00021-6. [DOI] [PubMed] [Google Scholar]
- 11.Harrisingh MC, Lloyd AC. Ras/Raf/ERK signaling and NF1. Cell Cycle. 2004;3:1255–1258. doi: 10.4161/cc.3.10.1182. [DOI] [PubMed] [Google Scholar]
- 12.Katz M, Amit I, Yarden Y. Regulation of MAPKs by growth factors and receptor tyrosine kinases. Biochim Biophys Acta. 2007;1773:1161–1176. doi: 10.1016/j.bbamcr.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffero F, et al. Different response of human glioma tumor-initiating cells to epidermal growth factor receptor kinase inhibitors. J Biol Chem. 2009;284:7138–7148. doi: 10.1074/jbc.M807111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong ML, Kaye AH, Hovens CM. Targeting malignant glioma survival signalling to improve clinical outcomes. J Clin Neurosci. 2007;14:301–308. doi: 10.1016/j.jocn.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351:289–305. [PMC free article] [PubMed] [Google Scholar]
- 16.Leicht DT, et al. Raf kinases: function, regulation and role in human cancer. Biochim Biophys Acta. 2007;1773:1196–1212. doi: 10.1016/j.bbamcr.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parsa AT, Holland EC. Cooperative translational control of gene expression by Ras and Akt in cancer. Trends Mol Med. 2004;10:607–613. doi: 10.1016/j.molmed.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Tatevossian RG, et al. MAPK pathway activation and the origins of pediatric low-grade astrocytomas. J Cell Physiol. 2010;222:509–514. doi: 10.1002/jcp.21978. [DOI] [PubMed] [Google Scholar]
- 19.Gwak J, et al. Protein-kinase-C-mediated beta-catenin phosphorylation negatively regulates the Wnt/beta-catenin pathway. J Cell Sci. 2006;119:4702–4709. doi: 10.1242/jcs.03256. [DOI] [PubMed] [Google Scholar]
- 20.Gwak J, et al. Stimulation of protein kinase C-α suppresses colon cancer cell proliferation by down-regulation of beta-catenin. J Cell Mol Med. 2009;13:2171–2180. doi: 10.1111/j.1582-4934.2008.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gartel AL, Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;1:639–649. [PubMed] [Google Scholar]
- 22.Marushige Y, et al. Modulation of growth and of morphological characteristics in glioma cells by nerve growth factor and glia maturation factor. Cancer Res. 1987;47:4109–4115. [PubMed] [Google Scholar]
- 23.Lee K, et al. Downregulation of GFAP, TSP-1, and p53 in human glioblastoma cell line, U373MG, by IE1 protein from human cytomegalovirus. Glia. 2005;51:1–12. doi: 10.1002/glia.20179. [DOI] [PubMed] [Google Scholar]
- 24.Leszczyniecka M, Roberts T, Dent P, Grant S, Fisher PB. Differentiation therapy of human cancer: basic science and clinical applications. Pharmacol Ther. 2001;90:105–156. doi: 10.1016/s0163-7258(01)00132-2. [DOI] [PubMed] [Google Scholar]
- 25.Escribá PV. Membrane-lipid therapy: a new approach in molecular medicine. Trends Mol Med. 2006;12:34–43. doi: 10.1016/j.molmed.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Barceló-Coblijn G, et al. Sphingomyelin and sphingomyelin synthase (SMS) in the malignant transformation of glioma cells and in 2-hydroxyoleic acid therapy. Proc Natl Acad Sci USA. 2011;108:19569–19574. doi: 10.1073/pnas.1115484108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Widemann BC, et al. Phase 1 trial and pharmacokinetic study of the farnesyl transferase inhibitor tipifarnib in children and adolescents with refractory leukemias: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2011;56:226–233. doi: 10.1002/pbc.22775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mollinedo F, et al. Lipid raft-targeted therapy in multiple myeloma. Oncogene. 2010;29:3748–3757. doi: 10.1038/onc.2010.131. [DOI] [PubMed] [Google Scholar]
- 29.Modrak DE, Leon E, Goldenberg DM, Gold DV. Ceramide regulates gemcitabine-induced senescence and apoptosis in human pancreatic cancer cell lines. Mol Cancer Res. 2009;7:890–896. doi: 10.1158/1541-7786.MCR-08-0457. [DOI] [PubMed] [Google Scholar]
- 30.Escribá PV, et al. Role of lipid polymorphism in G protein-membrane interactions: nonlamellar-prone phospholipids and peripheral protein binding to membranes. Proc Natl Acad Sci USA. 1997;94:11375–11380. doi: 10.1073/pnas.94.21.11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vögler O, et al. The Gβγ dimer drives the interaction of heterotrimeric Gi proteins with nonlamellar membrane structures. J Biol Chem. 2004;279:36540–36545. doi: 10.1074/jbc.M402061200. [DOI] [PubMed] [Google Scholar]
- 32.Barceló F, et al. Interaction of the C-terminal region of the Ggamma protein with model membranes. Biophys J. 2007;93:2530–2541. doi: 10.1529/biophysj.106.101196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guha A, Feldkamp MM, Lau N, Boss G, Pawson A. Proliferation of human malignant astrocytomas is dependent on Ras activation. Oncogene. 1997;15:2755–2765. doi: 10.1038/sj.onc.1201455. [DOI] [PubMed] [Google Scholar]
- 34.Ohgaki H, Kleihues P. Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci. 2009;100:2235–2241. doi: 10.1111/j.1349-7006.2009.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Omerovic J, Laude AJ, Prior IA. Ras proteins: paradigms for compartmentalised and isoform-specific signalling. Cell Mol Life Sci. 2007;64:2575–2589. doi: 10.1007/s00018-007-7133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holland EC. A mouse model for glioma: biology, pathology, and therapeutic opportunities. Toxicol Pathol. 2000;28:171–177. doi: 10.1177/019262330002800122. [DOI] [PubMed] [Google Scholar]
- 37.Kelly-Spratt KS, et al. Inhibition of PI-3K restores nuclear p27Kip1 expression in a mouse model of Kras-driven lung cancer. Oncogene. 2009;28:3652–3662. doi: 10.1038/onc.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Irvine RF. Nuclear lipid signalling. Nat Rev Mol Cell Biol. 2003;4:1–12. doi: 10.1038/nrm1100. [DOI] [PubMed] [Google Scholar]
- 39.Lefranc F, Facchini V, Kiss R. Proautophagic drugs: a novel means to combat apoptosis-resistant cancers, with a special emphasis on glioblastomas. Oncologist. 2007;12:1395–1403. doi: 10.1634/theoncologist.12-12-1395. [DOI] [PubMed] [Google Scholar]
- 40.Ferraro E, Cecconi F. Autophagic and apoptotic response to stress signals in mammalian cells. Arch Biochem Biophys. 2007;462:210–219. doi: 10.1016/j.abb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Jiang H, et al. The RB-E2F1 pathway regulates autophagy. Cancer Res. 2010;70:7882–7893. doi: 10.1158/0008-5472.CAN-10-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dalby KN, et al. Targeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer. Autophagy. 2010;6:322–329. doi: 10.4161/auto.6.3.11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.