Symbiosis is the intimate “living together” of different organisms (1), and symbiotic relationships range from mutually beneficial to neutral or parasitic. Indeed, many microbial–host symbioses can vary between these states, depending upon circumstances. Recent years have seen an explosion of discoveries revealing novel microbial–host relationships and interactions. In addition to “classic” nutritional symbioses (in which the microbe provides a limiting nutrient not normally available in the host’s diet), studies have shown that microbes can alter the reproductive mode of their hosts (e.g., induce development of unfertilized eggs, change sex, or cause sperm–egg incompatibilities), affect mate choice, and provide protection from natural enemies, including viruses, protozoa, and parasitic insects (2–4). Kikuchi et al. (5) report an extension of this repertoire of effects—bacteria in the genus Burkholderia impart protection against organophosphorous pesticides in stinkbugs.
Burkholderia is a genus of biologically diverse bacteria commonly found in soil and associated with plants (6). Although originally isolated as plant pathogens on onion, many Burkholderia are now known to provide benefits to plants, from involvement in nitrogen fixation to suppression of plant disease. Strains of Burkholderia cepacia can also cause chronic lung infections in cystic fibrosis patients. Stinkbug-associated Burkholderia are from a different group within the genus, and related strains are found both in soil and in the guts of stinkbugs (5).
Organophosphorus (OP) compounds are extensively used in agriculture, accounting for ∼38% of total pesticide use (7). These compounds inhibit the activity of acetylcholine esterase, resulting in neurotoxic effects in both insects and mammals. Hence, OPs are common causes of poisoning in people and livestock. Because of their heavy use, OP compounds are also significant contaminants in terrestrial and aquatic ecosystems.
Kikuchi et al. (5) bring together the findings of two different disciplines. For several decades researchers have been investigating how pesticides are degraded in the environment, discovering a major role of soil microorganisms, including bacteria in the genus Burkholderia (7, 8). Soil bacteria can metabolize OPs and use them as sources of carbon, phosphorus, or nitrogen, facilitating degradation of these compounds in the environment. Components of the genetic machinery for OP degradation have also been exchanged among diverse soil bacteria by lateral gene transfers. Motivation for the research includes possible use of soil microbes for environmental remediation of pesticides and application of the biochemical mechanisms to medical treatment of pesticide poisoning.
A separate research community has been busy exploring the microbial symbioses of insects. The resurgence of symbiosis research from its original heyday in the early to mid-20th century (9) has been spurred by the advent of molecular tools for characterizing previously intractable bacteria, as well as the discoveries of amazing and diverse phenotypic effects of symbionts on their hosts (2–4). In previous work, Kikuchi et al. (10) found that Burkholderia are common gut bacteria in a wide range of stinkbugs and relatives, encompassing a significant fraction of the insect order Heteroptera, or “true bugs.” Many are major pests in agriculture; for example, the bean bug Riptortus pedestris described in the present study. Burkholderia normally impart a growth advantage to the bugs, although the precise benefit provided is not yet known. Careful study also revealed that the bugs acquire Burkholderia from the environment each generation as nymphs (11), rather than from the mother (e.g., either within or on the eggs) or infectiously from other stinkbugs (Fig. 1). The bacteria colonize specialized structures in the posterior midgut referred to as “crypts” and can achieve upward of 108 cells per adult. Consistent with environmental acquisition, their phylogeny based on 16S ribosomal sequence indicate no separation of soil- vs. stinkbug-associated bacteria. Environmental acquisition of symbionts each generation has not been widely described in insects, although it occurs in some other systems, such as the squid–Vibrio (light organ), coral–algal (nutritional), and legume–rhizobium (nitrogen fixation) symbioses (12–14).
Fig. 1.
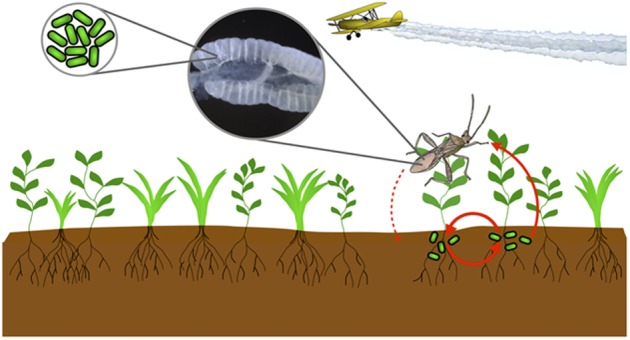
Burkholderia bacteria are found in the soil, where some genotypes are capable of detoxifying OP pesticides. Stinkbugs acquire bacteria from the environment, and Burkholderia preferentially colonize specialized structures (crypts) in the insect midgut. When stinkbugs are experimentally infected with pesticide-degrading bacteria, they have significantly increased resistance to pesticide treatment. It is unclear whether any Burkholderia genotypes have established a routine transmission cycle between bean bugs and the environment, which would enhance coadaptation of pesticide resistance in microbes and host.
In the present study, Kikuchi et al. (5) bring together their own observations on Burkholderia in stink bugs with the observations of Burkholderia involvement in pesticide degradation (7, 8) to develop the hypothesis that OP-degrading bacteria could impart pesticide resistance to the insects. Fenitrothion (FEN) is a commonly used OP pesticide, and FEN-degrading Burkholderia strains are found in soils where these pesticides are applied. Inoculating with FEN-degrading vs. nondegrading strains revealed that pesticide-degrading symbionts impart significant protection against the pesticide applied under laboratory conditions. Although FEN-degrading Burkholderia were uncommon in local bean fields, treatment of field soil brought to the laboratory with FEN greatly enhanced frequency of FEN-degrading Burkholderia, and the stinkbugs reared on soybeans from these soils showed elevated levels of FEN-degrading activity. Finally, much higher levels of FEN are used in sugar cane fields on some Japanese Islands to control the Oriental chinch bug Cavelerius saccharivorus. On one Island, FEN-degrading bacteria were detected in ∼8% of field-collected chinch bugs. The study shows the potential of symbiotic bacteria to impart pesticide resistance to insect hosts. However, as noted by the authors, it has not yet been shown that symbionts provide significant levels of protection in field populations.
Because OP compounds are used so widely in agriculture for pest control, symbiont detoxification could represent a rapid and previously unappreciated mechanism for pesticide resistance in insects (15). Given the general detoxification ability of microbes and their ability to evolve quickly, they could provide a potent means for rapid acquisition of pesticide resistance in hosts. However, the mode of microbe transmission to hosts will affect the likelihood that symbiont-mediated pesticide resistance evolves. For example, given that Burkholderia are acquired by stinkbugs each generation from a very large bacterial pool in soil, coadaptation of pesticide-degrading bacteria and their hosts will be difficult, unless particular Burkholderia genotypes
Bacteria in the genus Burkholderia impart protection against organophosphorous pesticides in stinkbugs.
establish a routine transmission cycle between environment and host (Fig. 1). It remains possible that stinkbug-adapted genotypes occur in some Burkholderia as “symbiosis islands,” or associated with plasmids. A clearer picture will emerge from whole-genome sequencing of different isolates.
Many insect symbionts can be transmitted from parent to offspring or are readily transferred between insects as “secondary” symbionts (2–4). These transmission modes would more readily “link” beneficial pesticide-degrading bacteria to their hosts, enhancing increase of both partners and therefore spread of the resistance phenotype.
The article by Kikuchi et al. (5) focuses our attention on a potential broad role for symbionts as chemical detoxifying agents in hosts. Surprisingly, the topic has been largely unstudied (but see refs. 16 and 17). Given the near ubiquity of microbial symbioses in nature, ranging from inherited microbes in insects to the gut microbiomes in humans (17, 18), detoxification by symbionts could be extremely important. For example, although our livers get much of the credit as a toxin-degrading organ, our gut microbiome is likely a major player as well (18). Finally, lateral gene transfers (LGTs) between symbionts and hosts are now known to be common (19). Therefore, it would not be surprising if microbe–eukaryote LGTs of detoxification genes have occurred during evolution in eukaryotic lineages.
Acknowledgments
I thank Michael Clark (University of Rochester) for producing the graphic for this article. Some images used in the graphic were kindly provided by Y. Kikuchi (Bioproduction Research Institute, Hokkaido Center, National Institute of Advanced Industrial Science and Technology).
Footnotes
The author declares no conflict of interest.
See companion article on page 8618.
References
- 1.de Bary A. The Phenomenon of Symbiosis. Strasbourg: Verlag Von Karl J. Trubner; 1879. [Google Scholar]
- 2.Haine ER. Symbiont-mediated protection. Proc Biol Sci. 2008;275:353–361. doi: 10.1098/rspb.2007.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Werren JH, Baldo L, Clark ME. Wolbachia: Master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 4.Moran NA. Symbiosis as an adaptive process and source of phenotypic complexity. Proc Natl Acad Sci USA. 2007;104(Suppl 1):8627–8633. doi: 10.1073/pnas.0611659104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kikuchi Y, et al. Symbiont-mediated insecticide resistance. Proc Natl Acad Sci USA. 2012;109:8618–8622. doi: 10.1073/pnas.1200231109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahenthiralingam E, Urban TA, Goldberg JB. The multifarious, multireplicon Burkholderia cepacia complex. Nat Rev Microbiol. 2005;3:144–156. doi: 10.1038/nrmicro1085. [DOI] [PubMed] [Google Scholar]
- 7.Singh BK. Organophosphorus-degrading bacteria: Ecology and industrial applications. Nat Rev Microbiol. 2009;7:156–164. doi: 10.1038/nrmicro2050. [DOI] [PubMed] [Google Scholar]
- 8.Felsot AS. Enhanced biodegradation of insecticides in soil: Implications for agroecosystems. Annu Rev Entomol. 1989;34:453–476. [Google Scholar]
- 9.Buchner P. Endosymbiosis of Animals with Plant Microorganisms. New York: Wiley Interscience; 1965. [Google Scholar]
- 10.Kikuchi Y, Hosokawa T, Fukatsu T. An ancient but promiscuous host-symbiont association between Burkholderia gut symbionts and their heteropteran hosts. ISME J. 2011;5:446–460. doi: 10.1038/ismej.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kikuchi Y, Hosokawa T, Fukatsu T. Specific developmental window for establishment of an insect-microbe gut symbiosis. Appl Environ Microbiol. 2011;77:4075–4081. doi: 10.1128/AEM.00358-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haygood MG. Light organ symbioses in fishes. Crit Rev Microbiol. 1993;19:191–216. doi: 10.3109/10408419309113529. [DOI] [PubMed] [Google Scholar]
- 13.Little AF, van Oppen MJ, Willis BL, Willis BL. Flexibility in algal endosymbioses shapes growth in reef corals. Science. 2004;304:1492–1494. doi: 10.1126/science.1095733. [DOI] [PubMed] [Google Scholar]
- 14.Simms EL, et al. An empirical test of partner choice mechanisms in a wild legume-rhizobium interaction. Proc Biol Sci. 2006;273:77–81. doi: 10.1098/rspb.2005.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whalon ME, Mota-Sanchez D, Hollingsworth R. Global Pesticide Resistance in Arthropods. Oxfordshire, UK: CAB International; 2008. [Google Scholar]
- 16.Saraswat S, Rai JPN. Mechanism of metal tolerance and detoxification in Mycorrhizal fungi. Biomanagement Metal-Contaminated Soils. 2011;20:225–240. [Google Scholar]
- 17.Vanhaecke L, et al. Isolation and characterization of human intestinal bacteria capable of transforming the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Appl Environ Microbiol. 2008;74:1469–1477. doi: 10.1128/AEM.02064-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill SR, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunning Hotopp JC, et al. Widespread lateral gene transfer from intracellular bacteria to multicellular eukaryotes. Science. 2007;317:1753–1756. doi: 10.1126/science.1142490. [DOI] [PubMed] [Google Scholar]


