Abstract
Deep brain stimulation (DBS) of the ventral capsule/ventral striatum (VC/VS) reduces symptoms of intractable obsessive-compulsive disorder (OCD), but the mechanism of action is unknown. OCD is characterized by avoidance behaviors that fail to extinguish, and DBS could act, in part, by facilitating extinction of fear. We investigated this possibility by using auditory fear conditioning in rats, for which the circuits of fear extinction are well characterized. We found that DBS of the VS (the VC/VS homolog in rats) during extinction training reduced fear expression and strengthened extinction memory. Facilitation of extinction was observed for a specific zone of dorsomedial VS, just above the anterior commissure; stimulation of more ventrolateral sites in VS impaired extinction. DBS effects could not be obtained with pharmacological inactivation of either dorsomedial VS or ventrolateral VS, suggesting an extrastriatal mechanism. Accordingly, DBS of dorsomedial VS (but not ventrolateral VS) increased expression of a plasticity marker in the prelimbic and infralimbic prefrontal cortices, the orbitofrontal cortex, the amygdala central nucleus (lateral division), and intercalated cells, areas known to learn and express extinction. Facilitation of fear extinction suggests that, in accord with clinical observations, DBS could augment the effectiveness of cognitive behavioral therapies for OCD.
Keywords: prefrontal cortex, anxiety disorders, posttraumatic stress disorder, phosphorylated extracellular signal-regulated kinase, accumbens
Deep brain stimulation (DBS) is a neurosurgical technique that has become the standard of care for movement disorders (1–3) and is under investigation in major depression (4–6). In DBS, chronic high-frequency stimulation of specific sites reduces symptoms in medically intractable illness. DBS of the ventral capsule and the adjacent ventral striatum (VC/VS) has been used to treat refractory obsessive-compulsive disorder (OCD) in the European Union and the United States (7–10). Little is known about how DBS acts in OCD, emphasizing the need for translational animal studies. A prominent feature observed in most OCD patients is repetitive avoidance behaviors to perceived threats (11, 12). The persistent avoidance in OCD suggests a deficit in circuits that regulate fear extinction (11). Given the strong parallels between fear circuits in rodents and humans (13), well-characterized rodent models of fear conditioning could shed light on DBS mechanisms in OCD and related illnesses that feature pathological anxiety.
The DBS target for OCD is the VC/VS (14), which contains fiber bundles interconnecting cortical areas implicated in fear extinction, such as the ventromedial prefrontal cortex, the dorsal anterior cingulate cortex, and the orbitofrontal cortex (OFC), with subcortical areas implicated in conditioned fear, such as the amygdala (15). Previous studies in anesthetized rats (16, 17) have shown that DBS of the VS modifies the excitability of the OFC as well as prelimbic (PL) and infralimbic (IL) prefrontal regions that modulate fear via projections to the amygdala (18). This finding suggests that DBS might regulate fear extinction; however, this hypothesis has never been tested. Along these same lines, it has been observed that DBS enables some OCD patients to respond to extinction-based therapies to which they previously failed to respond (9).
To investigate the impact of DBS on fear extinction, we administered 3 h of high-frequency stimulation of VS during extinction training in rats previously conditioned to freeze to tones paired with footshocks. We assessed the effects of DBS on freezing and the expression of phosphorylated extracellular signal-regulated kinase (pERK), a marker for plasticity. We describe discrete subregions of the VS in which DBS given concurrently with extinction can either facilitate or impair extinction memory. Furthermore, extinction-facilitating DBS induces a specific pattern of plasticity in the prefrontal-amygdala circuits that learn extinction, consistent with augmentation of extinction memory.
Results
DBS of Specific Regions Within the VS Either Enhances or Impairs Extinction.
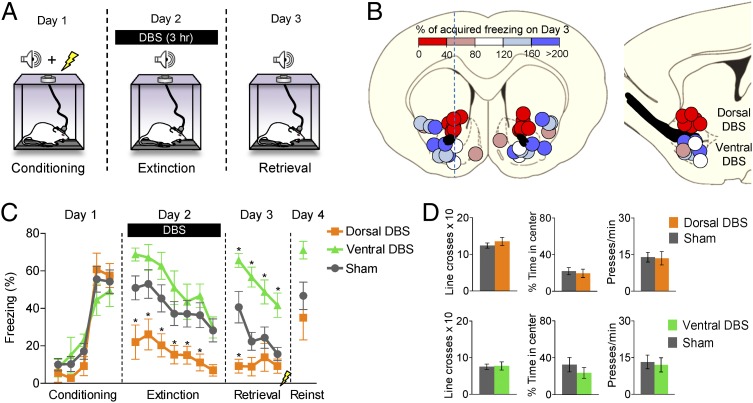
Rats were tested over 3 d: on day 1 they were conditioned with tones paired with footshock, on day 2 they were extinguished with tones in the absence of footshock, and on day 3 they were given additional extinction trials to test for extinction memory. DBS-like high-frequency stimulation (130 Hz) was delivered to the VS on day 2, continuously for 3 h: 1 h before, 1 h during, and 1 h after extinction training (see Fig. 1A for behavioral protocol). Combining all striatal placements, there was no effect of DBS on fear expression or extinction retrieval [F(1, 28) = 0.20; P = 0.66]. We next examined whether the effect of DBS varied with electrode position. We used a stimulus current of 100–200 μA, and a pulse width of 0.1 ms, which is estimated to activate fibers within a sphere of 0.3- to 0.5-mm radius (19). Fig. 1B shows electrode placements color-coded for extinction memory (percentage of acquired freezing expressed at retrieval). Placements resulting in reduced freezing during extinction retrieval (facilitated extinction, Fig. 1B, red circles) were located in the dorsomedial portion of the VS, whereas placements resulting in increased freezing at retrieval (impaired extinction, Fig. 1B, blue circles) were located in more ventral and lateral parts of the VS.
Fig. 1.
DBS in the VS can either facilitate or impair extinction depending on the site of stimulation. (A) Schematic of the behavioral protocol used to assess the effects of DBS, which was delivered for 3 h on the extinction training day (1 h preextinction, 1 h during, 1 h postextinction). (B Left) Coronal view. Electrode placements color-coded for extinction memory. Circle diameter indicates the estimated spread of current from electrode tip. (Right) Sagittal view. Dorsal DBS was dorsal to anterior commissure (black structure in B) and Ventral DBS was ventrolateral to anterior commissure. Placements lateral to Dorsal DBS were excluded from subsequent analyses. (C) Freezing plots for Sham (n = 16), Dorsal DBS (n = 6), and Ventral DBS (n = 8) groups. Dorsal DBS reduced freezing on day 2 and facilitated extinction recall on day 3. Ventral DBS had the opposite effects. Data are shown in blocks of two trials. (D) Dorsal DBS did not affect locomotion (line crosses) or anxiety (time in center) in the open-field task (Sham, n = 6; Dorsal DBS, n = 9) or the rate of pressing for food (Sham, n = 7; Dorsal DBS, n = 9). Ventral DBS did not affect locomotion or anxiety in the open-field task (Sham, n = 10; Ventral DBS, n = 8) or the rate of pressing for food (Sham, n = 7; Dorsal DBS, n = 7). Data are shown as mean and SEM. *P < 0.05.
Using the anterior commissure as a reference, we identified three subgroups: those stimulated dorsal to the commissure (Dorsal DBS), those stimulated ventral to the commissure (Ventral DBS), and those not stimulated (Sham; Fig. 1B). The three groups did not differ from each other during conditioning [F(2, 27)= 0.06; P = 0.94], but did differ during extinction training [F(2, 27) = 5.44; P = 0.01] and extinction retrieval [F(2, 27) = 10.97; P < 0.001; Fig. 1C]. During extinction training (in the presence of stimulation), rats receiving Dorsal DBS showed significantly less freezing than Sham rats did in the first six trial blocks (Tukey post hoc tests, all P < 0.05; Movie S1). During extinction retrieval in the absence of stimulation, Dorsal DBS and Ventral DBS groups were significantly lower and higher, respectively, than the Sham group in the first trial block (both P < 0.001) and showed a significant interaction between treatment and trial block [F(6, 81) = 2.94; P = 0.01]. Thus, Dorsal DBS strengthened memory for tone extinction, whereas Ventral DBS impaired it. Dorsal DBS also appeared to strengthen extinction to the context because the Dorsal DBS group showed reduced freezing before the first tone [H(2, 30) = 7.76; P = 0.02; Mann–Whitney P = 0.04], whereas the Ventral DBS group did not (Mann–Whitney P = 0.21). DBS of the dorsal or ventral sites did not alter spontaneous behaviors, such as the rate of bar-pressing, locomotion, or avoidance of an open field, when tested 1 wk after fear testing (all P > 0.3; Fig. 1D and Movie S2).
At the conclusion of the retrieval test on day 3, rats were given two unsignaled footshocks to reinstate their extinguished fear. The following day (day 4), there was no longer any difference between Dorsal DBS and Sham groups (Fig. 1C), indicating that DBS did not eliminate the original fear memory. The Ventral DBS group was significantly higher than the Dorsal DBS group, [F(2, 27) = 3.72, P = 0.038; post hoc P = 0.042], but not when compared with the Sham group (post hoc P = 0.065).
DBS Interacts Directly with Extinction Training.
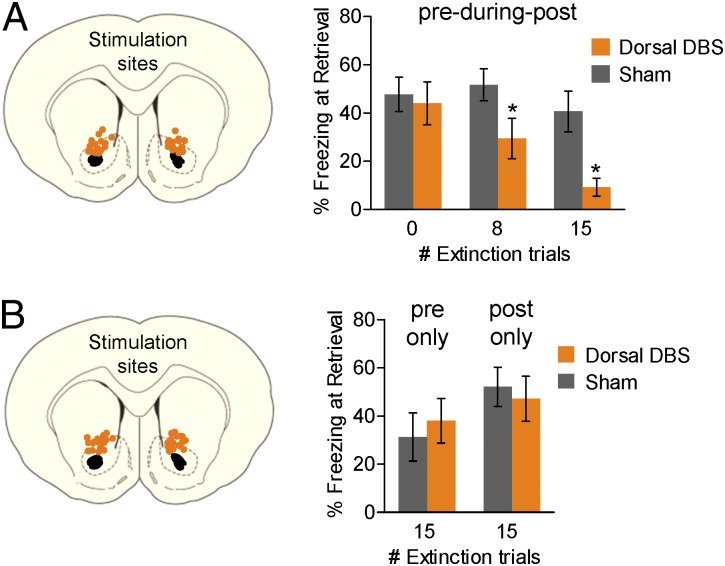
DBS during extinction training improves subsequent retrieval of extinction, but is extinction training required for DBS’s effects? To address this question, we administered Dorsal DBS during either partial extinction (eight trials) or no extinction (zero trials, home cage) for the equivalent period (3 h). The effect of DBS on extinction memory varied directly with the extent of extinction training. As shown in Fig. 2A, DBS in the absence of extinction training had no effect on freezing the following day (first block: Sham, 48%; Dorsal DBS, 44%; t(11) = 0.33; P = 0.75), whereas rats given 15 extinction trials during DBS showed robust facilitation of extinction memory by DBS (first block: Sham, 41%; Dorsal DBS, 9%; t(20) = 2.23; P = 0.038; Fig. 1C). Rats given an intermediate level of extinction showed an intermediate effect of DBS (first block: Sham, 52%; Dorsal DBS, 29%; t(21) = 2.11; P = 0.047; Fig. 2A).
Fig. 2.
DBS interacts with extinction training. (A Left) Placement of DBS electrode tips within the Dorsal DBS site. (Right) Percentage freezing during extinction retrieval on Day 3. DBS (3 h) in the absence of extinction training (0 trials) did not facilitate extinction memory (Sham, n = 8; Dorsal DBS, n = 5). DBS administered during (pre-during-post) an 8-trial extinction session facilitated extinction memory (Sham, n = 12; Dorsal DBS, n = 11); the same stimulation with a 15-trial extinction session facilitated extinction memory more robustly (data are from Fig. 1). (B Left) Placement of DBS electrode tips within the Dorsal DBS site. (Right) Dorsal DBS (3 h) confined to preextinction (pre only; Sham, n = 9; Dorsal DBS, n = 10) or postextinction (post only; Sham, n = 8; Dorsal DBS, n = 7) periods did not facilitate extinction memory on day 3. Data are shown in blocks of two trials with mean and SEM. *P < 0.05.
In the preceding experiments, DBS was administered during preextinction, extinction, and postextinction periods, leaving in doubt the critical period for DBS’s effects. We therefore repeated the experiment with the same duration of DBS but confined it to either 3 h before extinction or 3 h after extinction. As shown in Fig. 2B, neither procedure facilitated extinction memory (preextinction: t(17) = −0.49; P = 0.63; postextinction: t(13) = 0.40; P = 0.69). Thus, once turned off, DBS alone does not have a lasting effect on extinction, nor does DBS affect consolidation of extinction. These data also suggest that DBS must be given concurrently with extinction training to facilitate extinction. Altogether, these results suggest that Dorsal DBS does not itself induce a memory for extinction but strengthens plasticity induced by extinction training.
DBS Does Not Act via Inhibition of Striatal Neurons.
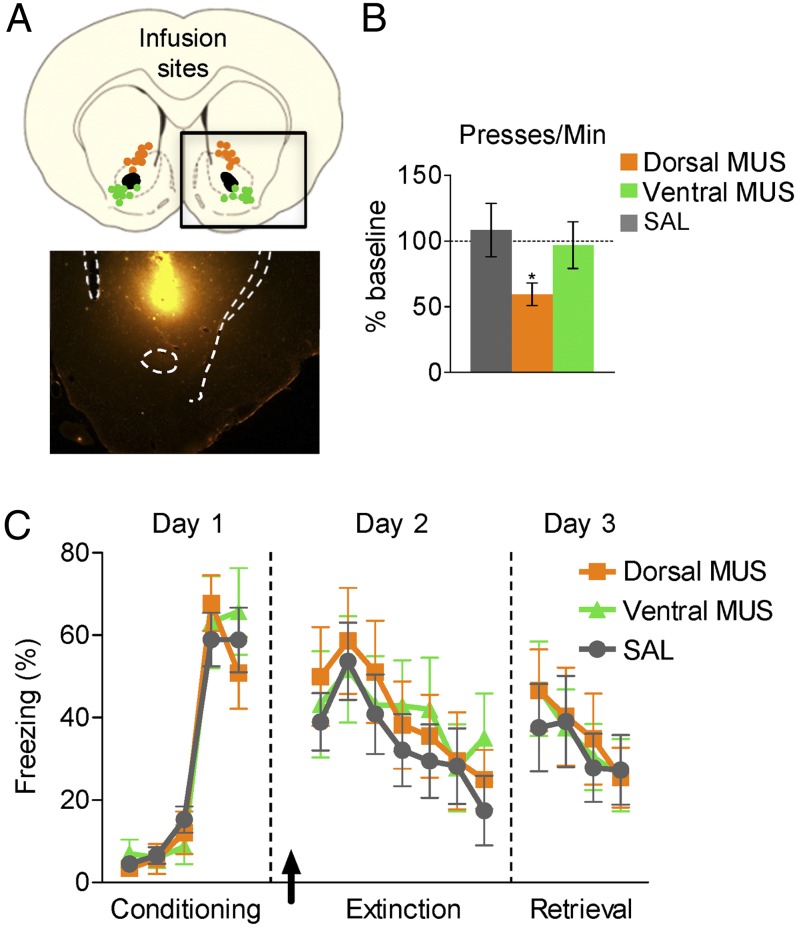
DBS of the VS could modulate extinction via local effects, such as increased GABAergic inhibition, as demonstrated in motor systems (20–24). To determine whether increased inhibition within the VS would be sufficient for facilitating extinction, we infused fluorescently labeled muscimol (MUS), a GABAA agonist, into the Dorsal or Ventral VS sites shortly before extinction training. An infusion volume of 0.1–0.2 μL approximated the spread of DBS current (Fig. 3A). Consistent with previous findings (25, 26), inactivation of the Dorsal DBS site significantly reduced the rate of bar-pressing for food (t16 = 2.25; P = 0.039; Fig. 3B), whereas inactivation of the Ventral DBS site did not (t16 = 0.89; P = 0.39). Despite this result, MUS in either the Dorsal or Ventral DBS sites had no effect on extinction training [F(2, 26) = 0.184; P = 0.83] or extinction retrieval [F(2, 26) = 0.054; P = 0.95; Fig. 3C]. Thus, similar to results found in investigations of DBS in prefrontal cortex (27), we were unable to obtain the behavioral effects of DBS with local pharmacological inactivation. Together with the use of short pulse widths to selectively activate fibers (28–30), these findings suggest that DBS facilitates extinction by acting on targets and/or origins of fibers passing through the VS.
Fig. 3.
The effects of DBS cannot be obtained with pharmacological inactivation of VS. (A Upper) Placement of cannula tips within the Dorsal (orange circles) and Ventral (green circles) DBS sites. (Lower) Micrograph showing the spread of fluorescently labeled MUS (4× magnification), similar to the current spread of DBS (Fig. 1B). (B) MUS infused into the Dorsal DBS site, but not into the Ventral DBS site, significantly reduced the rate of pressing for food (compared with preinfusion baseline). (C) MUS in either Dorsal or Ventral sites did not, however, reduce fear expression or facilitate extinction memory [saline (SAL), n = 11; Dorsal MUS, n = 9; Ventral MUS, n = 9]. Data are shown in blocks of two trials with mean and SEM. *P < 0.05.
DBS Induces Plasticity in Extinction Circuitry.
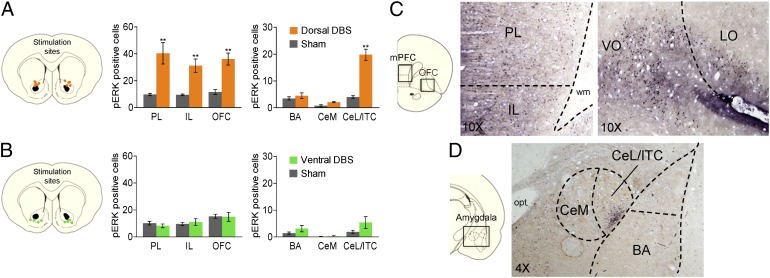
To evaluate the underlying circuitry that might mediate DBS facilitation of extinction, we performed an immunocytochemical analysis for pERK, a marker of cell plasticity (see ref. 31 for a review). Untrained rats were given either Dorsal DBS or Ventral DBS for 3 h and then killed immediately. We used untrained rats to assess the effects of DBS on pERK independently of extinction-induced pERK expression (32–34). We restricted our analysis to the mPFC and the amygdala, the two main regions involved in extinction learning (18), as well as the OFC, a region implicated in OCD (35). Compared with Sham-operated controls, Dorsal DBS significantly increased the number of pERK-labeled neurons in the mPFC and the OFC (effect of treatment: F(5, 46) = 66.17; P < 0.001), with post hoc tests revealing highly significant increases in PL (P < 0.001), IL (P = 0.004), and ventrolateral OFC (P = 0.005) subregions (Fig. 4A). The amygdala also showed significant changes in pERK [F(5, 42) = 54.13; P < 0.001], with post hoc tests revealing a significant increase in the lateral division of the central nucleus and intercalated cells (CeL/ITC; P < 0.001). There were no changes, however, in the basal nucleus of the amygdala (BA; P = 0.98) or medial division of the central nucleus (CeM; P = 0.93). In contrasts to Dorsal DBS, Ventral DBS did not alter pERK expression in any of these areas (all post hoc Tukey tests, P > 0.2; Fig. 4B). Thus, DBS that facilitated extinction memory also induced plasticity in PL, IL, and CeL/ITC, areas implicated in fear regulation and extinction (18, 36, 37), and sites of extinction-induced pERK (32–34).
Fig. 4.
Dorsal DBS induces plasticity within extinction circuits. (A) Dorsal DBS increased pERK in PL, IL, OFC, and CeL/ITC, but not in BA or CeM. (B) Ventral DBS did not increase pERK in PL, IL, OFC, CeL/ITC, BA, or CeM. (C) Representative micrographs showing pERK-labeled neurons in IL and PL regions of mPFC (10× magnification; Left) and OFC (10× magnification; Right) in rats administered Dorsal DBS. (D) Representative micrograph showing pERK-labeled neurons in the CeL/ITC in rats administered Dorsal DBS (4× magnification). LO, lateral OFC; wm, white matter; opt, optic tract; VO, ventral OFC. Data are shown as mean and SEM. **P < 0.01.
Discussion
We used a rodent model to assess the effects of DBS on extinction of conditioned fear, targeting a striatal region homologous to the VS/ventral capsule target for OCD. We observed that DBS applied just dorsal to the anterior commissure (Dorsal DBS) facilitated extinction, whereas DBS applied ventral to the commissure (Ventral DBS) impaired extinction. The degree of facilitation of extinction with DBS correlated with the amount of extinction training. Dorsal DBS induced expression of the plasticity marker pERK in areas previously implicated in extinction, such as the mPFC (PL/IL), OFC, and amygdala (CeL/ITC), consistent with augmentation of extinction memory. Given that extinction is deficient in anxiety disorders, including OCD (38), these findings suggest that clinical DBS may act to restore faulty extinction circuitry.
The most straightforward mechanism for these effects is that DBS acts locally on striatal neurons. A local mechanism of action has been reported for the subthalamic nucleus, where DBS augments inhibitory circuits to dampen neuronal activity (20–22). Several factors, however, argue against a local mechanism in the present study: (i) we observed opposite effects of DBS with small variations in electrode placement across the core of the nucleus accumbens, (ii) we observed that pharmacological inhibition with the GABAA agonist MUS did not alter fear expression or extinction retrieval (ref. 39 but see ref. 40), and (iii) 3 h of DBS of VS was previously shown to increase gamma activity in prefrontal and orbitofrontal areas but not locally in the striatum (16, 17).
Consistent with an extrastriatal locus of action, DBS of the dorsal site increased pERK labeling in the IL and PL cortices as well as in the CeL/ITC. IL is a critical site of plasticity for extinction (18), and neurons in IL increase their tone responses and bursting after extinction (41, 42). PL is important for expression of conditioned fear (43, 44), and PL tone responses are inversely correlated with extinction retrieval (42). IL and PL project to the CeL/ITC and BA, respectively (45). CeL/ITC contains inhibitory circuitry that regulates fear expression by gating CeM output (36, 37, 46, 47). Similar to the pattern we observed with DBS, extinction training has been shown to activate pERK in this same set of structures (32–34), and blocking pERK activity in either mPFC or amygdala prevents the development of extinction memory (48, 49). Thus, plasticity induced by Dorsal DBS may shift the balance of IL/PL activity to favor fear inhibition via CeL/ITC. This circuit likely involves the OFC, which is hyperactive in OCD subjects (50–52) and projects robustly to the mPFC and the amygdala (53).
By what mechanism could striatal DBS facilitate prefrontal-amygdala circuits? McCracken and Grace have proposed that antidromic activation of descending OFC fibers by striatal DBS potentiates inhibition within the OFC (16) and increases the coherence between the mPFC and OFC (17). In this way, DBS could decrease the excitatory drive within the fear circuit. Alternatively, DBS could facilitate the development of extinction-related plasticity. DBS in the absence of tones induced a pattern of pERK labeling similar to that observed with extinction training, but DBS alone was not sufficient to induce extinction. Therefore, DBS may serve to prime extinction circuits so that extinction is more readily learned and retrieved. This priming could occur, for example, by triggering pERK cascades that converge with tone inputs, thereby lowering the threshold for NMDA-dependent potentiation of those inputs. Further experiments are needed to determine the extent to which striatal DBS inhibits fear-promoting structures (PL and OFC) and/or potentiates fear-inhibiting structures (IL and CeL/ITC). This rodent model should facilitate subsequent analyses of molecular mechanisms involved in the therapeutic effects of VC/VS DBS for OCD.
Our findings could shed light on the mechanism by which DBS ameliorates symptoms of OCD. There is a high degree of homology of fear circuits in rodents and humans, especially in the prefrontal cortex and amygdala (13, 54–57). Fibers from the ventromedial prefrontal cortex (a homolog of IL) and the dorsal anterior cingulate cortex (a homolog of PL) pass through the VS and ventral capsule on their way to the amygdala and thalamus (15). Clinical DBS of the VC/VS site shows a similar dorsal–ventral pattern to what we observed here: stimulation ventral to the most clinically effective site can induce panic and fear (58, 59). Moreover, extinction is the basis of cognitive-behavior therapy for OCD, known as exposure with response prevention (ERP) (60, 61). Patients who failed ERP before neurosurgery are able to respond to ERP during DBS (9), perhaps reflecting a facilitation of extinction learning. In addition to strengthening extinction memory, our findings suggest that DBS might make ERP more tolerable by reducing fear and anxiety during the sessions. The delayed onset of symptom reduction of DBS (9) could reflect delays associated with initiating and completing a course of ERP. Combining DBS with extinction-based therapies may be necessary to achieve the full clinical benefit of DBS for OCD as well as for related anxiety and mood disorders.
Materials and Methods
Subjects.
One hundred twenty-nine male Sprague–Dawley rats (∼325 g; Harlan Laboratories) were housed and handled as previously described (62). Briefly, rats were fed standard rat chow in a restricted manner (18 g/d) to facilitate pressing a bar for food on a variable interval schedule of reinforcement (VI-60), which maintained a constant baseline against which freezing could be reliably measured (62). All procedures were approved by the Institutional Care and Use Committee from the University of Puerto Rico School of Medicine, in compliance the National Institutes of Health.
Surgery.
Rats were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (12 mg/kg, i.p.) and stereotaxically implanted with concentric bipolar stimulating electrodes (NEX-100; Rhodes Medical Instruments) as previously described (63). Initially, we targeted a large area of VS, but after the first set of experiments (Fig. 1), electrodes were aimed at a Dorsal DBS site (−6.5 mm dorsoventral, ±2.0 mm mediolateral, and +1.2 mm anteroposterior) and a Ventral DBS site (−6.5 mm dorsoventral, ±2.0 mm mediolateral, and +1.2 mm anteroposterior). Electrodes were fixed to the skull with anchoring screws and acrylic cement. Rats were allowed 5–7 d of recovery before behavioral testing initiated.
Behavior.
Fear conditioning and extinction.
After recovering from surgeries, rats were connected to a cable and commutator for 2 d before fear conditioning to allow rats to habituate to the cable. Rats underwent bar-press training, auditory fear conditioning, and extinction in standard operant chambers (Coulbourn Instruments) inside sound-attenuating boxes (Med Associates). Further details regarding the apparatus have been previously described (62). On day 1, rats were presented with five nonreinforced tones (4 kHz, 78 dB, 30 s; habituation) followed by seven tones that coterminated with footshocks (0.5 s, 0.56 mA; conditioning). On day 2, rats were presented with either 15 tones in the absence of footshocks, 8 tones, or 0 tones. DBS was conducted for 3 h with the extinction session occurring during the second hour. Another set of rats received 3 h of DBS terminating 30 min before, or initiating immediately after, a 15-tone extinction session. DBS outside the extinction session was conducted in the home cage, and there was no interruption of stimulation when animals were transferred from one environment to the other. An additional group received infusions of the GABAA agonist MUS (0.1–0.2 μL of fluorescent MUS; BODIPY TMR-X Conjugate; Sigma-Aldrich), or physiological saline at 15 min before a 15-tone extinction session. On day 3, all rats were presented with 15 tones in the absence of footshocks (extinction retrieval). At the end of the extinction - retrieval session, rats were presented with two unsignaled footshocks (0.5 s, 0.56 mA) to reinstate their fear to the extinguished tone on day 4 (reinstatement test; two tones). During all phases of the experiment, the intertone interval was variable (∼3 min), and food was available on a variable interval schedule (VI-60). For all experiments, sham control groups were implanted with electrodes and connected to the stimulation cable but never stimulated.
Open-field and spontaneous bar-pressing tasks.
After fear-conditioning experiments, a subset of rats was completely extinguished with 20 tones and tested 1 wk later for DBS effects on locomotion and anxiety. Motivation to press a bar for food was also assessed in the operant chambers. Average press rate was 14 (± 6.43) presses per min. Rats pressing <3 or >40 presses per min either before or after inactivation were eliminated from the data set (n = 2). Rats were stimulated with DBS or infused with MUS in the same dorsal and ventral DBS sites that modulated extinction memory (Figs. 1 and 3). Activity in the open field was assessed for 5 min after a 5-min acclimation period. The open-field box (91.5 × 91.5 × 61 cm) was divided into peripheral (within 15.25 cm of the walls) and central (61 × 61 cm) regions of equal area.
DBS.
DBS was delivered through concentric bipolar electrodes (NEX-100; Rhodes Medical Instruments) similar to previous reports (16, 17) with contacts measuring 0.5 mm in length and separated by 0.5 mm. Stimulation was monophasic, with the deeper contact as negative. We used DBS parameters similar to those used in humans (100–200 μA, 0.1-ms pulse duration, 130 Hz), which have been efficacious in rat models of Parkinson disease and depression (27, 64). These DBS parameters were previously used to model DBS at the VS site in anesthetized rats (16, 17) and did not result in seizure discharges (65) (Movie S2). DBS was generated with a S88X stimulator (Grass Instruments) and a constant-current unit (SIC-C Isolation Unit; Grass Instruments).
Histology.
Electrode placement.
After behavioral experiments, rats were deeply anesthetized with sodium pentobarbital (450 mg/kg i.p.) and transcardially perfused (10 min) with saline (0.9%) followed by paraformaldehyde [10% (vol/vol)]. Brains were removed and stored in a 30% (wt/vol) sucrose solution for at least 48 h before sectioning.
Anatomical map of the effects of DBS on extinction memory.
After verifying the placement of each individual electrode tip, we collapsed all placements to one anterior–posterior section (+1.2 mm from bregma). A circle with a 0.35 mm radius was used to depict the area of fiber activation (19). Rats were stimulated with either 100 μA or 200 μA current.
Immunocytochemistry.
For immunocytochemistry experiments, naïve rats received DBS or sham stimulation for 3 h in their home cage. Immediately after DBS, animals were anesthetized with sodium pentobarbital (450 mg/kg i.p.) and perfused transcardially with 100 mL of saline (0.9%), followed by 500 mL of 4% (vol/vol) paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were postfixed for 3 h in the same fixative solution and transferred to a solution of 30% (wt/vol) sucrose in 0.1 M phosphate buffer at 4 °C for two nights. Brains were then frozen, and series of 40-μm sections were cut with a cryostat (CM 1850; Leica) in the frontal plane and collected at different levels from the mPFC to the amygdala. Alternate sections were processed for the phosphorylated form of the protein kinase pERK, a marker of neural plasticity (see ref. 31 for a review), or for Nissl bodies, to determine anatomical boundaries of each structure.
For the first series of sections, anti-pERK serum raised in rabbit (p-44/42MAPK, 4370L, lot no. 0007; Invitrogen) was used at a dilution of 1:100 overnight. The primary antiserum was localized by using a variation of the avidin-biotin complex system. Sections were incubated for 120 min at room temperature in a solution of biotinylated goat anti-rabbit IgG (Vector Laboratories) and then placed in the mixed avidin-biotin horseradish peroxidase complex solution (ABC Elite Kit; Vector Laboratories) for 90 min. Brown immunoreactive cytoplasm labeled for pERK were visualized after 10 min of exposure to a chromogen solution containing 0.02% 3,3′-diaminobenzidine tetrahydrochloride with 0.3% nickel-ammonium sulfate (DAB-Ni) in 0.05 M Tris buffer (pH 7.6) followed by incubation for 5 min in a chromogen solution with glucose oxidase [10% (wt/vol)] and d-glucose [10% (wt/vol)]. The reaction was stopped with potassium PBS (pH 7.4). All sections were then mounted in gelatin-coated slides, dehydrated, and covered with coverslips.
Immunoreactivity Quantification.
pERK-positive cells were counted at 20× magnification with an Olympus BX51 microscope equipped with a digital camera. Images were generated for PL and IL portions of the mPFC, BA, and the amygdala central nucleus (dividing into CeM and CeL). Counting was done in a fixed area of 1,385 mm2 in the same position across sections, for PL, IL and BA. For the amygdala central nucleus, the complete area was counted and quantified separately for CeL and CeM. pERK-like immunoreactive cells were identified with the following criteria: cytoplasm size, ranging from 100 to 500 μm2; shape, oval or round; and being distinct from the background at 20× magnification. pERK-positive cells were counted and averaged across two to three distinct rostrocaudal levels for each brain structure with quantitative image analysis software (Metamorph 6.1).
Data Collection and Analysis.
Behavior was recorded with digital video cameras. Freezing was scored by an observer blind with respect to experimental group. Freezing was defined as the absence of all movement except respiration (66). For parametric data, statistical significance was determined with Student’s two-tailed t tests, one-way ANOVA, or repeated-measures ANOVA, followed by Tukey post hoc analysis, when appropriate (STATISTICA; Statsoft). For nonparametric data, statistical significance was determined by Kruskal–Wallis test followed by Mann–Whitney test (STATISTICA; Statsoft).
Supplementary Material
Acknowledgments
We thank the members of the Silvio O. Conte Center for Research in OCD [supported by National Institutes of Health (NIH) Grant P50 MH086400]: Benjamin D. Greenberg and Suzanne N. Haber for comments on the manuscript and Barry W. Connors, Darin Dougherty, Emad N. Eskandar, Anthony A. Grace, Mohammed R. Milad, and Steven A. Rasmussen for useful discussions. We also thank Freddyson J. Martinez-Rivera, Jerry O. Rodriguez-Resto, and Ashley S. Sanchez-Ramos for technical assistance. This work was supported by NIH Grants R01 MH058883 and R01 MH081975 (to G.J.Q.) and P50 MH086400 (to S. N. Haber), the University of Puerto Rico President’s Office, and an American Psychological Association Diversity Program in Neuroscience Fellowship (to J.R.-R.). Infrastructure support was provided in part by grants from the National Center for Research Resources (2G12 RR003051) and the National Institute on Minority Health and Health Disparities (8G12 MD007600).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200782109/-/DCSupplemental.
References
- 1.Johnson MD, Miocinovic S, McIntyre CC, Vitek JL. Mechanisms and targets of deep brain stimulation in movement disorders. Neurotherapeutics. 2008;5:294–308. doi: 10.1016/j.nurt.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu H, Neimat JS. The treatment of movement disorders by deep brain stimulation. Neurotherapeutics. 2008;5:26–36. doi: 10.1016/j.nurt.2007.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins KL, Lehmann EM, Patil PG. Deep brain stimulation for movement disorders. Neurobiol Dis. 2010;38:338–345. doi: 10.1016/j.nbd.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Giacobbe P, Mayberg HS, Lozano AM. Treatment resistant depression as a failure of brain homeostatic mechanisms: Implications for deep brain stimulation. Exp Neurol. 2009;219:44–52. doi: 10.1016/j.expneurol.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 5.Hamani C, Nóbrega JN. Deep brain stimulation in clinical trials and animal models of depression. Eur J Neurosci. 2010;32:1109–1117. doi: 10.1111/j.1460-9568.2010.07414.x. [DOI] [PubMed] [Google Scholar]
- 6.Blomstedt P, Sjöberg RL, Hansson M, Bodlund O, Hariz MI. Deep brain stimulation in the treatment of depression. Acta Psychiatr Scand. 2011;123:4–11. doi: 10.1111/j.1600-0447.2010.01625.x. [DOI] [PubMed] [Google Scholar]
- 7.Lipsman N, Neimat JS, Lozano AM. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: The search for a valid target. Neurosurgery. 2007;61:1–11, discussion 11–13. doi: 10.1227/01.neu.0000279719.75403.f7. [DOI] [PubMed] [Google Scholar]
- 8.Burdick A, Goodman WK, Foote KD. Deep brain stimulation for refractory obsessive-compulsive disorder. Front Biosci. 2009;14:1880–1890. doi: 10.2741/3348. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg BD, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: Worldwide experience. Mol Psychiatry. 2010;15:64–79. doi: 10.1038/mp.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mian MK, Campos M, Sheth SA, Eskandar EN. Deep brain stimulation for obsessive-compulsive disorder: Past, present, and future. Neurosurg Focus. 2010;29:E10. doi: 10.3171/2010.4.FOCUS10107. [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen SA, Eisen JL. The epidemiology and clinical features of obsessive compulsive disorder. Psychiatr Clin North Am. 1992;15:743–758. [PubMed] [Google Scholar]
- 12.Abed RT, de Pauw KW. An evolutionary hypothesis for obsessive compulsive disorder: A psychological immune system? Behav Neurol. 1998;11:245–250. doi: 10.1155/1999/657382. [DOI] [PubMed] [Google Scholar]
- 13.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: Ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberg BD, Rauch SL, Haber SN. Invasive circuitry-based neurotherapeutics: Stereotactic ablation and deep brain stimulation for OCD. Neuropsychopharmacology. 2010;35:317–336. doi: 10.1038/npp.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehman JF, Greenberg BD, McIntyre CC, Rasmussen SA, Haber SN. Rules ventral prefrontal cortical axons use to reach their targets: Implications for diffusion tensor imaging tractography and deep brain stimulation for psychiatric illness. J Neurosci. 2011;31:10392–10402. doi: 10.1523/JNEUROSCI.0595-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCracken CB, Grace AA. High-frequency deep brain stimulation of the nucleus accumbens region suppresses neuronal activity and selectively modulates afferent drive in rat orbitofrontal cortex in vivo. J Neurosci. 2007;27:12601–12610. doi: 10.1523/JNEUROSCI.3750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCracken CB, Grace AA. Nucleus accumbens deep brain stimulation produces region-specific alterations in local field potential oscillations and evoked responses in vivo. J Neurosci. 2009;29:5354–5363. doi: 10.1523/JNEUROSCI.0131-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: More than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ranck JB., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: A review. Brain Res. 1975;98:417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- 20.Benazzouz A, et al. Effect of high-frequency stimulation of the subthalamic nucleus on the neuronal activities of the substantia nigra pars reticulata and ventrolateral nucleus of the thalamus in the rat. Neuroscience. 2000;99:289–295. doi: 10.1016/s0306-4522(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 21.Beurrier C, Bioulac B, Audin J, Hammond C. High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol. 2001;85:1351–1356. doi: 10.1152/jn.2001.85.4.1351. [DOI] [PubMed] [Google Scholar]
- 22.Magariños-Ascone C, Pazo JH, Macadar O, Buño W. High-frequency stimulation of the subthalamic nucleus silences subthalamic neurons: A possible cellular mechanism in Parkinson’s disease. Neuroscience. 2002;115:1109–1117. doi: 10.1016/s0306-4522(02)00538-9. [DOI] [PubMed] [Google Scholar]
- 23.Boraud T, Bezard E, Bioulac B, Gross C. High frequency stimulation of the internal Globus Pallidus (GPi) simultaneously improves parkinsonian symptoms and reduces the firing frequency of GPi neurons in the MPTP-treated monkey. Neurosci Lett. 1996;215:17–20. doi: 10.1016/s0304-3940(96)12943-8. [DOI] [PubMed] [Google Scholar]
- 24.Dostrovsky JO, et al. Microstimulation-induced inhibition of neuronal firing in human globus pallidus. J Neurophysiol. 2000;84:570–574. doi: 10.1152/jn.2000.84.1.570. [DOI] [PubMed] [Google Scholar]
- 25.Moscarello JM, Ben-Shahar O, Ettenberg A. External incentives and internal states guide goal-directed behavior via the differential recruitment of the nucleus accumbens and the medial prefrontal cortex. Neuroscience. 2010;170:468–477. doi: 10.1016/j.neuroscience.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opioid, GABaergic, and dopaminergic modulation of palatable food motivation: Contrasting effects revealed by a progressive ratio study in the rat. Behav Neurosci. 2003;117:202–211. doi: 10.1037/0735-7044.117.2.202. [DOI] [PubMed] [Google Scholar]
- 27.Hamani C, et al. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol Psychiatry. 2010;67:117–124. doi: 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 28.Nowak LG, Bullier J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. I. Evidence from chronaxie measurements. Exp Brain Res. 1998;118:477–488. doi: 10.1007/s002210050304. [DOI] [PubMed] [Google Scholar]
- 29.Nowak LG, Bullier J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. II. Evidence from selective inactivation of cell bodies and axon initial segments. Exp Brain Res. 1998;118:489–500. doi: 10.1007/s002210050305. [DOI] [PubMed] [Google Scholar]
- 30.McIntyre CC, Grill WM. Excitation of central nervous system neurons by nonuniform electric fields. Biophys J. 1999;76:878–888. doi: 10.1016/S0006-3495(99)77251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Kim JH, Hamlin AS, Richardson R. Fear extinction across development: The involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. J Neurosci. 2009;29:10802–10808. doi: 10.1523/JNEUROSCI.0596-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parsons RG, Gafford GM, Helmstetter FJ. Regulation of extinction-related plasticity by opioid receptors in the ventrolateral periaqueductal gray matter. Front Behav Neurosci. 2010;4:44. doi: 10.3389/fnbeh.2010.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishikawa S, et al. Early postnatal stress alters extracellular signal-regulated kinase signaling in the corticolimbic system modulating emotional circuitry in adult rats. Eur J Neurosci. 2012;35:135–145. doi: 10.1111/j.1460-9568.2011.07921.x. [DOI] [PubMed] [Google Scholar]
- 35.Milad MR, Rauch SL. Obsessive-compulsive disorder: Beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16:43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amano T, Duvarci S, Popa D, Paré D. The fear circuit revisited: Contributions of the basal amygdala nuclei to conditioned fear. J Neurosci. 2011;31:15481–15489. doi: 10.1523/JNEUROSCI.3410-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graham BM, Milad MR. The study of fear extinction: Implications for anxiety disorders. Am J Psychiatry. 2011;168:1255–1265. doi: 10.1176/appi.ajp.2011.11040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwienbacher I, Schnitzler HU, Westbrook RF, Richardson R, Fendt M. Carbachol injections into the nucleus accumbens disrupt acquisition and expression of fear-potentiated startle and freezing in rats. Neuroscience. 2006;140:769–778. doi: 10.1016/j.neuroscience.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 40.Holtzman-Assif O, Laurent V, Westbrook RF. Blockade of dopamine activity in the nucleus accumbens impairs learning extinction of conditioned fear. Learn Mem. 2010;17:71–75. doi: 10.1101/lm.1668310. [DOI] [PubMed] [Google Scholar]
- 41.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 42.Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laurent V, Westbrook RF. Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learn Mem. 2009;16:520–529. doi: 10.1101/lm.1474609. [DOI] [PubMed] [Google Scholar]
- 45.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 46.Ciocchi S, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- 47.Tye KM, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herry C, Trifilieff P, Micheau J, Lüthi A, Mons N. Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur J Neurosci. 2006;24:261–269. doi: 10.1111/j.1460-9568.2006.04893.x. [DOI] [PubMed] [Google Scholar]
- 49.Hugues S, Deschaux O, Garcia R. Postextinction infusion of a mitogen-activated protein kinase inhibitor into the medial prefrontal cortex impairs memory of the extinction of conditioned fear. Learn Mem. 2004;11:540–543. doi: 10.1101/lm.77704. [DOI] [PubMed] [Google Scholar]
- 50.Evans DW, Lewis MD, Iobst E. The role of the orbitofrontal cortex in normally developing compulsive-like behaviors and obsessive-compulsive disorder. Brain Cogn. 2004;55:220–234. doi: 10.1016/S0278-2626(03)00274-4. [DOI] [PubMed] [Google Scholar]
- 51.Del Casale A, et al. Functional neuroimaging in obsessive-compulsive disorder. Neuropsychobiology. 2011;64:61–85. doi: 10.1159/000325223. [DOI] [PubMed] [Google Scholar]
- 52.Saxena S, Rauch SL. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin North Am. 2000;23:563–586. doi: 10.1016/s0193-953x(05)70181-7. [DOI] [PubMed] [Google Scholar]
- 53.Hoover WB, Vertes RP. Projections of the medial orbital and ventral orbital cortex in the rat. J Comp Neurol. 2011;519:3766–3801. doi: 10.1002/cne.22733. [DOI] [PubMed] [Google Scholar]
- 54.Kim JH, Richardson R. New findings on extinction of conditioned fear early in development: Theoretical and clinical implications. Biol Psychiatry. 2010;67:297–303. doi: 10.1016/j.biopsych.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 55.Myers KM, Carlezon WA, Jr, Davis M. Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology. 2011;36:274–293. doi: 10.1038/npp.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herry C, et al. Neuronal circuits of fear extinction. Eur J Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- 57.LeDoux J. The amygdala. Curr Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Shapira NA, et al. Panic and fear induced by deep brain stimulation. J Neurol Neurosurg Psychiatry. 2006;77:410–412. doi: 10.1136/jnnp.2005.069906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okun MS, et al. Deep brain stimulation in the internal capsule and nucleus accumbens region: Responses observed during active and sham programming. J Neurol Neurosurg Psychiatry. 2007;78:310–314. doi: 10.1136/jnnp.2006.095315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Franklin ME, Foa EB. Treatment of obsessive compulsive disorder. Annu Rev Clin Psychol. 2011;7:229–243. doi: 10.1146/annurev-clinpsy-032210-104533. [DOI] [PubMed] [Google Scholar]
- 61.Rasmussen SA, Eisen JL. Treatment strategies for chronic and refractory obsessive-compulsive disorder. J Clin Psychiatry. 1997;58(Suppl 13):9–13. [PubMed] [Google Scholar]
- 62.Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang JY, Shi LH, Luo F, Zhang WM, Woodward DJ. Studies of the neural mechanisms of deep brain stimulation in rodent models of Parkinson’s disease. Neurosci Biobehav Rev. 2007;31:643–657. doi: 10.1016/j.neubiorev.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 65.Saucier DM, Corcoran ME. Characteristics of dorsal and ventral striatal kindling in rats. Epilepsy Res. 1992;11:131–139. doi: 10.1016/0920-1211(92)90047-w. [DOI] [PubMed] [Google Scholar]
- 66.Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol. 1969;68:129–135. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.