Abstract
Fungi are a newly emerging source of peptide antibiotics with therapeutic potential. Here, we report 17 new fungal defensin-like peptide (fDLP) genes and the detailed characterization of a corresponding synthetic fDLP (micasin) from a dermatophyte in terms of its structure, activity and therapeutic potential. NMR analysis showed that synthetic micasin adopts a “hallmark” cysteine-stablized α-helical and β-sheet fold. It was active on both Gram-positive and Gram-negtive bacteria, and importantly it killed two clinical isolates of methicillin-resistant Staphylococcus aureus and the opportunistic pathogen Pseudomonas aeruginosa at low micromolar concentrations. Micasin killed approximately 100% of treated bacteria within 3 h through a membrane nondisruptive mechanism of action, and showed extremely low hemolysis and high serum stability. Consistent with these functional properties, micasin increases survival in mice infected by the pathogenic bacteria in a peritonitis model. Our work represents a valuable approach to explore novel peptide antibiotics from a large resource of fungal genomes.
Keywords: ascomycota, methicillin-resistant Staphylococcus aureus, plectasin, mouse peritonitis model
Antimicrobial peptides (AMPs), typically 12–80 amino acids in size, are key effectors of innate immunity in multicellular organisms (1). They comprise the first line of defense to rapidly clear pathogens in the early stage of infection through a multitarget mode of action, which may serve to prevent or delay evolution of microbial resistance. Their diverse action modes include disruption of membrane integrity, impairment of nucleic acid and protein synthesis, inhibition of chaperone-assisted protein folding, interruption of cell-wall biosynthesis pathway, and even targeting of membrane biogenesis (2–4). Some vertebrate AMPs (e.g., human cathelicidins and defensins) have also evolved a multifunctional, immunomodulatory ability in bridging the innate and adaptive immune systems (5).
The unique properties of AMPs make them attractive candidates for the development of antiinfective drugs with reduced risk of resistance (1, 6), especially when used in combination. To date, some such peptides and their derivatives are in clinical trials (7–9; however, they have proven difficult to reach the market as systemic (parenteral and oral) therapeutics owing to limitations including nonspecific toxicity, suboptimal efficacy, and lability to serum proteolysis (7, 8). Studies have shown that when systemically applied, some mammalian AMPs produce side effects, probably due to their immunomodulatory properties interfering with the normal immune network and inducing an exacerbating inflammatory response. Turning to nonmammalian sources of AMPs offers the potential to develop new classes of antimicrobial agents. Plectasin, the first defensin isolated from the saprophytic fungus Pseudoplectania nigrella (Ascomycota: Pezizomycota), is of particular interest as it selectively targets Gram-positive bacteria with particular activity against Streptococcus pneumoniae (10). Its lack of induction of the inflammatory cytokine interleukin-8 (IL-8) has also been confirmed (11). Following its discovery, plectasin-related peptides in fungi have also been reported recently using bioinformatics approaches (12).
Here, we describe 17 fungal defensin-like peptide (DLP) genes and the detailed characterization of a DLP (herein referred to as micasin) from the dermatophytic fungus Microsporum canis using the synthetic peptide. Our results show that fungal genomes are a source of antiinfective therapeutic agents that can be rapidly characterized by an integrated computational and experimental approach. The potential applications of dermatophytes in developing drugs against skin-derived bacterial infections (e.g., Staphylococcus aureus and P. aeruginosa) are highlighted.
Results
Fungal Genomes Are a New Source of Defensin-Like Peptides.
From recently sequenced fungal genomes, we identified 17 DLP genes. These DLPs can be grouped into four different families based on their sequence similarity, three of which are classified into known families (fDEF1, fDEF2 and fDEF5) (12) (Fig. 1). In comparison with fDEF1, fDEF2 has a smaller, less anionic propeptide, whereas the precursors of fDEF5 and the family fDEF7 lack a propeptide. Peptides in fDEF7 have a unique structural characteristic in that their amino-termini are longer and rich in histidine compared with other DLPs.
Fig. 1.
Multiple sequence alignment of fungal DLP families. (A) fDLP1; (B) fDLP2; (C) fDLP5; (D) fDLP7. Signal peptides, propeptides, and mature peptides are boxed in black, red, and green, respectively. Cysteines are shadowed in yellow; basic and acidic residues are shown in blue and red, respectively. The pairing pattern of the three disulfide bridges and secondary structural elements (cylinder: α-helix; arrow: β-strand) were extracted from the coordinates of plectasin (Protein Data Bank entry 1ZFU). HRR, histidine-rich region. Introns are indicated by arrows or small boxes. a, peptide size; b, identity to plectasin; c, net charges, estimated at pH 7.0 using protein calculation V3.3 (http://www.scripps.edu/~cdputnam/protcalc.html). Sequences described previously (10, 12) are indicated by an asterisk.
The fDEF1 members belong to ancient invertebrate-type defensins (AITDs) (12) and they all originated from Pezizomycota (Ascomycota) classified into three genera: Ajellomyces, Arthroderma, and Trichophyton (Table S1). Species derived from the latter two genera belong to dermatophytic fungi. The mature DLPs share 30% to 60% sequence identity with plectasin, and they all carry net positive charges from +1.2 to +5.2 at physiological pH (Fig. 1A), providing a possibility to bind polyanionic bacterial membranes by electrostatic interaction. Despite the overall net positive charge nature, these peptides have a net negatively charged N-terminal loop (n-loop), as in the case of plectasin. Aside from six conserved cysteines, the fDEF1 family has three identical residues (Ser17, Gly19, and Gly23, numbered according to micasin) whose conservation is across the alignment. The fDEF2 members belong to classical invertebrate-type defensins (CITDs) (Fig. 1B). However, unlike other known CITDs (12), these fungal CITDs carry net charges of -0.6 to -4.8, a characteristic previously observed in the tick scasin-type defensins (13). Except labisin in Basidiomycota (Agaricomycotina), a sister group to the Ascomycota, all other members come from Ascomycota (Pezizomycotina). Analysis of exon-intron structures of the newly discovered DLPs revealed that despite low sequence similarity among families 1, 2, and 7, some of them share a conserved phase-0 intron within the α-helical region (Fig. 1), suggesting common ancestral origin for these DLP families.
M. canis DLPs.
M. canis is the only species whose genome encodes two DLPs (micasin and micasin-1) with low sequence similarity. These two DLPs are functional genes, as evidenced by cDNA cloning (Fig. 2A; Fig. S1). In a Neighbor-Joining (NJ) tree, micasin-1 is separated as a single clade with a large distance from micasin, whereas micasin and other members constitute another orthologous clade (Fig. 2B), suggesting that these two M. canis DLPs originated by an early gene duplication preceding speciation of Pezizomycotina. In the subsequent evolution, the orthologue of this gene lost in other fungal lineages. Because of evolutionary retention in genomes of many fungal species hints functional importance of micasin and its orthologues, we thus selected micasin as a representative for detailed structural and funtional investigations.
Fig. 2.
M. canis DLPs. (A) Amplification of micasin and micasin-1 cDNAs by nested PCR. Bands corresponding to correct products are shown by red arrows. (B) Micasin and micasin-1 stemmed from an early gene duplication event (indicated by a red circle), as revealed by a Neighbor-Joining tree constructed based on amino acid sequences of the fDLP1 family by MEGA. Nodal support was estimated using the bootstrap approach with 1000 replicates. The scale bar shows total amino acid divergence. (C) Oxidative refolding of chemically synthetic micasin. RP-HPLC showing retention time (TR) difference between the reduced (R) and oxidized (O) peptides. Inset, MALDI-TOF MS of the oxidized peptide. (D) The CD spectrum of micasin, measured at 25 °C from 260 to 190 nm by using a quartz cell of 1.0-mm thickness. Data were collected at 1-nm intervals with a scan rate of 50 nm/ min.
Micasin shares 49% to 62% sequence identity with defensins from oysters, ticks, scorpions, and dragonflies, with four variable regions located at three loops and the carboxyl-terminus (Fig. S2A). We chemically synthesized the reduced form of micasin and then folded it by air oxidation in an alkaline environment. The oxidized product was purified to homogeneity by RP-HPLC, where it was eluted at the retention time (TR) of 21 min, earlier than its reduced form (TR of 23.2 min) (Fig. 2C), indicating that more hydrophobic residues are less accessible. To verify disulfide bond formation, we analyzed the sample using MALDI-TOF MS and determined an experimental molecular weight (MW) of 4055.7 Da (Fig. 2C), 5.98 Da less than the theoretical MW (4061.68 Da) calculated from its primary sequence, indicating that six hydrogen atoms in the cysteines of the reduced peptide have been removed when three disulfide bridges are formed. We then studied the structural features of micasin by circular dichroism and found that it displayed a minimum at 208 nm and a maximum at 198 nm (Fig. 2D), demonstrating that it has folded into a native-like conformation similar to other peptides with a cysteine-stabilized α-helical and β-sheet (CSαβ) fold (14).
Micasin Adopts a Typical CSαβ Fold.
The three-dimensional structure of micasin was determined by NMR spectroscopy. The 1D 1H NMR spectrum of micasin showed good dispersion (< 2 ppm) in the amide region, indicative of a highly structured peptide. Two-dimensional spectra were recorded at temperatures ranging from 283 to 303 K and a full assignment was obtained, except that no amide resonances could be detected for Arg30, Ala31 or Thr32 at any temperature studied and the amide resonance for Leu29 was only observed in spectra obtained at 283 K. This lack of signals suggests that this region of the peptide is highly flexible and its proton resonances are broadened beyond detection. Determination of translational diffusion coefficients from pulse field gradient NMR experiments indicate that the peptide is monomeric under the same conditions as used for the structure calculations (Table S2). A summary of nuclear Overhauser effect (NOE) data is displayed in Fig. 3A and Hα secondary shifts used to locate elements of secondary structure (15) are provided in Fig. S3.
Fig. 3.
Three-dimensional structure of micasin. (A) Summary of NOE data. The thickness of the bars indicates the intensity of the NOEs; slowly exchanging amide protons are denoted with black circles. (B) A family of 20 lowest energy structures superimposed over the backbone atoms of residues 1–27 and 33–38. (C) A ribbon representation with disulfide connectivities shown in ball and stick format. The termini are labeled with N-ter and C-ter, and the cysteine residues are labeled with their residue numbers. The n-, m-, and c-loops as shown represent the amino-terminal, middle, and carboxyl loops, respectively. Diagrams were generated using MOLMOL. (D) A space-filling model showing the location of different types of amino acids (green, hydrophobic; blue, positively charged; and red, negatively charged). (B) and (D) were displayed by Weblab Viewlite 4.2 and (C) by MOLMOL.
A number of resonances were observed for more than 20 h after the peptide was dissolved in deuterated water. This finding strongly suggests that these 10 amide protons (Cys11, Ala13, His14, Cys15, Leu16, Ser17, Ile18, Phe24, Ala26, and Thr34) are involved in significant hydrogen bonds. The three-dimensional structure of micasin was calculated with a total of 265 distance restraints, 21 dihedral angle restraints, and 11 hydrogen bond restraints using a simulated annealing protocol in CNS. The disulfide connectivities (Cys4-Cys25, Cys11-Cys33, Cys15-Cys35) were fully consistent with the NOE data and were included as restraints in the structure calculations. The resulting family of structures (Fig. 3B) had good structural and energetic statistics, as shown in Table S3. A ribbon representation of the secondary structures is shown in Fig. 3C. Analysis of the structures with PROCHECK (16), excluding the undefined region Leu29-Thr32, showed that 100% of the residues were in the most favored or additionally allowed regions of the Ramachandran plot. Analysis of the structures with PROMOTIF (17) identified an α-helix between residues 7–18 and a β-sheet consisting of two antiparallel strands between residues 22–26 and 32–36. A short sequence of only three residues (20–22) links the helix to strand I. The loop connecting the two β-strands (residues 27–31) is highly flexible, with no dominant turn type defined. The Cys4-Pro5 amide bond adopts an unusual cis-conformation, as observed in two Mollusca-derived AITDs (MGD-1 and Cg-DEF). The three disulfide bonds are arranged as in a typical CSαβ motif; that is, the Cys4-Cys25 disulfide bridge connects the N-terminus to the latter part of strand I, and the Cys11-Cys33, Cys15-Cys35 disulfide bridges connect the helix to strand II. Overall, micasin lacks amphipathic architecture, as identified by its cationic and hydrophobic residues scattered on the structural surface (Fig. 3D).
As expected, micasin shares the highest structural similarity to three AITDs (plectasin, MGD-1 and Cg-Def) with a root mean square deviation (rmsd) of 2.73–4.11 Å (Fig. S2B). Micasin and plectasin have different structurally flexible regions, and the functional significance of these regions have been highlighted in other defensins (18). In micasin, a highly flexible loop exists between two rigid β-strands, while in plectasin the variable regions are located in the first three amino-terminal residues and the loop following the α-helix. Although the presence of the CSαβ has been shown to be a prerequisite for structure and function of defensins, a strictly defined structure is not often associated with high antimicrobial activity (18). In micasin, the flexible loop is structurally located in a previously recognized “γ-core” region that is a common functional domain of many AMPs with phylogenetically diverse origins (19).
Micasin Kills Bacteria with a New Mode of Action.
When assayed for in vitro antimicrobial effect on a broad spectrum of microorganisms, micasin showed potent activity against both G+ and G- bacteria (Table 1), but had no effect on five filamentous fungi tested or on the yeast Saccharomyces cerevisiae (Table S4). Of all the sensitive bacteria, micasin displayed the strongest activity against B. megaterium with a lethal concentration (CL) of 54 nM, and it also killed two clinical isolates of MRSA and the opportunistic pathogen P. aeruginosa at low micromolar concentrations. P. aeruginosa causes significant nosocomial infections, and in most cases it is rather difficult to treat as it has an additional outer membrane permeability barrier (4). The killing kinetics of micasin against B. megaterium showed that it killed this bacterium more rapidly than vancomycin (Fig. 4A), an antibiotic inhibiting bacterial cell-wall biosynthesis. The extent of bacterial death caused by micasin and vancomycin is approximately 100% and 78.5%, respectively, after 3 h of incubation with the antibacterial agents. The ability of micasin to permeabilize the bacterial membrane was also assessed with the fluorescent nucleic acid-binding dye propidium iodide. Vancomycin and meucin-18 (a pore-forming peptide from scorpion venom) were used as negative and positive control (20, 21). No fluorescence increase was observed over 10 min after B. megaterium were exposed to micasin and vancomycin at 5× CL or 10× CL (Fig. 4B), indicating the bacterial membrane integrity was not destroyed. On the contrary, meucin-18 caused an immediate fluorescence increase upon exposure of the peptide. These observations are in agreement with features of these two control molecules previously reported (20, 21). At a concentration up to 20× CL, micasin only led to weak dye uptake after 6 min. The lack of membrane permeability to the dye in the range of lethal concentrations suggests that micasin is not a membrane disruptive AMP, consistent with its overall nonamphiphilic architecture (Fig. 3D).
Table 1.
Lethal concentrations (CL) of micasin on sensitive bacteria
| Microorganism | CL (μM) |
| Gram-positive bacteria | |
| Bacillus megaterium CGMCC 1.0459 | 0.054 |
| Bacillus subtilis CGMCC 1.2428 | 2.03 |
| Staphylococcus aureus MRSA P1386 | 2.46 |
| Staphylococcus aureus MRSA P1374 | 3.35 |
| Staphylococcus epidermidis PRSE P1389 | 11.55 |
| Staphylococcus MRCNS P1369 | 12.28 |
| Staphylococcus aureus CGMCC1.89 MSSA | 17.53 |
| Staphylococcus epidermidis PSSE P1111 | 23.42 |
| Micrococcus luteus CGMCC 1.0290 | NA |
| Bacillus cereus CGMCC 1.1626 | NA |
| Gram-negative bacteria | |
| Pseudomonas aeruginosa CCTCC AB 91095 | 0.94 |
| Agrobacterium tumefaciens CCTCC AB 92026 | 4.24 |
| Escherichia coli ATCC 25922 | NA |
NA, no activity, indicating that no inhibition zone was observed at 1.0 nmol peptide/well.
Fig. 4.
Effect of micasin on B. megaterium. (A) Killing kinetics of micasin. The cells treated with micasin or vancomycin at 5× CL. The CL value of vancomycin to B. megaterium is 0.119 μM, which is about twofold higher than that of micasin. Medium is without peptide. (B) Effect of micasin on bacterial membrane integrity. In each run, micasin at 5×, 10× or 20× CL was added when the basal fluorescence remained constant for 200 s. Vancomycin and meucin-18 (CL: 0.25 μM) were used as negative and positive control, respectively. (C) Scanning and (D) transmission electron microscopic observations of B. megaterium cells in the absence or presence of micasin. The cells were incubated with micasin at 5× CL for 90 min at 37 °C. The particle-like materials are indicated by white arrows.
To further explore micasin’s antibacterial mode of action, we employed electron microscopy techniques to visualize any potential damage caused by the peptide on the morphology and cellular structures of sensitive bacteria. Scanning electron microscopy revealed no obvious morphological change in B. megaterium treated with micasin at 5× CL after 90 min when compared with the normal cells (Fig. 4C), which is different from plectasin that changes bacterial morphology due to inhibiting cell-wall biosynthesis by directly binding to its precursor (21). However, treatment with micasin at this dose led to an effect on the bacterial intracellular components as revealed by transmission electron microscopy. The micasin-treated cells accumulated some internal protein particle-like materials that were not observed with untreated B. megaterium cells (Fig. 4D), suggesting that micasin kills bacterial cells via an intracellular action mode to affect protein folding. A similar mode of action was previously proposed for several insect AMPs whose killing activity is associated with the inhibition of chaperone-assisted protein folding (3). A plant defensin NaD1 with the CSαβ motif was reported to enter fungal cells and enhance killing via interacting with intracellular targets (22). The detailed mechanism of action of micasin awaits further investigation.
Micasin Is a New Antiinfective Peptide with Therapeutic Potential.
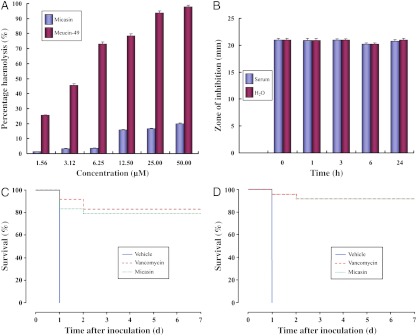
Micasin caused no notable hemolysis for mammalian erythrocytes at concentrations up to 50 μM (Fig. 5A). Moreover, it exhibited high serum stability as identified by no decrease of antibacterial activity, even over 24 h of incubation in mouse serum, where the activity was comparable with that in H2O (Fig. 5B). In contrast, incubation in serum completely inactivated the activity of vancomycin after 24 h. The potential therapeutic efficacy of micasin in providing protection against two lethal infections from S. aureus MRSA P1386 and P. aeruginosa was evaluated by a mouse peritonitis model. In these assays, all eight mice in the vehicle-treated control group died after 24 h of inoculation of S. aureus MRSA P1386 or P. aeruginosa, whereas most of the mice treated with micasin survived the 7-day study period with a survival rate of 79.5 ± 5.7% for S. aureus MRSA P1386 (Fig. 5C) and 91.7 ± 5.7% for P. aeruginosa (Fig. 5D). All the deaths occurred before the third day of inoculation. Overall, the protection efficiency of micasin is comparable with vancomycin.
Fig. 5.
Evaluation of therapeutic potential of micasin. (A) Hemolytic effect of micasin on mouse red blood cells. Meucin-49, a scorpion venom-derived cytolytic peptide of 49 residues, was used as a positive control (this peptide will be published elsewhere). Cells were incubated with different concentrations of peptides for 30 min at 37 °C. Controls for 0 and 100% heamolysis were determined by PBS buffer and 1% Triton X-100, respectively. Percentage haemolysis (%) are expressed as mean ± SD (n = 3). (B) Serum stability of micasin. The peptide was incubated at 37 °C in fresh normal mouse serum for 0, 1, 3, 6, and 24 h. Residual activities were measured by inhibition-zone assays with B. megaterium. (C) and (D) Survival percentage of mice after peritoneal infection with S. aureus MRSA P1386 (C) and P. aeruginosa (D). Treatment with micasin or vancomycin was initiated 1 h after inoculation. In these two models, 0.9% NaCl was used in the vehicle group whose survival fraction was 0 out of 8 mice (P ≤ 0.0001).
Discussion
Penicillin is the first antibiotic isolated from the fungus Penicillium chrysogenum (23). From 1930 to 1962, more than 20 unique classes of antibiotics were produced and marketed. However, since then the world has made only two new classes of antibiotics (24, 25). The discovery of peptide antibiotics in diverse fungal species is thus of considerable importance, as it demonstrates that fungi are a rich resource of human medicines to be exploited. The protection from infections of S. aureus and P. aeruginosa by micasin indicates that relative to mammalian AMPs (7, 26), fungal DLPs have more advantages as human drugs owing to their potent therapeutic efficacy associated with the high antibiotic activity and serum stability as well as low toxicity.
In the initial stage of this study, we attempted to isolate micasin as well as micasin-1 from the liquid culture of M. canis by traditional biochemical techniques, but failed. This result was probably due to their low abundance in the culture because their relatively rare trascripts can be only amplified by two rounds of nested PCR. In fact, plectasin was also identified by a genetic approach, instead of biochemical purification, to trap cDNA clones encoding a signal peptide by a transposon-assisted signal trapping system (10). However, such a genetic approach depends on large-scale random sequencing. Alternatively, the resource of sequenced fungal genomes will accelerate our study in that it can rapidly recognize DLPs in a given fungal species, and then a series of molecular and biochemical experiments as well as functional assays can be performed. We found that nearly all the DLPs identified here are derived from Pezizomycotina (Ascomycota). Such a species-specific phylogenetic distribution should not be due to sampling bias, because there are only six species of Pezizomycotina whose genomes were completely sequenced; in Saccharomycotina (Ascomycota), the number is thirteen. It is known that Ascomycota is the largest phylum of Fungi, comprising almost 50% of all known fungal species, and Pezizomycotina is the largest subphylum of Ascomycota, containing more than 32,000 described species (27), which constitute a tremendous resource library of templates for molecular design. For example, current fungal geneome projects include at least 74 Pezizomycotina species whose genomes are being sequenced.
As Pezizomycotina species occur in numerous ecological niches and occupy virtually all terrestrial and aquatic ecosystems, it remains an open question as to why Pezizomycotina rather than other subphylums of Ascomycota retains DLPs during evolution. The antibacterial spectrum of micasin could provide clues to answer this question. Molecular detection has identified seven bacterial species (S. epidermidis, S. aureus, Streptococcus mitis, Propionibacterium acnes, Corynebacterium spp., Acinetobacter johnsonii, and P. aeruginosa) frequently inhabiting human skin, which provide protection from other microbial infections (28). One such example is that P. aeruginosa in human skin produces compounds to kill and inhibit fungal growth (28). In addition, S. epidermidis was also found to generate phenol-soluble antimicrobial agents to contribute to normal defense at the epidermal interface (29). In this case, we assume that as common pathogens of animal and human skin (30), dermatophytes firstly need to fight against the bacterial flora by producing defensins to successfully survive on human skin. Consistent with this assumption, among the skin bacteria, three (S. epidermidis, S. aureus and P. aeruginosa) were used in this study and all are sensitive to micasin; in contrast, it had no effect on M. canis itself and the gut-derived Escherichia coli. This finding supports a potential role of micasin in competition with bacterial flora and suggests that dermatophytic defensins (i.e., micasin, tritosin, trivesin and trirusin) could be particularly useful in developing drugs against human skin-derived opportunistic pathogens.
Materials and Methods
The database search strategy for finding new defensin-like genes has been described previously (12). Briefly, amino acid sequences of plectasin and related defensins were first used as a query to perform TBLASTN search of fungal genomic database (http://www.ncbi.nlm.nih.gov) under the default parameters. This database includes 134 species of fungi with 26 genomes completely sequenced. The presence of a signal peptide combined with a “hallmark” defensin cysteine motif was used to filter the hits.
Additional experimental procedures are provided in SI Materials and Methods
Supplementary Material
Acknowledgments.
We thank the Lab of Bio-imagine, the Institute of Biophysics for our electron microscopy work, and we are grateful to Lei Sun for her help in making TEM samples and taking TEM images. We also thank Dr. Dongming Li (Beijing University, China) for kindly providing M. canis. This work was supported by the National Natural Science Foundation of China (Grant 30730015 and 30921006) and the National Basic Research Program of China (Grant 2010CB945300). D.J.C. is a National Health and Medical Research Council (Australia) Professorial Fellow.
Footnotes
Conflict of interest statement: Based on this work, a patent application (“Novel fungus-derived antiinfective defensins”) has been filed.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (http://www.ncbi.nlm.nih.gov/) [accession nos. JN014007 (micasin) and JN014008 (micasin-1)]. The atomic coordinates of micasin have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 2LR5)
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201263109/-/DCSupplemental.
References
- 1.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 2.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–350. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 3.Hale JD, Hancock RE. Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Rev Anti Infect Ther. 2007;5:951–959. doi: 10.1586/14787210.5.6.951. [DOI] [PubMed] [Google Scholar]
- 4.Srinivas N, et al. Peptidomimetic antibiotics target outer-membrane biogenesis in Pseudomonas aeruginosa. Science. 2010;327:1010–1013. doi: 10.1126/science.1182749. [DOI] [PubMed] [Google Scholar]
- 5.Easton DM, Nijnik A, Mayer ML, Hancock RE. Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol. 2009;27:582–590. doi: 10.1016/j.tibtech.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peschel A, Sahl HG. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol. 2006;4:529–536. doi: 10.1038/nrmicro1441. [DOI] [PubMed] [Google Scholar]
- 7.Vaara M. New approaches in peptide antibiotics. Curr Opin Pharmacol. 2009;9:571–576. doi: 10.1016/j.coph.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Marr AK, Gooderham WJ, Hancock RE. Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr Opin Pharmacol. 2006;6:468–472. doi: 10.1016/j.coph.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Eckert R. Road to clinical efficacy: challenges and novel strategies for antimicrobial peptide development. Future Microbiol. 2011;6:635–651. doi: 10.2217/fmb.11.27. [DOI] [PubMed] [Google Scholar]
- 10.Mygind PH, et al. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature. 2005;437:975–980. doi: 10.1038/nature04051. [DOI] [PubMed] [Google Scholar]
- 11.Hara S, et al. Plectasin has antibacterial activity and no affect on cell viability or IL-8 production. Biochem Biophys Res Commun. 2008;374:709–713. doi: 10.1016/j.bbrc.2008.07.093. [DOI] [PubMed] [Google Scholar]
- 12.Zhu S. Discovery of six families of fungal defensin-like peptides provides insights into origin and evolution of the CSαβ defensins. Mol Immunol. 2008;45:828–838. doi: 10.1016/j.molimm.2007.06.354. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Zhu S. The defensin gene family expansion in the tick Ixodes scapularis. Dev Comp Immunol. 2011;35:1128–1134. doi: 10.1016/j.dci.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Gao B, Zhu L, Zhu S. A naturally-occurring carboxyl-terminally truncated alpha-scorpion toxin is a blocker of sodium channels. Biochem Biophys Res Commun. 2011;411:673–678. doi: 10.1016/j.bbrc.2011.06.178. [DOI] [PubMed] [Google Scholar]
- 15.Wishart DS, Bigam CG, Holm A, Hodges RS, Sykes BD. 1H, 13C and 15N random coil NMR chemical shifts of the common amino acids. I. Investigations of nearest-neighbor effects. J Biomol NMR. 1995;5:67–81. doi: 10.1007/BF00227471. [DOI] [PubMed] [Google Scholar]
- 16.Laskowski RA, Rullmannn JA, MacArthur MW, Kaptein R, Thornton JM. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J Biomol NMR. 1996;8:477–486. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- 17.Hutchinson EG, Thornton JM. PROMOTIF--a program to identify and analyze structural motifs in proteins. Protein Sci. 1996;5:212–220. doi: 10.1002/pro.5560050204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landon C, Barbault F, Legrain M, Guenneugues M, Vovelle F. Rational design of peptides active against the gram positive bacteria Staphylococcus aureus. Proteins. 2008;72:229–239. doi: 10.1002/prot.21912. [DOI] [PubMed] [Google Scholar]
- 19.Yeaman MR, Yount NY. Unifying themes in host defence effector polypeptides. Nat Rev Microbiol. 2007;5:727–740. doi: 10.1038/nrmicro1744. [DOI] [PubMed] [Google Scholar]
- 20.Gao B, Sherman P, Luo L, Bowie J, Zhu S. Structural and functional characterization of two genetically related meucin peptides highlights evolutionary divergence and convergence in antimicrobial peptides. FASEB J. 2009;23:1230–1245. doi: 10.1096/fj.08-122317. [DOI] [PubMed] [Google Scholar]
- 21.Schneider T, et al. Plectasin, a fungal defensin, targets the bacterial cell wall precursor Lipid II. Science. 2010;328:1168–1172. doi: 10.1126/science.1185723. [DOI] [PubMed] [Google Scholar]
- 22.van der Weerden NL, Hancock RE, Anderson MA. Permeabilization of fungal hyphae by the plant defensin NaD1 occurs through a cell wall-dependent process. J Biol Chem. 2010;285:37513–37520. doi: 10.1074/jbc.M110.134882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleming A. On the antibacterial action of cultures of a Penicillium, with special reference to their use in the isolation of B. influenza. Br J Exp Pathol. 1929;10:226–236. [Google Scholar]
- 24.Coates A, Hu Y, Bax R, Page C. The future challenges facing the development of new antimicrobial drugs. Nat Rev Drug Discov. 2002;1:895–910. doi: 10.1038/nrd940. [DOI] [PubMed] [Google Scholar]
- 25.Coates A, Halls G, Hu Y. Novel classes of antibiotics or more of the same? Br J Pharmacol. 2011;163:184–194. doi: 10.1111/j.1476-5381.2011.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hancock RE, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 27.Kirk PM, Cannon PF, David JC, Stalpers JA. Ainsworth and Bisby’s dictionary of the Fungi. 9th Ed. Wallingford, UK: CAB International; 2010. [Google Scholar]
- 28.Cogen AL, Nizet V, Gallo RL. Skin microbiota: a source of disease or defence? Br J Dermatol. 2008;158:442–455. doi: 10.1111/j.1365-2133.2008.08437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cogen AL, et al. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J Invest Dermatol. 2010;130:192–200. doi: 10.1038/jid.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hainer BL. Dermatophyte infections. Am Fam Physician. 2003;67:101–108. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.