Abstract
Imaging protein assemblies at molecular resolution without affecting biological function is a long-standing goal. The diffraction-limited resolution of conventional light microscopy (∼200–300 nm) has been overcome by recent superresolution (SR) methods including techniques based on accurate localization of molecules exhibiting stochastic fluorescence; however, SR methods still suffer important restrictions inherent to the protein labeling strategies. Antibody labels are encumbered by variable specificity, limited commercial availability and affinity, and are mostly restricted to fixed cells. Fluorescent protein fusions, though compatible with live cell imaging, substantially increase protein size and can interfere with their biological activity. We demonstrate SR imaging of proteins tagged with small tetracysteine motifs and the fluorescein arsenical helix binder (FlAsH-PALM). We applied FlAsH-PALM to image the integrase enzyme (IN) of HIV in fixed and living cells under experimental conditions that fully preserved HIV infectivity. The obtained resolution (∼30 nm) allowed us to characterize the distribution of IN within virions and intracellular complexes and to distinguish different HIV structural populations based on their morphology. We could thus discriminate ∼100 nm long mature conical cores from immature Gag shells and observe that in infected cells cytoplasmic (but not nuclear) IN complexes display a morphology similar to the conical capsid. Together with the presence of capsid proteins, our data suggest that cytoplasmic IN is largely present in intact capsids and that these can be found deep within the cytoplasm. FlAsH-PALM opens the door to in vivo SR studies of microbial complexes within host cells and may help achieve truly molecular resolution.
Keywords: superresolution microscopy, virology
To better understand cellular processes and their interactions with pathogens, it is important to visualize specific proteins in their cellular context and, preferably, in living cells. Fluorescence microscopy is an invaluable tool for cell biology and has helped clarify key steps in pathogen infection; however, it remains challenging to image proteins at or near molecular resolution without interfering with their biological functions. Existing imaging methods face limitations preventing them from simultaneously satisfying these two demands. Conventional fluorescence light microscopy has a resolution limited by diffraction to ∼200–300 nm that is too large for small biological structures such as viruses with typical sizes from 25 to 300 nm (1). Several SR microscopy techniques have been developed that overcome this limit (2) including photoactivated localization microscopy (PALM) (3) and stochastic reconstruction optical microscopy (STORM) (4). In PALM, STORM, and related techniques (5–7), subdiffraction resolution images are assembled by computational localization in thousands of images of single molecules switching stochastically between dark and fluorescent states.
However, these methods rely on antibodies coupled to organic dyes (4, 8) or on fusions with fluorescent or fluorophore-binding proteins (3, 7, 9–11) and have restrictions inherent to these labeling strategies. Antibodies suffer from variable specificity depending on fixation conditions and from limited commercial availability. Although organic dyes have large photon outputs, theoretically enabling nanometer-scale localization accuracies (12), the resolution remains restricted by antibody size, especially in secondary immunolabeling, and by limited binding affinity (9). Finally, antibodies confine imaging to fixed and permeabilized cells or to extracellular proteins (4, 5, 8). Protein fusions, in contrast, allow highly specific labeling and are compatible with live cell imaging (7, 9). Unfortunately, these fusions substantially increase protein size (by ∼25 kDa for GFP) and can strongly interfere with their cellular localization and activity. This has proven problematic for a number of studies that imaged pathogen invasion of host cells. For example, GFP-labeling interferes with the delivery of bacterial effector proteins into host cells via type III secretion (13), and fluorescent protein fusions of the HIV-1 gag or pol can impair the production of infectious virions unless the labeled protein or wild-type Gag-Pol are provided in trans (14–16).
An alternative labeling strategy uses tetracysteine motifs inserted in the protein of interest that are bound with high affinity and specificity by the fluorescein arsenical helix binder (FlAsH) (17). These small motifs (six amino acids, 0.7 KDa) preserve the function of proteins that do not tolerate large protein fusions (13, 18). Furthermore, because FlAsH remains nonfluorescent until binding to tetracysteines, robust and controlled labeling efficiency is ensured. Contrary to antibodies, FlAsH-labeling is not subject to variable antigenic affinity, does not require sample permeabilization, and allows live cell imaging. FlAsH labeling has been used to study the localization and trafficking of several HIV-1 proteins in infected cells (18–23). Although fluorescein can exhibit photoswitching (5), to our knowledge the use of FlAsH has not been demonstrated for SR imaging. Here, we demonstrate FlAsH-based localization microscopy with a spatial resolution of ∼30 nm. We show the technique’s merit for cell biology of microbial proteins by SR imaging of the integrase enzyme (IN) of HIV-1 in fixed and live infected cells, a feat that has not been possible with previous techniques.
Results
Stochastic Fluorescence of Single FlAsH-Labeled Tetracysteine Peptides.
We first asked whether single FlAsH-labeled tetracysteine-containing molecules could be stochastically activated and localized with subdiffraction accuracy. Although initial implementations of PALM and STORM used special photoswitchable proteins or dyes (3, 4), ordinary fluorophores are now employed successfully (5, 6, 8) in the presence of a buffer containing oxygen scavengers, which reduces photobleaching and increases the lifetime of dark states thereby enabling localization of individual molecules. In addition, high-energy laser pulses, such as near-ultraviolet light, can accelerate return from dark states to the excitable ground state (5). Therefore, we subjected a diluted solution of tetracysteine-tagged peptides in the presence of FlAsH to an oxygen scavenger buffer. We then continuously excited the sample with blue light (488 nm), which initially caused the emitted fluorescence intensity (green light, 532 nm) to rise sharply then decay. After fluorescence stabilized, we repeatedly pulsed violet laser light (405 nm; pulse duration: 10 ms; typical frequency: 2 Hz) and acquired 5,000 time-lapse images (100 ms exposure time). We observed the repeated transient appearance of a small number of isolated diffraction-limited spots (see Movie S1), which most likely originated from individual fluorophores, allowing us to localize them computationally. The detected positions fell into distinct spatial clusters, and the temporal clustering of detections within these confirmed that they originated mostly from single fluorophores (Fig. S1 B–D). According to our estimates, almost all peptides were detected, the mean number of photons per detection event was ∼3,000 (SD ∼ 1,700), and the localization accuracy, as estimated by superposing position clusters, was characterized by a full width at half maximum (FWHM) ∼30 nm (Fig. S1 E–H).
FlAsH-PALM Recovers Subdiffraction Virion Morphology.
These properties immediately opened the perspective of FlAsH-based SR localization microscopy (FlAsH-PALM). To demonstrate FlAsH-PALM on a biological system, we chose to image HIV using the insertion of a tetracysteine tag into IN, the enzyme that mediates integration of viral DNA into the host genome (18). Labeling IN allows fluorescence detection of HIV as an extracellular virion and intracellular complex during all early steps of HIV replication because this protein is present in virions until integration of the viral genome into host chromatin, the hallmark of a productive infection. In HIV particles, IN is enclosed in structural shells whose morphology, as determined by electron microscopy (EM), changes upon virus maturation. In immature virions, the viral genome and associated enzymes, such as IN, are enclosed in spherical shells formed by the Gag structural polyprotein with an inner diameter of ∼65–90 nm (24), whereas capsids of mature virions have a conical shape with a length of ∼100–150 nm and a breadth of ∼40–60 nm (25–29). These sizes fall below the resolution of diffraction-limited microscopy but are potentially within reach of FlAsH-PALM. Because each virus contains ∼125–250 copies of IN (30, 31), we hypothesized that the spatial distribution of IN determined by FlAsH-PALM might reveal information about the capsid morphology if the protein explores a sufficient fraction of the capsid volume and sufficient numbers of complexes can be combined.
Determining viral morphology in this manner is potentially complicated by several factors: projection artifacts, whereby complexes at different depths can merge and elongated complexes tilted relative to the focal plane can appear round in 2D images; varying number and spatial distribution of IN molecules within each viral complex random localization errors and multiple detections of the same molecule. We first used Monte Carlo simulations accounting for these effects to verify theoretically if viral morphologies can be discriminated (Figs. S2 and S3). Briefly, we placed 1,000 simulated viral complexes of four different shapes (cylinders and cones of width 54 nm and length 120 nm, respectively, and spheres of diameter 54 nm and 120 nm) in 3D space with random positions and orientations, randomly distributed a variable number of molecules within the complex volume, added random localization errors with multiple detections of the same molecule, and projected the 3D data to create artificial 2D FlAsH-PALM images (Figs. S2 A–C).
We then analyzed the simulated images computationally. Briefly, our automated procedure (i) detected isolated clusters of points using the same algorithm as for the peptide data above, (ii) rotated individual clusters to align their principal axes, and then (iii) grouped clusters of similar morphology into families using hierarchical clustering (Figs. S2 D and E). Finally, we obtained an average probability density map of positions within each family by superposing all corresponding clusters (Figs. S2F). We applied this analysis to the artificial images generated for each of the four shapes (Fig. S3 A–D). Although a variable (3, 4) number of families was obtained, a single family always contained a large majority (79–98%) of all clusters. The shape and size of the probability density of this majority family was characteristic of each simulated shape, allowing, e.g., to distinguish conical from cylindrical or spherical structures (Fig. S3 A–D). Moreover, when we simulated mixtures of shapes, e.g., cones mixed with cylinders or cones mixed with spheres, this analysis yielded two large families with probability densities matching these two shapes, and the shape of each cluster could be recovered with good to high statistical certainty (Fig. S2 E and F).
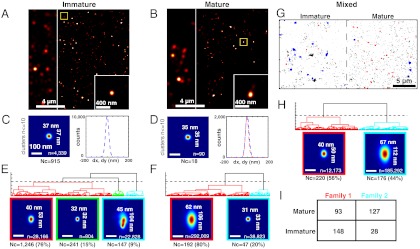
We proceeded to test the ability to discriminate viral morphologies experimentally using images of mature and immature virions. We performed FlAsH-PALM imaging of free HIV particles on coverslips in absence and in presence of ritonavir, which prevents virus maturation by inhibiting the HIV protease that cleaves the Gag-Pol polyprotein. We administered FlAsH in vitro followed by ultracentrifugation of labeled virions to remove unbound fluorophores (Fig. S4A) thus leading to specific labeling of tetracysteine-tagged virions (Fig. S4B). Virion maturation was assessed by the detection of Gag processing using Western blotting (Fig. S4C). We acquired ∼30,000 raw images of IN-FlAsH-labeled virions. As for the peptides, we observed stochastic fluorescence events (see Movie S2) enabling localization microscopy. Reconstructed images had higher resolution than widefield images and displayed hundreds of clusters of FlAsH-IN (Fig. 1 A and B).
Fig. 1.
FlAsH-PALM imaging of immature and mature HIV-1 virions reveals their distinct subdiffraction shapes. (A, C, E) Immature virions obtained by treating producer cells with ritonavir. (B, D, F) Mature virions. (A, B) FlAsH-PALM images of IN in free virions; the stripe on the left shows a reconstructed widefield image for comparison. The bottom right inset is a zoomed view of the yellow-boxed area. (C, D) Resolution estimates from superposed position clusters containing ≤ 10 positions. 2D histograms (left) and 1D histograms (right) of position coordinates x and y relative to cluster mass centers; 2D histograms are visualized as heat maps from dark blue (low probability) to red (high probability); indicated sizes are FWHM measured along x and y; Nc is the number of clusters; n is the total number of positions from all clusters. (E, F, H) Morphological families. Dendrograms show the hierarchical clustering of aligned clusters based on similarity of their probability densities; dashed horizontal lines show the threshold used to define families. 2D histograms below the dendrograms show the distribution of positions in superposed aligned clusters for each family (frame color matches corresponding subtree in the dendrogram). n and Nc as above, with the percentage of clusters indicated in brackets. (G–I) Discriminating mature from immature virions in a mixed population. Dendrogram and 2D histogram in (H) were obtained by analyzing a merged FlAsH-PALM image containing immature and mature virions, a portion of which is shown in (G). (I) Confusion matrix indicating how many clusters from the mature or immature virion images were assigned to each family (Families 1 and 2 correspond to the red and blue families in (G), respectively). All scale bars are 100 nm except where indicated otherwise.
Next, we analyzed these clusters using the same procedure as for the simulated images. To estimate the resolution, we superposed position clusters containing 10 or fewer localization events, which likely corresponded to individual IN molecules or small aggregates. The superposed clusters had a size (FWHM) of 35–37 nm indicating a resolution roughly similar to that estimated from peptides above (Fig. 1 C and D). We then aligned and grouped clusters in families as demonstrated on the simulated data. This analysis yielded three families for the immature virions and two families for the mature virions (data pooled from two independent experiments in each condition) (Fig. 1 E and F). In both cases, one family contained a large majority of clusters (76–80%), as in the simulations (red in Fig. 1 E and F). The probability densities of these two majority families displayed markedly distinct subdiffraction sizes and shapes. While immature virions displayed a roughly circular pattern with a size of ≈40 × 53 nm (width × length, as measured by FWHM perpendicular and parallel to the axis of alignment) (Fig. 1E), mature virions yielded an approximately conical shape of 62 × 106 nm (Fig. 1F). Although the spatial distribution of IN inside virions is not known, these data are consistent with the distinct morphologies of mature capsid cores and immature Gag shells as previously determined by EM (25–29). The measured diameter for immature virions is somewhat smaller than the previously estimated diameter of the inner Gag shell (∼60–95 nm) (24). This may be explained by the fact that in the presence of protease inhibitor, IN is located at the C-terminus of the unprocessed Gag-Pol polyprotein, which, like Gag, is radially oriented with the C-terminus facing the interior of the particle. Models suggest that the Pol domains may be located together with the RNA in the central portion of the virion and occupy a volume three-eighths the diameter of the whole virion (i.e., ∼40–50 nm) in good agreement with our measurements (32). The position patterns in the minority families were close to the estimated resolution limit, and likely caused by single IN molecules or fluorescent impurities (15–20%); or, in the case of immature virions, displayed an ill-defined elongated shape (9%) likely caused by fluorescent debris or the close proximity of distinct virions (Fig. 1 E and F).
Finally, we artificially mixed mature and immature virions by stitching the images from both datasets (Fig. 1G). Analysis of this mixed image identified two families: one characterized by a nearly spherical pattern of size ≈40 × 50 nm, very similar to the majority family of immature virions and another one by a conical pattern of width ≈67 nm and length ≈112 nm, very similar to the majority pattern of the mature virion (Fig. 1H). We therefore tested if mature virions could be discriminated from immature virions in the mixed population based on their morphology alone. We found that 82% of the clusters from the “conical” family originated from the mature virion image, whereas 61% of the “spherical” family clusters originated from the immature virion image (Fig. 1I). This shows that, much as in simulations, viral complexes of different morphologies can indeed be distinguished with good statistical confidence in a mixed population.
Thus, FlAsH-PALM can discriminate among morphologies of virions below the resolution of conventional microscopes.
SR Imaging of HIV in Infected Cells.
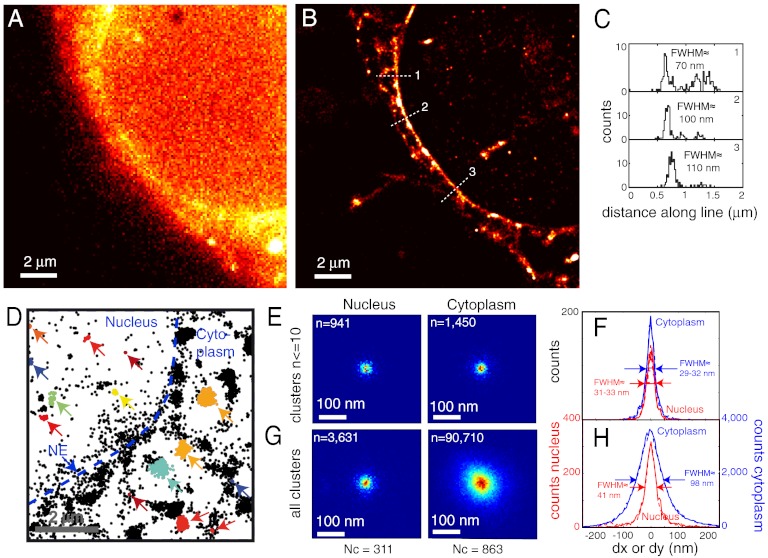
We next demonstrate SR FlAsH-PALM imaging of IN inside infected cells. Infection experiments were performed using HIV-1 pseudotyped with the Vesicular Stomatitis Virus glycoprotein (VSV-G), which is more stable than wild-type HIV envelope and enables a full recovery of viral infectivity after the ultracentrifugation step required to remove unbound FlAsH after labeling (18). The extracellular labelling strategy minimized background signal allowing specific visualization of IN by widefield fluorescence microscopy (Fig. S5A) (18).
We acquired 15,000–30,000 widefield images of FlAsH-labeled IN in target cells fixed 2 h postinfection. Again, we observed stochastic appearance of isolated fluorescent spots compatible with FlAsH-PALM (see Movie S3 and Fig. S5 B and C). The density of detected positions outside cells was negligible (< 5%) compared to that inside cells, indicating high specificity (Fig. S6). Reconstructed FlAsH-PALM images clearly had higher resolution than the corresponding widefield image (Fig. 2 A and B). IN signal was visible as discrete clusters appearing throughout the cell and as a partly punctate accumulation at the nuclear envelope (NE) (Fig. 2 A, B, and D). This accumulation reflects the docking of viral complexes to nuclear pore complexes that act as bottlenecks in the early steps of HIV replication (33). Intensity profiles across the NE confirmed subdiffraction (< ∼ 70–100 nm) resolution (Fig. 2C). Superposing position clusters containing 10 or fewer localization events yielded tighter resolution estimates with a FWHM of 29–33 nm in the nucleus and the cytoplasm similar to the resolution estimated from peptides and free virions above (Fig. 2 E and F) (data pooled from n = 12 cells in three independent experiments).
Fig. 2.
FlAsH-PALM imaging of infectious HIV-1 IN in fixed cells. (A) Widefield image. (B) Superresolution reconstruction of the same area computed from 159,229 positions in 20,000 frames. (C) Histograms of spot positions projected along three line segments crossing the NE [dotted segments in (B); positions were taken from 300 nm wide rectangles centered on these segments]. The approximate FWHM is indicated. (D) FlAsH-IN clusters. Detected positions in this area are shown as small black stars; positions in automatically defined clusters are shown by colored stars and indicated by arrows. (E–G) Resolution and IN complex size in the nucleus vs. cytoplasm. (E, H) 2D histograms of superposed clusters in the nucleus (left) and cytoplasm (right). (F, H) Histograms of position coordinates relative to the cluster mass center (solid lines: x, dashed lines: y, red: nucleus, blue: cytoplasm). (E, F) Clusters containing 10 or fewer detected positions for estimate of resolution. (G, H) Superposition of all clusters, irrespective of detection counts.
Thus, FlAsH-PALM enables ∼30 nm resolution imaging of intracellular structures that do not tolerate labeling by fluorescent proteins without compromising their function.
FlAsH-PALM Resolves Size Differences Between Cytoplasmic and Nuclear HIV Complexes.
HIV can replicate in nondividing cells and must, therefore, cross the NE to gain access to the host DNA, most likely, by transiting through nuclear pores. Because the capsid size exceeds the diameter of the nuclear pore lumen (26 nm) (34), the virus must shed its capsid in the cytoplasm prior to entering the nucleus. Increasing evidence suggests that this uncoating does not occur randomly in the cytoplasm and is critical for infection; but, the exact timing and location of uncoating has been debated (35–38). Although biochemical analyses of intracellular HIV initially concluded that capsids were lost immediately postfusion (39, 40), EM data revealed intact HIV capsids at or near nuclear pores (33) indicating that capsid uncoating does not necessarily precede viral trafficking to the NE. This is further supported by studies showing that premature uncoating upon retroviral restriction leads to abortive infection (41–43), and mutations in capsid proteins influence the requirement for nuclear pore components (35, 44–46). Recent work suggests that the trigger for uncoating is linked to the reverse transcription process (33, 36). Whether large or small proportions of capsids remain intact during cytoplasmic transport has yet to be determined.
We analyzed the morphology and subcellular location of all detected IN-FlAsH position clusters (irrespective of position counts) in the HIV-1 infected cells. The size and density of these clusters were not homogeneous but displayed a marked difference between the cytoplasmic and nuclear compartments. The FWHM of superposed clusters was 98 nm in the cytoplasm, compared to ≈41 nm in the nucleus (Fig. 2 G and H). This difference stands in contrast to the ∼30 nm resolution estimated above both in the nucleus and cytoplasm. Nuclear clusters are only ∼25% larger than this estimated resolution and could, thus, correspond to single IN molecules, tetramers, or other small molecular aggregates associated with the ends of viral DNA. In contrast, aggregated cytoplasmic clusters have a size comparable with the HIV capsid suggesting that a large portion of all cytoplasmic viral complexes may contain intact capsids.
Morphology of IN clusters and Dual-Color Imaging Confirm Capsid Presence in Cytoplasm.
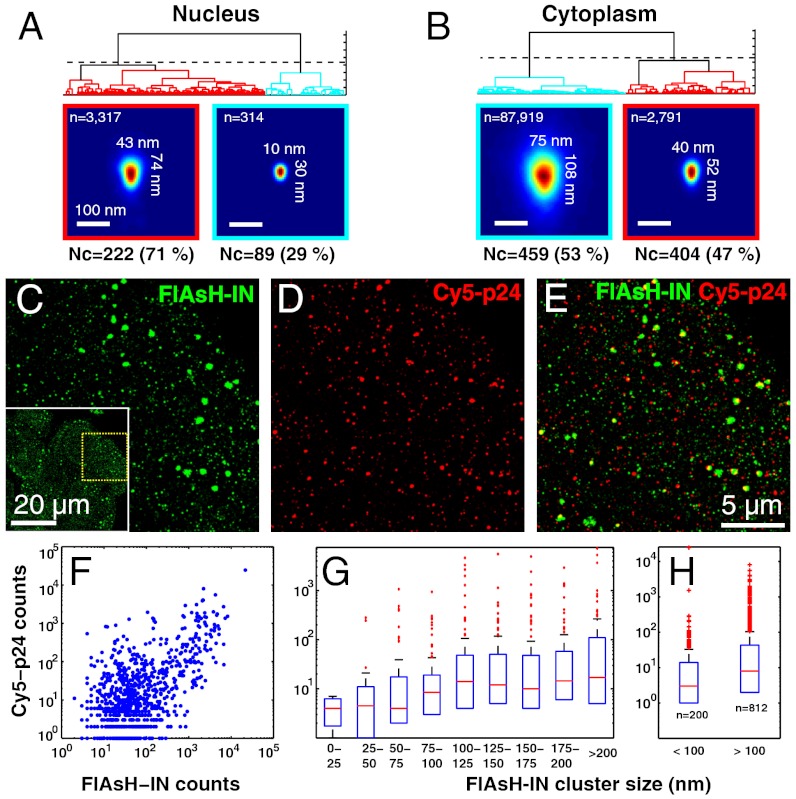
To further characterize the spatial distribution of IN in nuclear vs. cytoplasmic clusters, we analyzed cluster families as for the free virions above. This analysis resulted in two morphological families for nuclear and cytoplasmic clusters. Despite significant variability among individual clusters, the probability density of the majority family (53% of clusters and 97% of positions) displayed an approximately conical shape of ∼108 nm in length and ∼75 nm in width, approximately similar to the shape determined above in capsids of mature virions (Fig. 1F) and in agreement with the EM data (25–29) (Fig. 3A). This stood in contrast to the much smaller pattern (43 × 74 nm) of the majority family in the nucleus (71%). Although we cannot rule out that more complex mixtures of nonconical complexes may also be consistent with the data, our observations suggests that the bulk of cytoplasmic IN, 2 h postinfection, resides in intact capsids.
Fig. 3.
Optical evidence for intact HIV capsids in the cytoplasm. (A–B) Morphology families of nuclear (A) and cytoplasmic (B) FLASH-IN clusters. The majority family of cytoplasmic clusters exhibits a conical probability density similar to the intact HIV capsid in contrast to the much smaller structure in the nucleus. (C–H) Dual-color experiments show association of capsid protein p24 with large IN clusters. (C) FlAsH-PALM image of IN. The region shown corresponds to the yellow dotted box of the entire field of view shown in the inset (saturated to better show cell boundaries). (D) STORM image of p24 labeled with antibodies coupled to Cy5. (E) Merged image indicating partial colocalization of FlAsH-IN with p24-Cy5. (F–H) Quantitative analysis of 1,012 automatically detected FlAsH-IN clusters. Number of Cy5-p24 positions in 500 nm circles enclosing FlAsH-IN position clusters plotted vs. the number of FlAsH-IN positions in these circles (F) or vs. the size (G, H) of FlAsH-IN clusters. Boxplots indicate the median (red bar) and 25% and 75% percentiles (blue bars); whiskers show the data range except for outliers (red crosses). The size of FlAsH-IN clusters is measured by FWHM = 2.35√(σxσy), where σx and σy are the standard deviations of FlAsH-IN coordinates x and y.
To confirm the presence of capsid around IN clusters, we performed dual-color FlAsH-PALM/STORM imaging of FlAsH-tagged IN and of the capsid shell protein p24, which was labeled with antibodies coupled to the organic dye Cy5. In the dual-color images, signal from FlAsH-IN and Cy5-p24 was visible as clusters of different sizes and displayed partial colocalization (Fig. 3 C–E). In 500 nm diameter circles enclosing automatically defined FlAsH-IN clusters, the number of p24-Cy5 localizations correlated positively with the number of FlAsH-IN localizations (Fig. 3F) indicating that p24 is significantly associated with IN (Pearson’s r = 0.73, P < 10-100). We also analyzed the number of p24-Cy5 positions in these circles as a function of the size (FWHM) of the FlAsH-IN cluster. This number was roughly constant for sizes either smaller or larger than 75–100 nm (Fig. 3G) but was significantly larger above 100 nm than below (Wilcoxon P < 10-6) (n = 1,012 clusters in n = 22 cells in three independent experiments) (Fig. 3H). Thus, capsid proteins accumulate at IN clusters larger than ∼100 nm.
These data strengthen the case that the bulk of cytoplasmic IN proteins are not diluted in the cytoplasm upon viral entry but are maintained in the volume of intact capsids. In addition, our data indicate that these complexes can be found at any distance between the nuclear and cellular membranes (Fig. S7). Thus, FlAsH-PALM can reveal previously inaccessible subdiffraction information on the size and shape of intracellular HIV complexes and the subcellular location of uncoating throughout infected cells.
Live Cell FlAsH-PALM Imaging.
An appealing advantage of FlAsH-labeling is its applicability to in vivo imaging (13, 18). Live cell localization microscopy has previously been achieved with photoswitchable fluorescent proteins (9) or with proteins bound to small organic dyes (7, 11). An important issue in live experiments is cellular damage caused by intense laser illumination (9). In FlAsH-PALM, this is potentially compounded by toxicity of the photoswitching buffer.
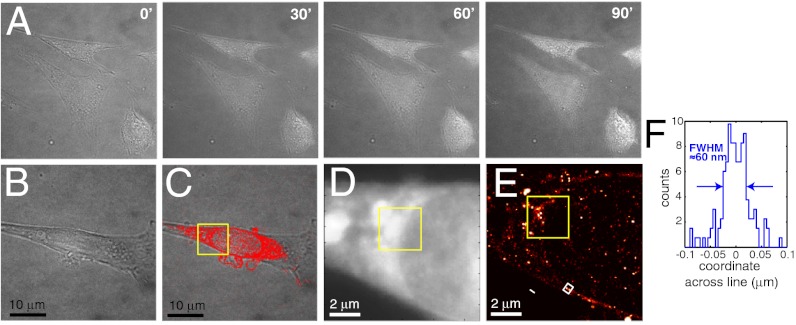
We analyzed buffer- and laser-induced toxicity in HIV infected and noninfected HeLa and 3T3 cells using brightfield imaging, which can detect alterations in cellular morphology. 3T3 cells are more photoresistant than HeLa cells and were previously used to demonstrate live PALM (9). We observed significant buffer-induced toxicity in HeLa cells. In contrast, 3T3 cells exposed to the buffer and 488 nm laser excitation levels used in FlAsH-PALM revealed no signs of toxicity even after 90 min (Fig. 4A). We, therefore, tested FlAsH-PALM on living HIV-infected 3T3 cells during acquisition times of 30–50 min with temperature kept constant at 37 °C. HIV was pseudotyped with a VSV-G envelope thus permitting viral entry of mouse cells. Brightfield images obtained before and after FlAsH-PALM imaging revealed no noticeable change of cell morphology (Fig. 4 B and C). FlAsH-PALM images generated from 25,000 consecutive frames showed a discrete accumulation of IN at the NE of live 3T3 cells much as observed on fixed HeLa cells, which was not readily apparent in the summed widefield image (Fig. 4 D and E). Resolution, as estimated by a line profile through the NE was ∼60 nm or better (Fig. 4F).
Fig. 4.
Live cell FlAsH-PALM on HIV-infected 3T3 cells. (A) Time-lapse brightfield images of cells exposed to the photoswitching buffer and FlAsH-PALM irradiation at 488 nm. The cells maintain normal morphology during the entire experiment, or 90 minutes. (B) Brightfield image of a cell at 10 h after infection taken before FlAsH-PALM imaging. (C) Superresolution image obtained from the 25,000 first frames, superimposed on a brightfield image taken after 108 minutes of FlAsH-PALM imaging. The cell shows no signs of toxicity. (D) Summed widefield image of the boxed region in (C). (E) FlAsH-PALM image of the same region obtained from the first 25,000 frames. (F) Histogram of positions across the NE computed from the white rectangle in (E).
To test dynamic live cell imaging, we generated time series of FlAsH-PALM images by aggregating positions from bins of 5,000 consecutive raw image frames (Fig. S8). Although limiting the temporal resolution to 500 s, this binning achieved the best possible spatial resolution according to the density-based Nyquist criterion (9). The improved spatial resolution of individual live FlAsH-PALM images compared to widefield was clearly apparent (Fig. S8 A, B, D–H) and allowed, for example, to observe a ∼200 nm structure moving towards the NE (Fig. S8 B–D) and to discern subdiffraction features including a structure resembling a viral capsid (Fig. S8H). Although the dynamics of this structure appear much slower than previously observed for individual viral complexes (18), these data illustrate that FlAsH-PALM allows dynamic SR imaging in live cells without apparent alteration of cellular physiology.
Discussion
We demonstrated FlAsH-based localization microscopy and applied it to SR imaging of HIV-1 as free virions and as intracellular complexes in fixed and living cells.
The fragility of the HIV capsid has, thus far, precluded the isolation and study of the composition of native viral replication complexes in the infected cell (43). In situ imaging is the only noninvasive alternative but has traditionally been limited by the diffraction barrier. In contrast, FlAsH-PALM allowed us to resolve IN localization and virion morphology at ∼30 nm resolution and to detect a substantial change in virus size between cytoplasmic and nuclear complexes. Although EM had previously shown the presence of intact capsids at nuclear pores (33), our results suggest that an important fraction of the cytoplasmic viral material remains encapsidated and that these capsids can be found throughout the cytoplasm in agreement with a recent report (36). Of note, all infection experiments were performed with VSV-G pseudotyped HIV-1 whose entry pathway involves endosomal fusion (47); however, there is no evidence that the kinetics of uncoating differ whether viral entry is mediated by VSV-G or wild-type HIV-1 envelope (33, 36). Furthermore, our data indicate that IN can occupy most of the capsid volume at 2 h postinfection, a time point when most intracellular HIV complexes still contain RNA rather than DNA (48) suggesting that, at this stage, IN molecules are randomly distributed inside the capsid volume. The ability of FlAsH-PALM to measure viral morphologies, in combination with its possible use in vivo, pave the way for resolving many unexplored steps in the replication cycles of intracellular microbes. Crucially, our data highlight that FlAsH-PALM preserves the biological function of a protein that would have been disrupted by fusion with fluorescent proteins or distorted by antibody binding. As such, our method should be of wide interest especially for the study of pathogen proteins whose functions are often compromised by conventional labels.
Several improvements of FlAsH-PALM can be envisaged. First, whereas the 2D data obtained here was sufficient to reveal statistical information on viral morphology, more detailed analyses will require extensions of FlAsH-PALM to 3D imaging (11). Another interesting perspective is to use ReAsH, the red counterpart of FlAsH, that allows light and EM visualization of the same specimen and proteins (49). We have shown how FlAsH-PALM can provide SR visualizations of slow dynamics in live cells. Although the much faster viral motions observed previously (18) may pose a challenge for localization microscopy, the raw images obtained with FlAsH-PALM could still be used for high-density tracking of individual molecules for extensive analyses of viral dynamics (50). Further optimizations in speed will be needed to achieve the full potential of live FlAsH-PALM.
Finally, the much smaller size of FlAsH labels compared to protein fusions or antibodies combined with the larger photon output of fluorescein relative to proteins may open the door to resolutions of ∼1 nm or less (12). We expect that our results will expand the applicative scope of FlAsH and facilitate subdiffraction imaging of delicate protein assemblies in fixed and living cells without perturbing their native function.
Materials and Methods
For a detailed description, please refer to SI Materials and Methods. Briefly, production of viruses and FlAsH labeling was performed as in (18). Localization microscopy setup, protocol, and computational image reconstruction were similar to those previously used (3, 4) (Figs. S9 and S10). To enable FlAsH photoswitching, we used the previously described buffer (8). Live cell microscopy was done at a constant temperature of 37 °C. In-house algorithms were used to create artificial FlAsH-PALM images and to automatically detect, align, and group experimental and simulated position clusters.
Supplementary Material
Acknowledgments.
We thank H. Wong for help with simulations, H. Marie-Nelly for suggesting the mixture analysis of Fig. 1 I and C, v. Middendorff and J. Enninga for critical reading of the manuscript, E. Doris for supplying the FlAsH fluorophores, and X. Darzacq for technical help. This work was funded by Institut Pasteur, Fondation pour la Recherche Médicale, C’Nano Région Ile-de-France, Sidaction, and the Centre National de la Recherche Scientifique.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013267109/-/DCSupplemental.
References
- 1.Knipe DM, Howley PM. Fundamental Virology. Philadelphia: Lippincott Williams and Wilkins; 2001. [Google Scholar]
- 2.Hell SW. Microscopy and its focal switch. Nat Methods. 2009;6:24–32. doi: 10.1038/nmeth.1291. [DOI] [PubMed] [Google Scholar]
- 3.Betzig E, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–1645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 4.Rust MJ, Bates M, Zhuang X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM) Nat Methods. 2006;3:793–795. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fölling J, et al. Fluorescence nanoscopy by ground-state depletion and single-molecule return. Nat Methods. 2008;5:943–945. doi: 10.1038/nmeth.1257. [DOI] [PubMed] [Google Scholar]
- 6.Baddeley D, Jayasinghe ID, Cremer C, Cannell MB, Soeller C. Light-induced dark states of organic fluochromes enable 30 nm resolution imaging in standard media. Biophys J. 2009;96:L22–24. doi: 10.1016/j.bpj.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wombacher R, et al. Live-cell super-resolution imaging with trimethoprim conjugates. Nat Methods. 2010;7:717–719. doi: 10.1038/nmeth.1489. [DOI] [PubMed] [Google Scholar]
- 8.Heilemann M, et al. Subdiffraction-resolution fluorescence imaging with conventional fluorescent probes. Angew Chem Int Ed. 2008;47:6172–6176. doi: 10.1002/anie.200802376. [DOI] [PubMed] [Google Scholar]
- 9.Shroff H, Galbraith CG, Galbraith JA, Betzig E. Live-cell photoactivated localization microscopy of nanoscale adhesion dynamics. Nat Methods. 2008;5:417–423. doi: 10.1038/nmeth.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein T, et al. Live-cell dSTORM with SNAP-tag fusion proteins. Nat Methods. 2011;8:7–9. doi: 10.1038/nmeth0111-7b. [DOI] [PubMed] [Google Scholar]
- 11.Jones SA, Shim SH, He J, Zhuang X. Fast, three-dimensional super-resolution imaging of live cells. Nat Methods. 2011;8:499–505. doi: 10.1038/nmeth.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pertsinidis A, Zhang Y, Chu S. Subnanometre single-molecule localization, registration and distance measurements. Nature. 2010;466:647–651. doi: 10.1038/nature09163. [DOI] [PubMed] [Google Scholar]
- 13.Enninga J, Mounier J, Sansonetti P, Van Nhieu GT. Secretion of type III effectors into host cells in real time. Nat Methods. 2005;2:959–965. doi: 10.1038/nmeth804. [DOI] [PubMed] [Google Scholar]
- 14.Muller B, et al. Construction and characterization of a fluorescently labeled infectious human immunodeficiency virus type 1 derivative. J Virol. 2004;78:10803–10813. doi: 10.1128/JVI.78.19.10803-10813.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albanese A, Arosio D, Terreni M, Cereseto A. HIV-1 pre-integration complexes selectively target decondensed chromatin in the nuclear periphery. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherer NM, et al. Visualization of retroviral replication in living cells reveals budding into multivesicular bodies. Traffic. 2003;4:785–801. doi: 10.1034/j.1600-0854.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 17.Griffin BA, Adams SR, Tsien RY. Specific covalent labeling of recombinant protein molecules inside live cells. Science. 1998;281:269–272. doi: 10.1126/science.281.5374.269. [DOI] [PubMed] [Google Scholar]
- 18.Arhel N, et al. Quantitative four-dimensional tracking of cytoplasmic and nuclear HIV-1 complexes. Nat Methods. 2006;3:817–824. doi: 10.1038/nmeth928. [DOI] [PubMed] [Google Scholar]
- 19.Rudner L, et al. Dynamic fluorescent imaging of human immunodeficiency virus type 1 gag in live cells by biarsenical labeling. J Virol. 2005;79:4055–4065. doi: 10.1128/JVI.79.7.4055-4065.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gousset K, et al. Real-time visualization of HIV-1 GAG trafficking in infected macrophages. PLoS Pathog. 2008;4:e1000015. doi: 10.1371/journal.ppat.1000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell EM, Perez O, Anderson JL, Hope TJ. Visualization of a proteasome-independent intermediate during restriction of HIV-1 by rhesus TRIM5alpha. J Cell Biol. 2008;180:549–561. doi: 10.1083/jcb.200706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turville SG, Aravantinou M, Stossel H, Romani N, Robbiani M. Resolution of de novo HIV production and trafficking in immature dendritic cells. Nat Methods. 2008;5:75–85. doi: 10.1038/nmeth1137. [DOI] [PubMed] [Google Scholar]
- 23.Pereira CF, et al. Labeling of multiple HIV-1 proteins with the biarsenical-tetracysteine system. PLoS One. 2011;6:e17016. doi: 10.1371/journal.pone.0017016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright ER, et al. Electron cryotomography of immature HIV-1 virions reveals the structure of the CA and SP1 Gag shells. EMBO J. 2007;26:2218–2226. doi: 10.1038/sj.emboj.7601664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briggs JAG, Wilk T, Welker R, Kräusslich HG, Fuller SD. Structural organization of authentic, mature HIV-1 virions and cores. EMBO J. 2003;22:1707–1715. doi: 10.1093/emboj/cdg143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganser BK, Li S, Klishko VY, Finch JT, Sundquist WI. Assembly and analysis of conical models for the HIV-1 core. Science. 1999;283:80–83. doi: 10.1126/science.283.5398.80. [DOI] [PubMed] [Google Scholar]
- 27.Höglund S, et al. Spatial visualization of the maturing HIV-1 core and its linkage to the envelope. AIDS Res Hum Retroviruses. 1992;8:1–7. doi: 10.1089/aid.1992.8.1. [DOI] [PubMed] [Google Scholar]
- 28.Welker R, Hohenberg H, Tessmer U, Huckhagel C, Krausslich HG. Biochemical and structural analysis of isolated mature cores of human immunodeficiency virus type 1. J Virol. 2000;74:1168–1177. doi: 10.1128/jvi.74.3.1168-1177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benjamin J, Ganser-Pornillos BK, Tivol WF, Sundquist WI, Jensen GJ. Three-dimensional structure of HIV-1 virus-like particles by electron cryotomography. J Mol Biol. 2005;346:577–588. doi: 10.1016/j.jmb.2004.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Briggs J, et al. The stoichiometry of Gag protein in HIV-1. Nat Struct Mol Biol. 2004;11:672–675. doi: 10.1038/nsmb785. [DOI] [PubMed] [Google Scholar]
- 31.Layne SP, et al. Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology. 1992;189:695–714. doi: 10.1016/0042-6822(92)90593-e. [DOI] [PubMed] [Google Scholar]
- 32.Vogt VM. Retroviral Virions and Genomes. In: Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. NY: Cold Spring Harbor; 1997. [PubMed] [Google Scholar]
- 33.Arhel NJ, et al. HIV-1 DNA Flap formation promotes uncoating of the pre-integration complex at the nuclear pore. EMBO J. 2007;26:3025–3037. doi: 10.1038/sj.emboj.7601740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen TD, Cronshaw JM, Bagley S, Kiseleva E, Goldberg MW. The nuclear pore complex: mediator of translocation between nucleus and cytoplasm. J Cell Sci. 2000;113:1651–1659. doi: 10.1242/jcs.113.10.1651. [DOI] [PubMed] [Google Scholar]
- 35.Arhel N. Revisiting HIV-1 uncoating. Retrovirology. 2010;7:85–95. doi: 10.1186/1742-4690-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hulme AE, Perez O, Hope TJ. Complementary assays reveal a relationship between HIV-1 uncoating and reverse transcription. Proc Natl Acad Sci USA. 2011;108:9975–9980. doi: 10.1073/pnas.1014522108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Briones MS, Dobard CW, Chow SA. Role of human immunodeficiency virus type 1 integrase in uncoating of the viral core. J Virol. 2010;84:5181–5190. doi: 10.1128/JVI.02382-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dismuke DJ, Aiken C. Evidence for a functional link between uncoating of the human immunodeficiency virus type 1 core and nuclear import of the viral preintegration complex. J Virol. 2006;80:3712–3720. doi: 10.1128/JVI.80.8.3712-3720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bukrinsky MI, et al. Association of integrase, matrix, and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc Natl Acad Sci USA. 1993;90:6125–6129. doi: 10.1073/pnas.90.13.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fassati A, Goff SP. Characterization of intracellular reverse transcription complexes of human immunodeficiency virus type 1. J Virol. 2001;75:3626–3635. doi: 10.1128/JVI.75.8.3626-3635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stremlau M, et al. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc Natl Acad Sci USA. 2006;103:5514–5519. doi: 10.1073/pnas.0509996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perron MJ, et al. The human TRIM5alpha restriction factor mediates accelerated uncoating of the N-tropic murine leukemia virus capsid. J Virol. 2007;81:2138–2148. doi: 10.1128/JVI.02318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Black LR, Aiken C. TRIM5alpha disrupts the structure of assembled HIV-1 capsid complexes in vitro. J Virol. 2010;84:6564–6569. doi: 10.1128/JVI.00210-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee K, et al. Flexible use of nuclear import pathways by HIV-1. Cell Host Microbe. 2010;7:221–233. doi: 10.1016/j.chom.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krishnan L, et al. The requirement for cellular transportin 3 (TNPO3 or TRN-SR2) during infection maps to human immunodeficiency virus type 1 capsid and not integrase. J Virol. 2010;84:397–406. doi: 10.1128/JVI.01899-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matreyek KA, Engelman A. The Requirement for Nucleoporin NUP153 during Human Immunodeficiency Virus Type 1 Infection Is Determined by the Viral Capsid. J Virol. 2011;85:7818–7827. doi: 10.1128/JVI.00325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aiken C. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J Virol. 1997;71:5871–5877. doi: 10.1128/jvi.71.8.5871-5877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farnet CM, Haseltine WA. Integration of human immunodeficiency virus type 1 DNA in vitro. Proc Natl Acad Sci USA. 1990;87:4164–4168. doi: 10.1073/pnas.87.11.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaietta G, et al. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296:503–507. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- 50.Manley S, et al. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat Methods. 2008;5:155–157. doi: 10.1038/nmeth.1176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.