Abstract
The γ-secretase complex, composed of presenilin, anterior-pharynx-defective 1, nicastrin, and presenilin enhancer 2, catalyzes the intramembranous processing of a wide variety of type I membrane proteins, including amyloid precursor protein (APP) and Notch. Earlier studies have revealed that nicastrin, a type I membrane-anchored glycoprotein, plays a role in γ-secretase assembly and trafficking and has been proposed to bind substrates. To gain more insights regarding nicastrin structure and function, we generated a conformation-specific synthetic antibody and used it as a molecular probe to map functional domains within nicastrin ectodomain. The antibody bound to a conformational epitope within a nicastrin segment encompassing residues 245–630 and inhibited the processing of APP and Notch substrates in in vitro γ-secretase activity assays, suggesting that a functional domain pertinent to γ-secretase activity resides within this region. Epitope mapping and database searches revealed the presence of a structured segment, located downstream of the previously identified DAP domain (DYIGS and peptidase; residues 261–502), that is homologous to a tetratricopeptide repeat (TPR) domain commonly involved in peptide recognition. Mutagenesis analyses within the predicted TPR-like domain showed that disruption of the signature helical structure resulted in the loss of γ-secretase activity but not the assembly of the γ-secretase and that Leu571 within the TPR-like domain plays an important role in mediating substrate binding. Taken together, these studies offer provocative insights pertaining to the structural basis for nicastrin function as a “substrate receptor” within the γ-secretase complex.
Alzheimer’s disease (AD), a progressive neurodegenerative disease, is the most prevalent cause of dementia in humans. The principal neuropathological hallmark of AD is the presence of senile plaques composed of dystrophic neurites surrounding extracellular aggregates of Aβ peptides (1). Aβ peptides are liberated from amyloid precursor proteins (APP) by the concerted action of β-site APP cleaving enzyme 1 and γ-secretase (2, 3). γ-Secretase is a macromolecular complex consisting of presenilin 1 or presenilin 2 (PS1 or PS2), anterior-pharynx-defective 1 (APH-1), nicastrin (NCT), and presenilin enhancer 2 (PEN-2) that catalyzes intramembranous proteolysis of several membrane-tethered substrates (4). Evidence has emerged to reveal the functions of each subunit: PS is the catalytic subunit (5); APH-1 serves as a scaffold for the complex assembly (6); NCT seems to be responsible for substrate binding (7); and PEN-2 promotes endoproteolytic cleavage of PS and “activation” of the enzyme complex (5, 8). Although γ-secretase cleaves a multiplicity of substrates at heterogeneous sites within individual transmembrane domains, the molecular mechanism(s) underlying substrate recognition and processing remain elusive.
NCT, a 709-aa type I transmembrane glycoprotein with a large, heavily glycosylated ectodomain (ECD), was first identified as a PS-interacting protein that modulates APP processing and Notch signaling (9). Early studies showed that the NCT ECD interacts with membrane-tethered substrates that are subject to intramembranous proteolysis by the γ-secretase complex, hence arguing for a role as a substrate “receptor” (7). In this case, glutamate 333 (E333) within the “DAP” domain (DYIGS and peptidase; residues 261–502) was shown to be critical for substrate binding and delivery to the catalytic site (7, 10). However, it has been argued that E333 may play an alternative role in enhancing NCT maturation through the secretory pathway (11). A recent study demonstrated that a Notch substrate was processed, albeit weakly, in NCT-deficient (NCT−/−) fibroblasts treated with a proteasome inhibitor, suggesting that NCT was not absolutely required for γ-secretase activity (12). On the other hand, a monoclonal antibody directed against the NCT ECD inhibited γ-secretase activity by apparently blocking a substrate-binding region (13). Taken together, although NCT is important in γ-secretase activity, the bona fide function of NCT remains unresolved.
To provide insights into the role of the NCT ECD in the regulation of γ-secretase activity, we sought to identify and dissect domains of the NCT ECD. We hypothesized that the NCT ECD may contain structured domains, in addition to the DAP domain, that are important for its function. Inspired by the work of Iwatsubo and colleagues (13, 14), we set out to generate conformation-specific antibodies that would potentially perturb the function of NCT. The rationale behind this approach is that such an antibody may bind to a surface of NCT critical for function, and we could discover a new domain by mapping the epitope of the antibody. In this work, we used recombinant antibody technology that is based on a powerful “reduced genetic code” phage display library (15).
We successfully identified two distinct synthetic antibodies in the Fab format, and one of these recognizes a structured region including a segment C-terminal to the DAP domain. Database searches suggested that this region is homologous to a tetratricopeptide repeat (TPR) domain (16) that is involved in peptide recognition. The antibody blocked γ-secretase activity in in vitro assays, and mutations that alter the α-helicity within the putative TPR-like domain also perturbed substrate binding and γ-secretase activity, suggesting the importance of the newly identified domain in NCT function.
Results
γ-Secretase with Minimally Glycosylated NCT Is Active.
To isolate NCT-specific Fabs for biophysical and structural studies, we thought it was essential to reduce the chemical heterogeneity of NCT resulting from glycosylation. NCT is predicted to harbor 16 potential N-linked glycosylation sites in the ECD, and mass spectrometric analysis confirmed that all of these sites were subject to posttranslational glycosylation events (Table S1), although the degree to which each site is glycosylated remains indeterminate.
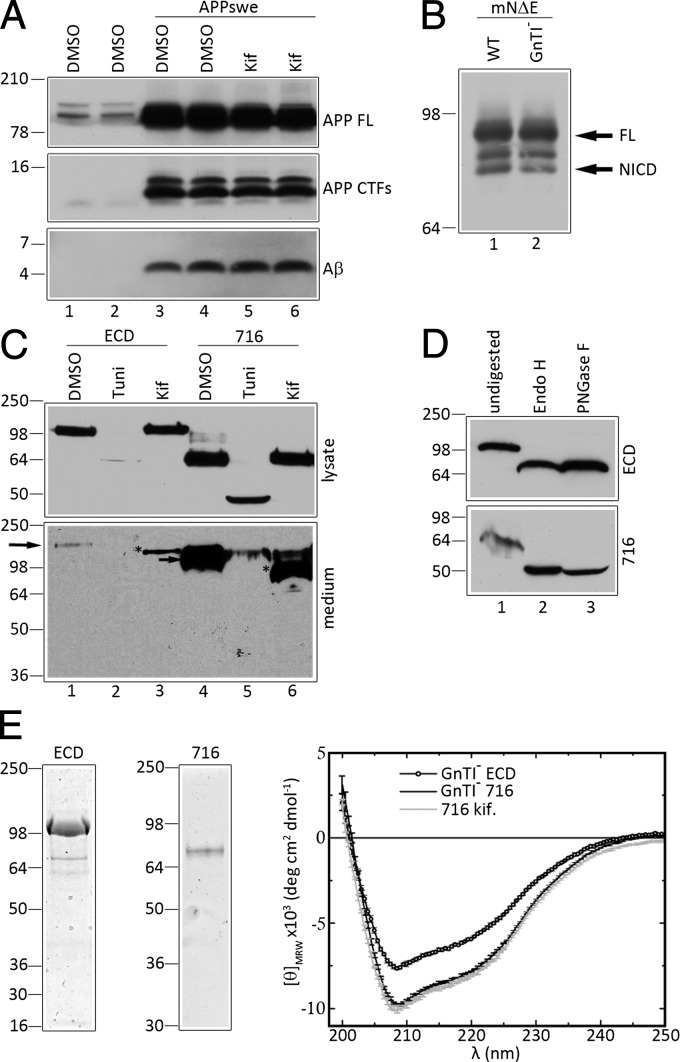
To produce minimally glycosylated NCT that retains γ-secretase activity, we treated HEK293 cells that overexpress human APP harboring the FAD-linked “Swedish” mutations (APPswe) with kifunensine, a potent inhibitor of α-mannosidase I, to suppress maturation of high mannose oligosaccharides. As expected, and as reported previously (17, 18), compared with vehicle-treated cells, kifunensine treatment affected neither the levels of APP-carboxyl-terminal fragments (CTFs) nor levels of secreted Aβ peptides (Fig. 1A, APP-CTFs and Aβ; compare lanes 3 and 4 with 5 and 6, respectively), suggesting that kifunensine-mediated inhibition of oligosaccharide maturation does not impair γ-secretase activity. Indeed, compared with the heterogeneous pattern of endogenous NCT species in HEK293 cells, we confirmed that ∼115- to 120-kDa immature NCT (imNCT) glycoforms accumulated in cells treated with kifunensine (Fig. S1A; compare lanes 3 and 4 with 5 and 6, respectively). In this case, the low levels of mature NCT (mNCT) in kifunensine-treated cells represent long-lived NCT species that were preexisting before drug treatment.
Fig. 1.
Analysis of deglycosylated NCT fragments. (A) HEK293 cells transiently transfected with either empty vector (lanes 1 and 2) or APPswe cDNA (lanes 3–6) were treated with either vehicle DMSO (lanes 1–4) or 1 μg/mL of kifunensine (kif). APP FL, CTF, and Aβ panels represent metabolites from either endogenous full-length APP (lanes 1 and 2) or overexpressed full-length APPswe (lanes 3–6). Duplicates are shown for each condition. (B) Analysis of Notch processing in naïve HEK293S cells (WT) or HEK293S GnTI− cells. Cells were transiently transfected with mouse NΔE cDNA, and full-length NΔE and NICD fragments are shown. (C) Analysis of ECD and 716 expressed in HEK293 cells. Western blots of lysates and medium of HEK293 cells stably expressing either ECD (lanes 1–3) or 716 (lanes 4–6) in the presence of vehicle DMSO, tunicamycin (Tuni), or kifunensine (kif). (D) Analysis of secreted ECD and 716 fragments from GnTI− cells in the presence or absence of Endo H (lane 2) or PNGaseF (lane 3). (E) Coomassie Blue staining of purified ECD and 716 fragments and circular dichroism spectroscopy of ECD and 716 from GnTI− cells or 716 from kifunensine-treated cell medium.
We also examined γ-secretase activity in HEK293S cells that lack N-acetyl glucosaminyl transferase I (GnTI− cells) (19), wherein we expected that NCT would harbor even simpler mannose side chains. To test γ-secretase activity in these cells, we transiently transfected HEK293S GnTI− cells with cDNA encoding a well-established γ-secretase substrate, termed mouse Notch 1 lacking the ECD segment (mNΔE) (20). The γ-secretase–dependent generation of the Notch intracellular domain (NICD) in this cell line was as efficient as in naïve HEK293S cells (Fig. 1B), suggesting that γ-secretase activity is unimpaired in cells lacking GnTI activity. We confirmed that compared with the heterogeneous pattern of endogenous NCT-related polypeptides in HEK293S cells, ∼110-kDa imNCT species accumulated in GnTI− cells (Fig. S1B, lanes 3 and 6, respectively). In the case of GnTI− cells, imNCT was sensitive to digestion with both Endo H and Peptide: N-Glycosidase F (PNGaseF) to generate a fully deglycosylated ∼80-kDa NCT species (Fig. S1B, lanes 4 and 5). Thus, two methods of reducing oligosaccharide maturation did not altered processing of APP or mNΔE to generate Aβ or NICD, respectively, suggesting that NCT lacking mixed oligossacharides does not impair γ-secretase activity. Thus, we believed that NCT fragments prepared from the medium of kifunensine-treated cells or GnTI− cells are compatible for studies of NCT function and structure.
Expression and Analysis of NCT Ectodomain Fragments.
In view of studies indicating that the substrate recognition function of NCT lies within the ECD segment that includes a highly conserved DAP domain (7), we chose to use water-soluble forms of the ECD for antibody generation. We stably transfected HEK293 cells with cDNA encoding full-length NCT ECD (termed “ECD”; 669 aa) and a fragment encoded by exons 7–16 of the NCT gene (termed “716”; amino acids 245–669), each fused to a C-terminal His-tag (Fig. 2A). Both ECD and 716 fragments were secreted into the conditioned medium (Fig. 1C, medium, lanes 1 and 4, respectively), and kifunensine treatment reduced the apparent molecular weights of these polypeptides (Fig. 1C, medium, lanes 3 and 6, respectively). In contrast, cells treated with tunicamycin, an inhibitor of N-linked oligosaccharide modification, fully inhibited secretion of ECD and 716 (Fig. 1C, lanes 2 and 5, respectively), suggesting that core glycosylation is essential for folding and/or secretion of these polypeptides. Stable expression of cDNAs encoding the ECD and 716 fragments in GnTI− cells lead to secretion of ECD (Fig. 1D, Upper) and 716 (Fig. 1D, Lower) fragments that were sensitive to digestion with both Endo H and PNGaseF (Fig. 1D, lanes 2 and 3), indicating that the secreted NCT fragments from these cells do not contain hybrid or complex polysaccharide chains. Purified ECD and 716 polypeptides (Fig. 1E, Left) exhibited similar circular dichroism spectra (Fig. 1E, Right), but the 716 fragment had larger negative mean ellipticity than ECD, suggesting that 716 contained more ordered structure per mass, which in turn suggests that the segment encoded by exons 1–6 of the NCT gene (amino acids 1–244) is not highly ordered.
Fig. 2.
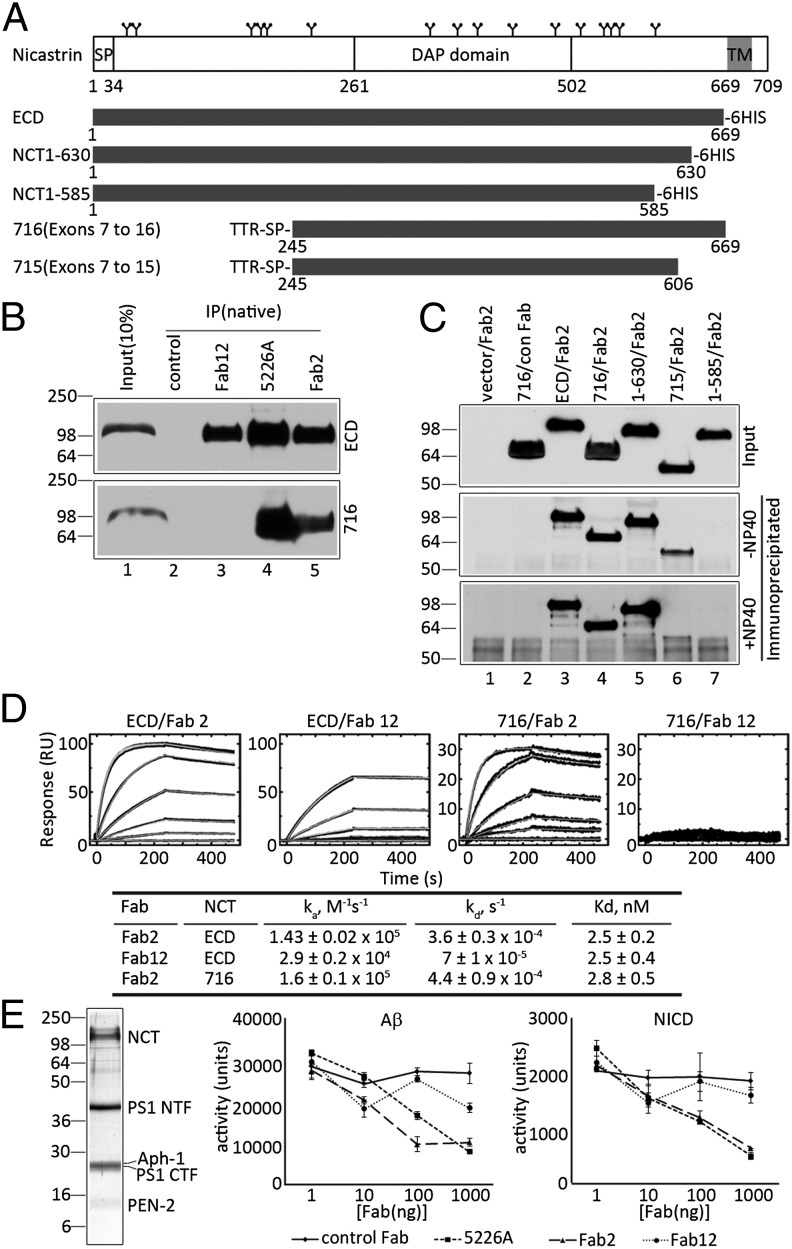
Potent NCT-specific Fab2 inhibits γ-secretase activity in vitro. (A) Schematic illustration of full-length human NCT and truncated NCT variants. SP, signal peptide; TM, transmembrane; TTR, transthyretin; 6HIS, His tag. (B) Immunoprecipitation of ECD (Upper) or 716 (Lower) from HEK293 cells with Fab12 (lanes 3), Mab5226A (lanes 4), or Fab2 (lanes 5). (C) Fab2 immunoprecipitation of secreted NCT fragments in medium of GnTI− cells (Top) in the absence (Middle) or presence (Bottom) of 0.1% Nonidet P-40. “con Fab” is a negative control Fab raised gainst an unrelated target. (D) SPR sensorgrams of Fab2 (ECD/Fab2 and 716/Fab2) or Fab12 (ECD/Fab12 and 716/Fab12) binding to immobilized ECD (Left and Center Left) or 716 (Right and Center Right); kinetic values are shown in the table. (E) Effects of Fabs on γ-secretase activity. Purified γ-secretase from HEK293S TAP-ANPP cells (Left). In vitro γ-secretase activity assays using purified γ-secretase preincubated with different Fabs; production of Aβ (Center) and NICD (Right) were monitored by ELISA. Data were represented as mean ± SEM, n = 3.
Generation of Synthetic Antibodies Directed to NCT ECD.
Applying phage-display technologies (15) to PNGaseF-treated ECD, we isolated two Fabs, termed Fab2 and Fab12. We also isolated a third Fab, Fab2-2 in the initial screen, but subsequent tests showed that it failed to bind to ECD. Thus, this antibody was used as a negative control for cell-surface labeling studies (see below; Fig. S2C). We then tested the ability of Fab2 and Fab12 to recognize the native form of NCT that is present in the γ-secretase complex. Membrane fractions were freshly prepared from HEK293S cells that stably overexpress cDNAs encoding APH-1as, NCT, PEN-2, and PS1 harboring an N-terminal Tandem Affinity Purification (TAP) tag [TAP-ANPP cells (21)]. Both Fab2 and Fab12 immunoprecipitated both mature and immature forms of full-length NCT (Fig. S2A, lanes 3 and 4), whereas an irrelevant Fab raised against an unrelated target did not (Fig. S2A, lane 2). We then asked whether Fab2 and Fab12 could detect native NCT on the plasma membrane. Because of the low levels of NCT on the plasma membrane of naïve 293 cells, we chose to use HEK293 cells that stably overexpress cDNAs encoding human Aph-1as, NCT, PS1, and PEN-2 [ANPP.8 cells (22)]. We show that when overexpressed, NCT and other components of the γ-secretase complex escape the normal endoplasmic reticulum (ER) and Golgi localization, leading to redistribution and accumulation on the cell surface (Fig. S2B), that we would suggest is the result of saturating Rer1p [Retrieval to ER 1 protein (23)] that is known to associate with NCT and APH-1 and regulates γ-secretase complex formation and trafficking (24). Using ANPP.8 cells, we show that both Fab2 and Fab12 bind to the surface of these cells, whereas the nonspecific Fab2-2 (see above) does not (Fig. S1C). Together, these results indicate that although Fab2 and Fab12 were generated using a deglycosylated form of NCT ECD, they both recognize and can immunoprecipitate fully glycosylated, full-length NCT from detergent cell extracts, as well as NCT in the γ-secretase complex.
To map the epitopes of the two Fabs, we performed immunoprecipitation experiments under native conditions using ECD and 716 purified from the conditioned medium of stable GnTI− cells. Both Fab12 and Fab2 immunoprecipitated ECD (Fig. 2B, Upper, lanes 3 and 5, respectively). In contrast, whereas Fab2 immunoprecipitated 716, Fab12 failed to do so (Fig. 2B, Lower, lanes 5 and 3, respectively), strongly suggesting that the Fab12 epitope is within a region encoded by exons 1–6 that is absent in the 716 fragment.
We extended this analysis to determine the boundaries of the Fab2 epitope. We transfected cDNAs encoding additional NCT fragments (Fig. 2A) and performed immunoprecipitation analysis (Fig. 2C). Fab2 immunoprecipitated ECD, 716, and NCT 1–630, but not NCT 1–585 (Fig. 2C, lanes 3–5 and 7, respectively). However, although Fab2 bound 715, corresponding to NCT amino acids 245–606 that are encoded by exons 7–15 (Fig. 2C, lane 6, −Nonidet P-40), the binding was abolished by the addition of 0.1% Nonidet P-40 (Fig. 2C, lane 6, +Nonidet P-40). These data strongly suggest that Fab2 binding requires a domain contained between amino acids 245 and 630.
We then characterized the interaction of Fab2 and Fab12 with NCT using surface plasmon resonance spectroscopy (SPR). These two Fabs bound to ECD with a single nanomolar dissociation constant (Fig. 2D). That Fab2 bound to ECD and 716 with the same affinity (Fig. 2D, ECD/Fab2 and 716/Fab2) indicates that the Fab2 epitope is fully contained within the 716 fragment. In contrast, binding of Fab12 to 716 was undetectable (Fig. 2D, 716/Fab12). Furthermore, the two Fabs can simultaneously bind to ECD, as demonstrated by serial binding experiments using SPR (Fig. S2D). These results, consistent with the immunoprecipitation studies described above (Fig. 2B), support the view that Fab2 and Fab12 bind to nonoverlapping epitopes.
A rationale for generating NCT-specific Fabs is to use them as molecular probes for delineating functional sites within the ECD that are critical for γ-secretase activity. To evaluate the effects of the Fabs on γ-secretase activity, we purified γ-secretase from TAP-ANPP cells (Fig. 2E) (21) and incubated this preparation with Fab2, Fab12, or a positive control, anti-NCT Mab5226A (13). The mixtures were then incubated with bacterially synthesized and purified APP or Notch substrates, and the production of Aβ40 or NICD, respectively, were monitored as readouts of γ-secretase activity (5, 25). Both Fab2 and Mab5226A caused a dosage-dependent inhibition of the processing of either APP or Notch (Fig. 2E, Center and Right), whereas Fab12 failed to do so. We interpret these results as indication that the 716 polypeptide includes a functional domain essential for γ-secretase activity and that the binding of Fab2 inhibits this function.
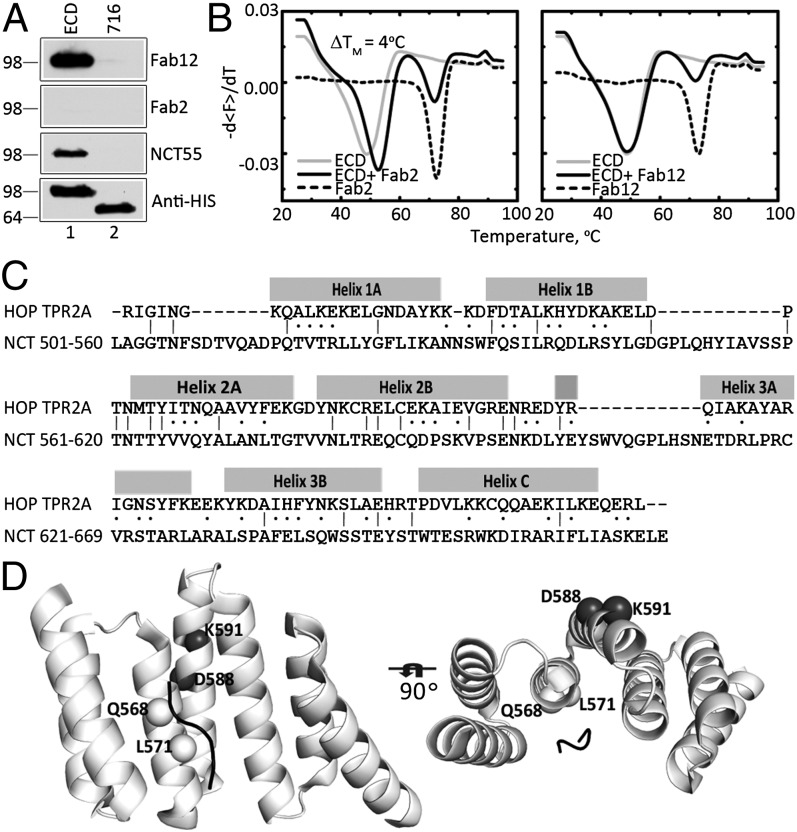
To further characterize the nature of the epitopes recognized by the two Fabs, we performed Western blotting studies under denaturing conditions. Fab12 detected ECD but not 716 (Fig. 3A, Fab12), suggesting that this Fab recognizes a linear epitope encoded by the first six exons. On the other hand, Fab2 failed to detect ECD or 716, suggesting that this Fab recognizes a conformational epitope (Fig. 3A, Fab2). To provide support for this conclusion, we used differential scanning fluorimetry (26). The binding of Fab2 to ECD resulted in a ΔTm of ∼4 °C, whereas the binding of Fab12 did not alter the ΔTm of ECD (Fig. 3B). These results further suggest that Fab2 binds selectively to the folded form of ECD, thereby stabilizing the polypeptide against thermal denaturation.
Fig. 3.
Sequence homology of a region of NCT ECD and TPR2A domain of HOP. (A) Western blot analysis of purified ECD and 716 probed with Fab12, Fab2, NCT55, and anti-6xHis antibodies. (B) Thermostability of ECD, Fab, and their complex was determined by differential scanning fluorimetry. (C) Alignment of NCT amino acid residues 501–669 to the TPR2A domain of HOP. The locations of helices of TPR2A domain are indicated above the alignment. (D) Homology modeling of TPR-like motif in ECD segment 501–669. Mutations in predicted α-helices in NCT 501–669 are labeled. The model is shown at two different angles to demonstrate the close proximity of L571 to the bound peptide (indicated as black line) in the model.
Identification of a TPR-Like Domain in NCT ECD.
The epitope mapping data described above (Fig. 2C) strongly suggested that Fab2 binding to ECD requires sequences between amino acids 245 and 630. Thus, we performed homology search in the Protein Data Bank against residues 501–669 of NCT, a segment between the DAP domain and the transmembrane region. The best hit was the TPR2A (tetratricopeptide repeat 2A) domain of human HOP (Hsp70/Hsp90 organizing protein) (27), a domain that binds to the C-terminal peptide of Hsp90 (27). Residues 501–669 of NCT and the TPR2A domain exhibited substantial sequence homology throughout the region (Fig. 3C), with higher homology observed for helices 2A and 2B of HOP TPR2A (Fig. 3C); helix 2A forms the center of the peptide-recognition interface of the TPR2A–peptide complex (27).
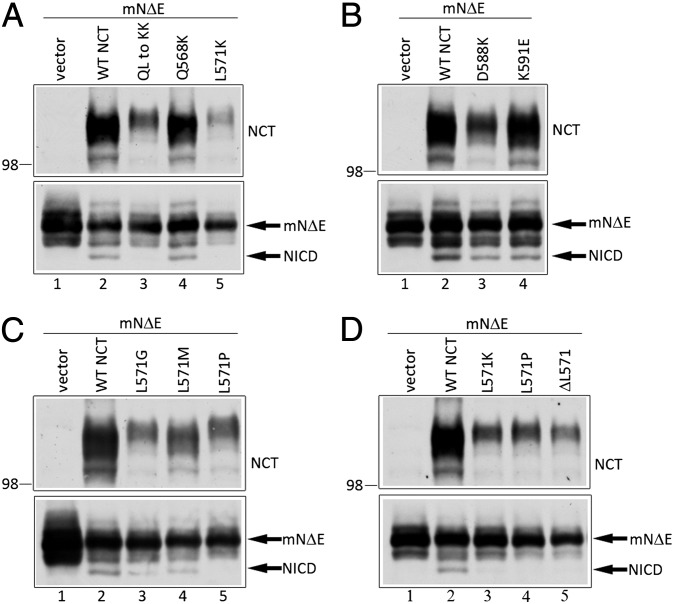
To validate our homology model of NCT 501–669, we performed mutagenesis experiments. The HOP TPR2A domain contains three TPR motifs, and each TPR motif is a helix-turn-helix structure. In the HOP TPR2A crystal structure, only the side chains of residues in the α-helices make direct contact with the peptide. In our model, residues 561–576 of NCT correspond to helix 2A, and Q568 and L571 are predicted to be in close contact with a bound peptide (Fig. 3C and D). Thus, if our model is accurate, mutations of these residues should affect γ-secretase function. Thus, we introduced the Q568K and L571K mutations singly or in combination in the context of full-length NCT and coexpressed these polypeptides with mNΔE in NCT−/− fibroblasts to assess γ-secretase activity. These mutations were designed to drastically alter electrostatic properties, and consequently intermolecular interactions, but minimally affect the helical propensity. As expected, mNΔE failed to be processed to NICD in NCT−/− cells (Fig. 4A, lane 1), but expression of WT NCT rescued γ-secretase activity (Fig. 4A, lane 2) (7). Although the expression of the Q568K NCT variant rescued γ-secretase activity (Fig. 4A, lane 4), neither the L571K nor Q568K/L571K NCT variants generated the NICD fragment (Fig. 4A, lanes 3 and 5). In addition, we generated two mutations at positions in helix 2B that are expected to be on the opposite side of the TPR domain from the predicted peptide-binding interface (Fig. 3D). These variants, D588K and K591E, efficiently rescued γ-secretase activity in NCT−/− cells (Fig. 4B, lanes 3 and 4). Together, these mutational analyses suggest the importance of L571 in NCT function.
Fig. 4.
The TPR-like motif and L571 of NCT are important for γ-secretase activity. (A–D) NCT−/− cells were transiently cotransfected with mNΔE and cDNAs encoding NCT or various NCT mutants. Upper: Steady-state expression of NCT and NCT variants. Lower: Full-length mNΔE substrate and γ-secretase–generated NICD fragments. The identities of NCT variants are indicated.
To further validate our model, we also tested mutations that should perturb the structure of the predicted TPR domain. We expressed NCT harboring the L571P mutation, which is predicted to break the α-helix, and the L571G and L571M NCT variants as controls; although glycine is generally considered to be a helix-destabilizing amino acid, it is frequently found in highly conserved positions within helices of TPR motifs (16), whereas methionine has a high α-helix-forming propensity and similar size and hydrophobicity as leucine. The L571P variant failed to rescue γ-secretase activity in NCT−/− cells, but the L571G and L571M mutants did (Fig. 4C, compare lanes 3 and 4 with lane 5, respectively). Moreover, we show that NCT with a deletion of L571 (ΔL571), predicted to cause a reorganization of the helix and disrupt peptide interaction(s), failed to rescue γ-secretase activity (Fig. 4D, lane 5). Taken together, these mutagenesis studies support our homology model of NCT residues 501–669 and argue for an important role of L571 in the maintenance of molecular interactions that are necessary for γ-secretase activity.
Unfortunately, our attempts to express the NCT segment (residues 503–669) in mammalian cells and bacteria did not produce sufficient quantities of soluble material, making it impossible to characterize the segment in detail. These results also suggest that folding and/or stability of the TPR-like domain may require interactions with regions more amino-terminal to residue 503.
L571 NCT Variants Assemble into γ-Secretase Complexes but Fail to Associate with Substrates.
An unexpected observation that emerged from our analyses of the L571 NCT variants that failed to rescue γ-secretase activity in NCT−/− was that the mutant NCT polypeptides accumulated to lower steady-state levels compared with WT NCT (NCT panels in Fig. 4 A–C). Hence, it was conceivable that the levels of NICD generation may in fact be similar to that generated by WT NCT if the mutant NCT levels were normalized to WT NCT expression levels. To test this possibility, we performed a titration experiment in which we transfected mNΔE cDNA together with differing levels of WT and mutant NCT cDNA and assayed both NICD generation and levels of mature NCT. Expression of the mutant NCT polypeptides at high doses, and at levels that mirrored those seen after expression of low levels of WT NCT, failed to generate NICD (Fig. S3A; compare lane 2 to lanes 9, 12, and 15). These results support our view that the reduction in γ-secretase activity exhibited by the NCT L571 variants is not the result of reduced steady-state levels of the mutant polypeptides.
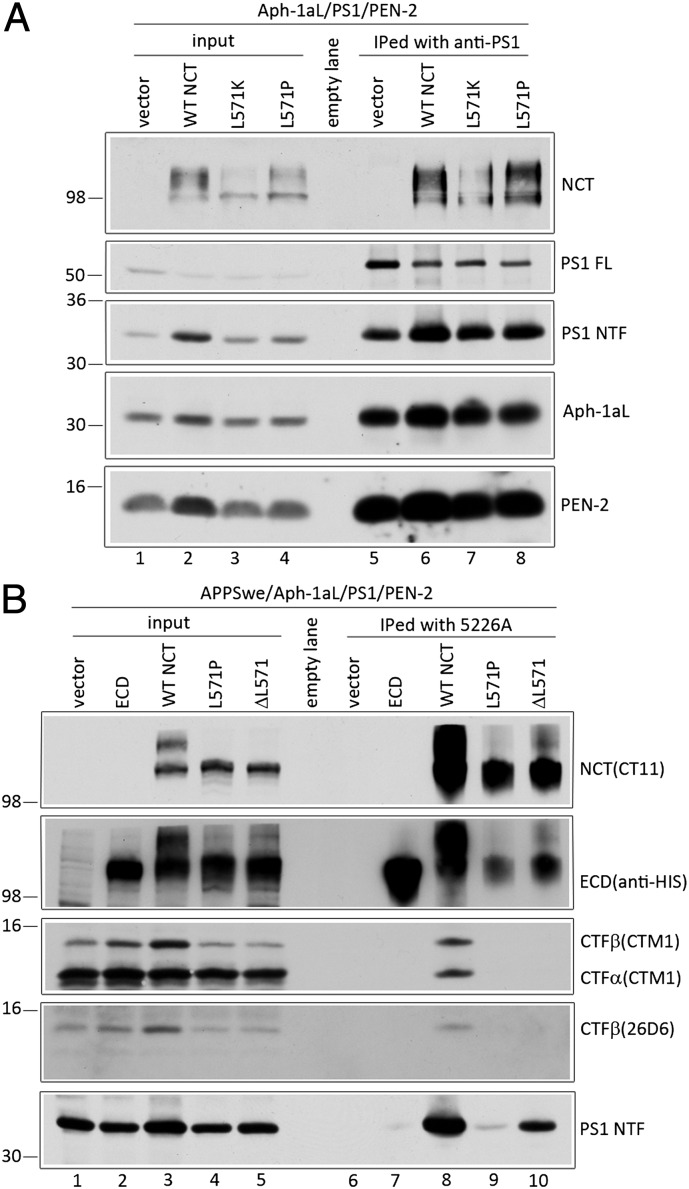
We entertained the notion that reduced steady-state levels of the NCT571 variants may be a reflection of failed assembly of the mutant polypeptides into the γ-secretase complex, leading to their degradation. To examine this possibility, we transiently cotransfected cDNA encoding human Aph-1aL, PS1, PEN-2, and either WT or NCT L571 variants into NCT−/− cells and assessed the oligosaccharide maturation of the NCT variants, a process that requires PS and is dependent on interactions with other components of the γ-secretase complex (28). Despite lower levels of accumulated NCT L571 variants, immature and mature forms of both WT and L571 mutant NCT accumulated at steady state (Fig. S3B, NCT panel). We also monitored the endoproteolytic maturation of PS1 in NCT−/− cells, wherein full-length PS1 normally accumulates to high levels (29). The expression of NCT L571 variants promoted the conversion of full-length PS1 into mature PS1 fragments at levels similar to that seen with WT NCT (Fig. S3B, PS1 panel; compare lanes 5 and 6 to lane 4, respectively). Finally, we tested the assembly of NCT L571 variants into γ-secretase complexes. Using anti-PS1 antibody for coimmunoprecipitation, we show that PS1 associated with WT NCT and the NCT L571 variants (Fig. 5A, lanes 6–8). Taken together, these findings lead us to conclude that the NCT L571 variants still assemble into the γ-secretase complex.
Fig. 5.
NCTs with the γ-secretase inactivating L571K or L571P mutations are recruited into the γ-secretase complex but fail to coimmunoprecipitate with APP CTFs. (A) NCT−/− cells were transiently cotransfected with Aph-1aL-Myc-His, PS1, PEN-2-CT11, and either WT or L571 mutant NCT cDNAs, and cell lysates were immunoprecipitated with anti-PS1 antibody. (B) NCT−/− cells were transiently cotransfected with APPswe, Aph-1aL-Myc-His, PS1, PEN2-CT11, and either WT, ECD, or L571 mutant NCT cDNAs. Anti-NCT Mab5226A was used to immunoprecipitate NCT and associated polypeptides. Immunoprecipitated NCT (NCT and ECD), APP CTFs (CTM1 and 26D6) and PS1 NTF are shown. Note that anti-His binding to full-length NCT in ECD panels is a residual signal from a reprobing of the CT11 immunoblot.
Despite being assembled with components of the γ-secretase complex, the L571P and L571K variants fail to rescue γ-secretase activity, suggesting that the L571 variants cannot associate with the substrate. To address this possibility, we coexpressed APPswe together with PS1, Aph-1aL, PEN-2, and either WT NCT or the NCT L571 mutants into NCT−/− cells and treated cells with the γ-secretase inhibitor l-685,458 and a membrane-permeable cross-linking agent, DSP. Immunoprecipitation studies with Mab5226A (13) revealed that WT NCT associates with APP-CTFs, but the L571P and ΔL571 NCT variants do not (Fig. 5B, lanes 8–10, CTF). Importantly, the Mab5226A antibody coimmunoprecipitated PS1 NTF from extracts of NCT−/− cells expressing WT NCT or the L571K and L571P mutants, albeit to lower levels in the latter instances (Fig. 5B, lanes 8–10, PS1 NTF), arguing that mutant NCTs that fail to promote γ-secretase processing still associate with the catalytic component of the complex. Collectively, these results strongly suggest that the newly identified TPR-like domain is directly involved in the recognition of γ-secretase substrates.
Discussion
The γ-secretase complex, composed of PS, PEN-2, Aph-1, and NCT, catalyzes intramembranous proteolysis of a number of membrane-tethered substrates, including APP and Notch. Although a consensus has emerged regarding the role of Aph-1, PS, and PEN-2 in assembly, catalysis, and activation, respectively, the function of NCT in the γ-secretase complex is less clear. Early studies demonstrated that the NCT ECD recognizes membrane-tethered substrates and delivers these species to the active site of the γ-secretase complex (7). A region of the NCT ECD, termed the DAP domain, and an essential glutamate (E333) within this domain was shown to be critical for the substrate binding, presumably via a salt-bridge interaction to the exposed amino terminus of the substrate (7). This model was challenged by the demonstration that expression of NCT harboring an E333A mutation in NCT−/− cells leads to inefficient oligosaccharide maturation of the NCT variant and reduced levels of mature γ-secretase complexes, but that the specific activity of the remaining complexes was no different from that of complexes containing WT NCT (11). However, the methods used to calculate specific activity in the latter report had several significant technical limitations, and a subsequent report by Dries et al. (10) established that complexes expressed in Sf9 cells that contained the E333A NCT were indeed inactive. These conflicting findings and the demonstration that mNΔE could be processed in NCT−/− cells, a reaction that was blocked with a γ-secretase inhibitor, led to the conclusion that NCT was dispensable for γ-secretase activity (12). On the other hand, the demonstration that an NCT ECD monoclonal antibody could inhibit γ-secretase activity in in vitro and in vivo settings by competing with substrate binding strongly argued for an essential role for NCT in substrate recognition (13, 14).
In view of the uncertainties regarding NCT function, we focused on better defining NCT architecture that may be functionally relevant. To this end, we exploited a phage display strategy to identify synthetic antibodies that detects conformational epitopes of the NCT ECD and also perturbs γ-secretase activity. A synthetic antibody, Fab2, satisfies these criteria, and it has enabled us to gain several insights regarding the functional role of the region that it recognizes. First, we demonstrate that Fab2 binds to a structured region of the ECD. Second, Fab2 detects native NCT that is present in the γ-secretase complex and inhibits γ-secretase–mediated processing of both APP and Notch substrates. Hence, we would argue that the NCT segment recognized by Fab2 is functionally important for enzymatic activity. Third, a database search revealed a previously unrecognized domain within the Fab2-binding region that is homologous to a TPR domain. On the basis of a homology model, we identified a critical residue (L571) within the predicted TPR-like domain that is essential for γ-secretase activity. Fourth, we demonstrate that compromised γ-secretase activity of the L571 variant is not the result of impairments in PS1 endoproteolysis or association with components of the γ-secretase complex, but rather the result of failed association with the substrate.
In summary, we now provide unequivocal evidence for a highly structured domain of NCT that is essential for substrate binding, which is consistent with the model that NCT participates in substrate recruitment. It is our expectation that future efforts to determine the 3D structure of the NCT ECD will provide important information pertaining to the molecular basis for substrate recognition that will ultimately offer new opportunities for the development of novel compounds that selectively target the binding and processing of APP substrates, but spare recognition of Notch and other substrates essential for cellular and organ physiology. We would argue that synthetic antibodies will be important tools for further delineating the role of NCT in γ-secretase function and for accelerating structural studies as “crystallization chaperones” (30).
Methods
To examine γ-secretase activity, we transiently transfected NCT−/− fibroblasts with a mouse NΔE-6xmyc construct and either WT or mutant NCT constructs. ECD and 716 fragments were purified by ammonium sulfate fractionation, Ni-NTA affinity purification, and Superdex 200 column gel-filtration. Synthetic antibody generation, Fab expression, and purification were described previously (15, 31). In vitro γ-secretase activity assay was performed as previously reported (5, 25). Circular dichroism spectroscopy and differential scanning fluorimetry were performed according to previous reports (26, 32). For SPR analysis, His-tagged ECD or 716 was immobilized on a Ni-NTA chip, and Fabs were flowed according to methods provided by the manufacturer. Coimmunoprecipitation of APP C-terminal fragments with γ-secretase complex was performed with γ-secretase inhibitor l-685,458 and cross-linking agent DSP-treated NCT−/− fibroblasts, which were transiently cotransfected with cDNAs encoding Aph-1aL-Myc-His, PS1, and PEN-2-CT11 together with NCT or NCT variants. An extended version of methods is provided in SI Methods.
Supplementary Material
Acknowledgments
We thank A. A. Kossiakoff for discussion and S. S. Sidhu for the antibody library. This work was supported by The Adler Foundation, Edward H. Levy Fund, Alzheimer’s Association, American Health Assistance Foundation, and Cure Alzheimer’s Fund (S.S.S.); National Institutes of Health (NIH) Grants U54-GM087519 and R01-GM072688 and the University of Chicago Comprehensive Cancer Center (S.K.); and NIH Grants P30 NS061777 and S10 RR022415 (to R.W.).
Footnotes
The authors declare no conflict of interest. S.S.S. is a paid consultant of Nociris Inc. and Eisai Research Labs Inc. but is not a shareholder in any company that is a maker or owner of a US Food and Drug Administration-regulated drug or device.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202691109/-/DCSupplemental.
References
- 1.Price DL, Sisodia SS. Mutant genes in familial Alzheimer’s disease and transgenic models. Annu Rev Neurosci. 1998;21:479–505. doi: 10.1146/annurev.neuro.21.1.479. [DOI] [PubMed] [Google Scholar]
- 2.Cole SL, Vassar R. The role of amyloid precursor protein processing by BACE1, the beta-secretase, in Alzheimer disease pathophysiology. J Biol Chem. 2008;283:29621–29625. doi: 10.1074/jbc.R800015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tolia A, De Strooper B. Structure and function of gamma-secretase. Semin Cell Dev Biol. 2009;20:211–218. doi: 10.1016/j.semcdb.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Edbauer D, et al. Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003;5:486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- 5.Ahn K, et al. Activation and intrinsic gamma-secretase activity of presenilin 1. Proc Natl Acad Sci USA. 2010;107:21435–21440. doi: 10.1073/pnas.1013246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goutte C, Tsunozaki M, Hale VA, Priess JR. APH-1 is a multipass membrane protein essential for the Notch signaling pathway in Caenorhabditis elegans embryos. Proc Natl Acad Sci USA. 2002;99:775–779. doi: 10.1073/pnas.022523499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah S, et al. Nicastrin functions as a gamma-secretase-substrate receptor. Cell. 2005;122:435–447. doi: 10.1016/j.cell.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Takasugi N, et al. The role of presenilin cofactors in the gamma-secretase complex. Nature. 2003;422:438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- 9.Yu G, et al. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and betaAPP processing. Nature. 2000;407:48–54. doi: 10.1038/35024009. [DOI] [PubMed] [Google Scholar]
- 10.Dries DR, et al. Glu-333 of nicastrin directly participates in gamma-secretase activity. J Biol Chem. 2009;284:29714–29724. doi: 10.1074/jbc.M109.038737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chávez-Gutiérrez L, et al. Glu(332) in the Nicastrin ectodomain is essential for gamma-secretase complex maturation but not for its activity. J Biol Chem. 2008;283:20096–20105. doi: 10.1074/jbc.M803040200. [DOI] [PubMed] [Google Scholar]
- 12.Zhao G, Liu Z, Ilagan MX, Kopan R. Gamma-secretase composed of PS1/Pen2/Aph1a can cleave notch and amyloid precursor protein in the absence of nicastrin. J Neurosci. 2010;30:1648–1656. doi: 10.1523/JNEUROSCI.3826-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi I, et al. Neutralization of the γ-secretase activity by monoclonal antibody against extracellular domain of nicastrin. Oncogene. 2012;31:787–798. doi: 10.1038/onc.2011.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi I, et al. Single chain variable fragment against nicastrin inhibits the gamma-secretase activity. J Biol Chem. 2009;284:27838–27847. doi: 10.1074/jbc.M109.055061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fellouse FA, et al. High-throughput generation of synthetic antibodies from highly functional minimalist phage-displayed libraries. J Mol Biol. 2007;373:924–940. doi: 10.1016/j.jmb.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 16.D’Andrea LD, Regan L. TPR proteins: The versatile helix. Trends Biochem Sci. 2003;28:655–662. doi: 10.1016/j.tibs.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Shirotani K, et al. Gamma-secretase activity is associated with a conformational change of nicastrin. J Biol Chem. 2003;278:16474–16477. doi: 10.1074/jbc.C300095200. [DOI] [PubMed] [Google Scholar]
- 18.Herreman A, et al. gamma-Secretase activity requires the presenilin-dependent trafficking of nicastrin through the Golgi apparatus but not its complex glycosylation. J Cell Sci. 2003;116:1127–1136. doi: 10.1242/jcs.00292. [DOI] [PubMed] [Google Scholar]
- 19.Reeves PJ, Callewaert N, Contreras R, Khorana HG. Structure and function in rhodopsin: High-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc Natl Acad Sci USA. 2002;99:13419–13424. doi: 10.1073/pnas.212519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 21.Kounnas MZ, et al. Modulation of gamma-secretase reduces beta-amyloid deposition in a transgenic mouse model of Alzheimer’s disease. Neuron. 2010;67:769–780. doi: 10.1016/j.neuron.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SH, Ikeuchi T, Yu C, Sisodia SS. Regulated hyperaccumulation of presenilin-1 and the “gamma-secretase” complex. Evidence for differential intramembranous processing of transmembrane subatrates. J Biol Chem. 2003;278:33992–34002. doi: 10.1074/jbc.M305834200. [DOI] [PubMed] [Google Scholar]
- 23.Füllekrug J, et al. Human Rer1 is localized to the Golgi apparatus and complements the deletion of the homologous Rer1 protein of Saccharomyces cerevisiae. Eur J Cell Biol. 1997;74:31–40. [PubMed] [Google Scholar]
- 24.Spasic D, et al. Rer1p competes with APH-1 for binding to nicastrin and regulates gamma-secretase complex assembly in the early secretory pathway. J Cell Biol. 2007;176:629–640. doi: 10.1083/jcb.200609180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Placanica L, Chien JW, Li YM. Characterization of an atypical gamma-secretase complex from hematopoietic origin. Biochemistry. 2010;49:2796–2804. doi: 10.1021/bi901388t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niesen FH, Berglund H, Vedadi M. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat Protoc. 2007;2:2212–2221. doi: 10.1038/nprot.2007.321. [DOI] [PubMed] [Google Scholar]
- 27.Scheufler C, et al. Structure of TPR domain-peptide complexes: Critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell. 2000;101:199–210. doi: 10.1016/S0092-8674(00)80830-2. [DOI] [PubMed] [Google Scholar]
- 28.Edbauer D, Winkler E, Haass C, Steiner H. Presenilin and nicastrin regulate each other and determine amyloid beta-peptide production via complex formation. Proc Natl Acad Sci USA. 2002;99:8666–8671. doi: 10.1073/pnas.132277899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang YW, et al. Nicastrin is critical for stability and trafficking but not association of other presenilin/gamma-secretase components. J Biol Chem. 2005;280:17020–17026. doi: 10.1074/jbc.M409467200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koide S. Engineering of recombinant crystallization chaperones. Curr Opin Struct Biol. 2009;19:449–457. doi: 10.1016/j.sbi.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rizk SS, et al. Allosteric control of ligand-binding affinity using engineered conformation-specific effector proteins. Nat Struct Mol Biol. 2011;18:437–442. doi: 10.1038/nsmb.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly SM, Jess TJ, Price NC. How to study proteins by circular dichroism. Biochim Biophys Acta. 2005;1751:119–139. doi: 10.1016/j.bbapap.2005.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.