Abstract
The mechanisms underlying tumor dormancy have been elusive and not well characterized. We recently published an experimental model for the study of human tumor dormancy and the role of angiogenesis, and reported that the angiogenic switch was preceded by a local increase in VEGF-A and basic fibroblast growth factor. In this breast cancer xenograft model (MDA-MB-436 cells), analysis of differentially expressed genes revealed that heat shock protein 27 (HSP27) was significantly up-regulated in angiogenic cells compared with nonangiogenic cells. The effect of HSP27 down-regulation was further evaluated in cell lines, mouse models, and clinical datasets of human patients with breast cancer and melanoma. Stable down-regulation of HSP27 in angiogenic tumor cells was followed by long-term tumor dormancy in vivo. Strikingly, only 4 of 30 HSP27 knockdown xenograft tumors initiated rapid growth after day 70, in correlation with a regain of HSP27 protein expression. Significantly, no tumors escaped from dormancy without HSP27 expression. Down-regulation of HSP27 was associated with reduced endothelial cell proliferation and decreased secretion of VEGF-A, VEGF-C, and basic fibroblast growth factor. Conversely, overexpression of HSP27 in nonangiogenic cells resulted in expansive tumor growth in vivo. By clinical validation, strong HSP27 protein expression was associated with markers of aggressive tumors and decreased survival in patients with breast cancer and melanoma. An HSP27-associated gene expression signature was related to molecular subgroups and survival in breast cancer. Our findings suggest a role for HSP27 in the balance between tumor dormancy and tumor progression, mediated by tumor–vascular interactions. Targeting HSP27 might offer a useful strategy in cancer treatment.
Keywords: NFκB, STAT3
More than one-third of the world’s population is diagnosed with cancer (invasive or in situ) during their lifetime (http://seer.cancer.gov/). Somewhat surprisingly, autopsy studies have demonstrated a higher prevalence (>90%) of microscopic cancer (1). These findings suggest that small tumors can remain dormant without progression into detectable disease (2, 3). Primary cancers or metastases usually present clinically after they become angiogenic and expand in mass (4, 5). We previously reported an experimental model for the study of human tumor dormancy and the critical role of angiogenesis (or lack thereof) in maintaining this disease state (6). Specifically, we reported that angiogenic and nonangiogenic populations isolated from human breast cancer cells were characterized by their ability to induce angiogenesis and tumor growth in SCID mice or to remain as microscopic (dormant) but actively proliferating tumors. Here we report that heat shock protein 27 (HSP27) is highly up-regulated in angiogenic breast cancer cells, and provide evidence that HSP27 plays a key role in the balance between tumor dormancy and expansive tumor growth associated with the onset of angiogenesis.
Heat shock proteins are constitutively expressed at low levels in all cells under physiological conditions (7). Their expression is rapidly induced by various stress factors, including heat, hypoxia, cytotoxic drugs, and radiation (7). HSP27 is a small heat shock protein with functions not limited to thermotolerance. Previous reports have suggested a role of HSP27 upstream of VEGF-A through HIF-1α, STAT3, and NFκB transcription factors (8–12). Furthermore, phosphorylated HSP27 has been shown to bind basic fibroblast growth factor (bFGF) and facilitate its transport over the plasma membrane (13, 14).
Studies indicate that HSP27 is also a downstream target of VEGF-A through VEGFR2 and the p38 signaling pathways (15). Here we present evidence that HSP27 is a key regulator of tumor dormancy, and that suppression of HSP27 induces long-term dormancy in a model of human breast cancer. Further, we demonstrate that the angiogenic switch is influenced by HSP27-dependent changes in VEGF-A, VEGF-C, and bFGF secretion from tumor cells. Expression analyses of dormant and angiogenic cells uncovered HSP27-regulated genes and a signature that predicts poor survival. Targeting this multifunctional cytoprotective protein might be a useful strategy in cancer treatment.
Results
HSP27 Is Overexpressed in Angiogenic Human Breast Cancer Cells Compared with Nonangiogenic Human Breast Cancer Cells (MDA-MB-436).
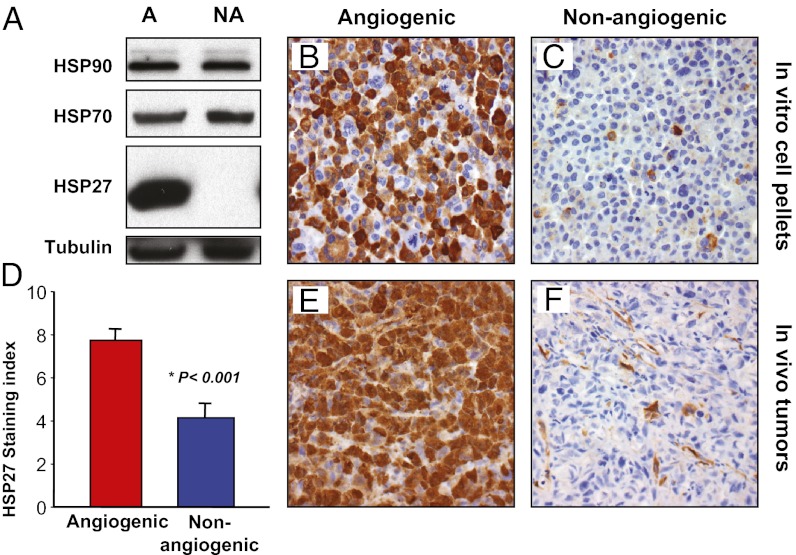
We previously characterized angiogenic (MDA-MB-436-A) and nonangiogenic (MDA-MB-436-NA) variants of human breast cancer cell line MDA-MB-436 (6). Subsequently, we compared gene expression profiles of angiogenic and nonangiogenic cells in vitro, and found that HSP27 (HSPB1) was the most overexpressed gene (by 33-fold) in angiogenic cells (Table S1). Western blot analysis revealed that angiogenic cells expressed higher levels of HSP27 protein in vitro compared with nonangiogenic cells, which expressed undetectable levels (Fig. 1A). Two other cancer-related heat shock proteins (HSP70 and HSP90) demonstrated no clear difference in expression. Immunohistochemistry of cell pellets revealed that >90% of angiogenic tumor cells were HSP27-positive, whereas <5% of nonangiogenic cells stained slightly positive (Fig. 1 B and C).
Fig. 1.
HSP27 is overexpressed in angiogenic human breast cancer cells compared with nonangiogenic human breast cancer cells. Differences in HSP27 expression between the angiogenic and nonangiogenic variants of the MDA-MB-436 cell line were validated. (A) Western blot analysis of HSP90, HSP70, and HSP27 protein expression in angiogenic (A) and nonangiogenic (NA) variants of the human breast cancer cell line. (B and C) Immunohistochemical staining for HSP27 protein in cell pellets containing angiogenic (B) or nonangiogenic (C) cells, grown under in vitro conditions. (D–F) Immunohistochemical staining of HSP27 protein in tumor xenografts was quantified using a staining index (D) and was significantly increased in angiogenic tumors (E) compared with nonangiogenic tumors (F). The sample shown in F is negative for HSP27 expression in tumor cells, representing one extreme end of the expression spectrum. In F, note the strong expression of HSP27 protein in some tumor-associated stromal (including endothelial) cells.
To confirm the difference in HSP27 expression in vivo, we analyzed 17 angiogenic and nonangiogenic size-matched breast cancer xenograft tumors harvested at various time points by immunohistochemistry (Fig. 1 E and F). Angiogenic tumors expressed 1.9-fold higher levels than nonangiogenic tumors (mean staining index [SI]: angiogenic, 7.8 ± 0.5; nonangiogenic, 4.2 ± 0.6; P < 0.001, t test) (Fig. 1D). These findings support the microarray data and indicate that HSP27 is overexpressed in angiogenic breast cancer cells compared with nonangiogenic breast cancer cells both in vitro and in vivo. In stromal cells, HSP27 protein expression did not differ significantly in HSP27KD-3 tumors and nontargeted (NT) control tumors (mean SI, 2.0 vs. 1.5).
Stable Down-Regulation of HSP27 in Angiogenic Human Breast Cancer Cells Induces Long-Term Dormancy in Vivo.
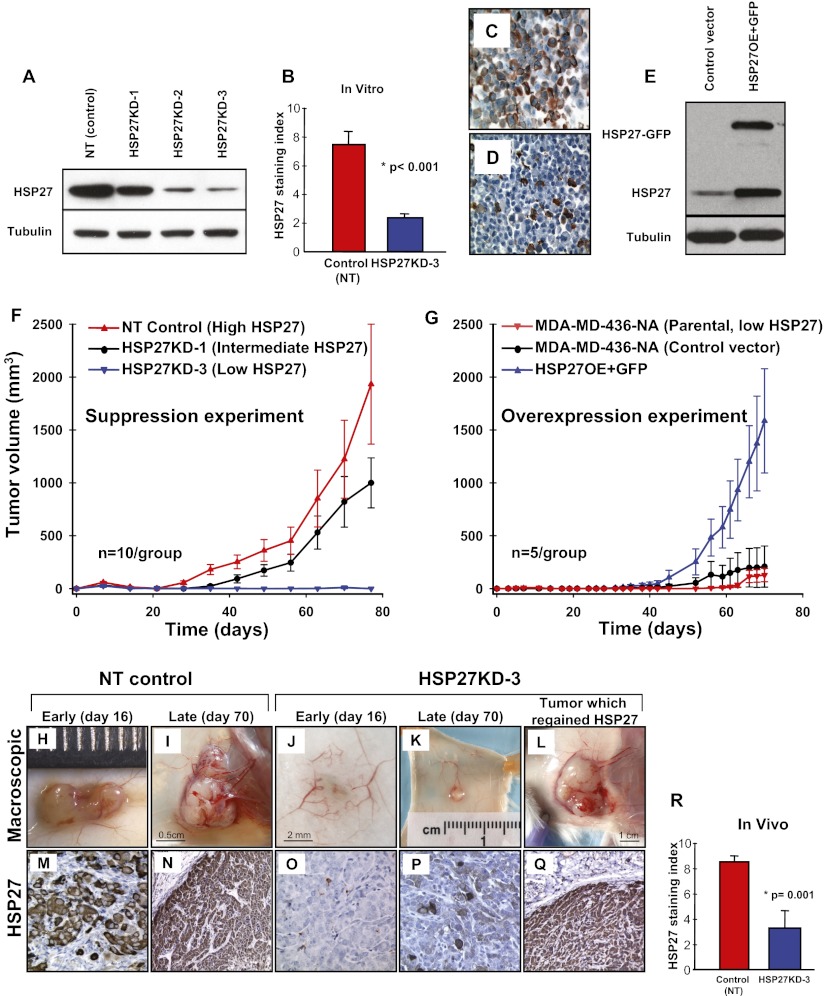
We hypothesized that if HSP27 was regulating the angiogenic potential of these breast cancer cells, then the angiogenic phenotype could be reverted to a nonangiogenic or dormant phenotype by down-regulation of HSP27. We further postulated that reverted, nonangiogenic tumors would be microscopic in size (16). MDA-MB-436-A cells were transduced with lentiviruses encoding three different shRNAs against HSP27 (HSP27KD-1, HSP27KD-2, and HSP27KD-3). Compared with the original angiogenic (MDA-MB-436-A) cell line, expression of HSP27 protein was 20% lower in HSP27KD-1 cells, 70% lower in HSP27KD-2 cells, 84% lower in HSP27KD-3 cells, and similar in NT control cells (Fig. 2A). NT control cells expressed threefold higher HSP27 levels compared with HSP27KD-3 cells (mean SI, 7.5 ± 0.9 vs. 2.4 ± 0.2; P < 0.001, χ2 test) as assessed by immunohistochemical analysis of cell pellets (Fig. 2 B–D).
Fig. 2.
Stable down-regulation of HSP27 in angiogenic cancer cells induces long-term dormancy in vivo, and overexpression of HSP27 in nonangiogenic cells induces expansive tumor growth in vivo. Five pools of the HSP27-expressing angiogenic breast cancer cell line transduced with different shRNA sequences against HSP27—HSP27KD-1, KD-2 and KD-3—were generated. (A) Their HSP27 protein expression was confirmed by Western blot and compared with the NT control. (B–D) Down-regulation of HSP27 protein expression in HSP27KD-3 cells was quantified by immunohistochemistry and compared with that in NT control cells using in vitro cell pellets (B). NT control cells are shown in C; HSP27KD-3 cells, in D. (E) HSP27 tagged with GFP was overexpressed (HSP27OE+GFP) in the parental nonangiogenic MDA-MB-436-NA (with intrinsically low HSP27 (Fig. 1A) cells and confirmed by Western blot analysis. (F) In vivo s.c. xenograft growth curves of HSP27KD-3, HSP27KD-1, and the NT control (estimated as mean ± SE tumor volume). (G) In vivo s.c. xenograft growth curves of HSP27OE+GFP, the control vector, and the parental unaltered nonangiogenic cells (estimated as mean ± SE tumor volume). (H and I) The NT control tumors showed vivid neo-angiogenesis within and around the growing tumors. (J and K) In contrast, unaffected normal-appearing s.c. vessels surround a small HSP27KD-3 tumor (J), whereas early angiogenic activity can be observed in a late (day 70) HSP27KD-3 tumor (K). (M–P) HSP27 protein staining was consistently high in the NT control tumors (M and N) but was low or absent in microscopic dormant tumors formed by the HSP27KD-3 cells (O and P). (L and Q) A mouse inoculated with HSP27KD-3 cells spontaneously initiated tumor growth (L), and was confirmed by immunohistochemistry to have regained HSP27 protein expression (Q). (R) The mean HSP27 staining index was 2.6-fold higher for the NT control tumors than for tumors formed by the HSP27KD-3 cells in vivo. (Original magnifications: 400× in C, D, M, O, and P; 200× in N and Q.) The Student t test was used to assess the statistical significance of differences (*).
To determine the effect of HSP27 suppression on tumor growth, SCID mice (n = 10 per group) were inoculated s.c. with NT control or HSP27KD cells. The mice were killed at early (16 d) or late (70 d) time points for microscopic evaluation; four mice per group remained by the end of the experiment. Whereas the control cells initiated exponential tumor growth at day 20 and reached a mean size of roughly 2,000 mm3 by day 77 (mean, 1,932 ± 1,132 mm3; n = 4) (Fig. 2 F, H, and I), the mice inoculated with HSP27KD-3 cells formed microscopic, nonpalpable tumors (<40 mm3) and persisted up to day 77 without switching to the angiogenic phenotype and with no evidence of exponential growth (Fig. 2 F, J, and K). Mice injected with HSP27KD-1 cells initiated tumor growth at around 10 d after the control group and demonstrated ∼50% inhibition of tumor growth by day 77 (SI Results).
We carried out a second, independent experiment with a larger number of mice (n = 20 per group), which confirmed our original findings (Fig. S1A). In this experiment, 17 of the 20 mice inoculated with HSP27KD-3 cells had nonpalpable and microscopic tumors for up to 112 d (Fig. S1B). Three HSP27KD-3 mice had spontaneously initiated tumor growth, at days 70, 90, and 112. In addition, one HSP27KD-3 mouse initiated tumor growth at day 77 in experiment 1 (Fig. 2 E, J, and O). Collectively, these four tumors all spontaneously regained expression of HSP27 during the in vivo experiment, to levels comparable to those seen in control cells (Fig. 2Q). These results support the hypothesis that HSP27 expression mediates the escape from tumor dormancy.
The in vivo stability of HSP27 knockdown in HSP27KD-3 cells was determined by immunohistochemistry of HSP27 protein in tumors from various time points. HSP27 expression was consistently strong in NT control tumors (Fig. 2 M and N) and low or absent in microscopic, dormant HSP27KD-3 tumors (Fig. 2 O and P). The mean HSP27 SI was 2.6-fold higher for NT control tumors compared with HSP27KD-3 tumors (8.6 ± 0.4 vs. 3.3 ± 1.3; P = 0.001, χ2 test) (Fig. 2R).
Stable Up-Regulation of HSP27 in Nonangiogenic Human Breast Cancer Cells Induces Expansive Tumor Growth in Vivo.
To further validate the role of HSP27 in tumor dormancy, we ectopically expressed a GFP-tagged HSP27 (HSP27-GFP) in the nonangiogenic MDA-MB-436NA cells with intrinsically low HSP27 (Fig. 1A), designated HSP27OE cells (Fig. 2E). We injected SCID mice (n = 5 per group) with HSP27OE cells s.c. and compared their growth with that of the negative vector control cells and parental nonangiogenic cells. HSP27OE cells initiated exponential tumor growth at day 40 and reached a mean size of 1,586 mm3 by day 70 (Fig. 2G). As expected, mice inoculated with parental cells formed microscopic, nonpalpable tumors (<40 mm3) and persisted up to day 70 without switching to the angiogenic phenotype, whereas the control vector cells reached a mean size of 209 mm3 by day 70. HSP27 protein staining by immunohistochemistry was significantly greater in the HSP27OE tumors compared with the control vector tumors (median, 6 vs. 2; P = 0.006, χ2 test). In addition, the mean vascular proliferation index was significantly higher in the HSP27OE tumors compared with control tumors (mean, 18.5% vs. 9%; P = 0.001, t test).
Down-Regulation of HSP27 in an Angiogenic Human Breast Cancer Cell Line Results in Gene Expression Patterns Similar to Those Seen in Nonangiogenic Cells.
To focus on potential targets of HSP27 responsible for affecting tumor progression, we performed gene expression analysis on control and HSP27KD cells by a significance analysis of microarrays. Significantly up-regulated or down-regulated genes and a subset of angiogenesis-related genes are listed in Table S1. HSP27 expression was suppressed 6.7-fold in HSP27KD-3 cells compared with control cells. Furthermore, gene expression of VEGF-A, VEGF-C, and bFGF was significantly reduced after HSP27 down-regulation (Table S1). Comparing HSP27KD-3 cells with angiogenic NT control cells using GSEA showed that HSP27 suppression resulted in a significant shift away from angiogenesis-related expression signatures, such as hypoxia, hypoxia-inducible factor 1 (HIF1) targets, and VEGF-A (Table S2).
Suppression of HSP27 Leads to Reduced Secretion of VEGF-A, VEGF-C, and bFGF.
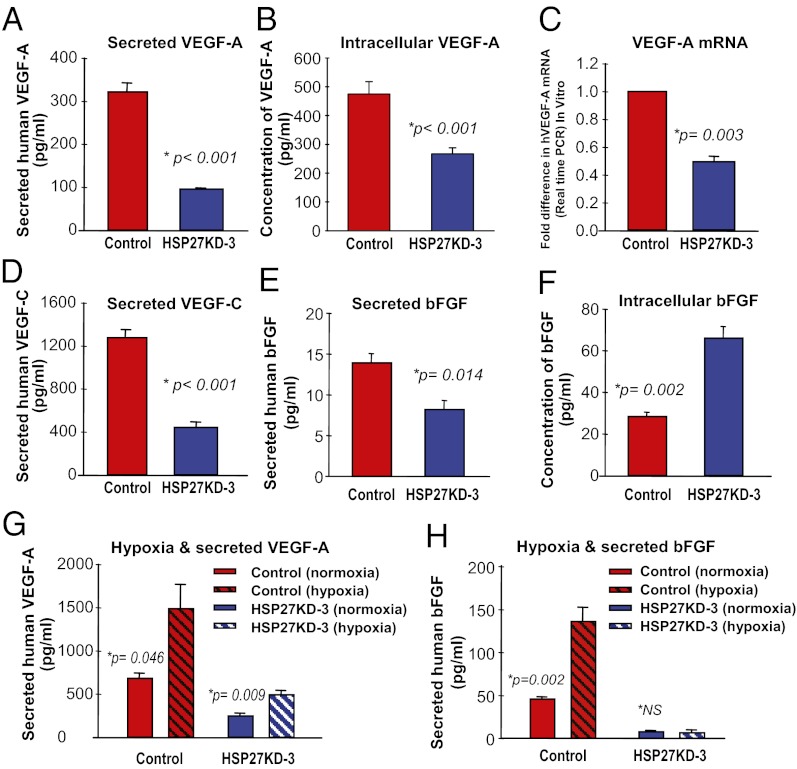
Along with gene expression screening, we specifically analyzed the secretion of important angiogenic factors by ELISA. Secretion of VEGF-A was 3.3-fold greater in NT control cells than in HSP27KD-3 cells (mean, 322.6 ± 19.6 pg/mL vs. 97.0 ± 1.5 pg/mL; P < 0.001, t test) (Fig. 3A). Intracellular levels of VEGF-A were 1.8 fold higher in control cells compared with HSP27KD-3 cells (475 ± 43 pg/mL vs. 267 ± 21 pg/mL; P < 0.001, t test) (Fig. 3B). RT-PCR revealed 2.1-fold higher levels of VEGF-A mRNA in NT control cells compared with HSP27KD-3 cells (Fig. 3C). In addition, HSP27KD-3 cells secreted 2.6-fold less VEGF-C protein compared with control cells (mean, 442.6 ± 18.1 pg/mL vs. 1264 ± 59.2 pg/mL; P < 0.001, t test) (Fig. 3D).
Fig. 3.
Suppression of HSP27 leads to reduced secretion of angiogenic factors. VEGF-A levels were quantified in the supernatant obtained from NT control (high HSP27) and HSP27KD-3 (low HSP27) cell lines. (A) The amount of human VEGF-A secreted into the media was 3.3-fold higher from the control cells compared with the HSP27KD-3 cells. (B) The concentration of intracellular VEGF-A was 1.8-fold higher in control NT cells. (C) Control NT cells contained twofold higher levels of VEGF-A mRNA compared with the HSP27KD-3 cells, as assessed by real-time qRT-PCR. (D) VEGF-C secretion was 2.6-fold higher in NT control cells compared with HSP27KD-3 cells, as assessed by ELISA. (E) bFGF secretion was 1.7-fold higher in NT control cells compared with HSP27KD-3 cells, as quantified by ELISA. (F) In contrast, bFGF concentration within the cell lysates (intracellular) was 2.3-fold greater in HSP27KD-3 cells compared with NT control cells. (G) Hypoxic conditions resulted in a significant increase in VEGF-A secretion from control cells and HSP27KD-3 breast cancer cells. (H) Hypoxic conditions induced significantly increased secretion of bFGF from the control cells, but not from HSP27KD-3 cells.
On ELISA, bFGF secretion was 1.7-fold greater in control cells compared with HSP27KD-3 cells (mean, 13.9 ± 0.9 pg/mL vs. 8.3 ± 0.9 pg/mL; P = 0.014, t test) (Fig. 3E). However, HSP27KD-3 cell lysates demonstrated a 2.3-fold increase in intracellular bFGF compared with control cells (66.3 ± 5.0 pg/mL vs. 28.8 ± 1.5 pg/mL; P = 0.002, t test) (Fig. 3F). These data demonstrate that down-regulation of HSP27 results in lower levels of secreted VEGF-A, VEGF-C, and bFGF.
Growth under hypoxic conditions (1% O2 overnight) resulted in 2.2-fold higher levels of VEGF-A secretion from both control cells and HSP27KD-3 cells despite the reduced expression in the latter (Fig. 3G) (SI Results). Secretion of bFGF was increased in control cells under hypoxia, but not in HSP27KD-3 cells (Fig. 3H).
To explore the role of angiogenesis-related transcription factors (8–12) as targets of HSP27, we quantified expression levels of STAT3 and NFκB in xenograft tumors by immunohistochemistry. The mean nuclear SI of phospho-STAT3 (ser727) was 2.3-fold higher in NT control tumors compared with HSP27KD-3 tumors (4.6 ± 0.4 vs. 2.0 ± 0.9; P = 0.007, t test), whereas total STAT3 staining was unaffected. Similarly, nuclear NFκB staining was reduced by 1.6-fold in HSP27-KD3 cells (2.4 ± 0.7 vs. 3.9 ± 0.3; P = 0.044, t test) (Fig. S2 A–F). Notably, STAT3 and NFκB activation signatures were significantly associated with HSP27 expression and KEGG pathways related to VEGF-A signaling, ECM receptor interactions, and focal adhesion (Table S2).
Suppression of HSP27 Affects Proliferation of Endothelial Cells, but Not of Tumor Cells.
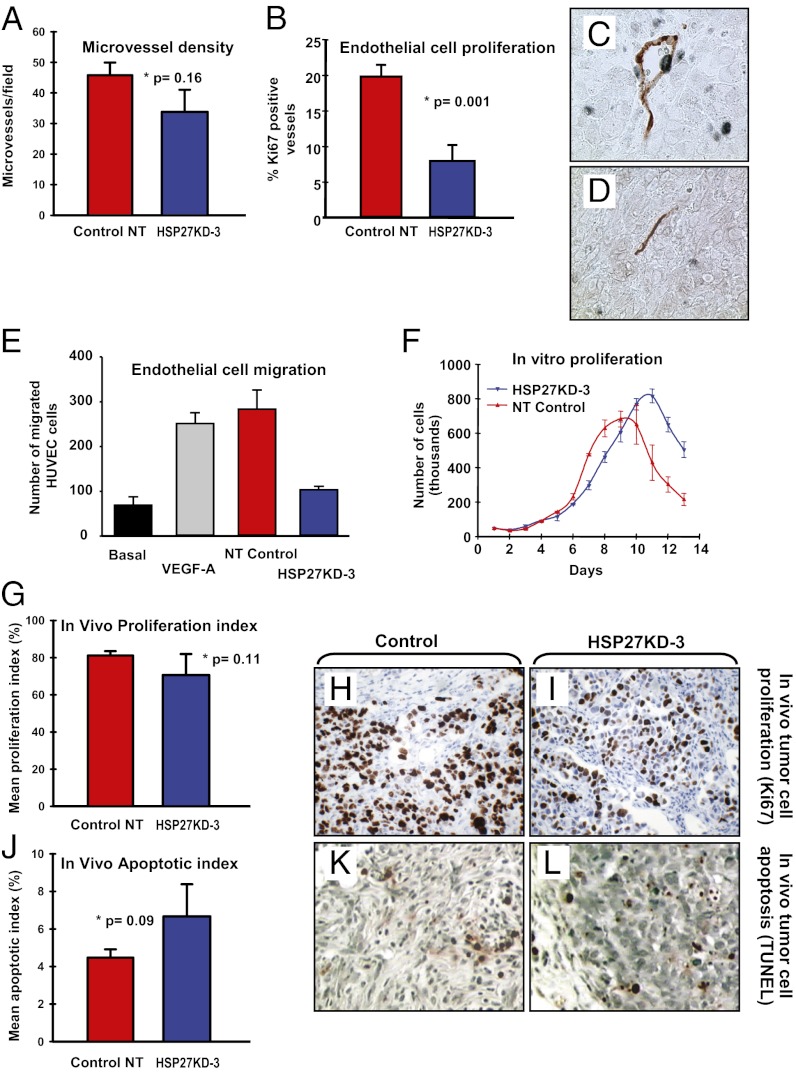
To determine whether tumor vascularity was altered in HSP27KD-3 compared with control tumors, we quantified microvessel density (MVD) and microvessel proliferation. Mean microvessel counts by CD34 expression was 1.3-fold higher in control tumors than in HSP27KD-3 tumors (45.7 ± 4.2 vessels/field vs. 33.8 ± 7.2 vessels/field; P = 0.16, t test) (Fig. 4A). Vascular proliferation was quantified by counting microvessels with evidence of proliferating endothelial cells (17); the percentage of Ki-67 positive vessels was 2.5-fold higher in control tumors compared with HSP27KD-3 tumors (P = 0.001, t test) (Fig. 4B) (SI Results). Vessels in control tumors often had open lumens containing erythrocytes (Fig. 4C), which were not seen in HSP27KD-3 tumors (Fig. 4D).
Fig. 4.
Suppression of HSP27 affects proliferation of endothelial cells, but not of tumor cells. (A) MVD, shown as microvessels per field of view (400× objective). (B) The ratio between tumor-associated vessels positive for Ki-67 in endothelial cells and all vessels was 2.5-fold higher within the control tumors. (C and D) CD34 (red) and Ki-67 (blue) dual immunostaining. In addition to high endothelial cell proliferation, the vessels found in the control tumors presented more frequently with open lumens containing erythrocytes (C), in contrast to compressed vessels within the HSP27KD-3 tumors (D). (E) HUVECs were exposed to VEGF-A or conditioned media from control cells or HSP27KD-3 cells grown under normoxia, and endothelial cell migration was quantified and compared with baseline migration. There number of endothelial cells migrating in response to conditioned media from control tumor cells was 2.7-fold greater. (F) There was no significant difference between the in vitro proliferation growth curves of the NT control cells and the HSP27KD-3 cells. (G–I) Tumor samples from the in vivo experiments showed no statistically significant difference in tumor cell proliferation (Ki-67) when NT control tumors were compared with tumors from HSP27KD-3 cells collected at comparable time points. Nonetheless, we observed a trend toward reduced tumor cell proliferation in the HSP27KD-3 tumors (P = 0.11). (J–L) Tumor cell apoptosis (TUNEL staining) was compared between NT-control cell line and HSP27KD-3 in tissue collected during the xenograft experiment. No significant difference in apoptotic rate was present, although a difference of borderline significance was found (P = 0.09) . (Original magnification: 400× for H, I, K, and L.)
We observed that migration of human umbilical vein endothelial cells (HUVECs) was affected by the presence or absence of HSP27. We exposed HUVECs to VEGF-A (3 ng/mL) or conditioned media from NT control or HSP27KD-3 cells under normoxic conditions and measured cell migration (18) compared with baseline migration (Fig. 4E). We observed a 2.7-fold decrease in endothelial migration in response to conditioned media from the HSP27KD-3 cells compared with control tumor cells (103 ± 8.3 vs. 282.7 ± 44; P = 0.016, t test). Endothelial cell migration stimulated by control cell medium had similar potency as VEGF-A–containing positive control cells (mean migrated cells, 251 ± 24; basal HUVEC migration, 69 ± 20 cells).
Notably, and in contrast to its effects on endothelial cells, we observed no statistically significant difference in the proliferation by Ki-67 expression of HSP27KD-3 tumor cells versus control NT cells either in vitro or in vivo (Fig. 4 F–I) (SI Results and Fig. S3). Nonetheless, in dormant tumors (HSP27KD-3), which remained at a microscopic size in vivo, tumor cells proliferated at a lower mean rate compared with control tumors (70.6% ± 11.3% vs. 81.2% ± 2.3%; P = 0.11, t test) (Fig. 4 G–I). Consistent with our observations on cellular proliferation, we found no differences in cyclin A or cyclin D1 protein expression (Fig. S4). In contrast to our findings with respect to proliferation, we observed an ∼50% higher mean apoptotic rate in HSP27KD-3 tumor cells compared with NT control tumor cells (6.7% ± 1.7% vs. 4.5% ± 0.4%; P = 0.09, t test) (Fig. 4 J–L). These data suggest that down-regulation of HSP27 inhibits primarily the angiogenic switch in our model, and that this is linked to growth inhibition and tumor dormancy.
Low Expression of HSP27 Protein Is Associated with a Less Aggressive Phenotype and Improved Survival in Human Patients with Breast Cancer and Cutaneous Melanoma.
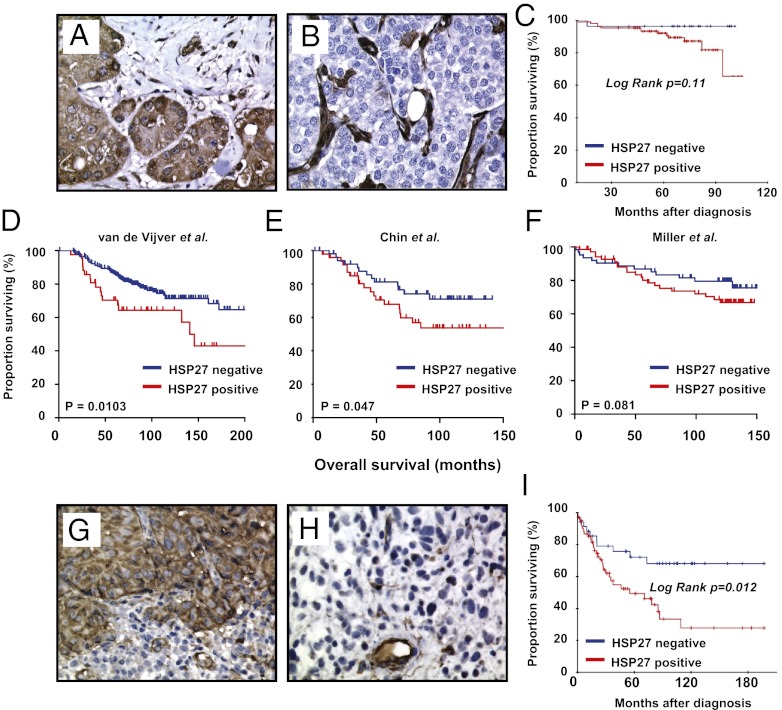
To evaluate whether our in vitro and xenograft results are clinically relevant, we analyzed tissues from human patients with breast cancer and melanoma. In breast cancer, tumor cell-associated HSP27 protein expression in tumor cells was significantly lower in screening-detected cancers compared with interval tumors (high expression, 16 of 59 vs. 36 of 74; P = 0.011), and in solitary tumors compared with multifocal tumors (41 of 115 vs. 10 of 16; P = 0.03). Cases with lower HSP27 expression (23%) tended to have improved patient survival (P = 0.11, log-rank test) (Fig. 5 A–C). Applying the signature extracted from HSP27-expressing breast cancer cells (109 genes by fold change >2; Table S3) to available breast cancer datasets, HSP27 expression was positively correlated with significantly reduced survival in 2 of 3 series (19–21) (Fig. 5 D–F). Positivity for the HSP27 signature was significantly associated with estrogen receptor-negative tumors, tumors with a basal-like signature, and HER2-positive tumors—that is, more aggressive tumor subsets (Fig. S5 C and D). In addition, the prognostic impact of the HSP27 expression signature was significant in estrogen receptor-positive, progesterone receptor-positive, and HER2-negative tumors in an independent dataset (20) (Fig. S5E).
Fig. 5.
Low expression of HSP27 protein is associated with a less aggressive phenotype and improved survival in human patients with breast cancer and melanoma. (A and B) HSP27 protein staining by immunohistochemistry was significantly stronger in interval breast cancers (A) compared with cancers detected by routine mammography scans (B); HSP27 protein-positive stromal cells serve as an internal control. (C) Patients with breast cancer with increased HSP27 expression also tended to have a poorer prognosis, although this trend was not statistically significant. (D–F) The HSP27 expression signature was applied on previously published breast cancer datasets. Patient samples designated as HSP27-positive were associated with a poorer cancer-specific survival in two of the three available datasets (van de Vijver dataset, P = 0.0103; Chin dataset, P = 0.047), with a trend toward poorer survival in the third dataset (Miller dataset, P = 0.081). (G–I) Patients with melanoma with high HSP27 protein expression by immunohistochemistry (G) had significantly lower cancer-specific overall survival (I) compared with those with low HSP27 expression (H).
In melanoma, HSP27 expression was low (SI <6) in 36% of the cases (Fig. 5 G and H). Increased staining was associated with strong expression of VEGF-A in tumor cells (P = 0.05, χ2 test) and strong expression of VEGFR2 in tumor cells (P = 0.016, χ2 test). No significant associations between HSP27 and tumor thickness, proliferation (by Ki-67), apoptotic index (by TUNEL assay), or MVD were detected. Low HSP27 expression was associated with improved patient survival (P = 0.012, log-rank test) (Fig. 5I). In multivariate analysis, HSP27 expression was an independent prognostic factor (Table S4).
Discussion
Current evidence suggests that some microscopic cancers may persist in a state of dormancy without expansion for long periods; however, little is known about the molecular mechanisms governing the maintenance and escape of dormancy (22). Here we identify HSP27 as an important molecular regulator of tumor dormancy in a model of human breast cancer. We demonstrate that HSP27 expression is significantly higher in rapidly growing angiogenic tumors, and that suppression of HSP27 induces long-term tumor dormancy in vivo. These dormant tumors were associated with reduced microvascular proliferation and lower secretion of VEGF-A and bFGF. In contrast, tumor cell proliferation and apoptotic rates were not significantly affected. By clinical validation, low levels of HSP27 protein and the HSP27 signature were associated with less aggressive tumors and improved patient survival in breast cancer and melanoma.
Ectopic overexpression of HSP27 in the parental nonangiogenic cell line mediated escape from tumor dormancy in concert with an angiogenic switch. In addition, overexpression was observed in all four HSP27KD-3 tumors that regained HSP27 protein expression and initiated tumor growth after a dormancy period of approximately 70 d or longer. These data support our hypothesis that HSP27 mediates the escape from tumor dormancy.
In a recent preclinical model of head and neck squamous cell carcinoma, targeting of HSP27 protein expression led to decreased migration and invasion of metastatic cancer cells (23). Moreover, a Phase I trial with OGX-427, a 2′methoxyethyl antisense oligonucleotide against HSP27, has demonstrated the clinical proof of concept for this target (24). OGX-427 was well tolerated and reduced tumor markers in patients with breast, prostate, lung, and ovarian cancers, and this experimental compound is now in Phase II development.
Recent studies indicate that malignant cells might achieve increased survival by an activated stress response involving heat shock factor 1 and heat shock proteins (7, 25). It has been suggested that the activation of these heat shock proteins is due to a nonspecific response, although our present data indicate that HSP27 plays a more direct role in the regulation of tumor angiogenesis and dormancy. Specifically, we have demonstrated in our model that specific silencing of HSP27 is sufficient to induce tumor dormancy in vivo.
Of note, our present data support the conclusion that HSP27 functions as an upstream regulator of VEGF-A, with transcription factors STAT3 and NFκB involved as mediators (8, 9). In addition to other regulatory pathways, transcription of both VEGF-A and bFGF is increased in response to hypoxia by an NFκB-dependent mechanism (11). As reported previously, HSP27 increases the proteasomal degradation of IκB, a cytosolic inhibitor of NFκB, thereby increasing nuclear relocalization of NFκB and transcriptional activity (12). Our data consistently support the reduced nuclear expression of NFκB after HSP27 knockdown. Along with HIF-1, STAT3 is a major transcription activator of VEGF-A (8), and STAT3 activation leads to increased angiogenesis in vivo (26). Importantly, downstream targets of STAT3 activation may be modulated by interactions with HSP27 (9). Consistent with these reports, we have demonstrated that nuclear expression of phospho-STAT3 is reduced after HSP27 knockdown. Thus, HSP27 might regulate the angiogenic potential of human tumor cells by modulating the activity of STAT3 and NFκB.
It has been reported that endothelial signaling by shear stress might induce phosphorylation of HSP27 via p38 MAP kinase (27), and that phosphorylated HSP27 binds bFGF and facilitates its transport across the plasma membrane (13). We found that secretion of bFGF was increased by hypoxic stress in the control cells only, and that down-regulation of HSP27 was associated with reduced secretion of bFGF and increased accumulation inside tumor cells. Moreover, HSP27 protein more frequently colocalized with bFGF within control NT cells, as evaluated by confocal microscopy (Fig. S4 D–I). Thus, our findings implicate interactions between HSP27 and bFGF at the protein level.
In the melanocytic system, HSP27 protects normal melanocytes from stressful influences (28), and HSP27 is phosphorylated in response to UVB radiation through p38 MAP kinase, leading to cytoplasmic–nuclear translocation (28). The role of HSP27 in human melanoma remains incompletely understood, however (29). The present study is the first to identify an independent prognostic value of HSP27 protein expression in patients with cutaneous melanoma. The association between HSP27 expression and VEGF-A expression found in our breast cancer model was present in the clinical melanoma samples as well. Regarding breast cancer, HSP27 expression was stronger in interval cancers than in low-grade screening-detected cancers. This observation may be relevant in light of the recent claim that some breast cancers detected by mammography might progress very slowly or represent dormant tumors (30).
As reviewed recently (31), the complex nature of human tumor dormancy is influenced by a multitude of regulatory steps, and our findings indicate that down-regulation of HSP27 in human breast cancer cells induces tumor dormancy in vivo. Significantly, our findings indicate that dormancy is mediated to a greater degree by impaired angiogenesis than by intrinsic alterations of tumor cell proliferation or apoptosis. Decreased tumor cell secretion of VEGF-A and bFGF might account for the reduced endothelial cell proliferation observed between HSP27 down-regulated tumors compared with controls. Thus, HSP27 appears to play a key role in the angiogenic switch in this model. By clinical validation, HSP27 expression levels were associated with tumor presentation and disease progression. These data may be relevant to the development of useful treatment strategies.
Materials and Methods
Cell lines, endothelial migration assays, xenografting and animal models are described in more detail in SI Materials and Methods. Immunohistochemistry, ELISA, and Western blot analysis were conducted using standard procedures detailed in SI Materials and Methods. Down-regulation by shRNA, constructs, gene expression analysis, RT-PCR, bioinformatics, statistical analyses, as well as collection of clinical samples followed established procedures as described in more detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Gerd Lillian Hallseth, Hua My Hoang, and Bendik Nordanger for excellent technical assistance and Marsha A. Moses and Alexis Mitsialis of Children’s Hospital Boston for helpful comments and experimental support. The work of O.S. was supported by Helse Vest, the Norwegian Cancer Society, and the Unger Vetlesen Foundation. T.S. was supported by the Dana-Farber Cancer Institute Claudia Adams Barr Program in Innovative Basic Cancer Research. G.I.S. was supported by Dana-Farber/Harvard Cancer Center Specialized Program of Research Excellence in Lung Cancer, National Institutes of Health (NIH) Grant P50 CA090578. The work of K.H.K. was supported by Research Council of Norway Grant 185676/V40, Helse Vest Grants 911401 and 911500, and Norwegian Cancer Society Grant HS02-2008-0188. The work of J.M.F. was funded by NIH Grant P01CA045548, the Breast Cancer Research Foundation, and Department of Defense Breast Cancer Innovator Award W81XWH-04-1-0316. L.A.A. was supported by Research Council of Norway Grants 154942/310, 161231/V40, and 163920/V50; Helse Vest Grants 15126600 and 911403; the Unger Vetlesen Foundation; and Norwegian Cancer Society Grants 94070 and 419328 71512-PR-2006-0356.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The annotated microarray data reported in this paper have been uploaded and formatted and deposited in ArrayExpress at the European Bioinformatics Institute, http://www.ebi.ac.uk/microarray, according to MIAME (Minimum Information About a Microarray Experiment) guidelines (accession no. E-TABM-883).
2Deceased January 14, 2008.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017909109/-/DCSupplemental.
References
- 1.Naumov GN, Folkman J, Straume O. Tumor dormancy due to failure of angiogenesis: Role of the microenvironment. Clin Exp Metastasis. 2009;26:51–60. doi: 10.1007/s10585-008-9176-0. [DOI] [PubMed] [Google Scholar]
- 2.Black WC, Welch HG. Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N Engl J Med. 1993;328:1237–1243. doi: 10.1056/NEJM199304293281706. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427:787. doi: 10.1038/427787a. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 5.Naumov GN, Akslen LA, Folkman J. Role of angiogenesis in human tumor dormancy: Animal models of the angiogenic switch. Cell Cycle. 2006;5:1779–1787. doi: 10.4161/cc.5.16.3018. [DOI] [PubMed] [Google Scholar]
- 6.Naumov GN, et al. A model of human tumor dormancy: An angiogenic switch from the nonangiogenic phenotype. J Natl Cancer Inst. 2006;98:316–325. doi: 10.1093/jnci/djj068. [DOI] [PubMed] [Google Scholar]
- 7.Garrido C, et al. Heat shock proteins 27 and 70: Anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- 8.Xu Q, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24:5552–5560. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- 9.Rocchi P, et al. Increased Hsp27 after androgen ablation facilitates androgen-independent progression in prostate cancer via signal transducers and activators of transcription 3-mediated suppression of apoptosis. Cancer Res. 2005;65:11083–11093. doi: 10.1158/0008-5472.CAN-05-1840. [DOI] [PubMed] [Google Scholar]
- 10.Song H, Ethier SP, Dziubinski ML, Lin J. Stat3 modulates heat shock 27kDa protein expression in breast epithelial cells. Biochem Biophys Res Commun. 2004;314:143–150. doi: 10.1016/j.bbrc.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 11.Crisostomo PR, et al. Human mesenchymal stem cells stimulated by TNF-α, LPS, or hypoxia produce growth factors by an NFκB- but not a JNK-dependent mechanism. Am J Physiol Cell Physiol. 2008;294:C675–C682. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]
- 12.Parcellier A, et al. HSP27 is a ubiquitin-binding protein involved in I-κBα proteasomal degradation. Mol Cell Biol. 2003;23:5790–5802. doi: 10.1128/MCB.23.16.5790-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piotrowicz RS, Martin JL, Dillman WH, Levin EG. The 27-kDa heat shock protein facilitates basic fibroblast growth factor release from endothelial cells. J Biol Chem. 1997;272:7042–7047. doi: 10.1074/jbc.272.11.7042. [DOI] [PubMed] [Google Scholar]
- 14.Cao H, et al. Novel role for STAT-5B in the regulation of Hsp27–FGF-2 axis facilitating thrombin-induced vascular smooth muscle cell growth and motility. Circ Res. 2006;98:913–922. doi: 10.1161/01.RES.0000216954.55724.a2. [DOI] [PubMed] [Google Scholar]
- 15.Rousseau S, Houle F, Landry J, Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997;15:2169–2177. doi: 10.1038/sj.onc.1201380. [DOI] [PubMed] [Google Scholar]
- 16.Holmgren L, O’Reilly MS, Folkman J. Dormancy of micrometastases: Balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 17.Arnes J, Stefansson I, Brunet J, Foulkes W, Akslen L. Vascular proliferation is a strong and independent prognostic factor in breast cancer. Virchows Arch. 2007;451:137. [Google Scholar]
- 18.Short SM, et al. Inhibition of endothelial cell migration by thrombospondin-1 type-1 repeats is mediated by beta1 integrins. J Cell Biol. 2005;168:643–653. doi: 10.1083/jcb.200407060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller LD, et al. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci USA. 2005;102:13550–13555. doi: 10.1073/pnas.0506230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chin SF, et al. Using array-comparative genomic hybridization to define molecular portraits of primary breast cancers. Oncogene. 2007;26:1959–1970. doi: 10.1038/sj.onc.1209985. [DOI] [PubMed] [Google Scholar]
- 21.van de Vijver MJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 22.Weinberg RA. The many faces of tumor dormancy. APMIS. 2008;116:548–551. doi: 10.1111/j.1600-0463.2008.001168.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Z, et al. Silencing heat shock protein 27 decreases metastatic behavior of human head and neck squamous cell cancer cells in vitro. Mol Pharm. 2010;7:1283–1290. doi: 10.1021/mp100073s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hotte SJ. Phase I trial of OGX-427, a 2′methoxyethyl antisense oligonucleotide (ASO), against heat shock protein 27 (Hsp27): Final results,(abstract) 2010 Journal of Clinical Oncology, 2010 ASCO Annual Meeting Proceedings (Post-Meeting Edition). Vol 28, No 15(Suppl) (May 20 Supplement), 2010:3077. [Google Scholar]
- 25.Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130:1005–1018. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei LH, et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22:1517–1527. doi: 10.1038/sj.onc.1206226. [DOI] [PubMed] [Google Scholar]
- 27.Gloe T, Sohn HY, Meininger GA, Pohl U. Shear stress-induced release of basic fibroblast growth factor from endothelial cells is mediated by matrix interaction via integrin α(v)β3. J Biol Chem. 2002;277:23453–23458. doi: 10.1074/jbc.M203889200. [DOI] [PubMed] [Google Scholar]
- 28.Shi B, Grahn JC, Reilly DA, Dizon TC, Isseroff RR. Responses of the 27-kDa heat shock protein to UVB irradiation in human epidermal melanocytes. Exp Dermatol. 2008;17:108–114. doi: 10.1111/j.1600-0625.2007.00641.x. [DOI] [PubMed] [Google Scholar]
- 29.Kang SH, et al. Heat shock protein 27 is expressed in normal and malignant human melanocytes in vivo. J Cutan Pathol. 2004;31:665–671. doi: 10.1111/j.0303-6987.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- 30.Retsky MW, Demicheli R, Hrushesky WJ, Baum M, Gukas ID. Dormancy and surgery-driven escape from dormancy help explain some clinical features of breast cancer. APMIS. 2008;116:730–741. doi: 10.1111/j.1600-0463.2008.00990.x. [DOI] [PubMed] [Google Scholar]
- 31.Akslen LA, Naumov GN. Tumor dormancy—from basic mechanisms to clinical practice. APMIS. 2008;116:545–547. doi: 10.1111/j.1600-0463.2008.01209.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.