Abstract
In principle, widespread polyandry (female promiscuity) creates potential for sexual selection in males both before and after copulation. However, the way polyandry affects pre- and postcopulatory episodes of sexual selection remains little understood. Resolving this fundamental question has been difficult because it requires extensive information on mating behavior as well as paternity for the whole male population. Here we show that in replicate seminatural groups of red junglefowl, Gallus gallus, polyandry eroded variance in male mating success, which simultaneously weakened the overall intensity of sexual selection but increased the relative strength of postcopulatory episodes. We further illustrate the differential effect of polyandry on pre- and postcopulatory sexual selection by considering the case of male social status, a key determinant of male reproductive success in this species. In low-polyandry groups, however, status was strongly sexually selected before copulation because dominants mated with more females. In high-polyandry groups, sexual selection for status was weakened and largely restricted after copulation because dominants defended paternity by mating repeatedly with the same female. These results reveal polyandry as a potent and dynamic modulator of sexual selection episodes.
Keywords: cryptic female choice, sperm competition, selection gradient, opportunity of selection
Polyandry is a taxonomically widespread consequence of sexual reproduction and a key modulator of fundamental evolutionary processes (1), including sociality (2, 3), sex allocation (4), selfish genetic elements (5), speciation (6), inbreeding (7), and evolutionary conflicts [e.g., between diploid and haploid genomes (8), between parents and offspring (9), and between the sexes (10)]. The realization of widespread polyandry has been particularly revolutionary for our understanding of sexual selection (11). Darwin (12) proposed that sexual selection operates on individual variation in the number of partners (i.e., mating success) and their reproductive quality (e.g., fecundity) to promote traits that confer an advantage in intrasexual competition (see also refs. 13–15). By inducing the ejaculates of multiple males to compete for fertilization, polyandry introduces an additional postcopulatory source of variation in male reproductive success—variation in the proportion of a set of eggs fertilized by different males—creating additional episodes of selection after copulation, namely sperm competition and cryptic female choice (11). Although the study of postcopulatory sexual selection has mushroomed over the last 30 y, the way in which polyandry affects the operation of sexual selection remains unclear. A number of studies have concluded that polyandry increases the strength of sexual selection on males by promoting variation in male reproductive success (e.g., refs. 16–19). Other studies, however, have suggested that the amount of variation in male reproductive success caused by polyandry is limited and that polyandry might in fact weaken sexual selection by reducing the mating skew among males (e.g., refs. 20–23). This lack of consensus means that we still do not fully understand how precopulatory sexual selection interacts with postcopulatory sexual selection caused by polyandry (24, 25). These fundamental issues remain unresolved because empirical efforts have largely been restricted to either mating or paternity data. However, the unbiased measurement of pre- and postcopulatory sexual selection requires extensive information on variation in both mating success and paternity for all of the males of a population (25, 26). Here we adopt an experimental approach to measure sexual selection in small seminatural replicate groups of polyandrous red junglefowl, Gallus gallus. The red junglefowl represents an appropriate model to study polyandry and sexual selection. In nature, red junglefowl live in small groups that are structured into social hierarchies (27, 28). Females are often polyandrous, although socially dominant males typically have privileged access to mating opportunities (29). Female fowl can store viable sperm for approximately 2 wk after insemination (30), creating potential for episodes of postcopulatory sexual selection (29). We investigated the impact of polyandry on (i) the opportunity and strength of precopulatory, postcopulatory, and overall sexual selection, and (ii) the way sexual selection operates on individual male traits.
Results
We measured sexual selection using experimental groups, a methodological approach that has been successfully implemented in the past (e.g., refs. 26, 31–35). We assembled 13 groups of three adult males and four adult females each, reflecting the size and sex ratio of natural social groups (27, 36). In each group, we monitored mating behavior for 10 consecutive days and assigned parentage of the resulting embryos (Materials and Methods). The average polyandry of a group (i.e., the number of males with whom each female mated averaged across all of the females of a group) ranged naturally from one to three. Variance in the reproductive success (T) of a focal male is determined by three constituents: male mating success (M, number of females mated), the average fecundity of these mates (N, i.e., the average number of eggs, or clutch size, of the females successfully mated by the focal male), his average paternity share (P, the proportion of embryos sired by the focal male out of the total number of eggs produced by all of the females with whom he successfully mated), such that T = (M × N × P) + ε, where ε represents an error term (37). To measure the impact of polyandry on sexual selection we first partitioned the variance in T into M, N, and P and the covariances in M and P, M and N, and N and P (16, 18). Variation in P was by far the largest source of variation in male reproductive success (46% of the variance of T), followed by the variance of M and by the covariance of P and M (Table 1), with variation in mate fecundity (N) having virtually no effect on T. An alternative quantitative approach (38), which does not consider covariance between M, N, and P, yielded qualitative very similar results, identifying P as the largest source of variance in T (Table S1). Together, these results indicate that polyandry plays a critical role in sexual selection in this species.
Table 1.
Contributions of male mating success (M), mate fecundity (N), paternity share (P), and their covariances to variance in total male reproductive success (T) in replicate groups of red junglefowl (16): P caused by polyandry represents the largest source of variance in T
| Source of variance | Unstandardized | Standardized | |
| Total variance in T | 50.83 | 1.20 | |
| Mating success, M | 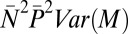 |
12.78 | 0.30 |
| Mate fecundity, N | 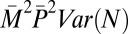 |
0.84 | 0.02 |
| Paternity share, P | 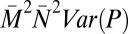 |
23.33 | 0.55 |
| Covariance M,N | 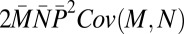 |
−1.46 | −0.03 |
| Covariance M,P | 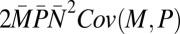 |
8.56 | 0.20 |
| Covariance N,P | 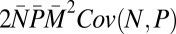 |
−0.08 | −0.002 |
| Error | 6.87 | 0.16 |
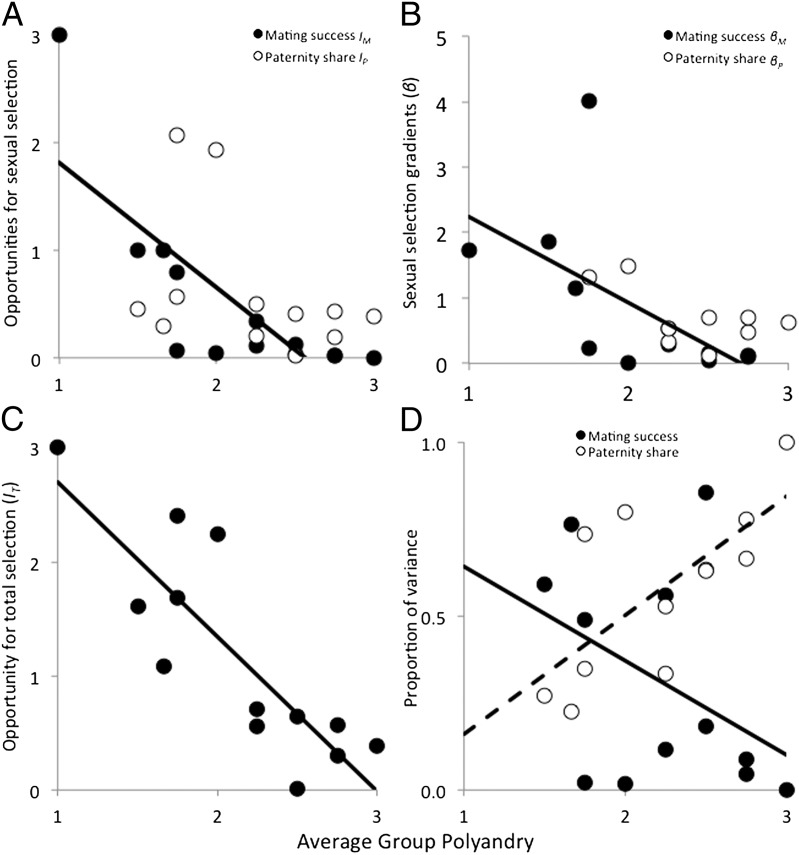
We then investigated the way polyandry affects sexual selection across individual groups and found that pre- and postcopulatory sexual selection are affected in strikingly different ways. The appropriate measurement of sexual selection is in itself nontrivial (39–41). We used two widely used measures of sexual selection, the opportunity of selection (I) and the selection gradient (β), which capture distinct and complementary aspects of the selective process (21, 41, 42). Whereas the opportunity for precopulatory sexual selection on mating success (IM) declined sharply in more polyandrous groups (where mating success was similarly high across all males), the opportunity for postcopulatory sexual selection on paternity share (IP) was unaffected by the average polyandry of a group (Fig. 1A). Similarly, polyandry affected differentially the gradient of pre- and postcopulatory sexual selection. Whereas the gradient of precopulatory sexual selection on mating success (βM) declined significantly with polyandry, the relationship between the gradient of postcopulatory sexual selection (βP) and polyandry was much weaker (Fig. 1B). As a result of this differential effect, polyandry was associated with a strong net reduction in the opportunity for total (i.e., pre- and postcopulatory) sexual selection (IT, Fig. 1C). Because variation in male reproductive success (T) was largely due to variation in mating success (M) and paternity share (P), the proportion of variance in T explained by variance in M tended to decline in more polyandrous groups. Simultaneously, the proportion explained by variance in P increased with increasing polyandry (Fig. 1D). In other words, in our study populations polyandry increased the importance of postcopulatory episodes of sexual selection relative to precopulatory episodes and simultaneously eroded the overall opportunity for, and strength of, sexual selection. Collectively, these results predict that sexual selection on a given male trait is reduced and disproportionally driven by postcopulatory episodes in more polyandrous populations.
Fig. 1.
Differential effect of polyandry on male sexual selection. (A) Opportunity for pre- (IM) and postcopulatory (IP) sexual selection in relation to average group polyandry. IM significantly decreased with polyandry (slope = −1.16 ± 0.26, t = −4.40, R2 = 0.60, df = 11, P = 0.001) but not IP (slope = −0.53 ± 0.39, t = −1.32, R2 = 0.06, df = 10, P = 0.218). A qualitatively similar pattern was obtained using alternative measures of IM and IP (38): IM (t = −4.40, R2 = 0.60, df = 11, P = 0.001); IP (t = −0.14, R2 = −0.09, df = 11, P = 0.892). (B) Multivariate gradients of pre- (βM) and postcopulatory (βP) sexual selection in relation to average group polyandry. βM significantly decreased with polyandry (slope = −1.32 ± 0.56, t = −2.42, R2 = 0.31, df = 10, P = 0.036), but this trend was much weaker for βP (slope = −0.66 ± 0.35, t = −1.90, R2 = −0.25, df = 7, P = 0.099). A qualitatively similar pattern was obtained using univariate measures of βM and βP based on ref. 38: βM (t = −6.30, R2 = 0.78, df = 10, P < 0.001); βP (t = −3.16, R2 = 0.45, df = 10, P = 0.010). (C) The opportunity for total sexual selection (IT) significantly decreased with group polyandry (−1.35 ± 0.26, t = −5.19, R2 = 0.68, df = 11, P < 0.001). (D) In more polyandrous groups, the proportion of the variance in total male reproductive success explained by male mating success tended to decline (solid line, t = −2.06, df = 11, P = 0.064), and simultaneously the proportion of male reproductive success explained by paternity share increased (shaded line, t = 1.50, df = 10, P = 0.019). Data points represent individual replicate groups.
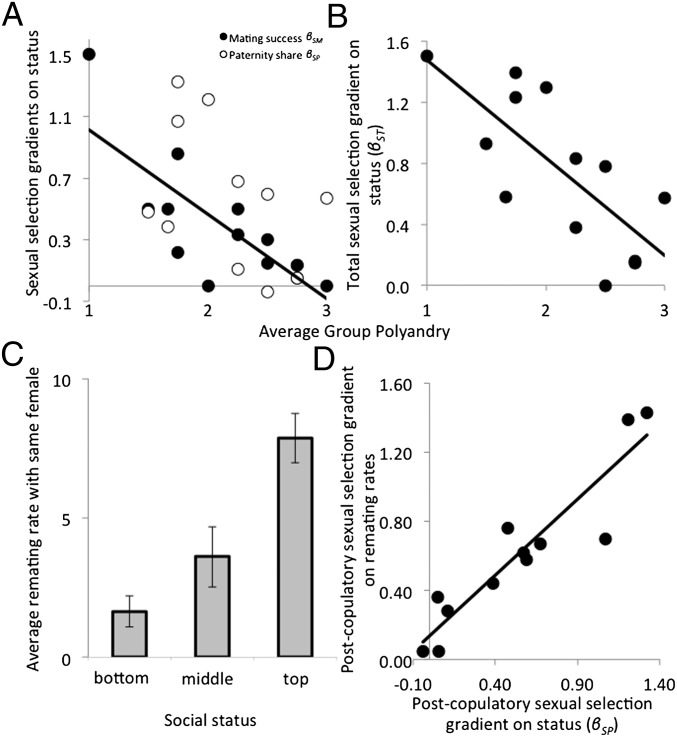
We tested this prediction by investigating how polyandry affects the operation of sexual selection on male social status, one of the best predictors of male reproductive success in fowl populations (27, 28). Consistent with expectations, we found that polyandry had a strongly differential effect on sexual selection on male status. In groups with relatively low polyandry, there was substantial precopulatory sexual selection on male status (i.e., high βSM) because dominant males mated with more females than their subordinates. In more polyandrous groups however, mating success was more equally distributed among males, and βSM was markedly weakened (Fig. 2A). In addition to mating with more females, dominant males also fertilized more of the eggs (i.e., high P) of the females they shared with subordinate males, resulting in a positive postcopulatory sexual selection gradient (βSP). In contrast with precopulatory βSM, however, postcopulatory sexual selection on social status βSP only marginally declined with the level of polyandry in a group (Fig. 2A). Therefore, polyandry weakened total sexual selection for male status by restricting it largely to postcopulatory episodes (Fig. 2B). This is partly intuitive: when dominant males are able to monopolize access to females, polyandry is low and dominant males enjoy a mating advantage. However, as females mate with more males, social status has a progressively limited impact on mating success (because all males have a similarly high mating success regardless of their status) but remains an important determinant of the outcome of sperm competition. At least two nonmutually exclusive mechanisms are likely to explain why status is favored by postcopulatory sexual selection under high polyandry. First, polyandrous female fowl can strongly bias the outcome of sperm competition in favor of the ejaculates of dominant males through cryptic female choice (43, 44). Second, dominant males might remate with the same females at a higher rate than their subordinates. This mating behavior would be favored by postcopulatory sexual selection if it enabled dominant males to better defend their paternity share. Consistent with this idea, we found that dominant males remated with the same females more often than subordinate males (Fig. 2C). Importantly, in groups where status was strongly sexually selected after copulation (i.e., had a strong effect on variation in paternity share), the rate at which a male remated with the same female was also strongly sexually selected after copulation (Fig. 2D). Sperm competition dynamics in red junglefowl follow a fair raffle and, all else being equal, males have a greater chance of paternity if they can replenish the sperm stored by a female by remating with her (45). This is particularly relevant for males such as dominant males, which on average tend to inseminate small ejaculates and which might suffer from low sperm velocity (46, 47).
Fig. 2.
Sexual selection on male social status. (A) Gradients of pre- (βSM) and postcopulatory (βSP) sexual selection on social status in relation to average group polyandry. βSM decreased with polyandry (slope = −0.55 ± 0.14, t = −4.05, R2 = 0.56, df = 11, P = 0.002), but this trend was nonsignificant for βSP (slope = −0.48 ± 0.26, t = −1.88, R2 = 0.19, df = 10, P = 0.090). (B) The total sexual selection gradient on social status (βST) decreased with polyandry (−0.64 ± 0.18, t = −3.64, R2 = 0.50, df = 11, P = 0.004). Data points represent individual replicate groups. (C) The rate of male remating with the same female was strongly status dependent (F = 11.11, df = 2, P < 0.001). (D) Postcopulatory sexual selection gradient on remating with the same female(s) increased significantly with the postcopulatory sexual selection gradient on social status [i.e., βSP (slope = 0.88 ± 0.11, t = 7.90, R2 = 0.85, df = 10, P < 0.001)]. Data points represent individual replicate groups.
Discussion
The impact of polyandry on the way sexual selection operates on males has been debated for decades. The results of our study contribute to resolving this debate by showing that polyandry affects differentially pre- and postcopulatory sexual selection: polyandry weakens the overall opportunity for, and strength of, sexual selection but simultaneously increases the relative importance of postcopulatory episodes of sexual selection. The differential impact of polyandry on pre- and postcopulatory sexual selection reveals a fundamental difference in the way mating and fertilization success contribute to variation in male reproductive success. In the measure of mating success (M), multiple individuals of the same sex can mate with the same partner and share mating success (i.e., partners are a public good). This means that as a group becomes more polyandrous, M becomes similarly high for all males, increasing average mating success 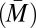 and simultaneously reducing its variance
and simultaneously reducing its variance 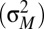 , which ultimately leads to a lower IM. In the measure of paternity share (P), on the other hand, fertilization by one male excludes automatically an egg from the reproductive success of his competitors. Therefore, an increase in polyandry is not necessarily expected to affect IP, because it can reduce both average share in paternity (
, which ultimately leads to a lower IM. In the measure of paternity share (P), on the other hand, fertilization by one male excludes automatically an egg from the reproductive success of his competitors. Therefore, an increase in polyandry is not necessarily expected to affect IP, because it can reduce both average share in paternity (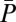 , because the paternity of a clutch is shared among more males) and its variance (
, because the paternity of a clutch is shared among more males) and its variance (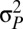 , because the number of males achieving paternity increases). In a mating system of strict lifetime monogamy (i.e., where the opportunity of sexual selection is restricted to individual variation in fecundity and reproductive investment), we expect that the introduction of some polyandry might initially increase the opportunity for sexual selection. This explains why in some socially monogamous birds a degree of polyandry (in the form of extrapair copulations) can represent an important additional source of sexual selection (e.g., refs. 16–19). However, our study demonstrates that in mating systems characterized by some degree of male reproductive skew, an increment in polyandry can erode sexual selection. This erosion is likely to cause dynamic fluctuations whereby sexual selection on male mating success (i.e., number of females mated, M) would increase polyandry, and increased polyandry might in turn weaken further sexual selection on male mating success (M). Such dynamic feedback loops may be more common than currently appreciated in sexual selection. Recently, similar dynamics have been theoretically demonstrated for sexual selection and maternal sex allocation (48). Finally, a widely recognized challenge that remains outstanding in sexual selection research is to establish the way episodes of pre- and postcopulatory sexual selection interact with each other (e.g., ref. 25). Recent work has shown that pre- and postcopulatory sexual selection tend to target different male traits (e.g., ref. 49). Our results reveal that pre- and postcopulatory sexual selection sometimes target the same male trait (e.g., social status) through alternative pathways. The level of polyandry of a population then plays a pivotal role in modulating how such traits are differentially targeted by pre- vs. postcopulatory selective episodes.
, because the number of males achieving paternity increases). In a mating system of strict lifetime monogamy (i.e., where the opportunity of sexual selection is restricted to individual variation in fecundity and reproductive investment), we expect that the introduction of some polyandry might initially increase the opportunity for sexual selection. This explains why in some socially monogamous birds a degree of polyandry (in the form of extrapair copulations) can represent an important additional source of sexual selection (e.g., refs. 16–19). However, our study demonstrates that in mating systems characterized by some degree of male reproductive skew, an increment in polyandry can erode sexual selection. This erosion is likely to cause dynamic fluctuations whereby sexual selection on male mating success (i.e., number of females mated, M) would increase polyandry, and increased polyandry might in turn weaken further sexual selection on male mating success (M). Such dynamic feedback loops may be more common than currently appreciated in sexual selection. Recently, similar dynamics have been theoretically demonstrated for sexual selection and maternal sex allocation (48). Finally, a widely recognized challenge that remains outstanding in sexual selection research is to establish the way episodes of pre- and postcopulatory sexual selection interact with each other (e.g., ref. 25). Recent work has shown that pre- and postcopulatory sexual selection tend to target different male traits (e.g., ref. 49). Our results reveal that pre- and postcopulatory sexual selection sometimes target the same male trait (e.g., social status) through alternative pathways. The level of polyandry of a population then plays a pivotal role in modulating how such traits are differentially targeted by pre- vs. postcopulatory selective episodes.
In conclusion, our results reveal fundamental properties of the relationship between sexual selection and polyandry and are expected to be broadly relevant to most polyandrous finite sexual populations with unitary sex ratios. Future work should investigate the way population size and sex ratio might interact to drive the dynamic relationship between polyandry and sexual selection.
Materials and Methods
Observations in Seminatural Conditions.
The study was conducted on a population of red junglefowl, between May and September 2007 and August and September 2008. All males and females hatched in March 2006, but for one female hatched in October 2007 and used in summer 2008. We studied 13 groups, each comprising three adult males and four adult females in outdoor pens of ∼50 m2. Before being introduced into an experimental group, each bird was sexually rested (i.e., prevented from delivering or receiving gametes): females, for at least 10 d to ensure that they did not store sperm from other males during a trial; and males for at least 2 d (30) to ensure that results were not influenced by sperm depletion due to previous mating. Each group was assembled by releasing males and females into a pen, and birds were allowed to familiarize with the new setting for at least 12 h, after which detailed observations of all mating interactions were recorded for 10 successive days, 4 to 5 h per day, at times of peak mating activity (50) (i.e., in the early morning and in the late afternoon until all females went to roost). Behaviors recorded included male- and female-initiated mating attempts, disruption of mating attempts by other males, and mating success following established criteria (50, 51). During the observation period, male social hierarchy was determined according to the outcome of competitive dyadic interactions following an established protocol (46). In each group, male status was ranked as 3 (top-ranking), 2 (intermediate), and 1 (bottom-ranking). All male hierarchies were linear. From the second day of observation and for the subsequent 10 d (i.e., day 2 to day 11 inclusive) all eggs were collected (between 6 and 28 eggs per group). One group departed from this pattern because observations and egg collection occurred from day 7 to day 16. Analyses were carried out with or without this group, and no significant difference was observed, so only results with this group are presented. In one of the groups a female died on day 5 of the observations, modifying the sex ratio of this group. However, the removal of this group did not change qualitatively any of the results reported.
Parentage Assignment.
Eggs were kept in a refrigerator during 0 to 7 d before being put in an incubator at 37 °C and 41% humidity. After 7 d of incubation eggs were opened, and embryos were collected and put in 1 mL of pure ethanol. Paternity and maternity were assigned for each egg laid during the observation period by parentage analysis. All samples were genotyped at seven variable microsatellite loci: ADL0299 (52), LEI0078 (53), LEI0196 (54), LEI0246 (55), MCW0123 (56), MCW0183 (57), and ROS0081 according to the methods outlined in ref. 58. All parental assignments were done in Cervus 3.0 (59) using the same procedure as in ref. 55. Both parents were assigned for all 164 embryos in 2007 and 90 embryos in 2008.
Statistical Analysis.
Male reproductive success (T) was measured as the number of embryos sired by each individual male. T has three constituents: the number of females successfully mated by a male (mating success, M), the average number of eggs produced by these females (mate fecundity, N), and the share of paternity of these eggs (P). M was measured as the total number of females with which a male was observed to have behaviorally successful copulations (50) during the 10 d of observation, plus females that were not observed copulating successfully with the male but that nonetheless produced offspring fathered by this male (overall, 102 females partners were attributed through observations, and 8 females partners were identified on the basis of molecular paternity data). N was measured as the average number of eggs (i.e., average clutch size) laid by all of the females successfully mated by a male. Finally, P was measured as the proportion of embryos sired by a male out of the total number of eggs produced by all of the females with whom he successfully mated. When a male did not mate with any female, there was no opportunity for this male to be selected for N nor P, thus he was given a score of 0 for M but was excluded from the analyses of N and P. Some males were used in more than one group (four used in two groups, four in three groups, and one in four groups). To test the potential effect of pseudoreplication, we measured the repeatability (60) of fitness components of these individual males across replicate groups. Repeatability was consistently low (T, r = 0.11; M, r = −0.04; N, r = −0.19; and P, r = 0.16), indicating that the reproductive success of these males was largely contigent on the dynamics of different replicate groups rather than consistently determined by inherent properties of the male. We therefore found it more appropriate to treat these few repeated observations of the same male over multiple replicate groups as independent data.
We measured two distinct but related aspects of sexual selection: the opportunity of sexual selection I (21, 61, 62) and the sexual selection gradient β (38, 63, 64). We measured the index of opportunity for sexual selection, I for the different components of male sexual selection (IT, IM, IN, IP) as follows:
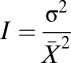 |
where 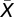 is the mean of the measure of reproductive success: T, M, N, or P and σ2 its variance. We also calculated the opportunity for selection of the different episodes according to equation 17 in ref. 38 and present these results in Table S1. Given our multiplicative model of total reproductive success: T = (M × N × P) + ε, where ε represents an error term, the variance of T is composed of three variance terms and a set of covariance terms (16, 37):
is the mean of the measure of reproductive success: T, M, N, or P and σ2 its variance. We also calculated the opportunity for selection of the different episodes according to equation 17 in ref. 38 and present these results in Table S1. Given our multiplicative model of total reproductive success: T = (M × N × P) + ε, where ε represents an error term, the variance of T is composed of three variance terms and a set of covariance terms (16, 37):
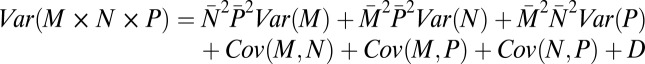 |
where M, N, and P are defined as previously, and D is an error term, which includes variance in ε. Variances and covariances were calculated for each group.
For calculation of all sexual selection gradients (β), data were standardized such that the responses had a mean of 1 and the predictors had a mean of 0 and an SD of 1 per group (64). When all of the males from a group had the same score for a predictor, the SD was null and no selection gradient could be obtained for that group. Multivariate selection gradients (βM, βP) were calculated as the partial regression slopes obtained from a multivariate model with T as the dependent variable and both M and P as independent variables, calculated in each group [lm in R 2.12.0 (65)]. Note that βM differs from the often-used (e.g., ref. 25) definition of a Bateman gradient because it was calculated from a multivariate model that included P, and it was calculated on standardized values. Multivariate selection gradients reduced the number of available data points (βP was calculated on 9 groups, βM on 12 groups) because both βM and βP are calculated in each group of three males. Thus, groups where a male did not mate successfully with at least one female lacked a βP. We also calculated univariate selection gradients equivalent to the selection differential (38) (Table S1). Sexual selection gradients on social status were measured with univariate regression of social status on T (βST), M (βSM), and P (βSP). If more polyandrous groups contained more fecund females, the relationships observed between polyandry and sexual selection indices could be due to female fecundity rather than polyandry. We controlled for this potential confound by entering female fecundity (the average number of eggs laid by each female in a group) as a covariate in all analyses testing the effect of polyandry. Because average female fecundity was never significantly associated with sexual selection indices, results are not presented here. All correlations between polyandry and sexual selection indices and between selection gradients themselves were calculated using nonstandardized data with a linear regression (65). Normal distribution of residuals was verified with a Shapiro-Wilk test, and data were transformed when necessary. We calculated the statistical significance of the selection gradients over all males of all replicate groups by bootstrapping on general mixed models (with male as a random effect) of T according to M (gradient = 0.86, P < 0.001), T according to P (gradient = 0.87, P < 0.001), T according to M and P (gradient for M = 0.28, PM = 0.15, gradient for P = 0.72, PP = 0.001), and also T according to social status (gradient = 0.76, P < 0.001), M according to social status (gradient = 0.42, P < 0.001), and P according to social status (gradient = 0.46, P < 0.001).
Supplementary Material
Acknowledgments
We thank two anonymous reviewers for their insightful comments, Dominic Cram and Reesha Sodha for help with observations, and David Wilson for excellent animal husbandry. This work was funded by a Natural Environment Research Council grant (to T.P. and D.S.R.), a Light Scholarship from St. Catherine’s College (Oxford), and a doctoral scholarship from the Biotechnology and Biological Sciences Research Council (to J.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200219109/-/DCSupplemental.
References
- 1.Birkhead TR, Pizzari T. Postcopulatory sexual selection. Nat Rev Genet. 2002;3:262–273. doi: 10.1038/nrg774. [DOI] [PubMed] [Google Scholar]
- 2.Hughes WOH, Oldroyd BP, Beekman M, Ratnieks FLW. Ancestral monogamy shows kin selection is key to the evolution of eusociality. Science. 2008;320:1213–1216. doi: 10.1126/science.1156108. [DOI] [PubMed] [Google Scholar]
- 3.Cornwallis CK, West SA, Davis KE, Griffin AS. Promiscuity and the evolutionary transition to complex societies. Nature. 2010;466:969–972. doi: 10.1038/nature09335. [DOI] [PubMed] [Google Scholar]
- 4.West SA. Sex Allocation. Princeton: Princeton Univ Press; 2009. [Google Scholar]
- 5.Price TAR, Hodgson DJ, Lewis Z, Hurst GDD, Wedell N. Selfish genetic elements promote polyandry in a fly. Science. 2008;322:1241–1243. doi: 10.1126/science.1163766. [DOI] [PubMed] [Google Scholar]
- 6.Martin OY, Hosken DJ. The evolution of reproductive isolation through sexual conflict. Nature. 2003;423:979–982. doi: 10.1038/nature01752. [DOI] [PubMed] [Google Scholar]
- 7.Michalczyk L, et al. Inbreeding promotes female promiscuity. Science. 2011;333:1739–1742. doi: 10.1126/science.1207314. [DOI] [PubMed] [Google Scholar]
- 8.Pizzari T, Foster KR. Sperm sociality: Cooperation, altruism, and spite. PLoS Biol. 2008;6:e130. doi: 10.1371/journal.pbio.0060130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spencer HG, Feldman MW, Clark AG. Genetic conflicts, multiple paternity and the evolution of genomic imprinting. Genetics. 1998;148:893–904. doi: 10.1093/genetics/148.2.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker GA. Sexual conflict over mating and fertilization: An overview. Philos Trans R Soc Lond B Biol Sci. 2006;361:235–259. doi: 10.1098/rstb.2005.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eberhard WG. Postcopulatory sexual selection: Darwin’s omission and its consequences. Proc Natl Acad Sci USA. 2009;106(Suppl 1):10025–10032. doi: 10.1073/pnas.0901217106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darwin C. The Descent of Man, and Selection in Relation to Sex. London: John Murray; 1871. [Google Scholar]
- 13.Andersson M. Sexual Selection. Princeton: Princeton Univ Press; 1994. [Google Scholar]
- 14.Arnold SJ. In: Mate Choice. Bateson PPG, editor. Cambridge, UK: Cambridge Univ Press; 1983. pp. 67–107. [Google Scholar]
- 15.Kokko H, Jennions MD, Brooks R. Unifying and testing models of sexual selection. Annu Rev Ecol Evol Syst. 2006;37:43–66. [Google Scholar]
- 16.Webster MS, Pruett-Jones S, Westneat DF, Arnold SJ. Measuring the effects of pairing success, extra-pair copulations and mate quality on the opportunity for sexual selection. Evolution. 1995;49:1147–1157. doi: 10.1111/j.1558-5646.1995.tb04441.x. [DOI] [PubMed] [Google Scholar]
- 17.Møller AP, Ninni P. Sperm competition and sexual selection: A meta-analysis of paternity studies of birds. Behav Ecol Sociobiol. 1998;43:345–358. [Google Scholar]
- 18.Webster MS, Tarvin KA, Tuttle EM, Pruett-Jones S. Promiscuity drives sexual selection in a socially monogamous bird. Evolution. 2007;61:2205–2211. doi: 10.1111/j.1558-5646.2007.00208.x. [DOI] [PubMed] [Google Scholar]
- 19.Albrecht T, et al. Extra-pair fertilizations contribute to selection on secondary male ornamentation in a socially monogamous passerine. J Evol Biol. 2009;22:2020–2030. doi: 10.1111/j.1420-9101.2009.01815.x. [DOI] [PubMed] [Google Scholar]
- 20.Jones AG, Walker DE, Kvarnemo C, Lindström K, Avise JC. How cuckoldry can decrease the opportunity for sexual selection: Data and theory from a genetic parentage analysis of the sand goby, Pomatoschistus minutus. Proc Natl Acad Sci USA. 2001;98:9151–9156. doi: 10.1073/pnas.171310198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shuster SM, Wade MJ. Mating Systems and Strategies. Princeton: Princeton Univ Press; 2003. [Google Scholar]
- 22.Whittingham LA, Dunn PO. Effects of extra-pair and within-pair reproductive success on the opportunity for selection in birds. Behav Ecol. 2005;16:138–144. [Google Scholar]
- 23.Lesobre L, et al. Absence of male reproductive skew, along with high frequency of polyandry and conspecific brood parasitism in the lekking Houbara bustard Chlamydotis undulata undulata. J Avian Biol. 2010;41:117–127. [Google Scholar]
- 24.Andersson M, Simmons LW. Sexual selection and mate choice. Trends Ecol Evol. 2006;21:296–302. doi: 10.1016/j.tree.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Jones AG. On the opportunity for sexual selection, the Bateman gradient and the maximum intensity of sexual selection. Evolution. 2009;63:1673–1684. doi: 10.1111/j.1558-5646.2009.00664.x. [DOI] [PubMed] [Google Scholar]
- 26.Lorch PD, Bussière L, Gwynne DT. Quantifying the potential for sexual dimorphism using upper the limits on Bateman slopes. Behaviour. 2008;145:1–24. [Google Scholar]
- 27.Sullivan MS. Flock structure in red junglefowl. Appl Anim Behav Sci. 1991;30:381–386. [Google Scholar]
- 28.Lill A. Some observations on social organisation and non-random mating in captive Burmese red junglefowl (Gallus gallus spadiceus) Behaviour. 1966;26:228–241. [Google Scholar]
- 29.Pizzari T, Froman DP, Birkhead TR. Pre- and post-insemination episodes of sexual selection in the fowl, Gallus g. domesticus. Heredity (Edinb) 2002;88:112–116. doi: 10.1038/sj.hdy.6800014. [DOI] [PubMed] [Google Scholar]
- 30.Etches RJ. Reproduction in Poultry. Wallingford, UK: CABI International; 1996. [Google Scholar]
- 31.Gwynne DT, Simmons LW. Experimental reversal of courtship roles in an insect. Nature. 1990;346:172–174. [Google Scholar]
- 32.Jirotkul M. Operational sex ratio influences female preference and male-male competition in guppies. Anim Behav. 1999;58:287–294. doi: 10.1006/anbe.1999.1149. [DOI] [PubMed] [Google Scholar]
- 33.Jones AG, Rosenqvist G, Berglund A, Arnold SJ, Avise JC. The Bateman gradient and the cause of sexual selection in a sex-role-reversed pipefish. Proc Biol Sci. 2000;267:677–680. doi: 10.1098/rspb.2000.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bjork A, Pitnick S. Intensity of sexual selection along the anisogamy-isogamy continuum. Nature. 2006;441:742–745. doi: 10.1038/nature04683. [DOI] [PubMed] [Google Scholar]
- 35.Lorenzi MC, Sella G. A measure of sexual selection in hermaphroditic animals: Parentage skew and the opportunity for selection. J Evol Biol. 2008;21:827–833. doi: 10.1111/j.1420-9101.2008.01513.x. [DOI] [PubMed] [Google Scholar]
- 36.Collias NE, Collias EC. A field study of the red jungle fowl in north-central India. Condor. 1967;69:360–386. [Google Scholar]
- 37.Bohrnstedt GW, Goldberger AS. On the exact covariance of products of random variables. J Am Stat Assoc. 1969;64:1439–1442. [Google Scholar]
- 38.Arnold S, Wade MJ. On the measurement of natural and sexual selection: Theory. Evolution. 1984;38:709–719. doi: 10.1111/j.1558-5646.1984.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 39.Klug H, Heuschele J, Jennions MD, Kokko H. The mismeasurement of sexual selection. J Evol Biol. 2010;23:447–462. doi: 10.1111/j.1420-9101.2009.01921.x. [DOI] [PubMed] [Google Scholar]
- 40.Fitze PS, Le Galliard JF. Inconsistency between different measures of sexual selection. Am Nat. 2011;178:256–268. doi: 10.1086/660826. [DOI] [PubMed] [Google Scholar]
- 41.Krakauer AH, Webster MS, Duval EH, Jones AG, Shuster SM. The opportunity for sexual selection: Not mismeasured, just misunderstood. J Evol Biol. 2011;24:2064–2071. doi: 10.1111/j.1420-9101.2011.02317.x. [DOI] [PubMed] [Google Scholar]
- 42.Mills SC, Grapputo A, Koskela E, Mappes T. Quantitative measure of sexual selection with respect to the operational sex ratio: A comparison of selection indices. Proc Biol Sci. 2007;274:143–150. doi: 10.1098/rspb.2006.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pizzari T, Birkhead TR. Female feral fowl eject sperm of subdominant males. Nature. 2000;405:787–789. doi: 10.1038/35015558. [DOI] [PubMed] [Google Scholar]
- 44.Dean R, Nakagawa S, Pizzari T. The risk and intensity of sperm ejection in female birds. Am Nat. 2011;178:343–354. doi: 10.1086/661244. [DOI] [PubMed] [Google Scholar]
- 45.Martin PA, Reimers TJ, Lodge JR, Dziuk PJ. The effect of ratios and numbers of spermatozoa mixed from two males on proportions of offspring. J Reprod Fertil. 1974;39:251–258. doi: 10.1530/jrf.0.0390251. [DOI] [PubMed] [Google Scholar]
- 46.Froman DP, Pizzari T, Feltmann AJ, Castillo-Juarez H, Birkhead TR. Sperm mobility: Mechanisms of fertilizing efficiency, genetic variation and phenotypic relationship with male status in the domestic fowl, Gallus gallus domesticus. Proc Biol Sci. 2002;269:607–612. doi: 10.1098/rspb.2001.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cornwallis CK, Birkhead TR. Changes in sperm quality and numbers in response to experimental manipulation of male social status and female attractiveness. Am Nat. 2007;170:758–770. doi: 10.1086/521955. [DOI] [PubMed] [Google Scholar]
- 48.Fawcett TW, Kuijper B, Weissing FJ, Pen I. Sex-ratio control erodes sexual selection, revealing evolutionary feedback from adaptive plasticity. Proc Natl Acad Sci USA. 2011;108:15925–15930. doi: 10.1073/pnas.1105721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Markow TA. Perspective: Female remating, operational sex ratio, and the arena of sexual selection in Drosophila species. Evolution. 2002;56:1725–1734. doi: 10.1111/j.0014-3820.2002.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 50.Løvlie H, Pizzari T. Sex in the morning or in the evening? Females adjust daily mating patterns to the intensity of sexual harassment. Am Nat. 2007;170:E1–E13. doi: 10.1086/518180. [DOI] [PubMed] [Google Scholar]
- 51.Løvlie H, Cornwallis CK, Pizzari T. Male mounting alone reduces female promiscuity in the fowl. Curr Biol. 2005;15:1222–1227. doi: 10.1016/j.cub.2005.05.060. [DOI] [PubMed] [Google Scholar]
- 52.Cheng HH, Crittenden LB. Microsatellite markers for genetic mapping in the chicken. Poult Sci. 1994;73:539–546. doi: 10.3382/ps.0730539. [DOI] [PubMed] [Google Scholar]
- 53.Gibbs M, et al. Chicken microsatellite markers isolated from libraries enriched for simple tandem repeats. Anim Genet. 1997;28:401–417. [PubMed] [Google Scholar]
- 54.Dawson D, McConnell S, Wardle A, Gibbs M, Burke T. Characterization and mapping of 15 novel chicken microsatellite loci. Anim Genet. 1998;29:159–160. [PubMed] [Google Scholar]
- 55.McConnell SKJ, Dawson DA, Wardle A, Burke T. The isolation and mapping of 19 tetranucleotide microsatellite markers in the chicken. Anim Genet. 1999;30:183–189. doi: 10.1046/j.1365-2052.1999.00454.x. [DOI] [PubMed] [Google Scholar]
- 56.Crooijmans RPMA, van Oers PAM, Strijk JA, van der Poel JJ, Groenen MA. Preliminary linkage map of the chicken (Gallus domesticus) genome based on microsatellite markers: 77 new markers mapped. Poult Sci. 1996;75:746–754. doi: 10.3382/ps.0750746. [DOI] [PubMed] [Google Scholar]
- 57.Crooijmans RPMA, Dijkhof RJM, van der Poel JJ, Groenen MAM. New microsatellite markers in chicken optimized for automated fluorescent genotyping. Anim Genet. 1997;28:427–437. doi: 10.1111/j.1365-2052.1997.00205.x. [DOI] [PubMed] [Google Scholar]
- 58.Worley K, et al. MHC heterozygosity and survival in red junglefowl. Mol Ecol. 2010;19:3064–3075. doi: 10.1111/j.1365-294X.2010.04724.x. [DOI] [PubMed] [Google Scholar]
- 59.Marshall TC, Slate J, Kruuk LEB, Pemberton JM. Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol. 1998;7:639–655. doi: 10.1046/j.1365-294x.1998.00374.x. [DOI] [PubMed] [Google Scholar]
- 60.Lessells CM, Boag PT. Unrepeatable repeatabilities—a common mistake. Auk. 1987;104:116–121. [Google Scholar]
- 61.Crow JF. Some possibilities for measuring selection intensities in man. Hum Biol. 1958;30:1–13. [PubMed] [Google Scholar]
- 62.Wade MJ. Sexual selection and variance in reproductive success. Am Nat. 1979;114:742–764. doi: 10.1086/424531. [DOI] [PubMed] [Google Scholar]
- 63.Arnold SJ, Duvall D. Animal mating systems: A synthesis based on selection theory. Am Nat. 1994;143:317–348. [Google Scholar]
- 64.Lande R, Arnold SJ. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 65.Crawley MJ. The R Book. Hoboken, NJ: John Wiley & Sons; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.