Abstract
The SNF1/AMP-activated protein kinases are αβγ-heterotrimers that sense and regulate energy status in eukaryotes. They are activated by phosphorylation of the catalytic Snf1/α subunit, and the Snf4/γ regulatory subunit regulates phosphorylation through adenine nucleotide binding. In Saccharomyces cerevisiae, the Snf1 subunit is phosphorylated on the activation-loop Thr-210 in response to glucose limitation. To assess the requirement of the heterotrimer for regulated Thr-210 phosphorylation, we examined Snf1 and a truncated Snf1 kinase domain (residues 1–309) that has partial Snf1 function. Snf1(1–309) does not interact with the β and Snf4/γ regulatory subunits, and its activity was independent of them in vivo. Phosphorylation of both Snf1 and Snf1(1–309) increased in response to glucose limitation in wild-type cells and in cells lacking β- and Snf4/γ-subunits. These results indicate that glucose regulation of activation-loop phosphorylation can occur by mechanism(s) that function independently of the regulatory subunits. We further show that the Reg1-Glc7 protein phosphatase 1 and Sit4 type 2A-like phosphatase are largely responsible for dephosphorylation of Thr-210 of Snf1(1–309). Together, these findings suggest that these two phosphatases mediate heterotrimer-independent regulation of Thr-210 phosphorylation.
The SNF1/AMP-activated protein kinase (AMPK) family is conserved from yeast to humans and has central roles in energy regulation and stress responses. In mammals, AMPK regulates energy balance at both cellular and organismal levels and has important roles in human physiology and disease (1, 2). In the budding yeast Saccharomyces cerevisiae, SNF1 is activated in response to glucose limitation and other stresses and facilitates adaptation through control of transcription and metabolic enzymes (3, 4).
SNF1 and AMPK are heterotrimers composing a catalytic subunit (Snf1/α) and two regulatory subunits (β and Snf4/γ). Phosphorylation of the activation-loop Thr of the catalytic subunit activates SNF1/AMPK. For SNF1, the protein kinases Sak1, Tos3, and Elm1 phosphorylate Thr-210 on the Snf1/α subunit in response to glucose limitation and other stresses (5–8), but there is no evidence that these Snf1-activating kinases are regulated (9, 10). Less is understood about the protein phosphatases that deactivate SNF1. Reg1-Glc7 protein phosphatase 1 (PP1) has been thought to be solely responsible for dephosphorylating Thr-210 (9–14), and it has been proposed that access of Reg1-Glc7 to Thr-210 is restricted during growth on limiting glucose (9). However, recent evidence has also implicated the type 2A-like protein phosphatase Sit4 in Thr-210 dephosphorylation (15). Both the reg1Δ and sit4Δ mutants have defects in dephosphorylation, but they also have elevated levels of glycogen, and abolishing glycogen synthesis restored Thr-210 dephosphorylation during growth on high levels of glucose (15). The reg1Δ sit4Δ double mutant is not viable due to inappropriate activation of SNF1, which precluded studies to demonstrate that both Reg1-Glc7 and Sit4 function in dephosphorylation of Thr-210.
Biochemical and genetic evidence supports a role for the heterotrimeric structure of SNF1/AMPK in regulation of the kinase. The Snf4/γ-subunit regulates activation-loop phosphorylation through adenine nucleotide binding. The AMPK γ-subunit binds AMP (16, 17), which causes allosteric activation (18), promotes phosphorylation of the activation loop of the α-subunit (19), and inhibits its dephosphorylation in vitro (20, 21); binding of ADP is also involved in regulation of AMPK phosphorylation (22, 23). In the case of SNF1, AMP does not cause allosteric activation (24, 25) or protect the activation loop from dephosphorylation (20), but ADP protects against dephosphorylation of SNF1 in vitro (26, 27). In addition, Snf4 is required for the protein kinase activity of SNF1 (25, 28) and counteracts autoinhibition by Snf1 sequences C-terminal to the catalytic domain (28–30). The β-subunits specify subcellular localization (31), affect access to substrates (32, 33), and contain glycogen-binding domains (34, 35). Glycogen binding is inhibitory to AMPK in vitro (36), whereas the glycogen-binding domain of the major SNF1 β-subunit is required for dephosphorylation of Thr-210 during growth of cells on high levels of glucose, independent of glycogen (15, 37). Finally, substitutions of residues at dispersed sites in all three subunits of SNF1 result in Thr-210 phosphorylation during growth on high levels of glucose, suggesting that the conformation of the heterotrimer is important to maintaining the dephosphorylated state (37, 38).
Two observations suggest that other mechanisms, independent of the heterotrimer, contribute to regulation of SNF1. First, our laboratory and others (39) have observed increased Snf1 Thr-210 phosphorylation in response to glucose limitation in cells lacking the regulatory subunits. Second, the N-terminal kinase domain of the Snf1 subunit, truncated after residue 309 and lacking the C-terminal region that interacts with the β and Snf4/γ regulatory subunits (29, 40, 41), has partial function and confers glucose-regulated SUC2 expression (28); however, it is not clear whether activity of this truncated kinase domain is regulated.
We examined the requirement of the SNF1 heterotrimer for regulating phosphorylation of Thr-210 by assaying phosphorylation of Snf1 and the truncated Snf1 kinase domain, Snf1(1–309), in vivo in the presence and absence of the β and Snf4/γ regulatory subunits. We further used Snf1(1–309) to assess the roles of the protein phosphatases Reg1-Glc7 and Sit4.
Results
Glucose-Regulated Phosphorylation of Thr-210 in the Absence of Snf4/γ- and β-Subunits.
To assess the requirement of the regulatory subunits for regulating phosphorylation of Thr-210 in vivo, we compared phosphorylation of Snf1 in wild-type cells and in cells lacking Snf4/γ- and/or β-subunits (Fig. 1A). Cultures were grown to exponential phase on high glucose, shifted to low glucose for 10 min, and then replenished with high glucose. Cell extracts were prepared, proteins were resolved by SDS/PAGE, and Thr-210 phosphorylation was detected by immunoblot analysis.
Fig. 1.
Glucose regulation of Thr-210 phosphorylation in the absence of the heterotrimer. Cells of the indicated genotype were grown on high (H) glucose, resuspended in low (L) glucose for 10 min, and replenished with 2% glucose (+G) for 15 min. Protein extracts were separated by SDS/PAGE and subjected to immunoblot analysis to detect phosphorylated Thr-210 (pT210). Membranes were reprobed to detect Snf1 polypeptides. (A) Differing amounts of protein extract were loaded, and Snf1 was detected with anti-polyhistidine. (B–D) Cells expressed Snf1-myc or Snf1(1–309)-myc from the native promoter on centromeric plasmids. Protein extract (4 μg) was used. (B) Lanes are from the same blot but longer exposures are shown for Snf1-myc. (D) SNF1-myc cells expressed Snf1-8xmyc from the genomic locus.
In the snf4Δ mutant, Snf1 protein levels were reduced, and fourfold more protein was loaded to compensate. As in wild-type cells, levels of Thr-210 phosphorylation were low during growth on high glucose, phosphorylation increased substantially in response to glucose depletion, and glucose replenishment resulted in rapid dephosphorylation (Fig. 1A). For low glucose, the intensities of the bands corresponding to phosphorylated Thr-210, normalized to Snf1 protein, were the same for wild-type and snf4Δ samples. Because of the low level of phosphorylation during growth on high glucose, it was difficult to quantify the fold increase in phosphorylation in response to glucose depletion. With this caveat, the fold increases were similar (28- to 31-fold for WT and snf4Δ cells in two experiments). In cells lacking the three β-subunits (Gal83, Sip1, and Sip2; called βΔ) and in snf4Δ βΔ cells, Snf1 protein levels were markedly reduced, and sevenfold more protein was loaded, indicating that the heterotrimer, notably the β-subunit, serves to stabilize Snf1. Clearer results were obtained using myc-tagged Snf1, expressed from the SNF1 promoter on a centromeric plasmid, in snf1Δ snf4Δ βΔ cells. Phosphorylation of Snf1-myc was glucose-regulated; Snf1-myc was also unstable in these cells, so a long exposure is shown (Fig. 1B). Thus, phosphorylation of Snf1 remained glucose-regulated in the absence of the other subunits, but its instability hindered efforts to examine this regulation.
Regulated Activation-Loop Phosphorylation of the Snf1(1–309) Kinase Domain in Response to Glucose Availability.
To further assess the requirement of the heterotrimer for glucose-regulated Thr-210 phosphorylation in vivo, we compared phosphorylation of the truncated Snf1 kinase domain (28), which lacks sequences that interact with Snf4 and β-subunits, to that of Snf1. We expressed myc-tagged Snf1(1–309) from the SNF1 promoter on a centromeric plasmid in snf1Δ cells. Thr-210 phosphorylation of Snf1(1–309)-myc, like that of Snf1-myc, was low during growth on high glucose and increased substantially in response to glucose depletion; glucose replenishment resulted in rapid dephosphorylation (Fig. 1C). Snf1(1–309)-myc was present at higher levels than Snf1-myc, but was phosphorylated to a lesser extent; incubation in low glucose for 5 or 20 min did not result in greater phosphorylation. Expression of Snf1(1–309)-myc in cells expressing Snf1-myc from the genomic SNF1-myc locus gave similar results (Fig. 1D), as did expression of Snf1(1–309)-myc in cells expressing native Snf1 (detected using anti-polyhistidine), and showed that the presence of the truncated protein did not interfere with regulation of Snf1. The fold increases in phosphorylation in response to glucose depletion for Snf1-myc and Snf1(1–309)-myc appeared similar (values ranged from 32 to 37 in Fig. 1 C and D). Phosphorylation of Snf1(1–309)-myc was also glucose-regulated in snf1Δ snf4Δ βΔ cells, and Snf1(1–309) was stable (Fig. 1B). From these immunoblots, it is apparent that mechanism(s) for glucose regulation of Thr-210 phosphorylation act on the isolated kinase domain of Snf1.
To assay the catalytic activity of Snf1(1–309)-myc, we immunopurified Snf1(1–309)-myc and Snf1-myc from extracts of snf1Δ cells grown on high or low glucose and measured phosphorylation of a synthetic peptide substrate. Activity was normalized to the amount of immunopurified protein, as judged by immunoblot analysis of a dilution series. Values for Snf1(1–309)-myc in the high and low glucose samples were 0.15 ± 0.03 and 1.0 ± 0.03 (in arbitrary units), respectively, whereas those for Snf1-myc were 5.3 ± 0.5 and 37 ± 2, respectively. Thus, the activity of Snf1(1–309) toward this substrate was glucose-regulated but was low relative to that of heterotrimeric SNF1. We note that fold changes in activity are somewhat lower than the apparent changes in Thr-210 phosphorylation.
Activity of Snf1(1–309) Is Independent of Snf4/γ- and β-Subunits in Vivo.
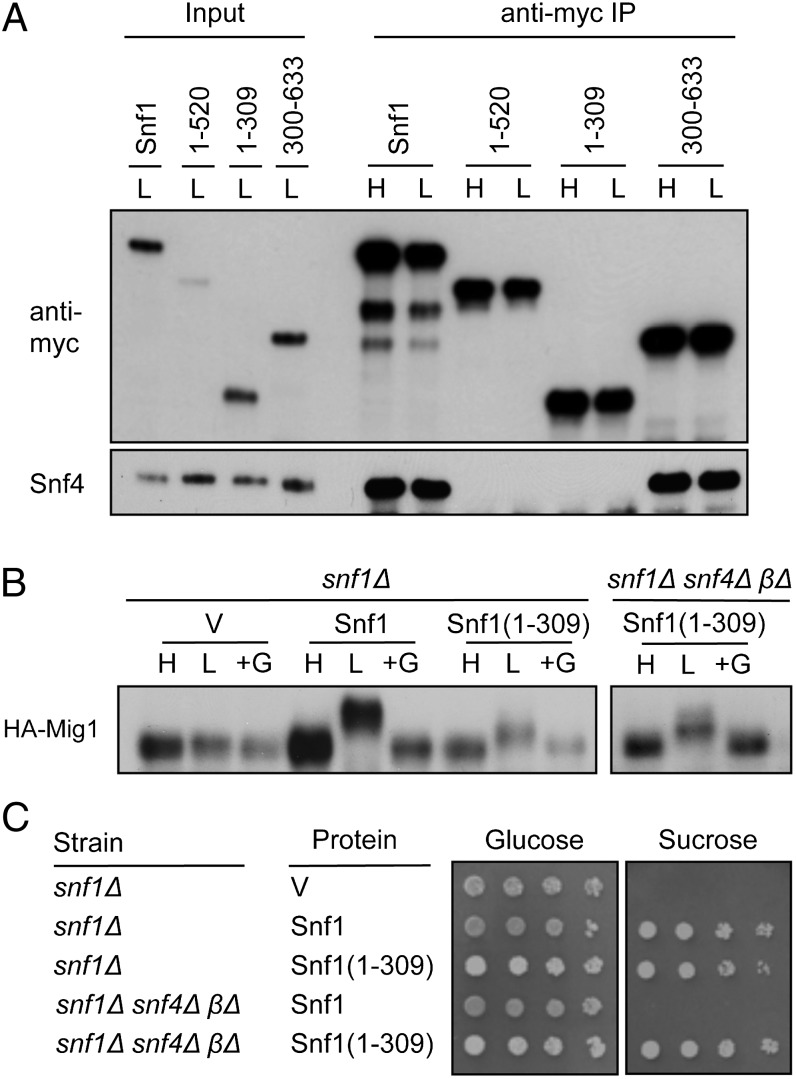
Previous studies provided no evidence that the truncated kinase domain interacts directly with the Snf4 or β subunits (29, 39, 41, 42). Correspondingly, we found that Snf4 coimmunopurified with myc-tagged Snf1 and Snf1(301–633), but not with Snf1(1–309) (Fig. 2A). Snf4 also did not copurify with Snf1(1–520), which lacks β-subunit–interacting sequences (40, 41), in accord with one study (39) but not with others (29, 42). We also found that Gal83 fused to green fluorescent protein (GFP) (31) did not coimmunopurify with Snf1(1–309)-myc from snf1Δ snf4Δ βΔ extracts. Finally, nuclear enrichment of Snf1-GFP in response to glucose depletion depends on the Gal83 β-subunit, as assayed by fluorescence microscopy (31), and Snf1(1–309)-GFP was not nuclear-enriched upon glucose depletion.
Fig. 2.
Function of Snf1(1–309) independent of the regulatory subunits. (A) myc-tagged Snf1 polypeptides containing the indicated residues were expressed from the native promoter on centromeric plasmids pYL199, pYL359 (43), pYL411, and pYL473 in snf1Δ cells. Proteins were immunopurified (IP) from cell extracts (0.2 mg) with anti-myc as described (43). Precipitated proteins and input (5%) were resolved by 9% SDS/PAGE and analyzed by immunoblotting with anti-myc and anti-Snf4. (B) snf1Δ or snf1Δ snf4Δ βΔ cells expressed 3xHA-Mig1 from the MIG1 promoter on a multicopy plasmid (44) and either expressed Snf1-myc or Snf1(1–309)-myc or carried vector (V). Preparation of cells was as in Fig. 1, and HA-Mig1 was detected by immunoblot analysis with anti-HA. The slower migrating forms of HA-Mig1 are phosphorylated (44, 45); expression from the multicopy plasmid resulted in variable levels of protein. No phosphorylation by Snf1-myc was detected in snf1Δ snf4Δ βΔ cells. (C) Cells expressing the indicated proteins, as in B, were grown overnight in SC + 2% glucose and spotted with serial fivefold dilutions on solid SC + 2% glucose or 2% sucrose plus the respiratory inhibitor antimycin A (1 μg/mL). Plates were incubated at 30 °C for 3 d and photographed.
Although Snf1 activity requires Snf4 (25, 28), Snf1(1–309) partially restored growth on Snf1-dependent carbon sources and expression of invertase from SUC2 in the snf4Δ mutant, and this partial function was independent of Snf4 (28, 46). To assess Snf1(1–309) activity in vivo more directly, we examined phosphorylation of a known SNF1 substrate, the Mig1 repressor, in response to glucose depletion. The SNF1 heterotrimer phosphorylates sites on Mig1 to release glucose repression of SUC2 and other genes (44, 45, 47, 48). Mig1 is nuclear in cells grown on high glucose (49). Although nuclear enrichment of SNF1 depends on Gal83, small proteins such as Snf1(1–309)-myc pass freely through the nuclear pore complex (50). We expressed Snf1(1–309)-myc or Snf1-myc and HA-tagged Mig1 in snf1Δ cells and analyzed HA-Mig1 by immunoblotting; reduced mobility reflects its phosphorylation (44, 45). Snf1(1–309)-myc partially phosphorylated HA-Mig1 in response to glucose limitation and did not require Snf4 or β-subunits (Fig. 2B). The reduced phosphorylation of HA-Mig1 most likely reflects low catalytic activity of Snf1(1–309), despite increased protein levels, and/or the lack of nuclear enrichment; however, Snf1(1–309) may also have altered substrate specificity and may only recognize some of the phosphorylation sites on Mig1. Snf1(1–309) also functioned independently of Snf4 and the β-subunits in growth assays (Fig. 2C), consistent with previous findings in the snf4Δ mutant (28, 46).
All evidence supports the view that the phosphorylation and function of Snf1(1–309) are independent of the Snf4 and β regulatory subunits. This enables use of Snf1(1–309) as a simple model for further genetic analysis of heterotrimer-independent regulation of Thr-210 phosphorylation.
Mutations That Cause Phosphorylation of the Heterotrimer During Growth on High Glucose Do Not Affect Snf1(1–309).
Several alterations in the Snf1 catalytic domain cause Thr-210 phosphorylation of SNF1 during growth on high glucose: substitution of Gly53 with Arg (G53R) and substitutions of Ala for Tyr106 (Y106A) in the αC helix or Leu198 (L198A) in the activation loop (38) (Fig. 3A). Substitution of Lys84 in the ATP-binding site with Arg (K84R) allowed low-level phosphorylation on high glucose (43). Previously, we proposed that such mutations, and others at dispersed sites in Gal83 and Snf4, perturb the SNF1 heterotrimer, and that the native conformation of the heterotrimer during growth on high glucose either prevents phosphorylation or promotes dephosphorylation (37, 38). Another possibility is that these kinase domain mutations affect the Thr-210 phosphorylation state by altering the structure of the kinase domain, perhaps affecting sensing of the glucose signal or interactions with Snf1-activating kinases or phosphatases. To determine whether these mutations affect regulation of Snf1(1–309)-myc, we expressed such mutant proteins in snf1Δ cells. K84R, L198A, and G53R did not cause phosphorylation during growth on high glucose, and Y106A had a minor effect (Fig. 3B). Thus, these kinase domain mutations affect phosphorylation primarily in the context of the heterotrimer, consistent with the idea that they perturb its conformation.
Fig. 3.
Effects of Snf1 kinase domain mutations on Thr-210 phosphorylation. (A) WT and mutant Snf1 proteins were expressed from the SNF1 promoter on centromeric plasmids pCE108 (28) and mutant derivatives (38) in snf1Δ cells carrying the GLC3 (WT) or glc3Δ (Δ) allele. Cells carrying glc3Δ, which abolishes glycogen synthesis, were tested to confirm that glycogen synthesis capability did not affect phosphorylation of the mutant Snf1 proteins. Cells were grown on high glucose and collected for immunoblot analysis; Snf1 was detected with anti-polyhistidine. (B) WT or mutant Snf1(1–309)-myc polypeptides were expressed in snf1Δ cells.
Each Snf1-Activating Kinase Recognizes Snf1(1–309) in Vivo.
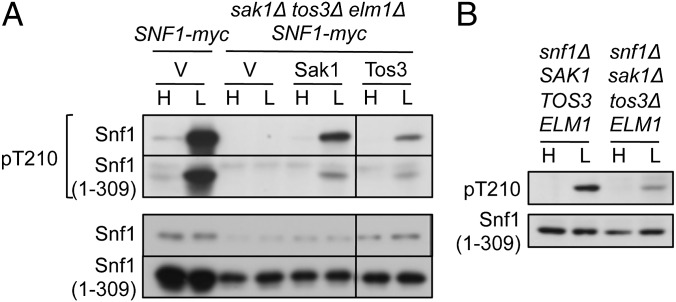
Each of the protein kinases Sak1, Tos3, and Elm1 phosphorylates SNF1 in vivo (5, 7). Sak1 is the primary upstream kinase (8, 51–53), and its C-terminal region interacts with the Snf1 kinase domain (43). To assess the role of the heterotrimer in determining interactions with activating kinases in vivo, we examined phosphorylation of Snf1(1–309). Snf1(1–309) was not phosphorylated in sak1Δ tos3Δ elm1Δ SNF1-myc cells (Fig. 4A), indicating that the heterotrimer does not serve to restrict phosphorylation by other protein kinases. Sak1 or Tos3 was sufficient for glucose-regulated phosphorylation (Fig. 4A), as was Elm1 (Fig. 4B). Hence, each of the Snf1-activating kinases recognizes the truncated kinase domain in vivo.
Fig. 4.
Phosphorylation of Snf1(1–309) by Snf1-activating kinases. Preparation of cells and immunoblot analysis were as in Fig. 1. (A) SNF1-myc cells and sak1Δ tos3Δ elm1Δ SNF1-myc cells expressed Snf1(1–309)-myc and Sak1-5xV5 or Tos3-5xV5 from the native promoters on centromeric plasmids pRS316-Sak1-5V5 (56) or pYL388 (43), respectively, or carried vector (V). All lanes are from the same blot. (B) snf1Δ sak1Δ tos3Δ ELM1 cells and control snf1Δ cells expressed Snf1(1–309)-myc. Control cells carrying vector showed no phosphorylation.
Effects of reg1Δ and sit4Δ on Dephosphorylation of Snf1(1–309).
Reg1-Glc7 PP1 and the type 2A-like Sit4 phosphatase have roles in dephosphorylation of Thr-210 of SNF1 (9, 10, 12–15, 54), and both Reg1 and Sit4 associate with Snf1 (12, 13, 15). The reg1Δ and sit4Δ mutants exhibited elevated Thr-210 phosphorylation during growth on high glucose, but glycogen levels were also elevated, and abolishing glycogen synthesis restored Thr-210 dephosphorylation; a partial defect in dephosphorylation upon glucose replenishment of glucose-limited cells was still evident in the absence of glycogen synthesis (15). Glycogen binding to the β-subunit was not involved (15, 37). These findings suggest that both protein phosphatases contribute to dephosphorylation of Snf1 on high glucose and that, in the absence of one phosphatase, elevated glycogen synthesis inhibits the other, perhaps by reducing glucose signaling, whereas in the absence of glycogen synthesis, the activity of a single phosphatase is sufficient (15). Inviability of the reg1Δ sit4Δ and reg1Δ sit4Δ glc3Δ mutants prevented us from verifying that both Reg1-Glc7 and Sit4 function in dephosphorylation of SNF1 during growth on high glucose.
To assess the effects of sit4Δ and reg1Δ in the absence of the SNF1 heterotrimer, we examined phosphorylation of Snf1(1–309)-myc in the mutants. As was the case for Snf1, Thr-210 phosphorylation of Snf1(1–309)-myc was elevated in sit4Δ snf1Δ cells on high glucose, and abolishing glycogen synthesis (glc3Δ) restored dephosphorylation, but a defect in dephosphorylation after glucose replenishment remained evident (Fig. 5A). In reg1Δ snf1Δ cells, no defect was observed (Fig. 5B), which was unexpected because Reg1 interacts with Snf1(1–392) in two-hybrid assays (12). We found, however, that Snf1(1–309) functions much less effectively than Snf1 for glycogen synthesis; during growth on high glucose, reg1Δ snf1Δ cells expressing Snf1-myc, Snf1(1–309)-myc, or vector had 48, 3.5, and 1.9 μg glycogen/108 cells, respectively (SD <10%). In contrast, the effect of sit4Δ on glycogen levels was Snf1-independent: the corresponding values for sit4Δ snf1Δ cells were 10, 4.7, and 8.1 μg glycogen/108 cells, a modest increase relative to 2.9 μg glycogen/108 cells in snf1Δ cells with Snf1-myc. These differences, and perhaps different effects of glycogen synthesis on Reg1-Glc7 and Sit4 function, may contribute to the different effects of reg1Δ and sit4Δ on Thr-210 phosphorylation.
Fig. 5.
Both Reg1-Glc7 and Sit4 phosphatases contribute to dephosphorylation of Snf1(1–309). (A–C) Snf1(1–309)-myc was expressed from pYL414 in cells of the indicated genotype. Preparation of cells and immunoblot analysis were as in Fig. 1, except that glucose replenishment (+G) was for 5 min. (C) Fourfold more protein was loaded for reg1Δ sit4Δ snf1Δ and reg1Δ sit4Δ glc3Δ snf1Δ samples than for snf1Δ samples. Several transformants of each type were analyzed.
Both Reg1-Glc7 and Sit4 Function in Dephosphorylation of Snf1(1–309).
The reg1Δ sit4Δ mutant is inviable, but further deletion of SNF1 rescues viability, indicating that inappropriate activity of SNF1 during growth on glucose is responsible for lethality. Because Snf1(1–309) provides only partial Snf1 function, we considered the possibility that reg1Δ sit4Δ snf1Δ cells expressing Snf1(1–309) might be viable and allow us to monitor Thr-210 phosphorylation in the absence of both phosphatases.
We were indeed able to express Snf1(1–309)-myc in reg1Δ sit4Δ snf1Δ and reg1Δ sit4Δ glc3Δ snf1Δ mutants. Snf1(1–309)-myc protein levels were low in these mutants, consistent with the view that Snf1 activity is deleterious (Fig. 5C; fourfold more protein was loaded for these samples than for snf1Δ samples). In the absence of both phosphatases, phosphorylation of Thr-210 was greatly elevated during growth on high glucose, and abolishing glycogen synthesis did not restore dephosphorylation (Fig. 5C; reg1Δ sit4Δ snf1Δ cells had 3.6 μg glycogen/108 cells). These effects of the reg1Δ sit4Δ double mutation reveal that both Reg1-Glc7 and Sit4 function in dephosphorylation of Snf1(1–309). Moreover, both strains exhibited only small further increases in Thr-210 phosphorylation in response to glucose limitation (1.6 ± 0.14- and 1.5 ± 0.06-fold increases, respectively), suggesting that these two phosphatases are largely responsible for Thr-210 dephosphorylation.
Discussion
The SNF1/AMPK family has a conserved heterotrimeric structure that is important for its regulation. The AMPK γ-subunit contributes to the regulation of activation-loop phosphorylation through nucleotide binding (16–23), and recent evidence supports a similar role for the Snf4 subunit of SNF1 (26, 27). In addition, mutations in all three SNF1 subunits cause Thr-210 phosphorylation during growth on high levels of glucose, suggesting that they perturb the heterotrimer and that its native conformation prevents Thr-210 phosphorylation or promotes its dephosphorylation (37, 38). In accord with this view, we have shown that mutations in the Snf1 kinase domain that cause phosphorylation in the context of the heterotrimer do not similarly affect Snf1(1–309).
We report here evidence that other mechanism(s), independent of the heterotrimer, contribute to regulation of the phosphorylation state of Thr-210. Snf1 exhibited glucose-regulated phosphorylation in the absence of the Snf4/γ- and β-subunits, although its stability was much reduced. The truncated Snf1(1–309) kinase domain, which does not interact with the Snf4 or β-subunits, also exhibited regulated phosphorylation. These findings show that substantial glucose regulation of activation-loop phosphorylation can occur by mechanisms that function independently of the regulatory subunits and the heterotrimer.
Such heterotrimer-independent regulation could occur at the level of the Snf1-activating kinases or the phosphatases that dephosphorylate Thr-210. Although the possibility of regulation of the activating kinases is not excluded, there is no evidence to support this idea; these kinases had similar activity when prepared from cells grown on high or low glucose (9), and expression of AMPK-activating kinases in yeast sufficed for glucose-regulated activation of SNF1 (10).
We show here that both Reg1-Glc7 PP1 and the type 2A-like phosphatase Sit4 function in dephosphorylation of Thr-210. Previous studies of single mutants implicated both phosphatases in dephosphorylation of SNF1 (15), but abolishing glycogen synthesis remedied their defects during growth on high glucose, and inviability of the reg1Δ sit4Δ double mutant precluded definitive genetic analysis. Here we were able to examine Thr-210 phosphorylation in the absence of one or both phosphatases by taking advantage of the diminished catalytic activity of Snf1(1–309); reg1Δ sit4Δ snf1Δ cells expressing Snf1(1–309) were viable. Snf1(1–309) was highly phosphorylated during growth of reg1Δ sit4Δ or reg1Δ sit4Δ glc3Δ cells on high glucose, but not in reg1Δ glc3Δ or sit4Δ glc3Δ mutants, indicating that both Reg1-Glc7 and Sit4 function in dephosphorylation of Thr-210. Moreover, glucose limitation increased Thr-210 phosphorylation less than twofold in reg1Δ sit4Δ mutants, suggesting that these two phosphatases are largely responsible for dephosphorylation on high glucose. These results further show that neither phosphatase requires the Snf4 or β-subunits to recognize Thr-210. We propose that regulation of Reg1-Glc7 and Sit4 activity is primarily responsible for the glucose-regulated phosphorylation of Snf1(1–309) (Fig. 6). Sit4 is most closely related to human PP6, which may be considered a candidate for an AMPK phosphatase.
Fig. 6.
Model for regulation of Thr-210 dephosphorylation by Reg1-Glc7 and Sit4. During growth on high levels of glucose, Reg1-Glc7 and Sit4 independently dephosphorylate Thr-210 of the truncated kinase domain Snf1(1–309); regulation could entail activation by a high-glucose signal, as shown, or inhibition by a low-glucose signal. In the absence of Sit4, elevated glycogen synthesis inhibits dephosphorylation by Reg1-Glc7, possibly by reducing glucose signaling as shown here, but when glycogen synthesis is abolished, Reg1-Glc7 function is sufficient for dephosphorylation.
Materials and Methods
Strains and Plasmids.
S. cerevisiae strains were constructed in the W303 genetic background; alleles were those used previously (15, 43). Cells were grown to midlog phase in selective synthetic complete medium (SC) containing 2% (wt/vol) glucose, unless otherwise specified. Plasmids pYL199 and pYL411 (43) express Snf1-8xmyc and Snf1(1–309)-8xmyc, respectively, from the SNF1 promoter on centromeric vector pRS313 (55) and were used unless otherwise noted; pYL414 expresses Snf1(1–309)-8xmyc on pRS314. pYL412, pYL418, and pYL419 are derivatives of pYL411 carrying the K84R, G53R, and Y106A mutations, respectively, and were constructed by transfer of a DNA fragment from the cognate mutant plasmid (38, 43). pYL417 is a similar derivative carrying L198A (38), which was constructed by transfer of a PCR-amplified fragment. pYL473 expresses Snf1(1–520)-8xmyc from the SNF1 promoter on pRS313. pAR38 is a centromeric plasmid, derived from pOV84 (31), that expresses Snf1(1–309)-GFP from the SNF1 promoter.
Growth of Cultures.
Cells were grown to midlog phase in SC + 2% (high) glucose, and an aliquot of the culture was collected by rapid filtration to preserve the phosphorylation state of Thr-210, and frozen in liquid nitrogen. Another aliquot was collected by rapid filtration and resuspended in SC + 0.05% (low) glucose for 10 min, or as otherwise specified, and collected by filtration and frozen. In some cases, after incubation in 0.05% glucose, an aliquot was replenished with 2% glucose for 15 min and collected.
Immunoblot Analysis.
Whole-cell extracts were prepared (15), and proteins were separated by SDS/PAGE on 7.5% polyacrylamide and analyzed by immunoblotting. Antibodies were anti-Thr(P)-172-AMPK (Cell Signaling Technologies), anti-myc (9E10; Santa Cruz), anti-polyhistidine (Sigma; Snf1 has a stretch of histidine residues), anti-HA (12CA5; Roche), anti-Snf4 (46), and anti-GFP (Clontech). Membranes were reprobed with anti-myc to detect Snf1 polypeptides unless otherwise noted. Before the membrane was reprobed, it was incubated in 0.2 M glycine, pH 2, for 10 min. ECL Plus (GE Healthcare) was used for visualization. Intensity of the bands was quantified using ImageJ software from the National Institutes of Health (57). Fold changes in phosphorylation were determined by comparing the intensity of bands corresponding to phosphorylated Thr-210, normalized to Snf1 polypeptide.
Assay for Catalytic Activity.
Whole-cell extracts were prepared (15), and proteins were immunopurified from extracts (0.25 mg) as described (43), except that extracts were precleared by incubation for 1 h with protein A agarose (Roche Applied Science), and the immunoprecipitation buffer was 50 mM Tris⋅HCl (pH 7.4), 150 mM NaCl, 50 mM NaF, 5 mM sodium pyrophosphate, 1 mM EDTA, 1 mM DTT, 1 mM PMSF, and Complete protease inhibitor (Roche Applied Science). Beads were resuspended in assay buffer, and Snf1 catalytic activity was assayed by measuring phosphorylation of the SAMS peptide (HMRSAMSGLHLVKRR) (58) as described (38); three dilutions were assayed for each sample. Activity (nmol of phosphate incorporated into peptide/min) was normalized to the amount of immunopurified protein, quantified by immunoblot analysis using a series of dilutions, and expressed in arbitrary units. Values are averages of four to seven assays of two to four independent cultures.
Determination of Glycogen Content.
Cells (10 mL) were harvested by centrifugation, and glycogen content was assayed (15, 59). Values are averages for four cultures, and SDs were <10%.
Acknowledgments
We thank Martin Schmidt for the Sak1 plasmid and Mark Johnston for comments on the manuscript. This work was supported by National Institutes of Health Grant GM34095 to M.C.
Footnotes
The authors declare no conflict of interest.
References
- 1.Hardie DG. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 3.Celenza JL, Carlson M. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science. 1986;233:1175–1180. doi: 10.1126/science.3526554. [DOI] [PubMed] [Google Scholar]
- 4.Hedbacker K, Carlson M. SNF1/AMPK pathways in yeast. Front Biosci. 2008;13:2408–2420. doi: 10.2741/2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong SP, Leiper FC, Woods A, Carling D, Carlson M. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc Natl Acad Sci USA. 2003;100:8839–8843. doi: 10.1073/pnas.1533136100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nath N, McCartney RR, Schmidt MC. Yeast Pak1 kinase associates with and activates Snf1. Mol Cell Biol. 2003;23:3909–3917. doi: 10.1128/MCB.23.11.3909-3917.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutherland CM, et al. Elm1p is one of three upstream kinases for the Saccharomyces cerevisiae SNF1 complex. Curr Biol. 2003;13:1299–1305. doi: 10.1016/s0960-9822(03)00459-7. [DOI] [PubMed] [Google Scholar]
- 8.Hong SP, Carlson M. Regulation of Snf1 protein kinase in response to environmental stress. J Biol Chem. 2007;282:16838–16845. doi: 10.1074/jbc.M700146200. [DOI] [PubMed] [Google Scholar]
- 9.Rubenstein EM, et al. Access denied: Snf1 activation loop phosphorylation is controlled by availability of the phosphorylated threonine 210 to the PP1 phosphatase. J Biol Chem. 2008;283:222–230. doi: 10.1074/jbc.M707957200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong SP, Momcilovic M, Carlson M. Function of mammalian LKB1 and Ca2+/calmodulin-dependent protein kinase kinase alpha as Snf1-activating kinases in yeast. J Biol Chem. 2005;280:21804–21809. doi: 10.1074/jbc.M501887200. [DOI] [PubMed] [Google Scholar]
- 11.Tu J, Carlson M. REG1 binds to protein phosphatase type 1 and regulates glucose repression in Saccharomyces cerevisiae. EMBO J. 1995;14:5939–5946. doi: 10.1002/j.1460-2075.1995.tb00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ludin K, Jiang R, Carlson M. Glucose-regulated interaction of a regulatory subunit of protein phosphatase 1 with the Snf1 protein kinase in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1998;95:6245–6250. doi: 10.1073/pnas.95.11.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanz P, Alms GR, Haystead TA, Carlson M. Regulatory interactions between the Reg1-Glc7 protein phosphatase and the Snf1 protein kinase. Mol Cell Biol. 2000;20:1321–1328. doi: 10.1128/mcb.20.4.1321-1328.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCartney RR, Schmidt MC. Regulation of Snf1 kinase. Activation requires phosphorylation of threonine 210 by an upstream kinase as well as a distinct step mediated by the Snf4 subunit. J Biol Chem. 2001;276:36460–36466. doi: 10.1074/jbc.M104418200. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz A, Xu X, Carlson M. Roles of two protein phosphatases, Reg1-Glc7 and Sit4, and glycogen synthesis in regulation of SNF1 protein kinase. Proc Natl Acad Sci USA. 2011;108:6349–6354. doi: 10.1073/pnas.1102758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott JW, et al. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113:274–284. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao B, et al. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449:496–500. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- 18.Davies SP, Helps NR, Cohen PT, Hardie DG. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C α and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- 19.Oakhill JS, et al. β-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK) Proc Natl Acad Sci USA. 2010;107:19237–19241. doi: 10.1073/pnas.1009705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403:139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suter M, et al. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem. 2006;281:32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- 22.Oakhill JS, et al. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011;332:1433–1435. doi: 10.1126/science.1200094. [DOI] [PubMed] [Google Scholar]
- 23.Xiao B, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchelhill KI, et al. Mammalian AMP-activated protein kinase shares structural and functional homology with the catalytic domain of yeast Snf1 protein kinase. J Biol Chem. 1994;269:2361–2364. [PubMed] [Google Scholar]
- 25.Woods A, et al. Yeast SNF1 is functionally related to mammalian AMP-activated protein kinase and regulates acetyl-CoA carboxylase in vivo. J Biol Chem. 1994;269:19509–19515. [PubMed] [Google Scholar]
- 26.Mayer FV, et al. ADP regulates SNF1, the Saccharomyces cerevisiae homolog of AMP-activated protein kinase. Cell Metab. 2011;14:707–714. doi: 10.1016/j.cmet.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandrashekarappa DG, McCartney RR, Schmidt MC. Subunit and domain requirements for adenylate-mediated protection of Snf1 kinase activation loop from dephosphorylation. J Biol Chem. 2011;286:44532–44541. doi: 10.1074/jbc.M111.315895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Celenza JL, Carlson M. Mutational analysis of the Saccharomyces cerevisiae SNF1 protein kinase and evidence for functional interaction with the SNF4 protein. Mol Cell Biol. 1989;9:5034–5044. doi: 10.1128/mcb.9.11.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang R, Carlson M. Glucose regulates protein interactions within the yeast SNF1 protein kinase complex. Genes Dev. 1996;10:3105–3115. doi: 10.1101/gad.10.24.3105. [DOI] [PubMed] [Google Scholar]
- 30.Leech A, Nath N, McCartney RR, Schmidt MC. Isolation of mutations in the catalytic domain of the Snf1 kinase that render its activity independent of the snf4 subunit. Eukaryot Cell. 2003;2:265–273. doi: 10.1128/EC.2.2.265-273.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vincent O, Townley R, Kuchin S, Carlson M. Subcellular localization of the Snf1 kinase is regulated by specific beta subunits and a novel glucose signaling mechanism. Genes Dev. 2001;15:1104–1114. doi: 10.1101/gad.879301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vincent O, Carlson M. Gal83 mediates the interaction of the Snf1 kinase complex with the transcription activator Sip4. EMBO J. 1999;18:6672–6681. doi: 10.1093/emboj/18.23.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt MC, McCartney RR. β-Subunits of Snf1 kinase are required for kinase function and substrate definition. EMBO J. 2000;19:4936–4943. doi: 10.1093/emboj/19.18.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polekhina G, et al. Structural basis for glycogen recognition by AMP-activated protein kinase. Structure. 2005;13:1453–1462. doi: 10.1016/j.str.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Wiatrowski HA, et al. Mutations in the Gal83 glycogen-binding domain activate the Snf1/Gal83 kinase pathway by a glycogen-independent mechanism. Mol Cell Biol. 2004;24:352–361. doi: 10.1128/MCB.24.1.352-361.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McBride A, Ghilagaber S, Nikolaev A, Hardie DG. The glycogen-binding domain on the AMPK β subunit allows the kinase to act as a glycogen sensor. Cell Metab. 2009;9:23–34. doi: 10.1016/j.cmet.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Momcilovic M, Iram SH, Liu Y, Carlson M. Roles of the glycogen-binding domain and Snf4 in glucose inhibition of SNF1 protein kinase. J Biol Chem. 2008;283:19521–19529. doi: 10.1074/jbc.M803624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Momcilovic M, Carlson M. Alterations at dispersed sites cause phosphorylation and activation of SNF1 protein kinase during growth on high glucose. J Biol Chem. 2011;286:23544–23551. doi: 10.1074/jbc.M111.244111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elbing K, Rubenstein EM, McCartney RR, Schmidt MC. Subunits of the Snf1 kinase heterotrimer show interdependence for association and activity. J Biol Chem. 2006;281:26170–26180. doi: 10.1074/jbc.M603811200. [DOI] [PubMed] [Google Scholar]
- 40.Amodeo GA, Rudolph MJ, Tong L. Crystal structure of the heterotrimer core of Saccharomyces cerevisiae AMPK homologue SNF1. Nature. 2007;449:492–495. doi: 10.1038/nature06127. [DOI] [PubMed] [Google Scholar]
- 41.Jiang R, Carlson M. The Snf1 protein kinase and its activating subunit, Snf4, interact with distinct domains of the Sip1/Sip2/Gal83 component in the kinase complex. Mol Cell Biol. 1997;17:2099–2106. doi: 10.1128/mcb.17.4.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amodeo GA, Momcilovic M, Carlson M, Tong L. Biochemical and functional studies on the regulation of the Saccharomyces cerevisiae AMPK homolog SNF1. Biochem Biophys Res Commun. 2010;397:197–201. doi: 10.1016/j.bbrc.2010.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Xu X, Carlson M. Interaction of SNF1 protein kinase with its activating kinase Sak1. Eukaryot Cell. 2011;10:313–319. doi: 10.1128/EC.00291-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Treitel MA, Kuchin S, Carlson M. Snf1 protein kinase regulates phosphorylation of the Mig1 repressor in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18:6273–6280. doi: 10.1128/mcb.18.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Treitel MA, Carlson M. Repression by SSN6-TUP1 is directed by MIG1, a repressor/activator protein. Proc Natl Acad Sci USA. 1995;92:3132–3136. doi: 10.1073/pnas.92.8.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Estruch F, Treitel MA, Yang X, Carlson M. N-terminal mutations modulate yeast SNF1 protein kinase function. Genetics. 1992;132:639–650. doi: 10.1093/genetics/132.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostling J, Ronne H. Negative control of the Mig1p repressor by Snf1p-dependent phosphorylation in the absence of glucose. Eur J Biochem. 1998;252:162–168. doi: 10.1046/j.1432-1327.1998.2520162.x. [DOI] [PubMed] [Google Scholar]
- 48.Smith FC, Davies SP, Wilson WA, Carling D, Hardie DG. The SNF1 kinase complex from Saccharomyces cerevisiae phosphorylates the transcriptional repressor protein Mig1p in vitro at four sites within or near regulatory domain 1. FEBS Lett. 1999;453:219–223. doi: 10.1016/s0014-5793(99)00725-5. [DOI] [PubMed] [Google Scholar]
- 49.De Vit MJ, Waddle JA, Johnston M. Regulated nuclear translocation of the Mig1 glucose repressor. Mol Biol Cell. 1997;8:1603–1618. doi: 10.1091/mbc.8.8.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wente SR, Rout MP. The nuclear pore complex and nuclear transport. Cold Spring Harb Perspect Biol. 2010;2:a000562. doi: 10.1101/cshperspect.a000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hedbacker K, Hong SP, Carlson M. Pak1 protein kinase regulates activation and nuclear localization of Snf1-Gal83 protein kinase. Mol Cell Biol. 2004;24:8255–8263. doi: 10.1128/MCB.24.18.8255-8263.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCartney RR, Rubenstein EM, Schmidt MC. Snf1 kinase complexes with different beta subunits display stress-dependent preferences for the three Snf1-activating kinases. Curr Genet. 2005;47:335–344. doi: 10.1007/s00294-005-0576-2. [DOI] [PubMed] [Google Scholar]
- 53.Elbing K, McCartney RR, Schmidt MC. Purification and characterization of the three Snf1-activating kinases of Saccharomyces cerevisiae. Biochem J. 2006;393:797–805. doi: 10.1042/BJ20051213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bozaquel-Morais BL, Madeira JB, Maya-Monteiro CM, Masuda CA, Montero-Lomeli M. A new fluorescence-based method identifies protein phosphatases regulating lipid droplet metabolism. PLoS ONE. 2010;5:e13692. doi: 10.1371/journal.pone.0013692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rubenstein EM, McCartney RR, Schmidt MC. Regulatory domains of Snf1-activating kinases determine pathway specificity. Eukaryot Cell. 2006;5:620–627. doi: 10.1128/EC.5.4.620-627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 58.Davies SP, Carling D, Hardie DG. Tissue distribution of the AMP-activated protein kinase, and lack of activation by cyclic-AMP-dependent protein kinase, studied using a specific and sensitive peptide assay. Eur J Biochem. 1989;186:123–128. doi: 10.1111/j.1432-1033.1989.tb15185.x. [DOI] [PubMed] [Google Scholar]
- 59.Parrou JL, François J. A simplified procedure for a rapid and reliable assay of both glycogen and trehalose in whole yeast cells. Anal Biochem. 1997;248:186–188. doi: 10.1006/abio.1997.2138. [DOI] [PubMed] [Google Scholar]