Abstract
The homeostasis of naive T cells is essential for protective immunity against infection, but the cell-intrinsic molecular mechanisms that control naïve T-cell homeostasis are poorly understood. Genetic ablation in lower organisms has revealed a critical role for Vps34, an evolutionary conserved class III phosphoinositide-3 kinase (PI3K), in regulating endocytosis and autophagy; however, the physiological function of Vps34 in the immune system, especially in T cells, is unclear. Here we report that Vps34 is required for the maintenance of naïve T cells, acting in a cell-intrinsic manner. T-cell–specific deletion of the gene encoding Vps34 resulted in reduced stability of Vps15 and Beclin-1, components of the class III PI3K complex, and impaired autophagy in T cells. Vps34 was dispensable for T-cell development but important for the survival of naïve T cells. Vps34-deficient T cells showed increased mitochondrial mass and accumulation of reactive oxygen species, consistent with deficient removal of damaged mitochondria. Thus, Vps34-dependent canonical autophagy plays a critical role in maintaining T-cell homeostasis by promoting T-cell survival through quality control of mitochondria.
Keywords: knockout, LC3B, chimera, MitoTracker
The homeostasis of naive T cells is crucial to ensure a diverse repertoire of T cells capable of protecting the host from pathogens and to prevent spontaneous T-cell activation, leading to autoimmunity (1, 2). The number of naïve T cells is kept constant by thymic output and by homeostatic mechanisms in the periphery, such as contact with self-peptide/MHC molecules and γc cytokines. But although much is known about these cell-extrinsic factors, the cell-intrinsic molecular mechanisms that control naïve T-cell homeostasis remain largely undefined.
The phosphoinositide-3 kinase (PI3K) Vps34, encoded by the Pik3c3 gene, was first identified in yeast in a screen for vacuolar protein sorting (Vps) mutants. Vps34 is the only member of the class III PI3K family of lipid kinases and is evolutionary conserved from lower eukaryotes to plants and mammals (3, 4). It phosphorylates phosphoinositides at the 3 hydroxy position, thereby generating phosphoinositide 3-phosphate [PI(3)P], which is essential for vesicular trafficking. Vps34 closely associates with Vps15, a protein required for Vps34 kinase activity in vivo. Distinct Vps34–Vps15 protein complexes exist inside the cell and differentially regulate vesicular trafficking (5–7). Genetic ablation of Vps34 in lower organisms has confirmed its essential role in endocytosis and autophagy. In mammalian cells, Vps34 so far has been studied mainly in cell lines and found to regulate endocytic trafficking, phagosome maturation, and autophagosome formation (3, 4).
Autophagy is an evolutionary conserved and fundamental catabolic cellular process (8–10). It plays an important role in cellular homeostasis by removing unwanted intracellular material (e.g., damaged organelles) and by providing nutrients during starvation. During (macro) autophagy, intracellular material is engulfed in double-membrane structures called autophagosomes and is degraded after fusion with lysosomes. Autophagosome biogenesis is a highly regulated process. During vesicle nucleation, the class III PI3K complex consisting of Vps34, Vps15, and Beclin-1 results in the production of PI(3)P and recruitment of effector proteins to the so-called “isolation membrane.” The next step, vesicle elongation, is mediated by two ubiquitin-like conjugation systems, Atg7–Atg10 and Atg7–Atg3. This leads to formation of the Atg5–Atg12–Atg16L complex, lipidation of LC3, and closure of the autophagosome.
Recent data suggest that regulated autophagy is important for naïve T-cell homeostasis (10–13). For example, T cells from Atg3, Atg5, and Atg7 KO mice have shown increased apoptosis and impaired TCR-induced proliferation in vitro (14–18). However, whether the effect of Atg5 or Atg7 deficiency on T-cell homeostasis is mediated through autophagy or nonautophagic functions of these genes is not clear. Thus, to clarify the requirement of autophagy for T-cell homeostasis, it is useful to examine mice with mutations in genes lying upstream of Atg5 and Atg7 in the autophagy pathway, for example, genes encoding the class III PI3K complex (Vps34–Vps15–Beclin-1). Similar to Atg5 and Atg7 KO mice, mice with a T-cell–specific deletion of Beclin-1 (Cd4-CreBecn1flox/flox mice) showed impaired T-cell homeostasis (19). In contrast, another study using Beclin-1–deficient mice derived from Rag1 blastocyst complementation reported a defect in T-cell development but a normal peripheral T-cell compartment (20). Therefore, the role of autophagy—specifically the role of the class III PI3K complex—in T-cell homeostasis remains unclear. In addition, at least in cell lines, instances of noncanonical autophagy have been reported (i.e., autophagy that is Vps34-/Beclin-1–independent) (21, 22). However, whether noncanonical autophagy exists in T cells and, if so, its function, remain unknown.
Genetic studies in T cells are needed to address these controversial areas conclusively. Pharmacologic inhibitors have been used to inhibit Vps34 catalytic activity; however, those studies have been hampered by a lack of specificity, given that inhibitors like wortmannin and LY294002 also inhibit class I and II PI3Ks, whereas the commonly used class III PI3K inhibitor 3-MA also is not entirely specific and has toxicity toward primary T cells at concentrations used in cell lines. Thus, we generated gene-targeted mice, allowing cell-type–specific deletion of Vps34 to define its physiological role in T cells. We found that Vps34-dependent canonical autophagy is essential for the homeostasis of naïve T cells in vivo by promoting T-cell survival through the removal of damaged mitochondria.
Results
Deletion of Vps34 Leads to Disruption of the Class III PI3K Complex in T Cells.
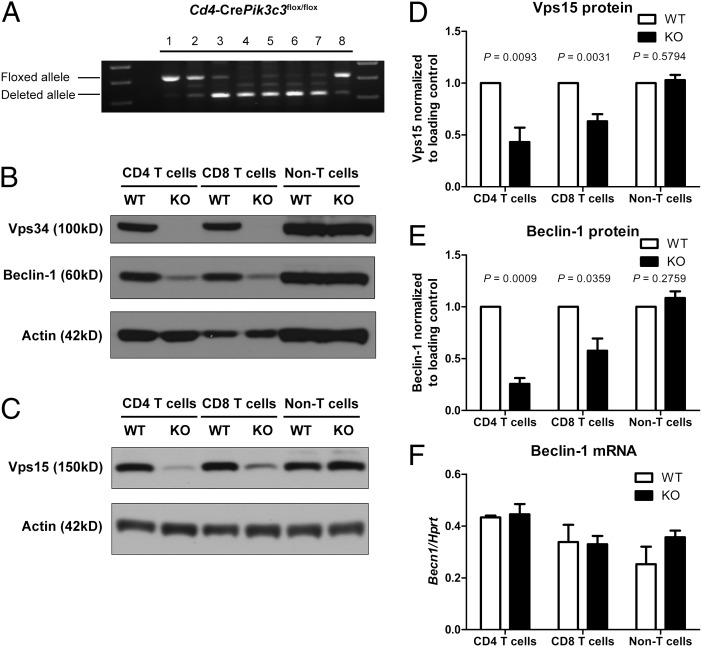
We generated conditional Pik3c3 KO mice by gene targeting to examine the function of Vps34 in T cells in vivo. Exon 4 of the Pik3c3 gene was flanked by LoxP sites to enable its excision by Cre recombinase (Fig. S1). Deletion of exon 4 leads to a frame shift and, if expressed, would result in a N-terminally truncated Vps34 protein lacking any functional domains. Pik3c3flox/flox mice were crossed to Cd4-Cre transgenic mice to delete Vps34 specifically in CD4 and CD8 T cells (23). The mice were then intercrossed to generate Cd4-CrePik3c3flox/flox (referred to as Vps34 KO) mice. Littermate Cd4-CrePik3c3+/+ or Pik3c3flox/flox (referred to as Vps34 WT) and Cd4-CrePik3c3flox/+ (referred to as Vps34 HET) mice were used as controls. The floxed Pik3c3 allele was efficiently deleted in double-positive (DP) thymocytes, CD4 and CD8 single-positive (SP) thymocytes, and peripheral CD4 and CD8 T cells from Vps34 KO mice, as demonstrated by genomic PCR (Fig. 1A). Deletion was T-cell specific, not occurring in non-T cells (mixture of B220+NK1.1+CD25+CD11b+CD11c+ cells) from Vps34 KO mice. Furthermore, Western blot analysis with antibodies against either the N- or C- terminus of Vps34 confirmed the absence of full-length Vps34 protein in CD4 and CD8 T cells, but not in non-T cells, from Vps34 KO mice (Fig. 1B).
Fig. 1.
Deletion of Vps34 leads to disruption of the class III PI3K complex in T cells. (A) PCR analysis of genomic DNA from various cell populations isolated from Cd4-CrePik3c3flox/flox mice. 1, tail; 2, DN; 3, DP; 4, CD4 SP; 5, CD8 SP thymocytes; 6, peripheral CD4 T cells; 7, peripheral CD8 T cells; 8, non-T cells (mixture of B220+ NK1.1+ CD25+ CD11b+ CD11c+ cells). (B and C) Western blot analysis of Vps34 and Beclin-1 (B) and Vps15 (C) protein expression in CD4 and CD8 T cells as well as non-T cells from Cd4-CrePik3c3+/+ or Pik3c3flox/flox (WT) and Cd4-CrePik3c3flox/flox (KO) mice. Actin was used as a loading control. (D and E) Densitometry of Vps15 (D) and Beclin-1 (E) protein expression in Vps34 WT and KO cells as determined by Western blot analysis (n = 4–6). Amounts of Vps15 and Beclin-1 protein were normalized to actin and are relative to amounts in WT cells. (E) Quantitative RT-PCR analysis of Beclin-1 mRNA expression in CD4 and CD8 T cells as well as non-T cells from Vps34 WT and KO mice (n = 3). mRNA expression was normalized to Hprt. Data are from one experiment (A), three independent experiments (F), or four to six independent experiments (B–E).
Vps34 is found in multiprotein complexes that differentially regulate autophagy versus endocytic trafficking. Therefore, we examined the effect of Vps34 deficiency on its main binding partners Vps15 and Beclin-1, two components of the class III PI3K complex. Western blot analysis showed that Vps34-deficient T cells had less Beclin-1 protein compared with WT cells (Fig. 1 B and E). The amount of Vps15 protein was also reduced in Vps34 KO T cells (Fig. 1 C and D). The reduction in Beclin-1 protein was due to a posttranscriptional mechanism, because Becn1 mRNA expression was not changed in the absence of Vps34 (Fig. 1F), similar to what has been reported in HeLa cells after Vps34 knockdown (24). These results suggest that Vps34 is required to maintain Beclin-1 protein expression in T cells, most likely by protecting Beclin-1 from degradation.
Vps34 Is Required for Autophagy in T Cells.
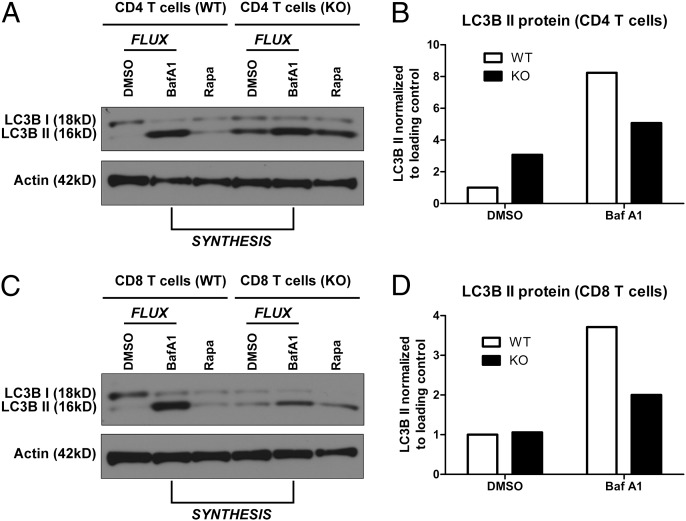
We next examined whether Vps34 deletion leads to defective autophagy in T cells. To begin, we analyzed the conversion of LC3B to its lipidated form, LC3B II, by Western blot analysis. After lipidation, LC3B is incorporated into the autophagosomal membrane and is currently the most specific known marker for autophagosomes, given that the amount of LC3B II is correlated with the number of autophagosomes (25). Activated CD4 cells (Fig. 2A) and CD8 T cells (Fig. 2C) from Vps34 KO mice were able to produce LC3B II in steady-state conditions, indicating that Vps34 is not required for LC3B lipidation. A similar observation has been made in HeLa cells, and it has been suggested that the presence of LC3B II in Vps34-deficient cells may be due to aberrant formation on nonautophagosomal membranes (24). We also examined the turnover of p62, an adaptor protein that links the ubiquitin system to the autophagy pathway and is a well-known autophagy substrate itself (25). In general, the amount of p62 protein is correlated with the autophagic activity in the cell. Resting Vps34-deficient CD8 T cells had slightly higher amounts of p62 protein measured on Western blot analysis (Fig. S2), although the difference did not reach statistical significance.
Fig. 2.
Autophagic activity is reduced in Vps34-deficient T cells. (A–D) Western blot analysis and densitometry of LC3B protein expression in activated CD4 T cells (A and B) and CD8 T cells (C and D) from Vps34 WT or KO mice. T cells were activated with α-CD3 and α-CD28 mAbs for 24 h in vitro. The lysosomal inhibitor BafA1 was added for the last 4 h of culture. DMSO was used as a solvent control. The nonlipidated (I) and lipidated (II) isoforms of LC3B are indicated. The difference in the amounts of LC3B II between BafA1-treated cells and DMSO-treated cells is a measure of LC3B II degradation, and thus of autophagic flux (compare ΔBafA1-DMSO LC3B II in WT and KO cells). The amount of LC3B II in the presence of BafA1 is a measure of LC3B II production and thus of autophagosome synthesis (compare LC3BII in BafA1-treated WT and KO cells). Data are representative of two independent experiments.
Precise assessment of cellular autophagic activity requires measurement of autophagic flux (25). Thus, we measured the amount of LC3B II in the absence or presence of bafilomycin A1 (BafA1), which inhibits the lysosomal degradation of LC3B II. The accumulation of LC3B II in the presence of BafA1 corresponds to the amount of LC3B II that is degraded in the lysosome, and thus serves a a measurement of autophagic flux (26). Vps34-deficient CD4 cells (Fig. 2 A and B) and CD8 T cells (Fig. 2 C and D) showed reduced amounts of degraded LC3B II (compare LC3B II in DMSO-treated cells and BafA1-treated cells), indicating reduced autophagic flux. Impaired autophagy can result from either impaired autophagosome synthesis or defective autophagosome maturation (26). To distinguish between these two possibilities, we measured autophagosome synthesis by comparing the amount of LC3B II in Vps34 WT cells and KO cells in the presence of BafA1. We found that LC3B II was reduced in Vps34-deficient cells compared with WT CD4 cells (Fig. 2 A and B) and CD8 T cells (Fig. 2 C and D) after BafA1 treatment, consistent with reduced LC3B II synthesis in cells lacking Vps34. The mammalian target of rapamycin (mTOR) pathway is a major regulator of autophagy, and Vps34 has been implicated in nutrient-induced mTORC1 activation, although this is controversial (27, 28). However, mTORC1 activation was similar in Vps34 WT and KO CD8 T cells in response to TCR stimulation, as assessed by phosphorylation of ribosomal protein S6 (Fig. S3). We conclude that Vps34 is required for autophagy, but is dispensable for mTOR activation in T cells.
Vps34 Is Essential for the Homeostasis of Naïve T Cells.
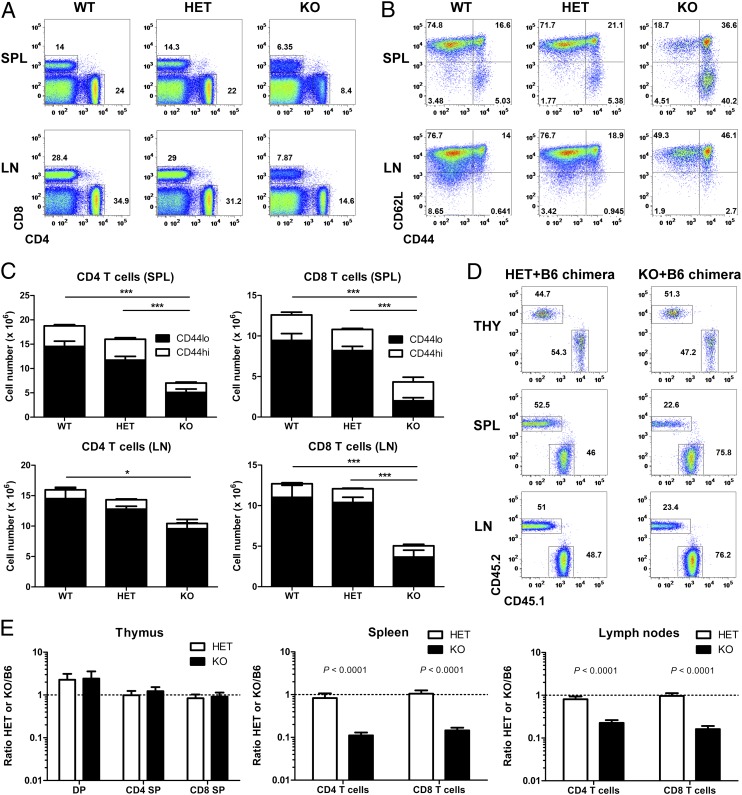
Having established that Vps34 is required for autophagy in T cells, we next investigated the effect of Vps34 deficiency on T-cell homeostasis in vivo. Analysis of T-cell populations by flow cytometry revealed a reduction of both CD4 and CD8 T cells in spleen and lymph node (LN) of Vps34 KO mice (Fig. 3 A and C). One allele of Pik3c3 was sufficient to maintain T-cell homeostasis, because Vps34 HET mice had similar frequencies of CD4 and CD8 T cells as WT mice (Fig. 3A). The reduced number of T cells in the Vps34 KO mice was due mainly to the loss of cells with a naïve (CD44lo) phenotype (Fig. 3 B and C). Overall, CD8 T cells were more affected by the lineage-specific deletion of Vps34 compared with their CD4+ counterparts; therefore, we focused on CD8 T cells in our analysis of the role of Vps34 in T-cell homeostasis.
Fig. 3.
Vps34 is essential for the homeostasis of naïve T cells. (A) Representative FACS analysis of spleen (SPL) and LN cells from Vps34 WT, HET, and KO mice. Numbers next to outlined areas indicate percentages of CD4 and CD8 T cells. (B) Representative FACS analysis of CD8 T cells from Vps34 WT, HET, and KO mice. Plots are gated on CD8+CD4− cells. (C) Numbers of CD4 and CD8 T cells from Vps34 WT, HET, and KO mice (n = 8–13). P < 0.0001 for spleen CD4 T cells, P = 0.0404 for LN CD4 T cells, P < 0.0001 for spleen CD8 T cells, P < 0.0001 for LN CD8 T cells (one-way ANOVA). P values determined by Tukey’s multiple comparison test are indicated by asterisks (*P < 0.05; ***P < 0.001). (D) Representative FACS analysis of CD8 SP thymocytes (THY) and spleen (SPL) and LN CD8 T cells from Vps34 HET/B6 and Vps34 KO/B6 mixed BM chimeras at 8 wk postreconstitution. Plots are gated on CD8+CD4− cells. Numbers next to outlined areas indicate percentages of donor BM-derived HET or KO (CD45.2+) and B6 (CD45.1+) cells. (E) Ratio of various T-cell populations from thymus (THY), spleen (SPL), and LN cells in Vps34 HET/B6 and Vps34 KO/B6 mixed BM chimeras (n = 15–17). Results are combined from three independent experiments.
The T-cell lymphopenia in Vps34 KO mice was not due to a defect in T-cell development or thymic egress, given that these mice had similar frequencies (Fig. S4A) and numbers (Fig. S4B) of DP and SP thymocytes. Furthermore, both CD4 and CD8 SP thymocytes showed normal maturation (Fig. S4 C and D). We further excluded any role of the thymus by using mice with postthymic deletion of Vps34, that is, Cd8a-CrePik3c3flox/flox mice (29). As expected, these mice showed deletion in peripheral CD8 T cells only (Fig. S5A), although Vps34 deletion was not as complete as in Cd4-CrePik3c3flox/flox mice (Fig. 1A). Similar to Cd4-CrePik3c3flox/flox mice, Cd8a-CrePik3c3flox/flox mice demonstrated a reduced number of peripheral CD8 T cells, but with normal T-cell development (Fig. S5 B and C). These findings indicate that Vps34 regulates peripheral T-cell homeostasis in a thymus-independent manner.
Next, we performed mixed BM chimera experiments to examine whether the impaired T-cell homeostasis is cell autonomous or due to an altered environment in Vps34 KO mice. Sublethally irradiated Rag1-deficient mice were reconstituted with a 1:1 mixture of Vps34 KO and WT C57BL/6 (B6) cells. Chimeras of Vps34 HET and WT B6 cells generated in an analogous manner were used as controls. The congenic marker CD45 was used to distinguish Vps34 HET or KO cells (CD45.2+) from WT B6 cells (CD45.1+). As expected, mixed Vps34 HET/WT B6 chimeras had approximately equal numbers of peripheral T cells at 8 wk postreconstitution (Fig. 3 D and E). In contrast, mixed Vps34 KO/WT B6 chimeras contained fewer peripheral T cells derived from Vps34 KO cells than from WT B6 BM cells despite equal numbers in the thymus (Fig. 3 D and E). This indicates that trans-acting factors provided by WT B6 cells are unable to rescue the defect in peripheral Vps34 KO T cells, demonstrating that T-cell lymphopenia in Cd4-CrePik3c3flox/flox mice is cell autonomous. Mixed BM chimera experiments also revealed that the increased frequency of CD44hi CD8 T cells in Vps34 KO mice (Fig. 3B) was related to a loss of naïve (CD44lo) cells, given the reduced frequency of CD44hi CD8 T cells in mixed Vps34+B6 chimeras, that is, in a nonlymphopenic environment (Fig. S6). In summary, we conclude that Vps34 is essential for the homeostasis of naïve T cells, acting in a cell-intrinsic manner.
Vps34 Promotes T-Cell Survival by Ensuring Mitochondrial Homeostasis.
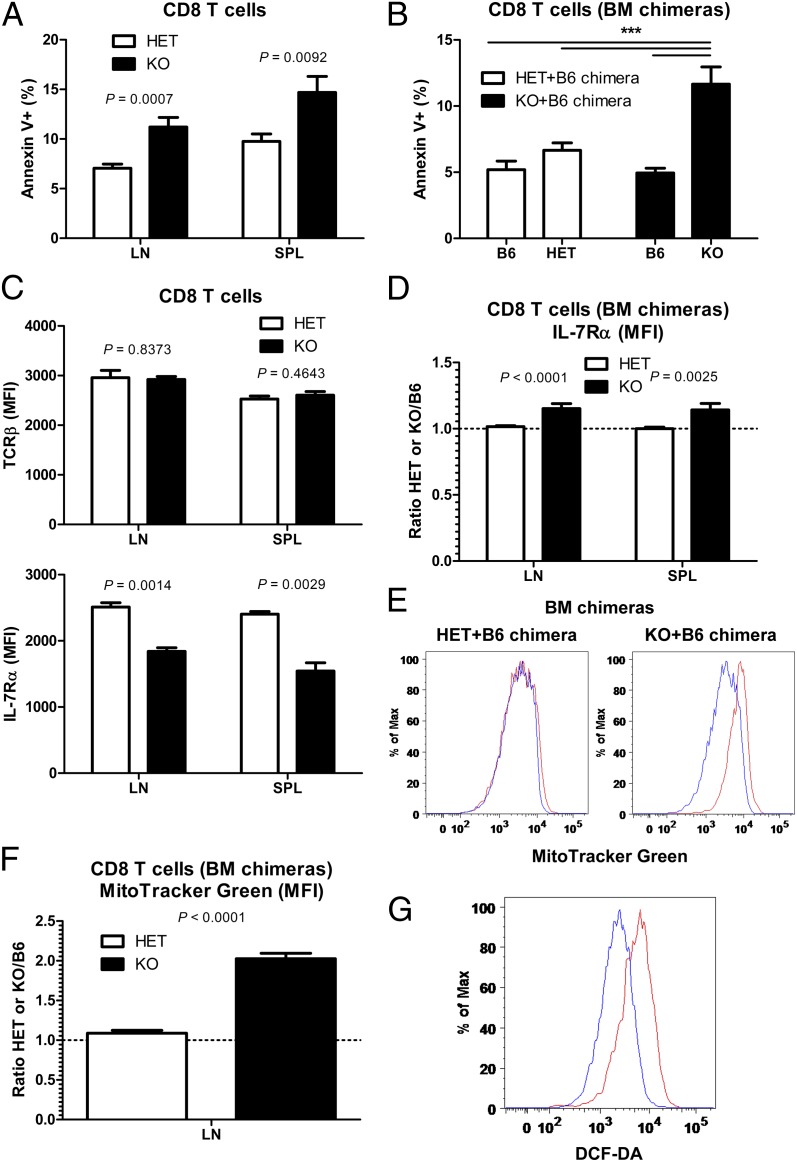
We next examined the molecular mechanism through which Vps34-dependent autophagy regulates T-cell homeostasis. In addition to thymic output, the size of the naïve T-cell compartment is controlled by homeostatic mechanisms in the periphery. Therefore, we hypothesized that the loss of naïve T cells in the absence of Vps34 is related to impaired survival. Consistent with this hypothesis, CD8 T cells from Vps34 KO mice demonstrated increased spontaneous apoptosis ex vivo, as detected by annexin V staining (Fig. 4A). To exclude cell-extrinsic effects (e.g., activation-induced cell death due to lymphopenia), we used mixed BM chimeras generated as described above. In these mixed chimeras, the cotransfer of WT B6 BM cells ensures a full peripheral lymphoid compartment. Again, Vps34-deficient CD8 T cells (from KO+B6 chimeras) exhibited increased apoptosis compared with Vps34-sufficient cells (from HET+B6 chimeras) (Fig. 4B). These data indicate a cell-intrinsic requirement for Vps34 in maintaining peripheral T-cell survival.
Fig. 4.
Vps34 promotes CD8 T-cell survival by ensuring mitochondrial homeostasis. (A) Frequency of apoptotic (Annexin V+) cells among total CD8 T cells from LN and spleen (SPL) of Vps34 HET and KO mice (n = 7–9). (B) Frequency of apoptotic (Annexin V+) cells among total B6 WT, Vps34 HET, and Vps34 KO LN CD8 T cells from Vps34 HET/B6 and Vps34 KO/B6 mixed BM chimeras (n = 16–17). P < 0.0001 (one-way ANOVA). P values determined by Tukey’s multiple comparison test are indicated by asterisks (***P < 0.001). (C) Cell surface expression of TCRβ and IL-7Rα by naïve (CD44lo) CD8 T cells from Vps34 HET and KO mice was determined using FACS (n = 3). MFI, mean fluorescence intensity. (D) Cell surface expression of IL-7Rα by LN and spleen (SPL) naïve (CD44lo) CD8 T cells from Vps34 HET/B6 and Vps34 KO/B6 mixed BM chimeras (n = 15–17). IL-7Rα expression (MFI) by Vps34 HET and KO CD8 T was normalized to expression by WT B6 cells in the same individual chimera (i.e., ratio of Vps34 Het or KO to WT B6). A ratio of 1.0 represents equal IL-7Rα expression by Vps34 HET or KO cells compared with WT B6 cells. (E) Representative FACS analysis of MitoTracker Green staining in LN CD8 T cells from Vps34 HET/B6 and Vps34 KO/B6 mixed BM chimeras. Blue and red histograms represent B6 and HET or KO cells, respectively. (F) Quantification of MitoTracker Green staining in LN CD8 T cells from Vps34 HET/B6 and Vps34 KO/B6 mixed BM chimeras (n = 16–17). Staining was normalized to WT B6 cells as described in D. (G) Representative FACS analysis of DCF-DA staining in LN CD8 T cells from Vps34 HET and KO mice. Blue and red histograms represent HET and KO cells, respectively. Data are from one experiment (C) or cumulative from two (A and G) or three (B and D–F) independent experiments.
Naïve T cells need contact with self-peptide/MHC molecules (TCR ligation) and IL-7 to survive. Therefore, we examined the cell surface expression of receptors for these survival signals by FACS. Although TCR expression did not differ between Vps34 HET and KO cells, IL-7Rα cell surface expression was slightly lower in Vps34-deficient CD4 cells (Fig. S7A) and CD8 T cells (Fig. 4C). However, this reduction was due to cell-extrinsic effects, because it did not occur in mixed BM chimeras, that is, in a nonlymphopenic environment (Fig. 4D and Fig. S7B). Most likely, lymphopenia in Cd4-CrePik3c3flox/flox mice results in increased IL-7 availability, which in turn leads to reduced IL-7Rα expression (30).
Autophagy is involved in the quality control of intracellular organelles, for example, the removal of damaged mitochondria (mitophagy). Furthermore, healthy mitochondria are crucial for cellular survival. This prompted us to examine mitochondrial homeostasis in Vps34 KO T cells. Vps34-deficient CD8 T cells had increased mitochondrial mass, as determined by staining with a cell-permeable mitochondrial dye (MitoTracker) in mixed BM chimeras (Fig. 4 E and F). Vps34-deficient CD4 T cells also showed an increase in mitochondrial mass, albeit to a lesser extent (Fig. S7 C and D). Increased mitochondrial mass in the absence of Vps34 is consistent with reduced removal of damaged mitochondria because of impaired mitophagy. Accumulation of damaged mitochondria should lead to increased production of reactive oxygen species (ROS). Consistent with this prediction, Vps34-deficient CD4 (Fig. S7E) and CD8 T cells (Fig. 4G) had increased amounts of ROS, as shown by staining with the hydrogen peroxide-sensitive dye DCF-DA. Overall, these findings suggest that Vps34-dependent autophagy is required for T-cell survival by regulating mitochondrial homeostasis.
Discussion
Here we provide genetic evidence for a critical role of Vps34-dependent canonical autophagy in maintaining T-cell homeostasis by removing damaged mitochondria through mitophagy. These results extend previous findings demonstrating a requirement for the autophagy genes Atg5 and Atg7 in T-cell homeostasis (14–16). Our study confirms that the requirement of these genes for T-cell homeostasis is not likely related to any nonautophagic function, given that the class III PI3K complex (Vps34) that acts upstream of Atg5 and Atg7 is also essential for T-cell homeostasis. Overall, our findings strengthen the concept that bona fide autophagy is crucial for naïve T-cell homeostasis.
The current study also clarifies the role of Beclin-1 in T-cell development versus peripheral T-cell homeostasis. Our data are in line with the reported requirement for Beclin-1 in peripheral T-cell maintenance in Cd4-CreBecn1flox/flox mice (19). Another study using Beclin-1 KO mice derived from Rag1 blastocyst complementation concluded that Beclin-1 is required for T-cell development, but not for the maintenance of peripheral T cells (20). The differences are likely related to the different approaches to generating Beclin-1–deficient T cells, for example, the developmental stage at which gene deletion occurs. In contrast to Atg5 and Atg7 KO mice, Vps34 KO mice demonstrate no defect in thymocyte survival. This is likely due to differences in the Cre lines used to achieve gene deletion, given that Lck-Cre (Atg5 and Atg7 KO mice) deletes at an earlier stage than Cd4-Cre (Vps34 KO mice) in thymocytes. Differences in mitochondrial content and turnover have been described in thymocytes versus peripheral T cells (20), which could explain a differential role of autophagy in developing (thymic) versus mature (peripheral) T cells.
Similar to what has been reported for Atg5- and Atg7-deficient T cells (14–16), we found that Vps34 is required for the removal of damaged mitochondria. Mitophagy thereby prevents the production of toxic ROS, which can lead to cell death. A recent study suggested an alternative mechanism in which autophagy regulates T-cell survival by the degradation of cell death proteins in TCR-activated T cells (19). It is conceivable that in resting T cells, basal autophagy maintains survival by mitophagy, whereas in activated T cells, autophagy operates by degrading apoptosis-promoting proteins that are induced by cellular activation. The possibility that autophagy has distinct functions in resting T cells and activated T cells warrants further study.
In addition, using primary T cells, we have demonstrated that Vps34 is required for autophagy in mammalian cells in vivo. A recent study that examined the function of Vps34 in sensory neurons using Advillin-CrePik3c3flox/flox mice concluded that the main function of Vps34 in neurons is related to its involvement in the endosomal pathway, but not the autophagic pathway (31). The differences between that study and the present study could be due to cell type-specific differences. Alternatively, different gene targeting strategies to generate conditional Vps34 KO mice could account for the observed differences. Zhou et al. (31) targeted exon 17/18 encoding the catalytic domain of Vps34, whereas we targeted exon 4 to delete as much of the Vps34 protein as possible. Although Beclin-1 protein expression was unaffected in their study, we observed reduced amounts of Beclin-1 protein in Vps34-deficient T cells. The rationale for our targeting approach is based on findings in yeast regarding the different phenotype of Vps34-null mutants versus mutants deficient in Vps34 lipid kinase activity (32). Although both mutants showed defects in endosomal trafficking (i.e., protein sorting to the vacuole), only the Vps34-null mutants demonstrated a growth defect at 37 °C and increased sensitivity to osmotic stress. The latter defects are likely due to impaired autophagy. Whereas both of the foregoing studies provide complementary data on the role of Vps34 in vivo, our data are in line with a plethora of previous studies in lower organisms in vivo and mammalian cells in vitro demonstrating a requirement for Vps34 in autophagy. Our results are also consistent with a contemporaneous study (using the same gene targeting strategy, i.e., targeting of exon 4) showing that Vps34 is required for autophagy in the heart and liver (33).
Another recent study (34), using the Vps34 conditional KO mice generated by Zhou et al. (31) (targeting of exon 17/18), concluded that Vps34 is required for T-cell homeostasis by regulating IL-7Rα cell surface expression but is dispensable for autophagy in T cells. We also observed reduced IL-7Rα surface expression in Vps34 KO T cells. However, this phenomenon is most likely due to the lymphopenia in Vps34 KO mice, because it did not occur in mixed BM chimera experiments with a full lymphocyte compartment. In the study of McLeod et al. (34), T cells were not studied in a nonlymphopenic environment. Furthermore, there was only a very partial rescue of T-cell homeostasis in Vps34 KO mice crossed to Bcl-2 transgenic mice, and similar experiments with IL-7Rα transgenic mice were not reported. Therefore, whether dysregulated IL-7Rα trafficking in the absence of Vps34 directly causes impaired T-cell homeostasis is not clear. Overall, in the studies reported to date, completely abolishing Vps34 protein expression (by targeting exon 4) leads to impaired autophagy [(33) and the present study], whereas autophagy seems to be preserved when targeting only the catalytic domain (exon 17/18) of Vps34 (31, 34).
We also show that Vps34-independent (noncanonical) autophagy is not sufficient to maintain T-cell homeostasis. So far, noncanonical autophagy (either Vps34-/Beclin-1– or Atg5-/Atg7-independent) has been reported mainly in cell lines in response to specific stimuli that might not be very physiological (21, 22, 35). However, Zhou et al. (31) described it in a subset of Vps34-deficient neurons. Again, cell type-specific differences could account for the observed differences. We favor the hypothesis that residual autophagy in T cells from Vps34 KO mice is due not to noncanonical autophagy, but rather to residual amounts of PI(3)P derived from Vps34-independent sources (3, 4). We attempted to quantify PI(3)P production in Vps34-deficient T cells, but current techniques do not allow specific and accurate monitoring of its production in primary T cells.
In summary, we have demonstrated that bona fide autophagy is required for T-cell homeostasis by providing evidence that the class III PI3K complex (Vps34), not only the Atg genes involved in vesicle elongation, are necessary for it. Furthermore, we show that canonical (Vps34-dependent) autophagy has a dominant function in T cells by promoting T-cell survival through mitophagy.
Materials and Methods
Conditional Pik3c3 KO Mice.
Pik3c3 KO mice were generated as described in SI Materials and Methods.
Flow Cytometry and Cell Sorting.
Single-cell suspensions were prepared from lymphoid organs and processed for flow cytometry as described in SI Materials and Methods.
Statistical Analysis.
The nonparametric Mann–Whitney U test was used to determine statistical significance between two groups (α = 0.05). For multigroup comparisons, one-way ANOVA was applied, along with post hoc testing using Tukey’s multiple comparison test (α = 0.05). Error bars represent SEM.
Supplementary Material
Acknowledgments
We thank A. Rongvaux for advice on gene targeting, L. Evangelisti for ES cell culture, J. Stein for screening of ES cells, C. Hughes for blastocyst injection, and L. Borelli for help with mouse breeding. We also thank S. Sanjabi and L. Zenewicz for critically reading the manuscript and F. Manzo for submitting the manuscript for publication. This work was supported by an Irvington Institute Postdoctoral Fellowship from the Cancer Research Institute (to T.W.). R.A.F. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1205305109/-/DCSupplemental.
References
- 1.Takada K, Jameson SC. Naive T cell homeostasis: From awareness of space to a sense of place. Nat Rev Immunol. 2009;9:823–832. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- 2.Sprent J, Surh CD. Normal T cell homeostasis: The conversion of naive cells into memory-phenotype cells. Nat Immunol. 2011;12:478–484. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backer JM. The regulation and function of class III PI3Ks: Novel roles for Vps34. Biochem J. 2008;410:1–17. doi: 10.1042/BJ20071427. [DOI] [PubMed] [Google Scholar]
- 4.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 5.Funderburk SF, Wang QJ, Yue Z. The Beclin 1–VPS34 complex—at the crossroads of autophagy and beyond. Trends Cell Biol. 2010;20:355–362. doi: 10.1016/j.tcb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonsen A, Tooze SA. Coordination of membrane events during autophagy by multiple class III PI3-kinase complexes. J Cell Biol. 2009;186:773–782. doi: 10.1083/jcb.200907014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noda T, Matsunaga K, Taguchi-Atarashi N, Yoshimori T. Regulation of membrane biogenesis in autophagy via PI3P dynamics. Semin Cell Dev Biol. 2010;21:671–676. doi: 10.1016/j.semcdb.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Virgin HW, Levine B. Autophagy genes in immunity. Nat Immunol. 2009;10:461–470. doi: 10.1038/ni.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh CM, Edinger AL. The complex interplay between autophagy, apoptosis, and necrotic signals promotes T-cell homeostasis. Immunol Rev. 2010;236:95–109. doi: 10.1111/j.1600-065X.2010.00919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7:767–777. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmid D, Münz C. Innate and adaptive immunity through autophagy. Immunity. 2007;27:11–21. doi: 10.1016/j.immuni.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pua HH, Guo J, Komatsu M, He YW. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. J Immunol. 2009;182:4046–4055. doi: 10.4049/jimmunol.0801143. [DOI] [PubMed] [Google Scholar]
- 15.Pua HH, Dzhagalov I, Chuck M, Mizushima N, He YW. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J Exp Med. 2007;204:25–31. doi: 10.1084/jem.20061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephenson LM, et al. Identification of Atg5-dependent transcriptional changes and increases in mitochondrial mass in Atg5-deficient T lymphocytes. Autophagy. 2009;5:625–635. doi: 10.4161/auto.5.5.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hubbard VM, et al. Macroautophagy regulates energy metabolism during effector T cell activation. J Immunol. 2010;185:7349–7357. doi: 10.4049/jimmunol.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia W, He YW. Temporal regulation of intracellular organelle homeostasis in T lymphocytes by autophagy. J Immunol. 2011;186:5313–5322. doi: 10.4049/jimmunol.1002404. [DOI] [PubMed] [Google Scholar]
- 19.Kovacs JR, et al. Autophagy promotes T-cell survival through degradation of proteins of the cell death machinery. Cell Death Differ. 2012;19:144–152. doi: 10.1038/cdd.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arsov I, et al. A role for autophagic protein beclin 1 early in lymphocyte development. J Immunol. 2011;186:2201–2209. doi: 10.4049/jimmunol.1002223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu CT, Zhu J, Dagda R. Beclin 1-independent pathway of damage-induced mitophagy and autophagic stress: Implications for neurodegeneration and cell death. Autophagy. 2007;3:663–666. doi: 10.4161/auto.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scarlatti F, Maffei R, Beau I, Ghidoni R, Codogno P. Non-canonical autophagy: An exception or an underestimated form of autophagy? Autophagy. 2008;4:1083–1085. doi: 10.4161/auto.7068. [DOI] [PubMed] [Google Scholar]
- 23.Lee PP, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 24.Itakura E, Kishi C, Inoue K, Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell. 2008;19:5360–5372. doi: 10.1091/mbc.E08-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubinsztein DC, et al. In search of an “autophagomometer.”. Autophagy. 2009;5:585–589. doi: 10.4161/auto.5.5.8823. [DOI] [PubMed] [Google Scholar]
- 27.Gulati P, et al. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 2008;7:456–465. doi: 10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juhász G, et al. The class III PI(3)K Vps34 promotes autophagy and endocytosis but not TOR signaling in Drosophila. J Cell Biol. 2008;181:655–666. doi: 10.1083/jcb.200712051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maekawa Y, et al. Notch2 integrates signaling by the transcription factors RBP-J and CREB1 to promote T cell cytotoxicity. Nat Immunol. 2008;9:1140–1147. doi: 10.1038/ni.1649. [DOI] [PubMed] [Google Scholar]
- 30.Park JH, et al. Suppression of IL7Rα transcription by IL-7 and other prosurvival cytokines: A novel mechanism for maximizing IL-7–dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 31.Zhou X, et al. Deletion of PIK3C3/Vps34 in sensory neurons causes rapid neurodegeneration by disrupting the endosomal but not the autophagic pathway. Proc Natl Acad Sci USA. 2010;107:9424–9429. doi: 10.1073/pnas.0914725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Günther J, et al. Generation and functional in vivo characterization of a lipid kinase defective phosphatidylinositol 3-kinase Vps34p of Candida albicans. Microbiology. 2005;151:81–89. doi: 10.1099/mic.0.27333-0. [DOI] [PubMed] [Google Scholar]
- 33.Jaber N, et al. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci USA. 2012;109:2003–2008. doi: 10.1073/pnas.1112848109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLeod IX, Zhou X, Li QJ, Wang F, He YW. The class III kinase Vps34 promotes T lymphocyte survival through regulating IL-7Rα surface expression. J Immunol. 2011;187:5051–5061. doi: 10.4049/jimmunol.1100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishida Y, et al. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 2009;461:654–658. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.