Abstract
ON bipolar cells are critical for the function of the ON pathway in the visual system. They express a metabotropic glutamate receptor (mGluR6) that, when activated, couples to the Go class of G protein. The channel that is primarily responsible for the synaptic response has been recently identified as the transient receptor potential cation channel subfamily M member 1 (TRPM1); TRPM1 is negatively coupled to the mGluR6/Go cascade such that activation of the cascade results in closure of the channel. Light indirectly opens TRPM1 by reducing transmitter release from presynaptic photoreceptors, resulting in a decrease in mGluR6 activation. Conversely, in the dark, binding of synaptic glutamate to mGluR6 inhibits TRPM1 current. Closure of TRPM1 by G-protein activation in the dark is a critical step in the process of ON bipolar cell signal transduction, but the precise pathway linking these two events is not understood. To address this question, we measured TRPM1 activity in retinal bipolar cells, in human ependymal melanocytes (HEMs) that endogenously express TRPM1, and in HEK293 cells transfected with TRPM1. Dialysis of the Gβγ subunit dimer, but not Gαo, closed TRPM1 channels in every cell type that we tested. In addition, activation of an endogenous G-protein–coupled receptor pathway in HEK293 cells that releases Gβγ without activating Go protein also closed TRPM1 channels. These results suggest a model in which the Gβγ dimer that is released as a result of the dissociation from Gαo upon activation of mGluR6 closes the TRPM1 channel, perhaps via a direct interaction.
Keywords: patch clamp, calcium imaging
Retinal ON bipolar cells are connected to photoreceptors through a sign-inverting synapse. At this synapse, glutamate binds to the metabotropic glutamate receptor mGluR6, which couples to the closure of a cation-selective transduction channel. Insight into the molecular identity of the transduction channel was gained from the observation that low levels of mRNA encoding the transient receptor potential ion channel TRPM1 was associated with a form of congenital stationary night blindness (CSNB) in a population of Appaloosa horses (1). Soon after, it was shown that ON bipolar cell function was absent or severely impaired in a mouse model that lacked TRPM1 expression (2–4), although there is evidence that one or more classes of cone-driven ON bipolar cells may use an additional channel (3). Mutations in TRPM1 have now been positively linked to CSNB in humans as well (5–7). Taken together, studies of mouse, horse, and human provide convincing evidence that the ON bipolar cell transduction channel is composed of TRPM1, either alone or in conjunction with other channels. However, all these studies do not provide insight into the mechanism by which activation of mGluR6 leads to closure of TRPM1.
The metabotropic receptor mGluR6 preferentially couples to the Go class of G protein, both in bipolar cells (8, 9) and expression systems (10, 11). Activation of mGluR6 and, correspondingly, Go, results in closure of TRPM1. However, it is unclear whether the Gαo subunit itself is necessary for closing TRPM1. In other examples of channel modulation by the Go class of G protein, the principle effecter is the Gβγ dimer, rather than the α subunit (12). These channels include the G-protein–gated inwardly rectifying K+ channel (GIRK), which is activated by Gβγ (13), and the high-voltage–activated Ca2+ channels, which are inhibited by Gβγ subunits (14, 15). This cascade is widely used by transmitter receptors, such as the GABAB receptor, that serve to regulate the excitability of presynaptic terminals (16). Interestingly, Koike et al. (4) recently expressed the TRPM1 channel in CHO cells and reported that the channel is closed by application of exogenous Gαo, but not by the Gβγ dimer (17).
Because of its importance, we have revisited this question by comparing the effect of exogenous Gβγ and Gαo on native TRPM1 channels expressed in ON bipolar cells. Slices of mouse retina were bathed in the mGluR6 agonist L-AP4, and TRPM1 channels were transiently opened either by brief puffs of an mGluR6 antagonist or with light stimulus. We also performed experiments on TRPM1-expressing cell lines. We find that Gβγ subunits decreased channel opening in all cell types, whereas activated Gαo had little effect. Our findings, although different from recent reports by Koike et al. (4, 17), support a mechanism whereby activation of mGluR6 closes TRPM1 directly through the effecter Gβγ, consistent with previously established mechanisms of the activity of this dimer on other ion channels.
Results
Exogenous Gβγ Inhibits TRPM1 Current in RBCs.
There is general agreement that activation of the mGluR6 receptor in ON bipolar cells results in closure of the downstream TRPM1 channel and that this effect is mediated by a G protein. TRPM1 is a cation-selective channel, and will therefore depolarize ON bipolar cells when it opens. One way to open TRPM1 is to use a pharmacological approach, as we have done in most of the experiments presented here. To close the TRPM1 channel, an mGluR6 agonist L-AP4, (4 µM), was included in the bath solution. This concentration is sufficient to fully activate mGluR6 and close TRPM1. A high concentration of the mGluR6 antagonist LY34149 (100 μM) was then “puffed” onto the dendrites of an ON bipolar cell, resulting in a transient displacement of L-AP4 from mGluR6, and the opening of TRPM1 channels. At physiological membrane potentials, the opening of TRPM1 produces an inward current, but we typically voltage clamp cells at positive voltages, as this tends to reduce run down of the response (18, 19), and so the current generated by the opening of TRPM1 is outward. An example of the TRPM1 current generated using this approach is shown in Fig. 1A. In this study, we targeted a specific class of ON bipolar cells that receive synaptic input from rods (RBCs). Morphologically, they can be distinguished by the length of their axons, which terminate in the deepest layer of the inner plexiform layer (20). An example of this type of cell, filled with Alexa 488, is also shown in Fig. 1A.
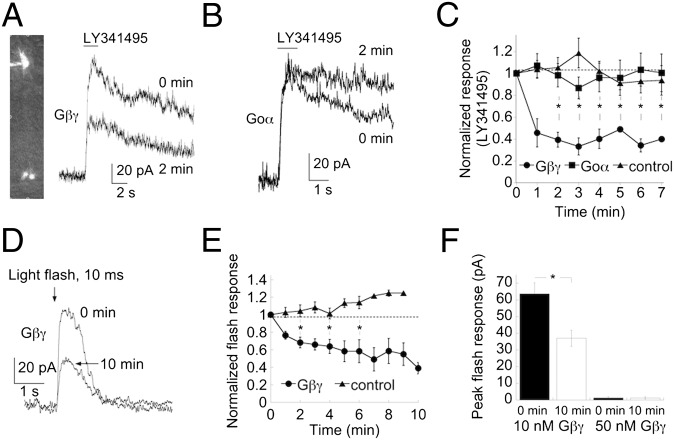
Fig. 1.
TRPM1 channels in mouse RBCs are negatively controlled by Gβγ subunits. (A) Representative RBC response to a puff of the mGluR6 receptor antagonist, 100 μM LY341495, when dialyzed with 50 nM Gβγ protein. (Upper) Break in response. (Lower) Response at 2 min later after break-in at holding potential of +40 mV. (B) LY341495 induced response from a representative RBC that was dialyzed with 100 nM Gαo protein. Responses are shown at break-in and after 2 min of dialysis, as indicated. Note the different kinetics of the decay responses. (C) Summary of the time course of the normalized LY341495-evoked response in RBCs that were dialyzed with Gβγ (filled circles), Gαo (filled squares), and in control cells (filled triangles). Asterisks indicate statistical significance (P < 0.01) between the normalized current amplitude of cells dialyzed with Gβγ compared with control cells at 2–7 min. (D) Representative light-evoked response of an RBC at break-in and after dialysis with 10 nM Gβγ; Vhold = +50 mV. (E) Time course of the light response (normalized to the response amplitude at break-in) in control conditions (filled triangles) and after dialysis of 10 nM Gβγ (filled circles). Asterisks indicate a significant difference (P < 0.01) between the normalized response of dialyzed cells versus the response of control cells at 2, 4, and 6 min of recording. (F) Summary of peak light-response amplitudes at break-in and after 10 min of dialysis of 10 and 50 nM Gβγ. Although the response in the presence of 10 nM Gβγ showed a clear decline, no response was detected at any time point in the presence of 50 nM Gβγ.
An important and unresolved issue in mGluR6 transduction is the mechanism by which activation of the G protein, thought to be a member of the Go family (8, 9), results in closure of TRPM1. One possibility is that the channel is closed by one or more of the G-protein subunits that couple to mGluR6. To test this we first dialyzed Gβγ dimers (50 nM) into RBCs through the recording pipette. After 2 min of recording, the TRPM1 current decreased by ∼60% (Fig. 1 A and C). Overall, dialysis with Gβγ protein reduced TRPM1 currents with an exponential time course, reaching steady state roughly 2 min after break-in. The average response decreased to 39.3 ± 7.0% of the response at break-in (Fig. 1C, *P < 0.001 at 2 min for Gβγ-dialyzed vs. control cells). The decrease of the TRPM1 response is not due to recording rundown, because we saw no such reduction in control cells that were not dialyzed with the Gβγ protein.
In seven other cells, all dialyzed with 100 nM Gβγ, we did not observe a discernible response to LY341495, even at break-in, suggesting the occurrence of rapid dialysis of the subunits. Such a lack of response to LY341495 was never observed in cells that were not dialyzed with Gβγ.
Our data are consistent with the idea that Gβγ can close TRPM1. However, an alternative hypothesis is that the other G-protein subunit, Gαo is responsible for closing TRPM1. TRPM1 channels expressed in CHO cells have been reported to be closed by the Gαo subunit (4, 17). In this scenario, dialysis of exogenous Gβγ binds to endogenous Gαo, preventing the α subunit from closing the channel, during activation of mGluR6. To test this possibility, we examined the baseline holding current in the presence of L-AP4 (i.e., when mGluR6 is activated). If Gβγ dialysis prevents closure of TRPM1 during mGluR6 activation, this should be observed as a progressive increase in holding current as the G-protein subunit is dialyzed into the cell. However, the average change in holding current was in the opposite direction than the predicted change (−6.5 ± 3.8 pA) and was not significantly different from no change at all (P = 0.11 compared with 0 pA). These data are consistent with the idea that Gβγ prevents the opening of the TRPM1 channel in the presence of mGluR6 antagonists rather than interfering with its closure.
As a more direct test of the idea that the Gαo subunit closes TRPM1, we dialyzed purified Gαo protein into RBCs. Gαo protein was first activated by incubation in 100 nM 5′-guanylyl imidodiphosphate (GMP-PNP) for 30 min at 4 °C. Dialysis of activated Gαo subunit had no effect on the amplitude of the TRPM1 current (Fig. 1 B and C, P = 0.78 for control vs. Gαo after 2 min of recording). Interestingly, Gαo had a significant effect on the rate of decay of the response to LY31495 (Fig. 1B, P = 0.003 compared with control cells) without any effect on rise time. The reason for this effect is unclear, but could result from the buffering of endogenous Gβγ by exogenous Gαo that was not bound to GMP-PNP.
As an independent approach, we tested the effects of Gβγ on the light-evoked RBC response. Fig. 1D illustrates the response of an RBC to a 10-ms full-field flash of 425 nm light delivering 3.6 photons/µm2, a flash strength that will activate only rods (21–23). At a concentration of 10 nM, dialysis of Gβγ resulted in a slow depression of the light response, reaching a steady-state value of 39.0 ± 6.5 of the response at break-in after 10 min of dialysis, (Fig. 1 D and E; P = 0.003). In contrast, the response of nondialyzed control cells showed no significant decline (Fig. 1E; response after 6 min of recording = 113.9% of the response at break-in). Dialysis of RBCs with a higher concentration of Gβγ (50 nM, n = 3, Fig. 1F) yielded no detectable light response, even at break-in, suggesting rapid dialysis of the dimer and complete inability of the channel to open.
TRPM1 Channels in HEK293 and HEM Cells Are Sensitive to Inhibition by Capsazepine.
It has been suggested that capsaicin can open the TRPM1 channel in RBCs (2). As a further test of this idea, we measured responses to capsaicin as well as LY341495 in mice lacking functional TRPM1 channels (trpm1tm1Lex). As expected, application of LY341495 did not produce a detectable response in RBCs (Fig. 2A, Left), as mGluR6 transduction is absent in this mouse model (2–4). Moreover, we were unable to detect responses to capsaicin in RBCs from trpm1tm1Lex mice (Fig. 2A, Right), suggesting that the action of capsaicin is specific to TRPM1 in this cell type. Similarly, capsaicin fails to evoke a response in mice lacking nyctalopin, a membrane-bound protein that is required for proper expression of TRPM1 (24). The observation that TRPM1 can be activated by capsaicin is critical as it paves the way for the pharmacological isolation of TRPM1 presented below.
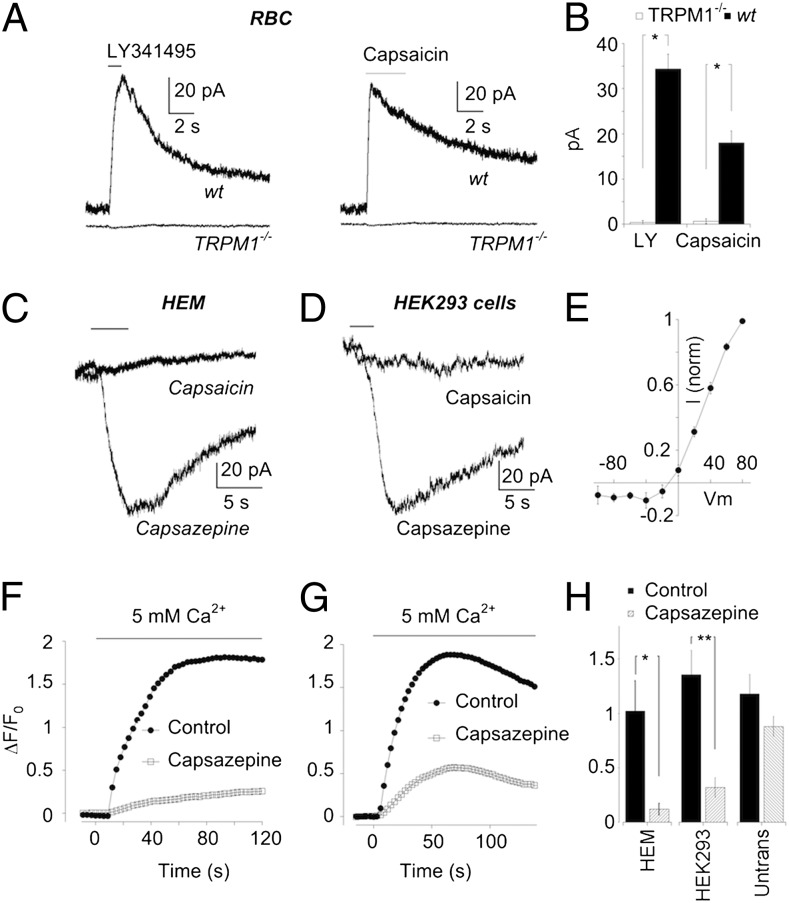
Fig. 2.
Functional isolation of TRPM1 using capsazepine as a pharmacological tool. (A) Representative records of RBC responses to LY341495 (Left) and capsaicin (Right) in wild-type and trpm1tm1Lex mice. Holding potential for all cells was +40 mV. (B) Summary of data in A. LY341495 response in wild type (n = 64) vs. trpm1tm1Lex (n = 6). Capsaicin responses in wild type (n = 46) vs. trpm1tm1Lex (n = 11), *P < 0.0001. (C) Representative responses to capsaicin and capsazepine (as indicted) in an HEM. Both traces were obtained from the same cell. (D) Similar experiment as in C using a HEK293 cell stably transfected with TRPM1 (HEK-TRPM1). (E) Averaged I–V relation obtained from three HEMs. (F) Typical example of Ca2+ imaging experiments showing the response of HEMs to increasing bath Ca2+ from 0 to 5 mM. Each trace represents changes in fluorescence intensity in an average of 5–10 cells in a single field of view in control solution (filled symbols) and in a solution containing 100 μM capsazepine (open symbols). The same cells were used for both conditions. (G) As in F except with HEK-TRPM1 cells. (H) Summary of Ca2+ imaging experiments in the absence and presence of capsazepine performed on HEMs (n = 6 coverslips; *P = 0.01) and both stably transfected (n = 7 coverslips; **P = 0.006) and untransfected (n = 8 coverslips) HEK293 cells.
TRPM1 is expressed in peripheral tissue as well as in ON bipolar cells, specifically in melanocytes (25, 26). We studied the properties of TRPM1 in neonatal human ependymal melanocytes (HEMs). Using whole cell recording, we found that HEMs were unresponsive to application of capsaicin (Fig. 2C). We have shown previously that the transient receptor potential cation channel subfamily V member 1(TRPV1) antagonist capsazepine blocks capsaicin-evoked currents in RBCs (2), providing for the possibility that capsazepine may also be an antagonist of the TRPM1 receptor. TRPM1 channels expressed in HEMs have been reported to open constitutively (25). If so, we reasoned that capsazepine might be able to elicit a current in HEMs by antagonizing TPRM1 and blocking the constitutive flow of current through the channel. In support of this idea, we observed that capsazepine reduced a standing current in the cells that were unresponsive to capsaicin. At a holding potential of +40 mV, the standing current was outward, and so its suppression by capsazepine generated an apparent inward current. The current–voltage (I–V) relationship that was suppressed by capsazepine displayed a strong outward rectification (Fig. 2E), comparable to that obtained previously in RBCs (2). These results are consistent with the idea that, capsazepine acts to inhibit the activity of constitutively open TRPM1 channels, although the mechanism for such inhibition is unclear. However, it does not rule out the possibility that capsazepine opens or closes a channel that is distinct from TRPM1. To address this we next expressed TRPM1 in HEK293 cells. Capsazepine elicited a response in HEK293 cells that stably expressed TRPM1 (HEK-TRPM1, Fig. 2D). On the basis of the finding that capsazepine evokes a current with the same properties as TRPM1 in both HEMs and transfected, but not untransfected HEK293 cells, we propose that capsazepine reduces, or completely inhibits TRPM1 current.
Because TRPM1 has been reported to be Ca2+ permeable (27, 28), we tested the possibility that changes in intracellular Ca2+ concentration could act as a readout for the presence of open TRPM1 channels. HEM or HEK-TRPM1 cells were incubated in Fluo-3 for 30 min and transferred to nominally 0 Ca2+ solution for imaging. Following addition of 5 mM Ca2+ to the bath, there was a readily discernible rise in intracellular Ca2+ levels. There are two lines of evidence that suggest that influx of Ca2+ through TRPM1 channels contributed to this rise. First, the magnitude of the Ca2+ signal in HEMs was dramatically reduced when capsazepine was present in the bath (Fig. 2F). Second, capsazepine strongly reduced the Ca2+ signal in HEK-TRPM1 cells (Fig. 2G), but had no significant effect in untransfected cells (data summarized in Fig. 2H). The modest effect of capsazepine on Ca2+ transients in untransfected cells may be due to blockade of endogenous Ca2+-permeable channels, or perhaps inhibition of Ca2+-activated Ca2+ release from stores.
Overexpression or Direct Application of Gβγ Subunits Closes TRPM1 in HEK293 Cells.
We further examined the regulation of TRPM1 currents by G-protein subunits using a cell line that does not natively express this channel. Addition of 100 μM GTPγS to HEK293 cells that were transfected with TRPM1 mRNA resulted in the closure of TRPM1 channels, indicated by the lack of a response to capsazepine (Fig. 3A, Upper), implying that the mobilization of endogenous G protein subunits was sufficient to close TRPM1. Dialysis of Gβγ subunits (100 nM) also resulted in complete suppression of TRPM1 current, as there was no response to capsazepine (Fig. 3A, Lower). These results are summarized in Fig. 3B. Thus, dialysis of Gβγ subunits into both HEMs and RBCs inhibits TRPM1 function.
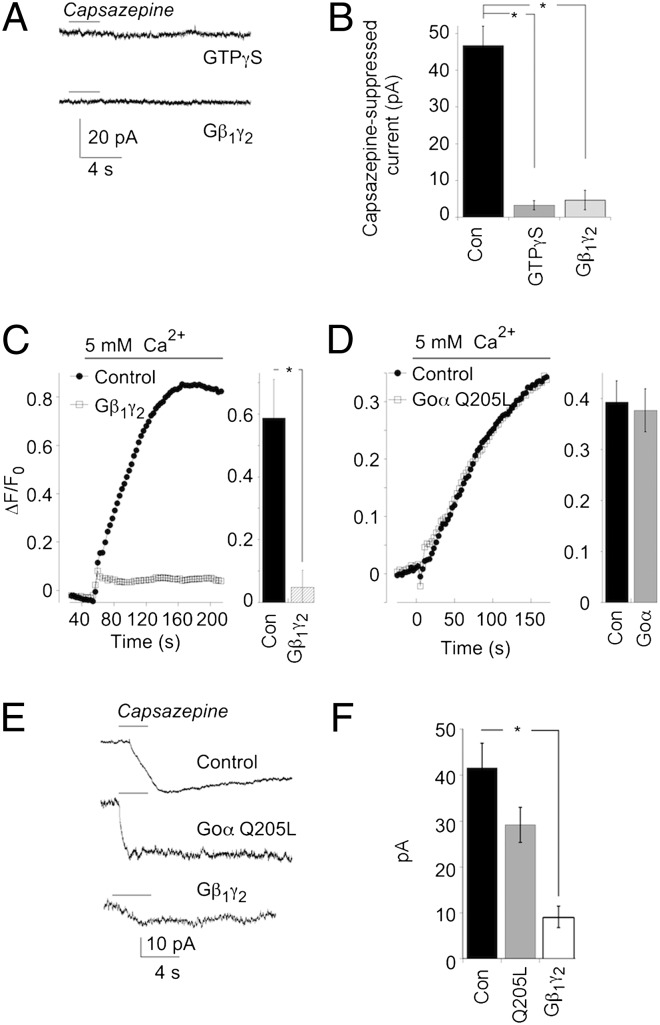
Fig. 3.
Application of exogenous Gβ1γ2 closes TRPM1 channels. (A) Representative responses to capsazepine in an HEK-TRPM1 cell dialyzed with 100 μM GTPγS (Upper), and an HEM dialyzed with 100 nM Gβ1γ2 (Lower). (B) Summary of experiments in A. Control data are pooled from both HEK-TRPM1 and HEMs (n = 24 cells). Experiments with dialysis of GTPγS (n = 7) were carried out on HEK-TRPM1 cells. Dialysis of Gβ1γ2 (n = 3) was carried out on HEMs. *P < 0.0001. (C, Left) example of a Ca2+ imaging experiment performed on control (filled symbols) and Gβ1γ2-transfected (open symbols) HEK-TRPM1 cells. (Right) Summary of the maximum ΔF/F0 for each group (control, n = 6 coverslips; Gβ1γ2, n = 9 coverslips; *P = 0.005. (D) As in C, except that cells were transfected with Gαo Q205L. (Right) Summary of the maximum ΔF/F0 for each group (control, n = 7 coverslips; Gαo Q205L-transfected cells, n = 9 coverslips). (E) Representative responses to capsazepine from an HEK-TRPM1 cell transfected with GFP alone (Top), Gαo Q205L and GFP (Middle), and Gβ1γ2 and GFP (Bottom). (F) Mean ± SEM of responses to capsazepine recorded in each condition (GFP only, n = 18; Gαo Q205L-transfected cells, n = 8; Gβ1γ2-transfected cells, n = 12). The difference between control and Gβ1γ2 (P < 0.0001), but not Gαo Q205L (P = 0.13), was highly significant.
To examine further the role of Gβγ in the regulation of TRPM1, we transfected HEK-TRPM1 cells with Gβγ subunits. Cells were cotransfected with DsRed for the identification of Gβγ+ cells. In the presence of the G-protein dimer, Ca2+ signals triggered by switching from a 0–5 mM Ca2+ solution were markedly reduced compared with those in untransfected cells or cells transfected with DsRed alone (Fig. 3C). This finding is consistent with the idea that the Gβγ subunit closes TRPM1 and reduces Ca2+ influx. We also tested the possibility that the Gαo subunit plays a direct role in closing TRPM1. HEK-TRPM1 cells were transfected with a constitutively activated form of Gαo (Gαo Q205L) along with DsRed. However, transfection of Gαo Q205L had no significant effect on the amplitude of Ca2+ transients compared with cells that were untransfected, or transfected with DsRed alone (Fig. 3D). Interpretation of the Ca2+ imaging experiments was supported by experiments with patch clamp recordings. TRPM1 currents were smaller in Gβγ-transfected cells (identified by contransfection with EGFP) than in control cells, and this difference was highly significant (P < 0.001), whereas the magnitude of currents recorded in cells transfected with Gαo Q205L were not significantly different from control (P = 0.13; Fig. 3 E and F).
Activation of an Endogenous Gαo-Independent Pathway Closes TRPM1.
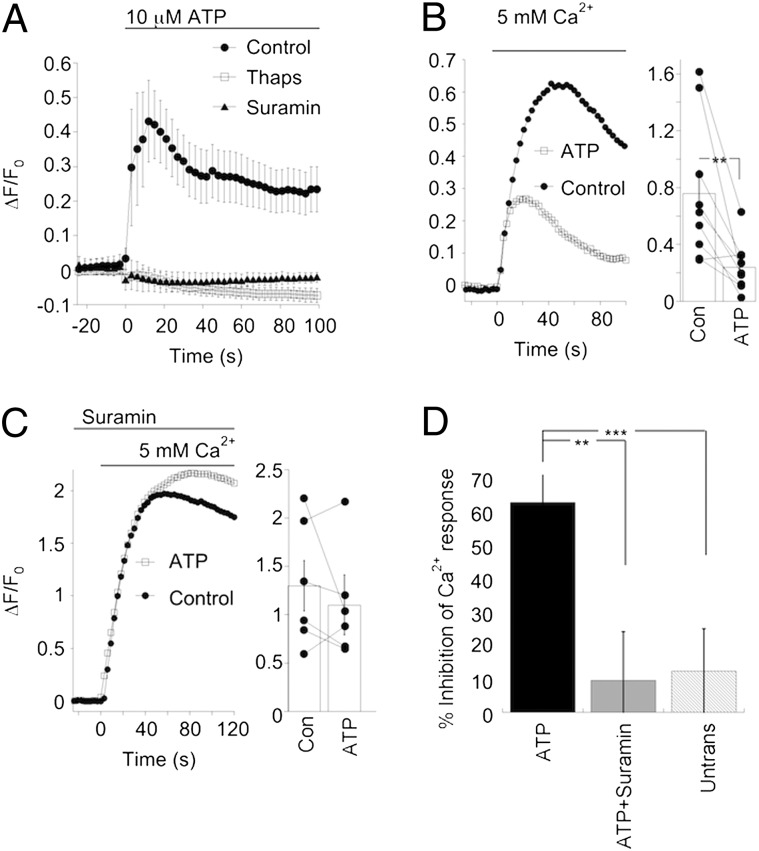
HEK293 cells express a variety of endogenous receptors, including the P2Y metabotropic purinergic receptors (29, 30), which couple predominantly to the Gq category of G protein (31). This class of G protein couples poorly to the mGluR6 receptor and is therefore unlikely to play a role in mGluR6 transduction cascade (10, 32). Activation of P2Y receptors should result in the liberation of endogenous Gβγ dimers from Gqα. We reasoned that if the Gβγ subunit is necessary to close TRPM1, then activation of the P2Y pathway should result in TRPM1 closure via a pathway that is independent of Gαo. To test this possibility, we first activated endogenous P2Y receptors in HEK-TRPM1 cells with 10 μM ATP, resulting in a rise in Ca2+ attributable to Gqα-mediated release from stores (Fig. 4A). This rise in Ca2+ was prevented by 100 μM suramin, a broad-spectrum P2 purinergic receptor antagonist, and also when stores were depleted by preincubation in 5 μM thapsigargin. Because store released Ca2+ would obscure the Ca2+ transient that resulted from influx through TRPM1 channels, experiments were performed in the presence of thapsigargin. Switching from nominally Ca2+-free solution to a solution containing 5 mM Ca2+ induced a rise in intracellular Ca2+ as expected. However, when cells were exposed to 10 μM ATP before increasing extracellular Ca2+, the Ca2+ transient was sharply reduced (Fig. 4B). When P2Y receptors were blocked with suramin, ATP had little effect on the transient response (Fig. 4C). Furthermore, ATP had almost no effect on Ca2+ transients elicited in untransfected HEK293 cells (data summarized in Fig. 3D). These results are consistent with the idea that free Gβγ dimers generated by activation of Gqα are capable of closing TRPM1, thereby reducing Ca2+ influx. Because this pathway does not couple to Gαo activation, this experiment provides further evidence that the Gαo subunit is not necessary to close TRPM1.
Fig. 4.
Stimulation of receptors that do not signal via Gαo can modulate TRPM1 function. (A) Time course of the change in intracellular Ca2+ concentration following bath application of the P2Y receptor agonist ATP alone (10 μM, filled circles) or in the presence of the broad-spectrum P2 receptor antagonist suramin (open circles), or 5 μM thapsigargin (filled triangles). The same cells were imaged in the absence and presence of 100 μM suramin (n = 6 coverslips). Responses to ATP after treatment with thapsigargin were measured in cells from separate coverslips (n = 9). (B, Left) Example of an experiment comparing Ca2+ transients evoked by increasing extracellular Ca2+ in the absence and presence of ATP. (Right) Summary of individual paired experiments as well as the mean ± SEM (P = 0.005, n = 9 coverslips). (C) Same experiment as in B except that suramin was added to the bath 2 min before the start of the experiment. In the presence of suramin, ATP did not significantly change the amplitude of the Ca2+ transient (P = 0.35, n = 6 coverslips). Thapsigargin was added to the bath to block release from Ca2+ stores in both B and C. (D) Summary of the effect of ATP on Ca2+ influx through TRPM1 channels, expressed as percent inhibition. Ca2+ influx was strongly reduced by ATP, but not in the presence of suramin (control; n = 9 coverslips, suramin; n = 6 coverslips, **P = 0.01) or in untransfected HEK293 cells (n = 12 coverslips, ***P = 0.006).
Discussion
Here we provide evidence that the Gβγ dimer plays a critical role in coupling mGluR6 activation with closure of the TRPM1 channel downstream. We favor a role for the Gβγ dimer rather than its counterpart Gαo for several reasons. First, the response to the mGluR antagonist LY341495, which is believed to be mediated largely by TRPM1 (2, 3), is robustly inhibited by delivery of exogenous Gβγ through the recording pipette. In principle, Gβγ can reduce the LY341495 by inhibiting TRPM1 or by constitutively opening the channel. The latter would also reduce the response to LY341495, but through an occlusion mechanism rather than channel inhibition. However, analysis of the baseline current indicated that the inhibition was due to closure of the channel by Gβγ, rather than as a result of the constitutive opening of TRPM1. On the other hand, we failed to see a significant effect of activated Gαo when it was delivered to RBCs under the same conditions. Second, we were able to observe clear evidence of TRPM1 channel closing in response to stimulation of an endogenous G-protein–coupled pathway that does not involve Gαo. A critical observation is that in unstimulated cells, the TRPM1 channel is open, indicating that under these conditions, endogenous G-protein activity is too low to close TRPM1. TRPM1 closed only when G-protein activity was mobilized by the addition of GTPγS, or by activation of the endogenous P2Y receptor. P2Y receptors are not known to couple to Go (31), providing further evidence that TRPM1 can be closed independently of Gαo.
Importantly, our findings differ from recent studies (4, 17) in which the authors concluded that it is Gαo rather than Gβγ that is responsible for closing TRPM1. They observed that application of Gαo to inside-out excised patches obtained from CHO cells reduced the open time of a channel believed to be TRPM1. These authors also show that glutamate suppresses a standing current in CHO cells coexpressing mGluR6, TRPM1, and Gαo, consistent with mGluR6-mediated suppression of TRPM1 current. At the present time, it is difficult to resolve the discrepancy between those studies and the findings described here. For example, the differences could result from the use of CHO cells as a model system, rather than bipolar cells. This explanation seems unlikely, however, as we observed nearly identical effects of Gβγ on TRPM1-L expressed in HEK293 cells and on the endogenous channel found in HEMs, as well as RBCs. There are also differences in the experimental approaches of those studies and the one described here. Most of the experiments with Gβγ described here made use of recombinant subunits, whereas the other studies were carried out using purified protein from brain. However, this is also unlikely to explain the difference in results, as we observed a robust effect of purified brain Gβγ on the light responses of RBCs.
We have previously suggested that capsazepine also antagonizes TRPM1 current in RBCs, and that capsaicin acts as an agonist (2). Recently Morgans et al. (3) reported a 50% reduction in the average response to capsaicin in ON bipolar cells of trpm1tm1Lex mice compared with wild type, but not complete elimination of the response. In the same study, it was reported that a subset of cone-driven ON bipolar cells may express other mGluR6-coupled channels in addition to TRPM1. If these channels are also sensitive to capsaicin, this could account for a residual capsaicin response in mice lacking functional TRPM1 receptors. We have chosen to target RBCs in the present study, and were unable to observe significant responses to capsaicin in trpm1tm1Lex mice. It is possible that the responses to capsaicin observed by Morgans et al. in trpm1tm1Lex mice were from cone-driven bipolar cells rather than RBCs, as this issue was not specifically addressed in their study.
Our results are consistent with known mechanisms of channel modulation by transmitters that couple to the Go pathway, specifically high-voltage–activated Ca2+ channel (14, 15) and GIRK channels (33, 34). In both examples, modulation of channel function is mediated directly by the βγ dimer, with the α subunit playing a supporting role (35–37). There are also several important differences that set G-protein regulation of the TRPM1 channel apart from other channels. GIRK channels are opened by G-protein activation, whereas the G-protein cascade activated by mGluR6 clearly closes a TRPM1. High-voltage–activated Ca2+ channels can be closed by Gβγ, but this effect is voltage dependent and can be reversed by strong depolarization (35, 36), whereas G-protein inhibition of TRPM1 appears to be independent of voltage.
ON bipolar cells do not fire action potentials, and are thought to be nearly isopotential. Thus, the dendrites can be considered as an electrical extension of the axon terminal. We propose that in darkness, Gβγ inhibits TRPM1, which hyperpolarizes the entire ON bipolar cell, including the axon terminal. Viewed in this way, the unique action of Gβγ in ON bipolar cells is therefore functionally analogous to the presynaptic regulation of excitability and transmitter release observed in a number of other central synapses.
Methods
Electrophysiological Recording and Data Analysis.
For recording from mouse RBCs, retinal slices were prepared from wild-type 4- to 6-wk-old C57BL/6 mice (Charles River) or trpm1tm1Lex mice from the same background (a gift from Ronald Gregg, University of Louisville, Louisville, KY, which was generated by Lexicon Genetics and obtained from the European Mouse Mutant Archive. More information is available at http://www.emmanet.org/). Experiments were conducted in accordance with the National Institutes of Health Guidelines, and the protocols were approved by the institutional animal care and use committee of Albert Einstein College of Medicine.
After euthanization, whole retinas were isolated and placed on a 0.65-μm cellulose acetate/nitrate membrane filter (Millipore), which was secured with vacuum grease to a glass slide adjacent to the recording chamber. Slices were cut to a thickness of 100 µm using a tissue slicer (Stoelting), transferred to the recording chamber, and viewed through a Nikon E600FN upright microscope with a water-immersion 40× objective and differential interference contrast optics (DIC), and were continuously perfused with Ames’ media bubbled with 95% O2/5% CO2.
Patch pipettes of resistance 7–9 MΩ were fabricated from borosilicate glass (WPI) using a two-stage vertical puller (Narishige), and filled with solution that contained 145 mM CsCl, 0.5 mM EGTA, 10 mM Hepes, 4 mM ATP, and 1 mM GTP (pH 7.4 by CsOH). The metabotropic receptor antagonist LY341495, or TRP channel reagents, were delivered to the retina from a pipette using positive pressure (2–4 psi) with a computer-controlled solenoid valve (Picospritzer; General Valve), and the mGluR6 agonist L-AP4 (4 μM) was added to the bath. Drugs and chemicals were purchased from Sigma-Aldrich, with the exceptions of L-AP4 and LY341495 (Tocris Bioscience).
In experiments where light responses were evoked from RBCs, the dissection was carried out under infrared illumination with an infrared converter mounted to the dissecting microscope (B. E. Meyers). Flashes of light were delivered to the slice from a 100-W mercury lamp through the objective lens. The duration, intensity, and wavelength of illumination were controlled by a computer-controlled shutter, neutral density, and interference filters in the light path. The internal solution contained 100 mM Cs-gluconate, 40 mM Hepes, 2.5 mM EGTA, 4 mM ATP, and 1 mM GTP; and L-AP4 was omitted from the bath solution.
For HEK293 or HEM recordings, the internal solution was composed of 145 mM KCl, 5.5 mM EGTA, 10 mM Hepes, and 4 mM MgATP (pH 7.2, KOH). The bath solutions used for recording were 145 mM NaCl, 5.4 mM KCl, 2 mM CaCl2, 11 mM glucose, and 10 mM Hepes (pH 7.4 with NaOH). Stocks of capsazepine and capsaicin (Sigma-Aldrich) were freshly diluted to the final concentration (50–100 µM) and then sonicated immediately before each experiment. Recordings were obtained with an Axopatch 1D amplifier, and an ITC-18 analog-to-digital converter. Data were filtered at 2 kHz and digitized at 5 kHz. To minimize voltage errors, 70–80% of the series resistance was compensated. I–V relations were obtained by step protocols from −80 mV to +80 mV. Data were analyzed offline with Excel (Microsoft), Axograph X, and KaleidaGraph (Synergy Software).
Gαo and Gβ1γ2 recombinant protein were purchased from Calbiochem/EMD Chemicals and Genway Biotech (10-783-79554) and diluted from frozen stocks on the day of recording. Gαo was preactivated by incubation in 100 nM GMP-PNP for 30 min at 4 °C. Gβ1γ2 recombinant protein was used in experiments with HEM cells and RBCs stimulated with LY341495. For examples on light-evoked responses in RBCs, Gβγ protein purified from brain was used (Calbiochem/EMD Chemicals). Identical results were observed for protein obtained from both sources, so they are collectively referred to as Gβγ in the text and figures.
Calcium Imaging.
TRPM1 activity was assessed in HEK293 and HEM cells by measuring changes in intracellular calcium ([Ca2+]i). Relative changes in [Ca2+]i were monitored by digital fluorescence imaging in cells loaded with Fluo-3. Cells were plated on 24-well chamber cover glass slides and studied 1–3 d after plating. Cells were loaded with 10 μM Fluo-3, AM (Invitrogen, Molecular Probes) in extracellular solution (120 mM NaCl, 2 mM KCl, 0.5 mM CaCl2, 1.0 mM MgCl2, 5 mM glucose, 23 mM NaHCO3, and 15 mM Hepes, adjusted to pH 7.4 with NaOH) for 30 min at 37 °C followed by washing in extracellular solution. During recordings extracellular Ca2+ was switched between 0 mM and 5 mM with or without 100 µM capsazepine. In some experiments, 5 µM thapsigargin was included during Fluo-3 loading to deplete calcium stores. Cells were imaged with a Retiga 2000× CCD camera (QImaging) attached to an inverted Nikon fluorescent microscope equipped with a 20× lens. Hardware control, data acquisition, and analysis were through MetaMorph software (Molecular Devices). Calcium responses were determined by averaging the change in Fluo-3 fluorescence (ΔF/F0) in each experiment. In a typical experiment, responses were measured in 5–8 randomly selected cells per field of view.
Transfection.
HEK293 cells (ATCC) were cultured in DMEM (Gibco/Invitrogen) and supplemented with 10% FBS. Neonatal HEMs were purchased from Cascade Biologics and cultured in Medium 254 (Gibco/Invitrogen) with human melanocyte growth supplement (Cascade Biologics). Cells were cultured on glass coverslips for imaging, and patch clamp recording experiments. HEK293 cells were transiently transfected with either a full-length mouse TRPM1 cDNA plasmid fused to an HA tag (a gift from Ronald Gregg, University of Louisville, Louisville, KY) using the CalPhos Mammalian Transfection kit (Clontech) according to the manufacturer’s instructions, or human TRPM1 cDNA plasmid (hTRPM1, a gift from Noga Vardi, University of Pennsylvania, Philadelphia, PA). Cells were plated at least 24 h before the transfection and grown to 50% confluence and were replaced with complete growth medium and cultured for another 24–48 h before experiments. A mouse TRPM1-expressing stable cell line (HEK-TRPM1) was established by selection in G418-containing media (500 μg/mL to 1 mg/mL) (Sigma). Individual colonies were isolated and TRPM1 expression was confirmed by electrophysiology and HA antibody (Covance; MMS-101P) labeling. In some experiments, HEK-TRPM1 cells were transfected with a constitutively activated Gαo mutant Q205 plasmid (Missouri S&T cDNA Resource Center) or a Gβ1 and Gγ2 plasmid (a gift from Stephen Ikeda, National Institutes of Health, Bethesda, MD). Transfected cells were identified by cotransfection with an EGFP plasmid for electrophysiology experiments or a DsRed plasmid for imaging experiments.
Acknowledgments
We thank Dr. Noga Vardi for her kind donation of the hTRPM1 plasmid and Dr. Ronald Gregg for the trpm1tm1Lex mice. This research was supported by National Eye Institute Grant EY010254 (to S.N.) and an unrestricted grant from Research to Prevent Blindness (to S.N.), National Natural Science Foundation of China Grant 81000395 (to Y.S.), State Key Laboratory of Medical Neurobiology, Fudan University 10-02 (Y.S.), and Luojia Young Scholars of Wuhan University (Y.S.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Bellone RR, et al. Differential gene expression of TRPM1, the potential cause of congenital stationary night blindness and coat spotting patterns (LP) in the Appaloosa horse (Equus caballus) Genetics. 2008;179:1861–1870. doi: 10.1534/genetics.108.088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen Y, et al. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J Neurosci. 2009;29:6088–6093. doi: 10.1523/JNEUROSCI.0132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgans CW, et al. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc Natl Acad Sci USA. 2009;106:19174–19178. doi: 10.1073/pnas.0908711106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koike C, et al. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci USA. 2010;107:332–337. doi: 10.1073/pnas.0912730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Genderen MM, et al. Mutations in TRPM1 are a common cause of complete congenital stationary night blindness. Am J Hum Genet. 2009;85:730–736. doi: 10.1016/j.ajhg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z, et al. Recessive mutations of the gene TRPM1 abrogate ON bipolar cell function and cause complete congenital stationary night blindness in humans. Am J Hum Genet. 2009;85:711–719. doi: 10.1016/j.ajhg.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Audo I, et al. TRPM1 is mutated in patients with autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2009;85:720–729. doi: 10.1016/j.ajhg.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhingra A, et al. Light response of retinal ON bipolar cells requires a specific splice variant of Galpha(o) J Neurosci. 2002;22:4878–4884. doi: 10.1523/JNEUROSCI.22-12-04878.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhingra A, et al. The light response of ON bipolar neurons requires G[alpha]o. J Neurosci. 2000;20:9053–9058. doi: 10.1523/JNEUROSCI.20-24-09053.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian L, Kammermeier PJ. G protein coupling profile of mGluR6 and expression of G alpha proteins in retinal ON bipolar cells. Vis Neurosci. 2006;23:909–916. doi: 10.1017/S0952523806230268. [DOI] [PubMed] [Google Scholar]
- 11.Weng K, et al. Functional coupling of a human retinal metabotropic glutamate receptor (hmGluR6) to bovine rod transducin and rat Go in an in vitro reconstitution system. J Biol Chem. 1997;272:33100–33104. doi: 10.1074/jbc.272.52.33100. [DOI] [PubMed] [Google Scholar]
- 12.Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 13.Wickman KD, et al. Recombinant G-protein beta gamma-subunits activate the muscarinic-gated atrial potassium channel. Nature. 1994;368:255–257. doi: 10.1038/368255a0. [DOI] [PubMed] [Google Scholar]
- 14.Holz GG, 4th, Rane SG, Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature. 1986;319:670–672. doi: 10.1038/319670a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott RH, Dolphin AC. Regulation of calcium currents by a GTP analogue: Potentiation of (-)-baclofen-mediated inhibition. Neurosci Lett. 1986;69:59–64. doi: 10.1016/0304-3940(86)90414-3. [DOI] [PubMed] [Google Scholar]
- 16.Padgett CL, Slesinger PA. GABAB receptor coupling to G-proteins and ion channels. In: Thomas PB, editor. Advances in Pharmacology. Vol 58. New York: Academic; 2010. pp. 123–147. [DOI] [PubMed] [Google Scholar]
- 17.Koike C, Numata T, Ueda H, Mori Y, Furukawa T. TRPM1: A vertebrate TRP channel responsible for retinal ON bipolar function. Cell Calcium. 2010;48:95–101. doi: 10.1016/j.ceca.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Rampino MAF, Nawy SA. Relief of Mg²+-dependent inhibition of TRPM1 by PKCα at the rod bipolar cell synapse. J Neurosci. 2011;31:13596–13603. doi: 10.1523/JNEUROSCI.2655-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snellman J, Nawy S. cGMP-dependent kinase regulates response sensitivity of the mouse on bipolar cell. J Neurosci. 2004;24(29):6621–6628. doi: 10.1523/JNEUROSCI.1474-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wässle H. Types of bipolar cells in the mouse retina. J Comp Neurol. 2004;469:70–82. doi: 10.1002/cne.10985. [DOI] [PubMed] [Google Scholar]
- 21.Deans MR, Volgyi B, Goodenough DA, Bloomfield SA, Paul DL. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 2002;36:703–712. doi: 10.1016/s0896-6273(02)01046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soucy E, Wang Y, Nirenberg S, Nathans J, Meister M. A novel signaling pathway from rod photoreceptors to ganglion cells in mammalian retina. Neuron. 1998;21:481–493. doi: 10.1016/s0896-6273(00)80560-7. [DOI] [PubMed] [Google Scholar]
- 23.Wang YV, Weick M, Demb JB. Spectral and temporal sensitivity of cone-mediated responses in mouse retinal ganglion cells. J Neurosci. 2011;31(21):7670–7681. doi: 10.1523/JNEUROSCI.0629-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearring JN, et al. A role for nyctalopin, a small leucine-rich repeat protein, in localizing the TRP melastatin 1 channel to retinal depolarizing bipolar cell dendrites. J Neurosci. 2011;31:10060–10066. doi: 10.1523/JNEUROSCI.1014-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oancea E, et al. TRPM1 forms ion channels associated with melanin content in melanocytes. Sci Signal. 2009;2:ra21. doi: 10.1126/scisignal.2000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller AJ, et al. Transcriptional regulation of the melanoma prognostic marker melastatin (TRPM1) by MITF in melanocytes and melanoma. Cancer Res. 2004;64:509–516. doi: 10.1158/0008-5472.can-03-2440. [DOI] [PubMed] [Google Scholar]
- 27.Nawy S. Regulation of the on bipolar cell mGluR6 pathway by Ca2+ J Neurosci. 2000;20:4471–4479. doi: 10.1523/JNEUROSCI.20-12-04471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu XZS, Moebius F, Gill DL, Montell C. Regulation of melastatin, a TRP-related protein, through interaction with a cytoplasmic isoform. Proc Natl Acad Sci USA. 2001;98:10692–10697. doi: 10.1073/pnas.191360198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schachter JB, Sromek SM, Nicholas RA, Harden TK. HEK293 human embryonic kidney cells endogenously express the P2Y1 and P2Y2 receptors. Neuropharmacology. 1997;36:1181–1187. doi: 10.1016/s0028-3908(97)00138-x. [DOI] [PubMed] [Google Scholar]
- 30.Fischer W, Franke H, Gröger-Arndt H, Illes P. Evidence for the existence of P2Y1,2,4 receptor subtypes in HEK-293 cells: Reactivation of P2Y1 receptors after repetitive agonist application. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:466–472. doi: 10.1007/s00210-005-1070-6. [DOI] [PubMed] [Google Scholar]
- 31.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 32.Beqollari D, Betzenhauser MJ, Kammermeier PJ. Altered G-protein coupling in an mGluR6 point mutant associated with congenital stationary night blindness. Mol Pharmacol. 2009;76:992–997. doi: 10.1124/mol.109.058628. [DOI] [PubMed] [Google Scholar]
- 33.Pfaffinger PJ, Martin JM, Hunter DD, Nathanson NM, Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985;317:536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- 34.Breitwieser GE, Szabo G. Uncoupling of cardiac muscarinic and beta-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985;317:538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- 35.Herlitze S, et al. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 36.Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 37.Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]