Abstract
We conducted a systematic review and meta-analysis to assess the evidence for the postulation that inappropriate tuberculosis (TB) regimens are a risk for development of multidrug-resistant (MDR)-TB.
MEDLINE, EMBASE and other databases were searched for relevant articles in January 2011. Cohort studies including TB patients who received treatment were selected and data on treatment regimen, drug susceptibility testing results and genotyping results before treatment and at failure or relapse were abstracted from the articles.
Four studies were included in the systematic review and two were included in the meta-analysis. In these two studies the risk of developing MDR-TB in patients who failed treatment and used an inappropriate treatment regimen was increased 27-fold (RR 26.7, 95% CI 5.0–141.7) when compared with individuals who received an appropriate treatment regimen.
This review provides evidence that supports the general opinion that the development of MDR-TB can be caused by inadequate treatment, given the drug susceptibility pattern of the Mycobacterium tuberculosis bacilli. It should be noted that only two studies provided data for the meta-analysis. The information can be used to advocate for adequate treatment for patients based on drug resistance profiles.
Keywords: Multidrug resistance, review, treatment, tuberculosis
Multidrug resistance remains a threat to tuberculosis (TB) control [1]. It is generally accepted that the development of drug resistance and multidrug-resistant (MDR)-TB is caused by inadequate treatment, i.e. regimens with an inadequate number of drugs to which the bacilli are susceptible, an inadequate dose or dosing frequency, an inadequate quality of the drugs, or an inadequate adherence to the regimen. The aim of this review was to assess the evidence for this hypothesis. As new anti-TB drugs become available, it is important to assess the best process for their introduction and use in TB regimens at an early stage. If the hypothesis holds, it will add to and strengthen the evidence that the introduction of new TB drugs, in TB treatment regimens, needs to be performed with great care to prevent the development of resistance against new TB drugs.
Assessing whether drug resistance develops when inadequate treatment is provided requires studies that provide information on: drug susceptibility testing (DST) before the start of treatment, the treatment regimen undertaken by each individual in the study, DST of specimens obtained from individuals that fail treatment or relapse, and genotyping information of the initial strain (collected before treatment) and of the strain that is present at failure or relapse.
Data from low- and high-prevalence areas show that patients can be re-infected with a new strain of TB during treatment or after successful treatment [2, 3]. Since patients might be re-infected with a resistant strain, “acquired” drug resistance can only be diagnosed if the possibility of re-infection is excluded. Re-infection can only be excluded by genotyping the strain that caused the initial episode and the strain that is present in the sample taken at failure or recurrence. The method most frequently used to collect information about the genotype is restriction fragment length polymorphism (RFLP) analysis [4]. van Embden et al. [4] first proposed this standard methodology for strain identification in 1993. Other methods include variable number of tandem repeats typing [5] or spoligotyping [6].
We conducted a systematic review and meta-analysis according to the Cochrane Handbook for Systematic Reviews and the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement [7, 8] to assess the risk for development of MDR-TB after the use of inappropriate TB regimens.
METHODS
Search strategy
To identify relevant studies we conducted a literature search in the bibliographic databases MEDLINE and EMBASE in January 2011. We searched for guidelines in the National Guideline Clearinghouse, and the National Institute for Clinical Excellence (NICE) and Scottish Intercollegiate Guidelines Network (SIGN) databases. In addition abstracts of conference proceedings were sought in the research database BIOSIS. Reviews and guidelines were searched for in the TRIP database. The World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) was evaluated for ongoing trials that might provide relevant data. Key words used in the search were determined in collaboration with the clinical librarian of the Dutch Cochrane Centre (Amsterdam, the Netherlands) and included “Tuberculosis” OR “TB” OR “Mycobacterium” AND for TB treatment “Prescriptions” OR “Treatment regimen” OR “Combination treatment” OR “Treatment strategy/-ies” OR “Drug supply” OR “Standard treatment/standard regimen” OR “Inappropiate use, appropriate use, rational use, irrational use, misuse” AND for drug resistance “drug or multidrug” OR “extensive or extensively” OR “drug and resistance or resistant”. We excluded case reports. The search strategy was supplemented by hand searching reference lists of identified articles and relevant review articles.
Selection of studies and quality assessment
We initially planned to include cohort studies investigating the use of inappropriate TB regimens as a risk factor for the development of drug resistance. However, none of the cohort studies, identified in the search, investigated the use of inappropriate TB regimens as a risk factor for the development of drug resistance. Therefore, we widened our selection and inclusion criteria to cohort studies that provided treatment to non-MDR-TB patients and measured drug resistance and genotype of the isolated Mycobacterium tuberculosis bacilli before treatment started, and drug resistance and genotype in failure and/or recurrent TB cases. Only studies that included TB patients meeting either the WHO definitions for “definite case” [9], or meeting the “possible”, “probable” or “definite” case definition as published by the European Commission in 2008 [10] were included. Articles published in Dutch, English, French, German, Italian, Portuguese, Spanish or Swedish were included.
Studies identified by the search strategy were reviewed for eligibility based on title and abstract by one investigator (M.J. van der Werf). If it could not be assessed with certainty whether there was reason for exclusion of a record the record was kept for the second selection step. Full manuscripts of the records kept based on title/abstract were assessed by one investigator (M.J. van der Werf). For both steps, a 10% random sample was assessed by a second investigator (M.W. Langendam) and compared with the assessment of the first reviewer. Inconsistencies in assessment were discussed and disagreements resolved by consensus. A complete double selection was planned if the 10% random sample assessment revealed relevant inconsistencies.
Data extraction
One reviewer (M.J. van der Werf) extracted all relevant data items from the included studies using a data extraction form. A second reviewer (M.W. Langendam) independently extracted the main results of the included studies and checked the other extracted results for a subsample of the articles. Inconsistencies were discussed to obtain consensus. The risk of bias of the individual studies was assessed by two reviewers independently using the Newcastle–Ottawa Scale (NOS) [11].
Data analysis and synthesis of results
For studies that were clinically and methodologically homogeneous, a meta-analysis was performed using Review Manager software version 5.0 (Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration, 2008). For other studies, the results were summarised qualitatively. If results were missing in a study (e.g. due to contamination of culture or because the sample was not collected) attempts were made to extrapolate the missing information from the information provided in the article or by contacting the authors.
For the analysis, appropriate treatment regimens were defined for TB patients with different drug resistance patterns. The WHO TB treatment guidelines were a source for defining the number and type of effective drugs required for the initial treatment phase and for the continuation phase (table 1) [9, 12]. Treatment regimens were considered appropriate if the patient had disease due to a pansusceptible strain and the regimen contained isoniazid and rifampicin and two other drugs in the intensive phase, and isoniazid and rifampicin in the continuation phase. If the patient had disease caused by a strain monoresistant to isoniazid the intensive phase needed to contain isoniazid and rifampicin and two other drugs. The continuation phase included isoniazid, rifampicin and ethambutol. Patients with non-MDR-TB that were susceptible to rifampicin needed a regimen with at least three drugs to which the strain is susceptible in both phases and patients with non-MDR-TB and resistance to rifampicin needed a regimen that included at least four drugs to which the strain was susceptible. All other regimens were considered inappropriate.
Table 1– Appropriate treatment regimens for tuberculosis (TB) patients with strains that have certain drug resistance patterns.
| Drug resistance pattern | Appropriate treatment regimen |
| Pansusceptible | HR and two other drugs in intensive phase and HR in the continuation phase |
| H | HR and two other drugs in intensive phase and HRE in the continuation phase# |
| Non-MDR-TB, R susceptible | At least three drugs to which the strain is sensitive in the intensive and continuation phase¶ |
| Non-MDR-TB, R resistant | At least four drugs to which the strain is sensitive in the intensive and continuation phase¶ |
Definitions of MDR-TB, acquired MDR-TB, recurrence, relapse and re-infection are provided in table 2 [9, 13]. In this study, acquired MDR-TB was defined as a case with an initial strain susceptible to at least isoniazid or rifampicin, which was then MDR-TB at the time of failure or disease recurrence and with a genotype pattern identical to the initial strain at the time of first diagnosis.
Table 2– Definitions of multidrug-resistant (MDR)-tuberculosis (TB), acquired MDR-TB, recurrence, relapse and re-infection.
| Definition | |
| MDR-TB | TB resistant to at least isoniazid and rifampicin. |
| Acquired MDR-TB | A case with an initial strain susceptible to at least isoniazid or rifampicin that developed MDR-TB and has a genotyping pattern identical to the strain at time of diagnosis. |
| Recurrence | A second episode of TB occurring after a first episode has been considered cured. |
| Relapse | A second episode of TB occurring after a first episode has been considered cured with the same Mycobacterium tuberculosis strain as the first episode. |
The quality of the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach [14].
RESULTS
Study selection
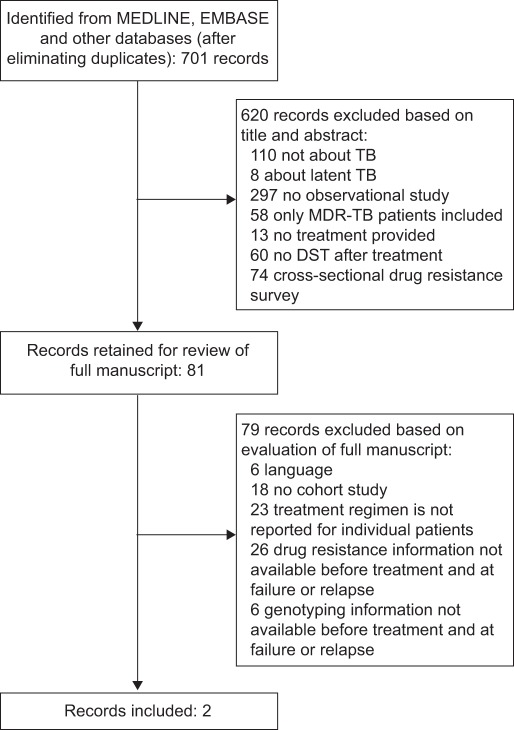
Of the 701 records identified from the MEDLINE and EMBASE searches, 81 were kept for evaluation of the full manuscript. Of these records, two studies fulfilled the eligibility criteria [15, 16]. figure 1 shows why records were excluded based on the assessment of title/abstract and full manuscripts. Three relevant reviews were identified in the MEDLINE and EMBASE search [17–19].
Figure 1–
Summary of literature search and study selection. TB: tuberculosis; MDR: multidrug resistant; DST: drug susceptibility testing.
The National Guideline Clearinghouse, NICE, SIGN, and BIOSIS databases did not provide relevant aggregated evidence. The WHO ICTRP did not include ongoing trials that might provide relevant data. The TRIP database provided 212 systematic reviews and 220 guidelines for the search terms “Tuberculosis” and “Treatment”. Two additional systematic reviews were considered relevant [20, 21]. Checking the reference lists of the identified reviews provided five potentially relevant primary studies [22–26]. None of the studies could be included as it was not possible to link individual treatment information with DST and genotyping information or the treatment regimen was not mentioned in sufficient detail.
Hand searching reference lists of identified articles provided two additional papers that fulfilled the eligibility criteria [27, 28].
Description of the included studies
All four included studies were cohort studies (tables 3 and 4). They included 233–2,901 TB patients at the start of treatment who were followed up until the end of treatment to identify failure cases [15, 27], or followed up for >1.5–3 yrs after treatment to identify cases that had recurrent TB [16, 28].
Table 3– General characteristics of the study populations.
| First author [ref.] | Age in median yrs or n (%) | Male % | HIV positive % | Type of TB | Prior TB treatment % |
| Sonnenberg [28] | <30: 35 (10.7) | Not reported | 46.3 | Culture-positive TB patients without multidrug resistance and cured of TB | 22.7 |
| 30–39: 163 (50.0) | |||||
| 40–49: 84 (25.8) | |||||
| ≥50: 44 (13.5) | |||||
| Quy [16] | 15–24: 359 (12.4) | 74.2 | Not reported | New smear-positive TB | 0 |
| 25–34: 920 (31.7) | |||||
| 35–44: 867 (29.9) | |||||
| 45–54: 399 (13.8) | |||||
| 55–64: 208 (7.2) | |||||
| ≥65: 148 (5.1) | |||||
| Cox [27] | Not reported | Not reported | Not reported | Smear-positive pulmonary TB | 45.3 |
| Matthys [15] | 29 (16–66) | 100 | 0 | Culture-positive TB patients | 73 |
TB: tuberculosis.
Table 4– Characteristics of the cohort study design, outcome and exposure.
| First author [ref.] | Country | Recruitment year | Setting | Inclusion | DST method | Genotyping method | Treatment adherence | Treatment regimen | Sample size |
| Sonnenberg [28] | South Africa | 1995 | Hospital serving four gold mines | Culture-positive TB patients without MDR and cured of TB | Bactec system or conventional proportional count method and Lowenstein–Jensen medium | IS6110 DNA fingerprinting | DOT | 2HRZE/4HR | 326# |
| Quy [16] | Vietnam | 1996–1998 | TB control programme | New smear- positive TB | Proportion method on Lowenstein–Jensen medium | IS6110 DNA fingerprinting | DOT | 2HRZS/6HE | 2901 |
| Cox [27] | Uzbekistan | 2001–2002 | DOTS programme | Smear-positive pulmonary TB patients | Lowenstein–Jensen media using proportion method or modified proportion method in Bactec 460TB | IS6610 DNA fingerprinting and spoligotyping | DOT | 2HRZE (with or without S)/4HR | 209 |
| 2HRZES/1HRZE/5HRE | 173 | ||||||||
| Matthys [15] | Russian Federation | 1997–1998 | TB treatment programme in a penitentiary hospital | Newly admitted patients diagnosed with TB through sputum smear and culture | Proportion method on Lowenstein–Jensen medium | IS6110 DNA fingerprinting | Daily strict supervision | 2HRZES/1HREZ/5HRE | 233 |
DST: drug susceptibility testing; TB: tuberculosis; MDR: multidrug resistant; DOTS: directly observed treatment, short course; H: isoniazid; R: rifampicin; Z: pyrazinamide; E: ethambutol; S: streptomycin. #: initial pre-treatment DST result missing for three TB patients.
The included studies did not provide sufficient detailed information about dose or dosing frequency, quality of the drugs, or adherence to the regimen to assess the association of these factors with the development of MDR-TB.
Sonnenberg et al. [28] included TB patients with an episode of TB that was proven by culture. Quy et al. [16] included new smear-positive TB patients (either two sputum smears with acid-fast bacilli or one positive smear and an abnormal chest radiograph consistent with TB). Cox et al. [27] included smear-positive pulmonary TB patients (at least one sputum sample reading >10 bacilli/100 fields in a sputum smear by direct microscopy). Matthys et al. [15] included patients diagnosed with TB through sputum smear and culture. All included patients fulfilled the definition of a definite TB case according to the WHO definition [9] and the definition of the European Commission [10].
Risk of bias assessment
table 5 presents the results of the risk of bias assessment of the four studies using the star template. The three studies that scored four stars for selection all included a sample of the general population [16, 27, 28]. However, none of these three studies were considered truly representative of the average population at risk for acquiring MDR-TB. The fourth study was in a selected group, prisoners with TB who were admitted to a referral penitentiary hospital and was, therefore, considered not representative of the general population [15]. In all four studies, the unexposed cohort (those who received an appropriate TB regimen) was drawn from the same community as the exposed cohort, so there was minimal risk of bias in the selection of the unexposed. Also, all four studies measured exposure (receiving an inappropriate TB regimen) with sufficient quality. We only selected studies that performed DST before treatment so the outcome of interest (MDR-TB) was not present at the start of the study. None of the four included studies assessed differences between individuals receiving appropriate treatment and inappropriate treatment, so an assessment of the comparability of the exposed and unexposed cohort was not performed.
Table 5– Risk of bias assessment using the Newcastle–Ottawa Scale star template# for cohort studies.
| First author [ref.] | Selection | Comparability¶ | Outcome |
| Sonnenberg [28] | **** | – | *** |
| Quy [16] | **** | – | ** |
| Cox [27] | **** | – | * |
| Matthys [15] | *** | – | *** |
#: a study can be awarded a maximum of one star for each numbered item within the “selection” and “outcome” category and a maximum of two stars can be given for “comparability”. For the “selection” category a star is awarded if the exposed cohort is representative; if the unexposed cohort is drawn from the same community as the exposed cohort; if exposure was ascertained by secure record or by a structured interview; and if it was demonstrated that outcome of interest was not present at the start of the study. For “comparability”, one star is awarded if the study controls for the most important factor and another star can be awarded if the study controls for any additional factor. For “outcome” a star is awarded if the assessment of the outcome is an independent blind assessment or by record linkage; if there was a long enough follow-up for the outcomes to occur; and if there was complete follow-up or if the fact that subjects were lost to follow-up is unlikely to introduce bias. ¶: no comparison was made between individuals receiving appropriate treatment and inappropriate treatment since this was not the interest of the authors.
As mentioned in the Methods section, the included studies were not designed to investigate the use of inappropriate TB regimens as a risk factor for drug resistance. Thus, the assessment of outcome was not influenced, because the authors did not look separately at the group of exposed and unexposed TB patients. We considered a follow-up until the end of treatment as adequate for identifying failure cases and a follow-up of a minimum of 1 yr for the identification of recurrence cases. In all four studies the follow-up was long enough for the outcome to occur.
In conclusion, study quality was moderate to high. Two studies provided data for acquired MDR-TB in failure cases [15, 27]. One study provided data for acquired MDR-TB in recurrence cases [28] and Quy et al. [16] provided information for acquired MDR-TB in both failure and recurrence cases.
Meta-analysis
All TB patients in the study by Quy et al. [16] received inappropriate treatment, as the continuation phase consisted of isoniazid and ethambutol and not isoniazid and rifampicin (table 6). Quy et al. [16] showed that the risk of acquiring MDR-TB was 1.5% for failure cases and 0.4% for recurrence cases, after correction for missing culture results. The study by Sonnenberg et al. [28] had both patients who received an appropriate TB regimen and patients who received an inappropriate TB regimen. However, this study did not have any events; i.e. none of the patients presenting with MDR-TB at recurrence had a strain with an identical genotype pattern to the initial strain at the time of diagnosis. As these two studies did not have both exposed and unexposed individuals and events, they could not be included in the meta-analysis and thus only two of the four studies were available for meta-analysis [15, 27].
Table 6– Data abstracted from the included studies for meta-analysis#.
| First author [ref.] | Treatment appropriate based on DST | Non-MDR-TB patients treated | Patients that failed treatment and acquired MDR-TB | Patients with recurrence with acquired MDR-TB |
| Sonnenberg [28] | Yes | 294 | 0 | |
| No | 29 | 0 | ||
| Quy [16] | Yes | 0 | 0 | 0 |
| No | 2551 | 38 | 10 | |
| Cox [27] | Yes | 240 | 1 | |
| No | 74 | 9 | ||
| Matthys [15] | Yes | 127 | 0 | |
| No | 62 | 5 |
Data are presented as n. DST: drug-susceptibility testing; MDR: multidrug resistance; TB: tuberculosis. #: the patients for whom it was unknown whether they acquired MDR-TB are not included as acquired MDR-TB.
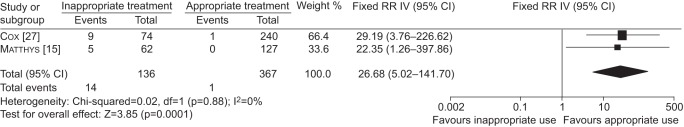
We used a fixed-effects model for the meta-analysis due to the low number of studies. Patients who received an inappropriate treatment regimen had a 27-fold increased risk of developing MDR-TB (RR 26.7, 95% CI 5.0–141.7) (fig. 2). For two patients it was not clear whether they had acquired MDR-TB or whether they were re-infected with an MDR strain. One of these patients was a patient who had MDR-TB at recurrence [27] and the other was diagnosed with MDR-TB at failure [15]. For the main analysis we included these two cases as re-infection cases and they were not counted as events. Including these two cases as acquired MDR-TB cases in the meta-analysis results in a slightly lower risk ratio (RR 17.7, 95% CI 4.1–77.6).
Figure 2–
Forest and meta-analysis of the two included studies showing the risk ratio (RR) of inappropriate treatment and risk of developing multidrug-resistant tuberculosis. IV: inverse variance.
Using the GRADE approach we started with low quality evidence as we included observational studies. There was no need to downgrade the quality of the evidence due to study limitations, imprecision, indirectness or inconsistency. Since the information collected for this review is mainly from studies that did not assess our research question we feel that the risk for publication bias is low, and so downgrading for publication bias was also not considered necessary. The effect identified in the meta-analysis is large (RR 26.7), therefore, we believe that the quality of the evidence should be upgraded by one level, from “low” to “moderate”. No plausible confounding factor that would change the effect was identified. Also, there was no dose–response gradient. The overall quality of the evidence as assessed by the GRADE approach is, therefore, moderate. This means that we are moderately confident in the effect estimate, and that the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
DISCUSSION
In this review we identified four studies that provided information for estimating the risk of developing MDR-TB after the use of a TB regimen that was inappropriate for a patient, considering the drug resistance pattern of the M. tuberculosis strain. Only two studies could be used for the meta-analysis. These two studies showed that the risk of developing MDR-TB was increased 27-fold in patients who were prescribed an inappropriate treatment regimen. This finding supports the general opinion that inadequate treatment is a risk factor for the development of drug-resistant TB and MDR-TB.
We chose to include only high quality studies in our review. Studies had to provide information about drug resistance in the initial and the recurrent episode, genotyping information of the TB strain in both episodes and information about individual TB treatment regimens. We did come across studies on acquired drug resistance that did not apply genotyping to exclude re-infection [29, 30]. One study included in our review provided information on the percentage of MDR-TB in failure or recurrent cases that is really acquired and not re-infection. This study by Matthys et al. [15] showed that more than 60% (nine out of 14) of new MDR-TB in failure cases could be attributed to re-infection. The study was conducted in a penitentiary hospital in central Siberia (Russia) where the transmission of TB and MDR-TB is believed to be high, and thus the chances of re-infection with a MDR-TB strain is also high. A review on re-infection or relapse not focussing on MDR-TB showed that the proportion of recurrences due to re-infection ranged between 0% and 100% [13]. These results are in themselves not indicative; however, together with the information from the included studies it shows that an unknown proportion of the cases with “acquired” MDR-TB in studies not performing genotyping are due to re-infection. It is, therefore, essential to have genotyping information to assess whether new MDR-TB is truly acquired or due to re-infection.
There are several aspects that need to be considered when interpreting DNA fingerprinting results: 1) changes in the insertion sequence pattern due to recombination events, 2) heterogeneity of the fingerprinting patterns in a given area, and 3) mixed infections. Although IS6110 fingerprint patterns of M. tuberculosis isolates have a high degree of stability, changes in insertion sequence patterns have been identified, especially when the time interval between obtaining the isolates increased [31, 32]. Thus, two strains may be wrongly identified as different. This provides an underestimation of the number of strains that acquire drug resistance.
In areas shown to have a low heterogeneity in fingerprinting patterns [33, 34], patients have a higher chance of becoming re-infected with an identical strain. If the strain that causes re-infection is resistant to TB drugs this may wrongly lead to the conclusion that the patient acquired drug resistance. Also, strains with a low number of IS6110 copies (five copies or less) cannot be differentiated between; as the level of discrimination is low, a strain with a low copy number at failure or recurrence might be incorrectly identified as the same strain. This would overestimate the percentage of patients that acquired MDR-TB. Spoligotyping of such RFLP low copy strains can be used to further differentiate strains within identical, low-copy RFLP patterns [35].
The extent of acquired MDR-TB can be overestimated if there is a low heterogeneity of circulating M. tuberculosis strains. A study [36], based on the same patient population as that described by Cox et al. [27], identified a high degree of strain diversity among the patient population. Specifically, 152 (40%) isolates were in clusters ranging in size from two to 21 isolates; i.e. 60% of strains were “unique”. Given that the level of strain diversity was relatively high, we believe that the risk that patients defined as having acquired MDR-TB were in fact re-infected with an identical strain that was already MDR-TB, is small. Information about the heterogeneity of strains was not provided by Matthys et al. [15]. In an earlier study in 1999 [37], strains from the same setting of Matthys et al. [15] were characterised and a high degree of strain homogeneity, as assessed by RFLP, was seen. The authors concluded that there was ongoing transmission of a few strains within the prison. This study was performed 8 yrs before the study by Matthys et al. [15] and transmission in prisons might have changed due to TB control activities in prisons. However, we cannot rule out the possibility that some cases that were identified with acquired MDR-TB were actually due to re-infection with an MDR strain with the same RFLP pattern. If this is the case the relative risk for developing acquired MDR-TB will be lower.
Until recently, evidence of mixed infection in a single host at a single time-point was infrequently observed, which probably reflected the insensitivity of DNA fingerprinting methods [38]. More sensitive methods have shown that TB patients in high-incidence settings often have different M. tuberculosis strains in the same sputum specimen [39, 40]. Patients with mixed infection may thus wrongly be diagnosed with acquired drug resistance [41]. Newer genotyping methods have since become available that increase the discriminatory power of strain genotyping [42], including whole genome typing of M. tuberculosis [43]. Use of these methods for studying acquired drug resistance might resolve some of the challenges discussed previously.
A further consideration, in assessing the acquisition of drug resistance, is that DST results must be interpreted with caution. A round of proficiency testing by the supranational TB reference laboratories showed that DST for rifampicin presented difficulties in correctly identifying resistance in these top level laboratories [44]. Also, results for ethambutol and streptomycin were poor. The studies included in our review used WHO recommended DST methods. Two studies had all DST testing performed by a supranational reference laboratory [15, 27], and one sent a random sample for quality assurance to a supranational reference laboratory [16]; no discordance was observed for isoniazid and rifampicin. The study in South Africa had all testing performed by Lancet Laboratories in Johannesburg [28].
A limitation of cohort studies for assessing the incidence of acquired drug resistance is that a number of included patients in the cohort may die. The cause of death could be the acquisition of MDR-TB and thus non-response to treatment. The difficulty in collecting samples from deceased patients to assess the acquisition of drug resistance means that these cases will commonly be missed in cohort studies on acquired drug resistance, thereby resulting in an underestimation of the incidence of acquired resistance.
Only quality TB drugs will ensure that patients obtain the required regimen and the required dosage. Content and stability of TB drugs have been reported to be substandard [45, 46] and patients receiving substandard drugs may develop drug resistance even when prescribed an adequate regimen. The drugs used in the study of Matthys et al. [15] were procured outside Russia and had certificates that guaranteed their quality. The other included studies did not provide information about the quality of the drugs.
Inadequate adherence to treatment can also be a form of inappropriate treatment and thus a risk factor for acquiring MDR-TB. Furthermore, full adherence to treatment is essential to ensure the patient is cured. Adherence to treatment was not used as a criterion in this review for appropriate treatment and risk of acquired MDR-TB; however, all included studies mentioned how adherence to treatment was supported. All indicated that directly observed treatment was provided. Quy et al., [15] Matthys et al., [16] and Sonnenberg et al. [28] all provided treatment under direct observation. In the study by Cox et al. [27] patients were hospitalised during the intensive phase of treatment (exposure) and received doses during the continuation phase, which were ostensibly administered under the direct observation by local healthcare workers.
With regard to the applicability of the evidence, the evidence is based on the best quality studies available. However, only two studies could be included in the meta-analysis. If more studies become available the review and meta-analysis can be updated.
CONCLUSIONS
This review provides evidence for the general opinion that the development of MDR-TB can be caused by treatment that is inadequate, given the drug susceptibility pattern of the M. tuberculosis strain. The information can be used to advocate the need for adequate treatment of patients, based on drug resistance profiles. Few cohort studies provided information on treatment regimens and drug resistance profiles before treatment and at failure or recurrence, or genotyping information at failure or recurrence. To monitor the development of acquired drug resistance we suggest that, given sufficient resources are available, TB treatment cohort studies or surveillance systems measure both the drug resistance profile and genotype information before starting treatment and at failure or recurrence. This would enable future larger studies to assess the risk of resistance development due to inappropriate treatment.
Acknowledgments
We acknowledge the help of the European Centre for Disease Prevention and Control (ECDC) staff. We also thank R. Spijker, the clinical librarian of the Dutch Cochrane Centre, who assisted us with developing the search strategy. We appreciate the provision of additional data by H. Cox (Médecins Sans Frontières, Cape Town, South Africa).
Footnotes
Previous articles in this series: No. 1: Langendam MW, van der Werf MJ, Huitric E, et al. Prevalence of inappropriate tuberculosis treatment regimens: a systematic review. Eur Respir J 2012; 39: 1012–1020. No. 2: van der Werf MJ, Langendam MW, Huitric E, et al. Knowledge of tuberculosis-treatment prescription of health workers: a systematic review. Eur Respir J 2012; 39: 1248–1255.
Support Statement
The study reported in this manuscript was financially supported by an ECDC Direct Service Contract (Publication Reference: OJ/2010/07/12-PROC/2010/034).
Statement of Interest
None declared.
REFERENCES
- 1.Wright A, Zignol M, Van DA, et al. Epidemiology of antituberculosis drug resistance 2002–07: an updated analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Lancet 2009; 373: 1861–1873 [DOI] [PubMed] [Google Scholar]
- 2.Dobler CC, Marks GB, Simpson SE, et al. Recurrence of tuberculosis at a Sydney chest clinic between 1994 and 2006: reactivation or reinfection?. Med J Aust 2008; 188: 153–155 [DOI] [PubMed] [Google Scholar]
- 3.Verver S, Warren RM, Beyers N, et al. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am J Respir Crit Care Med 2005; 171: 1430–1435 [DOI] [PubMed] [Google Scholar]
- 4.van Embden JD, Cave MD, Crawford JT, et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol 1993; 31: 406–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frothingham R, Meeker-O'Connell WA. Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem DNA repeats. Microbiology 1998; 144: 1189–1196 [DOI] [PubMed] [Google Scholar]
- 6.Goyal M, Saunders NA, van Embden JD, et al. Differentiation of Mycobacterium tuberculosis isolates by spoligotyping and IS6110 restriction fragment length polymorphism. J Clin Microbiol 1997; 35: 647–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgens JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 (updated February 2008). The Chochrane collaboration, 2008. Available from www.cochrane-handbook.org. [Google Scholar]
- 8.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151: W65–W94 [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Treatment of Tuberculosis Guidelines. 4th Edn. Geneva,World Health Organization, 2010. Available from: http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf.
- 10.European Centre for Disease Preventionand Controland World Health Organization Regional Office for Europe Tuberculosis surveillance in Europe 2008. Stockholm European Centre for Disease Prevention and Control, 2010
- 11.Wells GA, Shea B, O'Connell D, et al. The Newcastle Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. www.ohri.ca/programs/clinical_epidemiology\oxford.htm Date last accessed: May, 2011
- 12.World Health Organization Guidelines for the programmatic management of drug-resistant tuberculosis. Emergency update 2008. Geneva, World Health Organization, 2008. Available from: http://whqlibdoc.who.int/publications/2008/9789241547581_eng.pdf [Google Scholar]
- 13.Lambert ML, Hasker E, Van DA, et al. Recurrence in tuberculosis: relapse or reinfection?. Lancet Infect Dis 2003; 3: 282–287 [DOI] [PubMed] [Google Scholar]
- 14.Guyatt GH, Oxman AD, Kunz R, et al. What is “quality of evidence” and why is it important to clinicians?. BMJ 2008; 336: 995–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthys F, Rigouts L, Sizaire V, et al. Outcomes after chemotherapy with WHO category II regimen in a population with high prevalence of drug resistant tuberculosis. PLoS One 2009; 4: e7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quy HT, Lan NT, Borgdorff MW, et al. Drug resistance among failure and relapse cases of tuberculosis: is the standard re-treatment regimen adequate?. Int J Tuberc Lung Dis 2003; 7: 631–636 [PubMed] [Google Scholar]
- 17.Lew W, Pai M, Oxlade O, et al. Initial drug resistance and tuberculosis treatment outcomes: systematic review and meta-analysis. Ann Intern Med 2008; 149: 123–134 [DOI] [PubMed] [Google Scholar]
- 18.Menzies D, Benedetti A, Paydar A, et al. Standardized treatment of active tuberculosis in patients with previous treatment and/or with mono-resistance to isoniazid: a systematic review and meta-analysis. PLoS Med 2009; 6: e1000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menzies D, Benedetti A, Paydar A, et al. Effect of duration and intermittency of rifampin on tuberculosis treatment outcomes: a systematic review and meta-analysis. PLoS Med 2009; 6: e1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao XF, Li J, Yang ZW, et al. Rifapentine vs. rifampicin for the treatment of pulmonary tuberculosis: a systematic review. Int J Tuberc Lung Dis 2009; 13: 810–819 [PubMed] [Google Scholar]
- 21.Mwandumba HC, Squire SB. Fully intermittent dosing with drugs for treating tuberculosis in adults. Cochrane Database Syst Rev 2001; 4: CD000970. [DOI] [PubMed] [Google Scholar]
- 22.Driver CR, Munsiff SS, Li J, et al. Relapse in persons treated for drug-susceptible tuberculosis in a population with high coinfection with human immunodeficiency virus in New York City. Clin Infect Dis 2001; 33: 1762–1769 [DOI] [PubMed] [Google Scholar]
- 23.Benator D, Bhattacharya M, Bozeman L, et al. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet 2002; 360: 528–534 [DOI] [PubMed] [Google Scholar]
- 24.Li J, Munsiff SS, Driver CR, et al. Relapse and acquired rifampin resistance in HIV-infected patients with tuberculosis treated with rifampin- or rifabutin-based regimens in New York City, 1997–2000. Clin Infect Dis 2005; 41: 83–91 [DOI] [PubMed] [Google Scholar]
- 25.Yoshiyama T, Yanai H, Rhiengtong D, et al. Development of acquired drug resistance in recurrent tuberculosis patients with various previous treatment outcomes. Int J Tuberc Lung Dis 2004; 8: 31–38 [PubMed] [Google Scholar]
- 26.Burman W, Benator D, Vernon A, et al. Acquired rifamycin resistance with twice-weekly treatment of HIV-related tuberculosis. Am J Respir Crit Care Med 2006; 173: 350–356 [DOI] [PubMed] [Google Scholar]
- 27.Cox HS, Niemann S, Ismailov G, et al. Risk of acquired drug resistance during short-course directly observed treatment of tuberculosis in an area with high levels of drug resistance. Clin Infect Dis 2007; 44: 1421–1427 [DOI] [PubMed] [Google Scholar]
- 28.Sonnenberg P, Murray J, Glynn JR, et al. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet 2001; 358: 1687–1693 [DOI] [PubMed] [Google Scholar]
- 29.Gelmanova IY, Keshavjee S, Golubchikova VT, et al. Barriers to successful tuberculosis treatment in Tomsk, Russian Federation: non-adherence, default and the acquisition of multidrug resistance. Bull World Health Organ 2007; 85: 703–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seung KJ, Gelmanova IE, Peremitin GG, et al. The effect of initial drug resistance on treatment response and acquired drug resistance during standardized short-course chemotherapy for tuberculosis. Clin Infect Dis 2004; 39: 1321–1328 [DOI] [PubMed] [Google Scholar]
- 31.Yeh RW, Ponce de LA, Agasino CB, et al. Stability of Mycobacterium tuberculosis DNA genotypes. J Infect Dis 1998; 177: 1107–1111 [DOI] [PubMed] [Google Scholar]
- 32.Niemann S, Richter E, Rusch-Gerdes S. Stability of IS6110 restriction fragment length polymorphism patterns of multidrug-resistant Mycobacterium tuberculosis strains. J Clin Microbiol 1999; 37: 3078–3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hermans PW, Messadi F, Guebrexabher H, et al. Analysis of the population structure of Mycobacterium tuberculosis in Ethiopia, Tunisia, and The Netherlands: usefulness of DNA typing for global tuberculosis epidemiology. J Infect Dis 1995; 171: 1504–1513 [DOI] [PubMed] [Google Scholar]
- 34.van Soolingen D, Qian L, de Haas PE, et al. Predominance of a single genotype of Mycobacterium tuberculosis in countries of east Asia. J Clin Microbiol 1995; 33: 3234–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cowan LS, Crawford JT. Genotype analysis of Mycobacterium tuberculosis isolates from a sentinel surveillance population. Emerg Infect Dis 2002; 8: 1294–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cox HS, Kubica T, Doshetov D, et al. The Beijing genotype and drug resistant tuberculosis in the Aral Sea region of Central Asia. Respir Res 2005; 6: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Portaels F, Rigouts L, Bastian I. Addressing multidrug-resistant tuberculosis in penitentiary hospitals and in the general population of the former Soviet Union. Int J Tuberc Lung Dis 1999; 3: 582–588 [PubMed] [Google Scholar]
- 38.Richardson M, Carroll NM, Engelke E, et al. Multiple Mycobacterium tuberculosis strains in early cultures from patients in a high-incidence community setting. J Clin Microbiol 2002; 40: 2750–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warren RM, Victor TC, Streicher EM, et al. Patients with active tuberculosis often have different strains in the same sputum specimen. Am J Respir Crit Care Med 2004; 169: 610–614 [DOI] [PubMed] [Google Scholar]
- 40.Shamputa IC, Jugheli L, Sadradze N, et al. Mixed infection and clonal representativeness of a single sputum sample in tuberculosis patients from a penitentiary hospital in Georgia. Respir Res 2006; 7: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Rie A, Victor TC, Richardson M, et al. Reinfection and mixed infection cause changing Mycobacterium tuberculosis drug-resistance patterns. Am J Respir Crit Care Med 2005; 172: 636–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathema B, Kurepina NE, Bifani PJ, et al. Molecular epidemiology of tuberculosis: current insights. Clin Microbiol Rev 2006; 19: 658–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gardy JL, Johnston JC, Ho Sui SJ, et al. Whole-genome sequencing and social-network analysis of a tuberculosis outbreak. N Engl J Med 2011; 364: 730–739 [DOI] [PubMed] [Google Scholar]
- 44.Van Deun A, Wright A, Zignol M, et al. Drug susceptibility testing proficiency in the network of supranational tuberculosis reference laboratories. Int J Tuberc Lung Dis 2011; 15: 116–124 [PubMed] [Google Scholar]
- 45.Bhutani H, Mariappan TT, Singh S. The physical and chemical stability of anti-tuberculosis fixed-dose combination products under accelerated climatic conditions. Int J Tuberc Lung Dis 2004; 8: 1073–1080 [PubMed] [Google Scholar]
- 46.Laserson KF, Kenyon AS, Kenyon TA, et al. Substandard tuberculosis drugs on the global market and their simple detection. Int J Tuberc Lung Dis 2001; 5: 448–454 [PubMed] [Google Scholar]