Abstract
The presence of olfactory dysfunction in individuals at higher risk of Alzheimer's disease has significant diagnostic and screening implications for preventive and ameliorative drug trials. Olfactory threshold, discrimination and identification can be reliably recorded in the early stages of neurodegenerative diseases. The current study has examined the ability of various olfactory functions in predicting cognitive decline in a community-dwelling sample. A group of 308 participants, aged 46–86 years old, were recruited for this study. After 3 years of follow-up, participants were divided into cognitively declined and non-declined groups based on their performance on a neuropsychological battery. Assessment of olfactory functions using the Sniffin' Sticks battery indicated that, contrary to previous findings, olfactory discrimination, but not olfactory identification, significantly predicted subsequent cognitive decline (odds ratio=0.869; P<0.05; 95% confidence interval=0.764−0.988). The current study findings confirm previously reported associations between olfactory and cognitive functions, and indicate that impairment in olfactory discrimination can predict future cognitive decline. These findings further our current understanding of the association between cognition and olfaction, and support olfactory assessment in screening those at higher risk of dementia.
Keywords: Alzheimer's disease, cognition, cognitive decline, odor discrimination, olfactory dysfunction, smell sense
Introduction
Olfactory dysfunction has been reliably demonstrated in Alzheimer's disease (AD)1, 2, 3 and mild cognitive impairment.4, 5 Of note, olfactory impairment has also been reported in cognitively healthy individuals positive for apolipoprotein E ɛ4 (APOE-ɛ4) allele, the main genetic risk factor for AD,6, 7 as well as in those with another potential risk factor for AD, namely subjective memory complaints.8
Indeed, olfactory dysfunction has been significantly associated with the risk of future AD and AD neuropathology burden in the brain.9 AD-related neuropathological studies of animals and humans have indicated the following: (i) A negative association between amyloid-beta (β-amyloid) load in the brain and olfaction;10, 11, 12 (ii) A strong association between tau pathology in olfactory system, Braak staging of AD pathology and cognitive decline;13, 14 and (iii) Presence of oxidative damage in the olfactory epithelium in the early stages of AD.15, 16
Observational and clinical studies have found a significant association between olfactory impairment and subsequent cognitive decline. For example, a large-scale study (N=1920) on the relationship between olfactory identification ability and general cognitive functioning (as measured by Mini Mental State Examination (MMSE)) indicated that olfactory dysfunction at baseline was significantly predictive of future cognitive impairment after 5 years (odds ratio (OR)=6.62; confidence interval (CI)=4.36–10.04).17 Schubert et al.17 have also reported low sensitivity of 55.1% but high specificity (84.4%) for olfactory assessment in predicting cognitive decline.
However, the olfaction/cognition relationship has not been consistently found, particularly when more complicated olfactory assessment instruments including electrophysiological measures were used in addition to psychophysical methods.18 Indeed, the different methods utilized to assess olfaction may be responsible for the inconsistent findings.19 Olfactory abilities are primarily assessed by measuring threshold (lowest detectable concentration of odors), discrimination (ability to differentiate between odors) and identification (ability to identify odors).20 It has been suggested that olfactory threshold is strongly related to sensory capability, while olfactory discrimination and identification are more closely associated with higher cognitive functions, and thus may be more cognitively loaded.8, 21 A dated, but still informative, review has reported a strong association between cognitive functions and certain aspects of olfactory functioning, concluding that compared with the ability to detect odors, identification of odors is more challenging, perhaps due to a lack of access to verbal or visual representations of odors.22 Similarly, Schab23 noted that odor identification may represent a semantic memory function. Some researchers suggest that olfactory identification is primarily predictive of memory decline.24 It is interesting that while verbal and visual cues may affect olfactory information processing, olfactory memories usually last longer than memories formed through other sensory modalities and have more emotional valence.25, 26
There is strong evidence that neuroanatomical regions involved in episodic memory, including the medial temporal lobe, are also associated with olfactory functioning.27, 28 Interestingly, individuals with hippocampal lesions show significantly poorer olfactory recognition compared with odor threshold.29
Olfactory impairment is not confined to people with AD or AD-related cognitive decline, and it has been reported in individuals suffering from other neurodegenerative diseases such as Parkinson's disease, frontotemporal dementia and Lewy body dementia.30, 31, 32 Olfactory deficits have also been reported in psychiatric disorders including schizophrenia and depression.33, 34 As such, olfactory dysfunctions appear not to be specific to AD.35, 36 Further studies are needed to improve the sensitivity and specificity of olfactory screening to identify which olfactory domains are specifically affected at particular stages of preclinical AD.
We hypothesized that olfactory ability at baseline could predict altered cognitive function in a 3-year follow-up assessment. The specific questions examined by the current study were the following: (1) Is there any association between olfaction and cognition at baseline in this cohort of apparently healthy aging individuals? (2) Could baseline olfactory function predict future (3-year) cognitive decline? (3) Which olfactory domain(s) best predict cognitive alteration?
Materials and methods
Design
Participants were derived from a larger, longitudinal study, ‘the Western Australia Memory Study' (for more information on this cohort, see refs 8, 37, 38, 39). Participants were monitored for 3 years, and underwent annual neuropsychological, biochemical and physiological examinations. Herein, the baseline and final assessment (two-point data) results of this study will be examined. The study was approved by the Human Research Ethics Committees of the associated institutions, namely the University of Western Australia, Edith Cowan University and Hollywood Private Hospital. Participants provided informed consent before baseline assessments according to the guidelines of the National Health and Medical Research Council of Australia.
Participants
A total of 308 community-dwelling older adults aged 46–86 years old (68% female) were recruited from a larger study cohort. The volunteers' family members were also invited to participate. The APOE-ɛ4 genotype was available for 273 participants, of whom 34% were ɛ4 allele carriers (ɛ2-ɛ4, ɛ3-ɛ4, or ɛ4-ɛ4). The mean education level for the cohort was 13.5 years (±3.77), and inclusion criteria were a minimum of 6 years of education, age 45 and over, and fluency in English. Exclusion criteria for this study included: (i) a baseline score of ⩽24 in MMSE40 and ⩽81 in the Cambridge Cognitive Examination-Revised (CAMCOG-R);41 (ii) history of anosmia or any known olfactory problems; and (iii) history or formal diagnosis of medical, neurological or psychiatric diseases and disorders affecting olfactory capacities (for example, sinunasal diseases, upper respiratory tract infection, severe head injury, Parkinson's disease, schizophrenia, and so on).
Measures
Olfactory function was assessed using the ‘Extended Sniffin' Sticks' battery (Burghart, Wedel, Germany), in which odors are presented using felt-tip pen-like sticks.42 The Extended Sniffin' Sticks assess three domains of olfactory function, using threshold (T), discrimination (D) and identification (I) as three subscales and enabling calculation of a composite score, namely TDI.43, 44 In this study, the Sniffin' Sticks battery was administered in a triple forced-choice staircase method as outlined previously45 and also in the test manual (Burghart).
The CAMCOG-R46 and MMSE40 were used to assess general cognitive functioning. The CAMCOG-R measures orientation, language, memory, attention, abstract thinking, praxis and calculation abilities, and provides a total score of cognitive functioning. The MMSE is commonly used as a screening measure to assess general cognitive performance in clinical and research settings.47
APOE genotyping
Blood samples were collected into different blood collection tubes including serum, EDTA (containing prostaglandin E) and heparin (Interpath Services, West VIC, Australia). The DNA was isolated from leukocytes, and APOE genotype was determined using PCR amplification and restriction enzyme digestion as previously described.48, 49
Statistical analysis
All statistical analyses were performed using PASW Statistics 18 for Windows 7. Partial correlation was applied to control for the effects of age. Variables examined in this study including sex, APOE genotype, age at baseline, baseline cognitive function and olfactory performance, as measured by Sniffin' Sticks, were entered in a logistic regression model using the enter analysis model. After the completion of 3-year follow-up, participants were divided into two groups: cognitively declined and non-declined. Participants were considered to be ‘cognitively declined' if their score on the final cognitive assessment (as measured by CAMCOG-R) was ⩾1 s.d. below their baseline performance.
Results
Cohort demographics
For this study, 308 participants with a mean age of 63.06 (±7.25) years undertook neuropsychological testing. Demographic characteristics of the cohort are shown in Table 1. The scores of male/female and APOE-ɛ4 carrier and non-carrier groups on the MMSE, Sniffin' Sticks and CAMCOG-R at baseline (Table 1) were all above the cutoff scores, as required in the exclusion criteria (40,41,50, respectively).
Table 1. Baseline descriptive results (age, years of education, cognitive measures and olfactory function were assessed and data analyzed in terms of gender and APOE genotype; Total N=308).
| Variables | Male (N=99) | Female (N=209) | P | APOE-ɛ4anon-carrier (N=202) | APOE-ɛ4 carrier (N=99) | P | Total cohort |
|---|---|---|---|---|---|---|---|
| Age | 65.19 (±7.59) | 62.05 (±6.88) | 0.000* | 63.43 (±7.62) | 62.70 (±6.39) | 0.413 | 63.06 (±7.25) |
| Education years | 14.05 (±4.14) | 13.34 (±3.56) | 0.127 | 13.58 (±3.91) | 13.41 (±3.43) | 0.713 | 13.57 (±3.77) |
| MMSE | 29.05 (±1.12) | 29.20 (±1.09) | 0.281 | 29.16 (±1.17) | 29.11 (±0.96) | 0.729 | 29.15 (±1.10) |
| CAMCOG-R | 98.58 (±3.58) | 98.71 (±3.34) | 0.743 | 98.75 (±3.50) | 98.49 (±3.12) | 0.536 | 98.67 (±3.41) |
| SS-T | 7.03 (±2.58) | 7.58 (± 2.34) | 0.063 | 7.44 (±2.35) | 7.30 (±2.09) | 0.622 | 7.40 (±2.37) |
| SS-D | 11.31 (±2.25) | 11.88 (±2.43) | 0.058 | 11.83 (±2.37) | 11.49 (±2.41) | 0.260 | 11.69 (±2.39) |
| SS-I | 12.64 (±1.80) | 12.49 (±2.04) | 0.539 | 12.54 (±1.98) | 12.56 (±1.80) | 0.919 | 12.54 (±1.96) |
| SS-TDI | 30.67 (±4.53) | 31.72 (±4.63) | 0.061 | 31.54 (±4.58) | 31.08 (±4.53) | 0.414 | 31.38 (±4.62) |
Abbreviations: CAMCOG-R, The Revised Cambridge Cognitive Examination; MMSE, Mini Mental State Examination; SS-D, Sniffin' Sticks discrimination; SS-I, Sniffin' Sticks identification; SS-T, Sniffin' Sticks threshold; SS-TDI, Sniffin' Sticks composite score.
Apolipoprotein E ɛ4 allele.
*P⩽0.05.
Mackay-Sim et al.51 provide guidelines for the classification of differing levels of olfactory impairment using Sniffin' Sticks: (1) severe hyposmia (a score of ⩽23 on Sniffin' Sticks TDI for women and ⩽21 for men); (2) anosmia (a TDI score of ⩽16); and (3) mild hyposmia (in women a score of 23–27, and in men a score of 21–29). In our cohort, 6.5, 1.3 and 19.5% met these criteria, respectively.
In this cohort, 194 (62.98) participants were non-smokers, 105 (34.09) were ex-smokers (with at least 2 years of abstinence) and 9 (2.93) participants were smokers. However, as there were no significant differences between the non-smokers and other groups in any of the baseline assessments including age, education, cognitive function (as assessed by MMSE and CAMCOG-R) and olfactory assessments, we did not include smoking as a factor in this study.
Cognitive performance over time
There was no significant association between sex and cognitive decline (χ2(1)=0.179, P=0.672) or APOE genotypes and cognitive decline (χ2(1)=1.773, P=0.183). Participants who were classified as declined (n=58) performed more poorly on olfactory discrimination (t=3.14; df=286; P<0.05) and on cumulative olfactory performance (t=1.33; df=306; P<.05) as measured by Sniffin' Sticks TDI (Table 2). The declined group was older at baseline as compared with non-declined group (N=250; t=−2.39; df=306; P<0.05), but did not differ on other demographic variables including education and cognitive functioning as measured by MMSE and CAMCOG-R.
Table 2. Demographic information and baseline performance of cognitively declineda and non-declined participants.
| Variables | Age | Educ. yearsb | MMSE | CAMCOG-R | SS-T | SS-D | SS-I | SS-TDI |
|---|---|---|---|---|---|---|---|---|
| Non-declined (N=250) | 62.59 (±6.98) | 13.65 (±3.65) | 29.20 (±1.11) | 98.63 (±3.28) | 7.53 (±2.32) | 11.90 (±2.21) | 12.62 (±1.91) | 31.79 (±4.13) |
| Declined (N=58) | 65.10 (±8.08) | 13.22 (±4.25) | 28.95 (±1.05) | 98.84 (±3. 94) | 6.87 (±2.55) | 10.80 (±2.86) | 12.20 (±2.16) | 29.65 (±6.04) |
| P | 0.017* | 0.442 | 0.124 | 0.664 | 0.063 | 0.009*,c | 0.153 | 0.013*,c |
Abbreviations: CAMCOG-R, The Revised Cambridge Cognitive Examination; MMSE, Mini Mental State Examination; SS-D, Sniffin' Sticks discrimination; SS-I, Sniffin' Sticks identification; SS-T, Sniffin' Sticks threshold; SS-TDI, Sniffin' Sticks composite score.
Cognitive decline was considered as a score ⩾1 s.d. below baseline performance on CAMCOG-R in the last assessment.
Education years.
Equal variance not assumed.
*P⩽0.05.
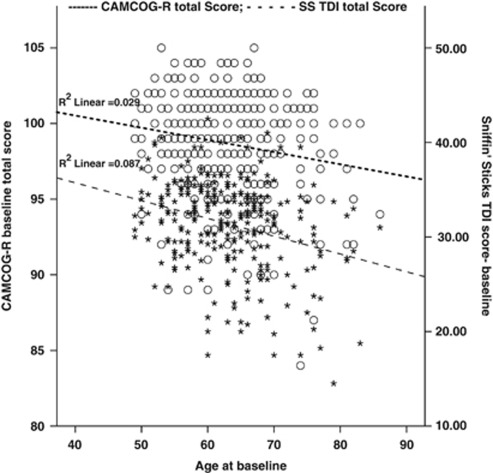
Analysis of the association between the variables indicated that age was negatively associated with baseline CAMCOG-R (r=−0.171; P<0.01), Sniffin' Sticks T (r=−0.193; P<0.01), D (r=−0.260; P<0.01), I (r=−0.188; P<0.01) and TDI (r=−0.296; P<0.01). The CAMCOG-R was significantly associated with baseline Sniffin' Sticks D (r=0.253; P<0.01), I (r=0.271; P<0.01) and TDI (r=0.228; P<0.01), but not with Sniffin' Sticks T (r=−0.006; P<0.92). To control the effects of age on the associations between various variables, partial correlation was performed. Partial correlation analysis indicated that even after controlling for the effects of age, baseline CAMCOG-R was still significantly associated with Sniffin' Sticks D (r=0.296, P<0.01), I (r=0.218, P<0.01) and TDI (r=0.277, P<0.01). The association between baseline olfactory functions with cognitive decline after 3 years was further explored using logistic regression analysis (Tables 3 and 4). The ‘D' scale was significantly associated with cognitive decline (OR=0.869; P<0.05; 95% CI=0.764−0.988); however, neither ‘T' (OR=0.916; P<0.192; 95% CI=0.803–1.045) nor ‘I' (OR=0.917; P<0.262; 95% CI=0.787–1.067) was significantly associated with cognitive decline as defined by CAMCOG-R performance in the last assessment (Table 3). Interestingly, baseline cognitive function (as measured by CAMCOG-R) and olfactory abilities showed similar trend of decline (Figure 1). However, other factors including sex, APOE genotype, age at baseline and baseline MMSE score were not significantly associated with cognitive decline in this study.
Table 3. Prediction of cognitive declinea using Sniffin' Sticks discrimination in multiple logistic regression analysis (N=282)b.
| Factors | B | s.e. | Wald | df | P* | OR |
95% CI
for OR |
|
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Sex | −0.005 | 0.340 | 0.000 | 1 | 0.988 | 0.995 | 0.511 | 1.937 |
| APOE genotypes | −0.518 | 0.323 | 2.579 | 1 | 0.108 | 0.596 | 0.316 | 1.121 |
| Age at baseline | 0.031 | 0.023 | 1.846 | 1 | 0.174 | 1.035 | 0.986 | 1.078 |
| Education years | −0.008 | 0.042 | 0.039 | 1 | 0.843 | 0.992 | 0.912 | 1.078 |
| MMSE | −0.140 | 0.132 | 1.119 | 1 | 0.290 | 0.870 | 0.617 | 1.127 |
| SS-D | −0.141 | 0.066 | 4.582 | 1 | 0.032* | 0.869 | 0.764 | 0.988 |
Abbreviations: CAMCOG-R, The Revised Cambridge Cognitive Examination; CI, confidence interval; MMSE, Mini Mental State Examination; OR, odds ratio; SS-D, Sniffin' Sticks discrimination.
Cognitive decline was considered as a score ⩾1 s.d. below baseline performance on the CAMCOG-R in the last assessment.
N was 308; however, only 282 were entered in the actual analysis.
*P< 0.05.
Table 4. Predictors of cognitive declinea using Sniffin' Sticks total, composite score in multiple logistic regression analysis (N=282)b.
| Factors | B | s.e. | Wald | df | P* | OR |
95% CI
for OR |
|
|---|---|---|---|---|---|---|---|---|
| Lower | Lower | |||||||
| Sex | 0.095 | 0.333 | 0.082 | 1 | 0.775 | 1.100 | 0.572 | 2.113 |
| APOE genotypes | −0.442 | 0.316 | 1.964 | 1 | 0.161 | 0.642 | 0.346 | 1.193 |
| Age at baseline | 0.034 | 0.022 | 2.296 | 1 | 0.130 | 1.035 | 0.990 | 1.081 |
| Education years | −0.008 | 0.042 | 0.035 | 1 | 0.851 | 0.992 | 0.914 | 1.077 |
| MMSE | −0.158 | 0.130 | 1.482 | 1 | 0.223 | 0.852 | 0.663 | 1.101 |
| SS-TDI | −0.073 | 0.033 | 4.842 | 1 | 0.028* | 0.930 | 0.872 | 0.992 |
Abbreviations: CAMCOG-R, The Revised Cambridge Cognitive Examination; CI, confidence interval; MMSE, Mini Mental State Examination; OR, odds ratio; SS-TDI, Sniffin' Sticks composite score.
Cognitive decline was considered as a score ⩾1 s.d. below baseline performance on the CAMCOG-R in the last assessment.
N was 308; however, only 282 were entered in the actual analysis.
*P<0.05.
Figure 1.
Dual axes graph showing the association between age, the CAMCOG-R and the Sniffin' Sticks composite score. ○ The Cambridge Cognitive Examination-Revised (CAMCOG-R) total score; *Sniffin' Sticks TDI (composite score). The dual axes graph with linear trend lines shows the baseline performance on cognitive measure (CAMCOG-R total score) and general olfactory function (as derived from the Sniffin' Sticks composite score). Both the CAMCOG-R (upper line) and SS TDI (below the first line) linear lines show a close trend of decline over time. The mean scores were used for this graph.
Logistic regression analysis indicated that higher Sniffin' Sticks TDI (composite) score was associated with lower risk of cognitive decline (OR=0.872; P<0.05; 95% CI=0.872−0.992) (Table 4). Other variables, including sex, APOE genotype, age, education and baseline MMSE score, did not indicate a significant predictive value with regards to cognitive decline.
Discussion
The major novel finding of the current study was that olfactory discrimination (as measured by Sniffin' Sticks D) was a significant predictor of future cognitive decline over a 3-year period. The study also confirmed a series of existing findings, demonstrating that age is associated with both cognitive and olfactory functions. However, there was a significant association between olfactory function and cognitive performance even after controlling for the effects of age, education years, APOE genotype and sex. Three olfactory functions including threshold, discrimination and identification were separately assessed in this study. Interestingly, we found that discrimination was the best predictor of cognitive decline over time. It is important to note that the cognitive changes observed were small and subtle with the study participants performing within the normal range on cognitive measures.
Interestingly, our findings do not support those of other researchers who have reported that impaired olfactory identification is a strong predictor of cognitive impairment.52, 53, 54, 55 Wilson et al.56 further reported that impairment in olfactory identification at baseline was significantly associated with the incidence of mild cognitive impairment. It has been reported that patients who were anosmic at baseline had twice the risk of developing AD compared with controls over a 2-year follow up, and if they had at least one APOE-ɛ4 allele, the risk increased to 4.9 times.7 It should be noted that while some researchers report a significant olfactory decline in individuals positive for APOE-ɛ4 allele,6 others have failed to find this relationship8, 57 and our current findings are consistent with these latter studies.
The reports on olfactory identification impairments in AD patients and in individuals at higher risk for pathological cognitive decline are not conclusive. That is, impairment in both olfactory identification and threshold have been reported as predictive of cognitive decline and AD.52, 55, 58, 59 One potential explanation is odor discrimination is primarily impaired in AD individuals while identification problems are more common among patients with semantic dementia, frontotemporal dementia or corticobasal dementia.60 Another explanation for our findings is that previous studies have focused primarily, often exclusively, on odor identification and might have not extensively assessed other olfactory domains including threshold and discrimination.61, 62 It has been suggested that olfactory discrimination, similar to identification, requires higher cognitive functions including working memory, judgment and decision making, and its dysfunction may represent generalized cognitive deterioration.63 Our findings support this interpretation. However, involvement of hippocampal regions in a network underlying odor discrimination64 increases the probability of a more specific memory-related role in discrimination. Clearly, more studies are needed to clarify the neural regions associated with odor discrimination and cognition. As demonstrated in the present study, controlled, systematic evaluation of the various olfactory domains would contribute to more powerful assessment of the cognitive elements of odor memory and recognition, and thus also possible links to cognitive function.
The association of age with both cognitive65 and olfactory25 functions is consistent with the reports indicating that age is a significant covariate. However, the findings reported in this study indicate that the association between cognitive functions and olfactory discrimination and identification is independent of the effects of age. It has been argued that the effects of age on olfaction can be explained by the effects of cognitive decline, not age or age-related hazards affecting olfaction.66 Age, per se, may not account for the so-called age-associated olfactory dysfunction or presbyosmia, as decline in olfactory function with healthy aging seems much lower than previously reported.67 Given this, our findings support previous reports suggesting that cognitive functions, specifically those with higher verbal component, are significantly associated with olfaction.66, 68
Another significant finding was the value of measuring multiple domains of olfactory functioning in predicting cognitive decline over 3 years in community-dwelling elderly individuals. Previous reports have mainly focused on the value of measuring specific olfactory domains such as identification in predicting pathological or age-related cognitive decline (for examples, see refs 17,56,69). The study reported here indicated that a comprehensive measure of olfactory function has significant power in predicting cognitive decline in healthy individuals. As various olfactory functions are to some degree reliant on threshold,70 it is necessary to assess the participants' olfactory acuity before further assessment with more specialized measures such as identification71 that may then be used for differential diagnosis or screening purposes.
The mechanisms underlying the association between olfaction and cognition have been extensively examined in the last 100 years both by psychophysics and neuroanatomical studies (72, 73, for a review of earlier work see Herz and Engen74). For example, psychophysics studies have found that olfactory identification was significantly associated with semantic verbal memory, implying that the two may share some cognitive domains.66, 75, 76, 77 Neuropathological studies have revealed that brain regions and subsystems involved in odor information processing, including the olfactory bulb, piriform and orbital prefrontal cortices, have direct projections to perirhinal and entorinal cortices. These, in turn, have extensive projections to the hippocampus,78, 79, 80 known as the primary brain region involved in initial memory formation,81, 82, 83 and also one of the first regions affected in AD neurodegeneration.84, 85 In addition, the anterior olfactory nucleus and olfactory bulb are the two primary brain regions commonly affected in AD.86, 87 Indeed, change in olfactory identification has been strongly associated with pathological changes in the medial temporal lobe structures.88 These studies strongly imply a primary role for olfactory dysfunction as an indicator of pathological cognitive decline and dementia.
The current study had some limitations that should be considered when interpreting the findings reported. First, participants were both physically and cognitively within the normal range, at least as far as it can be inferred from the MMSE, CAMCOG-R, years of education and various exclusion criteria applied during the participants recruitment phase. In addition, the participants were divided into declined/non-declined groups based on neuropsychological measures and not a formal diagnostic clinical interview. However, the CAMCOG-R is a comprehensive measure of cognitive function, and while it cannot be considered as a substitute for formal clinical evaluation of the participants, it has demonstrated high sensitivity to cognitive decline.89, 90 Ideally, any future research using this cohort will examine the consistency of the reported results against formal diagnostic criteria. Also, while this study observed a significant association between olfactory D and cognitive decline, it did not examine the underlying mechanisms involved in such a distinctive effect for odor differentiation as compared with other olfactory functions.
In conclusion, the association between the olfactory function and ongoing cognitive decline established in this study provides further evidence in support of the inclusion of a smell assessment alongside other neuropsychological measures in standard health screens for older adults. Many studies have reported olfactory impairments both in preclinical and clinical phases of AD. However, as noted by us and others, the predictive value of olfactory assessment in screening those at a higher risk for AD needs further research91, 92 to improve its sensitivity and specificity.
Acknowledgments
This research was funded by the Australian National Institute of Health (Grant# 7P01AG010491-12) and the McCusker's Alzheimer's Research Foundation (Project ID: 52070400). We would also like to thank A/Prof Jonathan Foster for assistance in data collection for this study.
HRS has performed neuropsychological assessments for Pfizer and previously for Wyeth. His PhD was partially supported by a scholarship from the University of Western Australia. RNM is the founder and owns stock in Alzhyme. SEG is a consultant for, owns stock/options in, and/or has received lecture fees from Amicus, Diagenic, Epix, Smart Pharma and Wyeth/Elan. SEG is also a member of the data safety monitoring board for the Alzheimer Immunotherapy Alliance and holds a grant from Amicus Pharmaceuticals. The findings of this study were partially reported at the Alzheimer's Association International Conference (AA-ICAD), 2011, Paris, France.
References
- Nordin S, Murphy C. Odor memory in normal aging and Alzheimer's disease. Ann N Y Acad Sci. 1998;855:686–693. doi: 10.1111/j.1749-6632.1998.tb10646.x. [DOI] [PubMed] [Google Scholar]
- Doty RL, Perl DP, Steele JC, Chen KM, Pierce JD, Jr, Reyes P, et al. Olfactory dysfunction in three neurodegenerative diseases. Geriatrics. 1991;46 (Suppl 1:47–51. [PubMed] [Google Scholar]
- Warner MD, Peabody CA, Flattery JJ, Tinklenberg JR. Olfactory deficits and Alzheimer's disease. Biol Psychiatry. 1986;21:116–118. doi: 10.1016/0006-3223(86)90013-2. [DOI] [PubMed] [Google Scholar]
- Djordjevic J, Jones-Gotman M, De Sousa K, Chertkow H. Olfaction in patients with mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2008;29:693–706. doi: 10.1016/j.neurobiolaging.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Lehrner J, Pusswald G, Gleiss A, Auff E, Dal-Bianco P. Odor identification and self-reported olfactory functioning in patients with subtypes of mild cognitive impairment. Clin Neuropsychol. 2009;23:818–830. doi: 10.1080/13854040802585030. [DOI] [PubMed] [Google Scholar]
- Murphy C, Bacon AW, Bondi MW, Salmon DP. Apolipoprotein E status is associated with odor identification deficits in nondemented older persons. Ann N Y Acad Sci. 1998;30:744–750. doi: 10.1111/j.1749-6632.1998.tb10654.x. [DOI] [PubMed] [Google Scholar]
- Graves AB, Bowen JD, Rajaram L, McCormick WC, McCurry SM, Schellenberg GD, et al. Impaired olfaction as a marker for cognitive decline: interaction with apolipoprotein E epsilon4 status. Neurology. 1999;53:1480–1487. doi: 10.1212/wnl.53.7.1480. [DOI] [PubMed] [Google Scholar]
- Sohrabi HR, Bates KA, Rodrigues M, Taddei K, Laws SM, Lautenschlager NT, et al. Olfactory dysfunction is associated with subjective memory complaints in community-dwelling elderly individuals. J Alzheimers Dis. 2009;17:135–142. doi: 10.3233/JAD-2009-1020. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA. Olfactory impairment in presymptomatic Alzheimer's disease. Int Symp Olfaction Taste: Ann N Y Acad Sci. 2009;1170:730–735. doi: 10.1111/j.1749-6632.2009.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Levy E, Nixon RA, Wilson DA. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer's disease mouse model. J Neurosci. 2010;30:505–514. doi: 10.1523/JNEUROSCI.4622-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs T, Cairns NJ, Lantos PL. Olfactory centres in Alzheimer's disease: olfactory bulb is involved in early Braak's stages. Neuroreport. 2001;12:285–288. doi: 10.1097/00001756-200102120-00021. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Tang Y, Bennett DA. The relation of cerebral Alzheimer's disease pathology to odor identification in old age. J Neurol Neurosurg Psychiatry. 2007;78:30–35. doi: 10.1136/jnnp.2006.099721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi Y, Wszolek ZK, Graff-Radford NR, Cookson N, Dickson DW. Tau pathology in the olfactory bulb correlates with Braak stage, Lewy body pathology and apolipoprotein epsilon4. Neuropathol Appl Neurobiol. 2003;29:503–510. doi: 10.1046/j.1365-2990.2003.00453.x. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Alzheimer pathology in the olfactory bulb. Neuropathol Appl Neurobiol. 2005;31:203. doi: 10.1111/j.1365-2990.2004.00619.x. [DOI] [PubMed] [Google Scholar]
- Ghanbari HA, Ghanbari K, Harris PL, Jones PK, Kubat Z, Castellani RJ, et al. Oxidative damage in cultured human olfactory neurons from Alzheimer's disease patients. Aging Cell. 2004;3:41–44. doi: 10.1111/j.1474-9728.2004.00083.x. [DOI] [PubMed] [Google Scholar]
- Forster S, Vaitl A, Teipel SJ, Yakushev I, Mustafa M, la Fougere C, et al. Functional representation of olfactory impairment in early Alzheimer's disease. J Alzheimers Dis. 2010;22:581–591. doi: 10.3233/JAD-2010-091549. [DOI] [PubMed] [Google Scholar]
- Schubert CR, Carmichael LL, Murphy C, Klein BE, Klein R, Cruickshanks KJ. Olfaction and the 5-year incidence of cognitive impairment in an epidemiological study of older adults. J Am Geriatrics Soc. 2008;56:1517–1521. doi: 10.1111/j.1532-5415.2008.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma K, Nakashima K, Takahashi K. Olfactory evoked potentials in Parkinson's disease, Alzheimer's disease and anosmic patients. Psychiatry Clin Neurosci. 1996;50:35–40. doi: 10.1111/j.1440-1819.1996.tb01660.x. [DOI] [PubMed] [Google Scholar]
- Mesholam RI, Moberg PJ, Mahr RN, Doty RL. Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer's and Parkinson's diseases. Arch Neurol. 1998;55:84–90. doi: 10.1001/archneur.55.1.84. [DOI] [PubMed] [Google Scholar]
- Eibenstein A, Fioretti AB, Lena C, Rosati N, Amabile G, Fusetti M. Modern psychophysical tests to assess olfactory function. Neurol Sci. 2005;26:147–155. doi: 10.1007/s10072-005-0452-3. [DOI] [PubMed] [Google Scholar]
- de Wijk RA, Cain WS. Odor quality: discrimination versus free and cued identification. Percept Psychophys. 1994;56:12–18. doi: 10.3758/bf03211686. [DOI] [PubMed] [Google Scholar]
- Richardson JT, Zucco GM. Cognition and olfaction: a review. Psychol Bull. 1989;105:352–360. doi: 10.1037/0033-2909.105.3.352. [DOI] [PubMed] [Google Scholar]
- Schab FR. Odor memory: taking stock. Psychol Bull. 1991;109:242–251. doi: 10.1037/0033-2909.109.2.242. [DOI] [PubMed] [Google Scholar]
- Swan GE, Carmelli D. Impaired olfaction predicts cognitive decline in nondemented older adults. Neuroepidemiology. 2002;21:58–67. doi: 10.1159/000048618. [DOI] [PubMed] [Google Scholar]
- Elsner RJF. Odor memory and aging. Aging Neuropsychol Cogn. 2001;8:284–306. [Google Scholar]
- Stevenson RJ, Case TI, Tomiczek C. Resistance to interference of olfactory perceptual learning. Psychol Rec. 2007;57:103–116. [Google Scholar]
- Dade LA, Zatorre RJ, Jones-Gotman M. Olfactory learning: convergent findings from lesion and brain imaging studies in humans. Brain. 2002;125 (Part 1:86–101. doi: 10.1093/brain/awf003. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D, Adolphs R. A specific role for the human amygdala in olfactory memory. Learn Mem. 2003;10:319–325. doi: 10.1101/lm.62303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DA, Hopkins RO, Squire LR. Impaired odor recognition memory in patients with hippocampal lesions. Learn Memory. 2004;11:794–796. doi: 10.1101/lm.82504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda JE. Olfactory system pathology as a model of Lewy neurodegenerative disease. J Neurol Sci. 2010;289:49–54. doi: 10.1016/j.jns.2009.08.042. [DOI] [PubMed] [Google Scholar]
- McShane R, Williams SS, Williams J, Combrinck M, Christie S, Smith AD. Olfactory impairment is more marked in patients with mild dementia with Lewy bodies than those with mild Alzheimer disease. J Neurol Neurosurg Psychiatry. 2009;80:667–670. doi: 10.1136/jnnp.2008.155895. [DOI] [PubMed] [Google Scholar]
- Haehner A, Boesveldt S, Berendse HW, Mackay-Sim A, Fleischmann J, Silburn PA, et al. Prevalence of smell loss in Parkinson's disease: a multicenter study. Parkinsonism Relat Disord. 2009;15:490–494. doi: 10.1016/j.parkreldis.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Smutzer GS, Trojanowski JQ, Moberg PJ. Cellular and molecular neuropathology of the olfactory epithelium and central olfactory pathways in Alzheimer's disease and schizophrenia. Ann N Y Acad Sci. 1998;855:762–775. doi: 10.1111/j.1749-6632.1998.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Arnold SE, Doty RL, Gur RE, Balderston CC, Roalf DR, et al. Olfactory functioning in schizophrenia: relationship to clinical, neuropsychological, and volumetric MRI measures. J Clin Exp Neuropsychol. 2006;28:1444–1461. doi: 10.1080/13803390500434409. [DOI] [PubMed] [Google Scholar]
- Hawkes C. Olfaction in neurodegenerative disorder. Mov Disord. 2003;18:364–372. doi: 10.1002/mds.10379. [DOI] [PubMed] [Google Scholar]
- Burns A. Might olfactory dysfunction be a marker of early Alzheimer's disease. Lancet. 2000;355:84–85. doi: 10.1016/S0140-6736(99)90402-6. [DOI] [PubMed] [Google Scholar]
- Bates KA, Sohrabi HR, Rodrigues M, Beilby J, Dhaliwal SS, Taddei K, et al. Association of cardiovascular factors and Alzheimer's disease plasma amyloid-beta protein in subjective memory complainers. J Alzheimer's Dis: JAD. 2009;17:305–318. doi: 10.3233/JAD-2009-1050. [DOI] [PubMed] [Google Scholar]
- Lau S, Bates KA, Sohrabi HR, Rodrigues M, Martins G, Dhaliwal SS, et al. Functional effects of genetic polymorphism in inflammatory genes in subjective memory complainers. Neurobiol Aging. 2012;33:1054–1056. doi: 10.1016/j.neurobiolaging.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Sohrabi HR, Bates KA, Rodrigues M, Taddei K, Martins G, Laws SM, et al. The relationship between memory complaints, perceived quality of life and mental health in apolipoprotein Eepsilon4 carriers and non-carriers. J Alzheimer's Dis: JAD. 2009;17:69–79. doi: 10.3233/JAD-2009-1018. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Roth M, Huppert FA, Mountjoy CQ, Tym E. Cambridge Cognitive Examination-Revised (CAMCOG-R) Cambridge University Press: Cambridge; 1999. [Google Scholar]
- Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‘Sniffin' sticks': olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22:39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- Eibenstein A, Fioretti AB, Simaskou MN, Sucapane P, Mearelli S, Mina C, et al. Olfactory screening test in mild cognitive impairment. Neurol Sci. 2005;26:156–160. doi: 10.1007/s10072-005-0453-2. [DOI] [PubMed] [Google Scholar]
- Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‘Sniffin' sticks': olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22:39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- Wolfensberger M, Schnieper I, Welge-Lussen A. Sniffin'Sticks: a new olfactory test battery. Acta Oto-Laryngol. 2000;120:303–306. doi: 10.1080/000164800750001134. [DOI] [PubMed] [Google Scholar]
- Roth M, Huppert FA, Mountjoy CQ, Tym E. CAMDEX-R. Cambridge University Press: Cambridge; 1998. [Google Scholar]
- Molloy DW, Standish TI.A guide to the standardized Mini-Mental State Examination Int Psychogeriatr 19979(Suppl 187–94.discussion 143–50. [DOI] [PubMed] [Google Scholar]
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- Wenham PR, Price WH, Blandell G. Apolipoprotein E genotyping by one-stage PCR. Lancet. 1991;337:1158–1159. doi: 10.1016/0140-6736(91)92823-k. [DOI] [PubMed] [Google Scholar]
- Hummel T, Kobal G, Gudziol H, Mackay-Sim A. Normative data for the ‘Sniffin' Sticks' including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. 2007;264:237–243. doi: 10.1007/s00405-006-0173-0. [DOI] [PubMed] [Google Scholar]
- Mackay-Sim A, Grant L, Owen C, Chant D, Silburn P. Australian norms for a quantitative olfactory function test. J Clin Neurosci. 2004;11:874–879. doi: 10.1016/j.jocn.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Yamamoto S, Umegaki H, Onishi J, Mogi N, Fujishiro H, et al. Smell identification test as an indicator for cognitive impairment in Alzheimer's disease. Int J Geriatr Psych. 2004;19:727–733. doi: 10.1002/gps.1161. [DOI] [PubMed] [Google Scholar]
- Stephenson R, Houghton D, Sundarararjan S, Doty RL, Stern M, Xie SX, et al. Odor identification deficits are associated with increased risk of neuropsychiatric complications in patients with Parkinson's disease. Mov Disord. 2010;25:2099–2104. doi: 10.1002/mds.23234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayudhan L, Lovestone S. Smell identification test as a treatment response marker in patients with Alzheimer disease receiving donepezil. J Clin Psychopharmacol. 2009;29:387–390. doi: 10.1097/JCP.0b013e3181aba5a5. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Tang Y, Bennett DA. Odor identification and decline in different cognitive domains in old age. Neuroepidemiology. 2006;26:61–67. doi: 10.1159/000090250. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Arnold SE, Tang YX, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiat. 2007;64:802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Michaels-Marston KS, Liu X, Pelton GH, Padilla M, Marder K, et al. Olfactory deficits in patients with mild cognitive impairment predict Alzheimer's disease at follow-up. Am J Psychiatry. 2000;157:1399–1405. doi: 10.1176/appi.ajp.157.9.1399. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Wiens S, Hedner M, Nilsson LG, Larsson M. Odor identification impairment in carriers of ApoE-varepsilon4 is independent of clinical dementia. Neurobiol Aging. 2010;31:567–577. doi: 10.1016/j.neurobiolaging.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Bacon AW, Bondi MW, Salmon DP, Murphy C. Very early changes in olfactory functioning due to Alzheimer's disease and the role of apolipoprotein E in olfaction. Ann N Y Acad Sci. 1998;855:723–731. doi: 10.1111/j.1749-6632.1998.tb10651.x. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Snowden JS, Neary D, Coccia M, Provinciali L, Lambon Ralph MA. Distinct patterns of olfactory impairment in Alzheimer's disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia. 2007;45:1823–1831. doi: 10.1016/j.neuropsychologia.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Olfactory system: functional organization and involvement in neurodegenerative disease. Neurology. 2010;75:1104–1109. doi: 10.1212/WNL.0b013e3181f3db84. [DOI] [PubMed] [Google Scholar]
- Albers MW, Tabert MH, Devanand DP. Olfactory dysfunction as a predictor of neurodegenerative disease. Curr Neurol Neurosci Rep. 2006;6:379–386. doi: 10.1007/s11910-996-0018-7. [DOI] [PubMed] [Google Scholar]
- Corwin J, Serby M, Rotrosen J. Olfactory deficits in AD: what we know about the nose. Neurobiol Aging. 1986;7:580–582. [Google Scholar]
- Martin C, Beshel J, Kay LM. An olfacto-hippocampal network is dynamically involved in odor-discrimination learning. J Neurophysiol. 2007;98:2196–2205. doi: 10.1152/jn.00524.2007. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. When does age-related cognitive decline begin. Neurobiol Aging. 2009;30:507–514. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D, Pedersen NL, Larsson M. Olfactory functioning and cognitive abilities: a twin study. J Gerontol B Psychol Sci Soc Sci. 2001;56:P226–P233. doi: 10.1093/geronb/56.4.p226. [DOI] [PubMed] [Google Scholar]
- Mackay-Sim A, Johnston AN, Owen C, Burne TH. Olfactory ability in the healthy population: reassessing presbyosmia. Chem Senses. 2006;31:763–771. doi: 10.1093/chemse/bjl019. [DOI] [PubMed] [Google Scholar]
- Larsson M, Finkel D, Pedersen NL. Odor identification: influences of age, gender, cognition, and personality. J Gerontol B-Psychol. 2000;55:304–310. doi: 10.1093/geronb/55.5.p304. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Yu L, Schneider JA, Arnold SE, Buchman AS, Bennett DA. Lewy bodies and olfactory dysfunction in old age. Chem Senses. 2011;36:367–373. doi: 10.1093/chemse/bjq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JC, Hall SB, Walker DB, Kendal-Reed MS, Hood AF, Niu XF. Human odor detectability: new methodology used to determine threshold and variation. Chem Senses. 2003;28:817–826. doi: 10.1093/chemse/bjg075. [DOI] [PubMed] [Google Scholar]
- Dulay MF, Murphy C. Olfactory acuity and cognitive function converge in older adulthood: support for the common cause hypothesis. Psychol Aging. 2002;17:392–404. [PubMed] [Google Scholar]
- Engen T, Pfaffmann C. Absolute judgments of odor quality. J Exp Psychol. 1960;59:214–219. doi: 10.1037/h0043912. [DOI] [PubMed] [Google Scholar]
- Campbell IM, Gregson RAM. Olfactory short term memory in normal, schizophrenic and brain-damaged cases. Aust J Psychol. 1972;24:179–185. [Google Scholar]
- Herz RS, Engen T. Odor memory: review and analysis. Psychon B Rev. 1996;3:300–313. doi: 10.3758/BF03210754. [DOI] [PubMed] [Google Scholar]
- Larsson M. Semantic factors in episodic recognition of common odors in early and late adulthood: a review. Chem Senses. 1997;22:623–633. doi: 10.1093/chemse/22.6.623. [DOI] [PubMed] [Google Scholar]
- Larsson M, Nilsson LG, Olofsson JK, Nordin S. Demographic and cognitive predictors of cued odor identification: evidence from a population-based study. Chem Senses. 2004;29:547–554. doi: 10.1093/chemse/bjh059. [DOI] [PubMed] [Google Scholar]
- Makowska I, Kloszewska I, Grabowska A, Szatkowska I, Rymarczyk K. Olfactory deficits in normal aging and Alzheimer's disease in the polish elderly population. Arch Clin Neuropsychol. 2011;26:270–279. doi: 10.1093/arclin/acr011. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Using olfaction to study memory. Ann N Y Acad Sci. 1998;855:657–669. doi: 10.1111/j.1749-6632.1998.tb10642.x. [DOI] [PubMed] [Google Scholar]
- Powell TP, Cowan WM, Raisman G. The central olfactory connexions. J Anat. 1965;99 (Part 4:791–813. [PMC free article] [PubMed] [Google Scholar]
- Savic I, Gulyas B, Larsson M, Roland P. Olfactory functions are mediated by parallel and hierarchical processing. Neuron. 2000;26:735–745. doi: 10.1016/s0896-6273(00)81209-x. [DOI] [PubMed] [Google Scholar]
- Riedel G, Micheau J. Function of the hippocampus in memory formation: desperately seeking resolution. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:835–853. doi: 10.1016/s0278-5846(01)00153-1. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- Wang SH, Morris RG.Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation Annu Rev Psychol 20106149–79.C1–4. [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Biol. 2011;3:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Averback P. Two new lesions in Alzheimer's disease. Lancet. 1983;2:1203. doi: 10.1016/s0140-6736(83)91256-4. [DOI] [PubMed] [Google Scholar]
- Ohm TG, Muller H, Braak E. Calbindin-D-28k-like immunoreactive structures in the olfactory bulb and anterior olfactory nucleus of the human adult: distribution and cell typology--partial complementarity with parvalbumin. Neuroscience. 1991;42:823–840. doi: 10.1016/0306-4522(91)90047-r. [DOI] [PubMed] [Google Scholar]
- Lojkowska W, Sawicka B, Gugala M, Sienkiewicz-Jarosz H, Bochynska A, Scinska A, et al. Follow-up study of olfactory deficits, cognitive function, and volume loss of medial temporal lobe structures in patients with mild cognitive impairment. Curr Alzheimer Res. 2011;8:689–698. doi: 10.2174/156720511796717212. [DOI] [PubMed] [Google Scholar]
- Heinik J, Solomesh I. Validity of the Cambridge Cognitive Examination-Revised new Executive Function Scores in the diagnosis of dementia: some early findings. J Geriatr Psychiatry Neurol. 2007;20:22–28. doi: 10.1177/0891988706297090. [DOI] [PubMed] [Google Scholar]
- Verhoeven CL, Schepers VP, Post MW, van Heugten CM. The predictive value of cognitive impairments measured at the start of clinical rehabilitation for health status 1 year and 3 years poststroke. Int J Rehabil Res. 2011;34:38–43. doi: 10.1097/MRR.0b013e32833ba577. [DOI] [PubMed] [Google Scholar]
- Bahar-Fuchs A, Moss S, Rowe C, Savage G. Olfactory performance in AD, aMCI, and healthy ageing: a unirhinal approach. Chem Senses. 2010;35:855–862. doi: 10.1093/chemse/bjq094. [DOI] [PubMed] [Google Scholar]
- Foster J, Sohrabi H, Verdile G, Martins R. Research criteria for the diagnosis of Alzheimer's disease: genetic risk factors, blood biomarkers and olfactory dysfunction. Int Psychogeriatr. 2008;20:853–855. doi: 10.1017/S1041610208006807. [DOI] [PubMed] [Google Scholar]