Abstract
Serotonin is a major neurotransmitter in the central nervous system (CNS). Dysregulation of serotonin transmission in the CNS is reported to be related to different psychiatric disorders in humans including depression, impulsive aggression and anxiety disorders. The most frequently prescribed antidepressants and anxiolytics target the serotonergic system. However, these drugs are not effective in 20–30% of cases. The causes of this failure as well as the molecular mechanisms involved in the origin of psychological disorders are poorly understood. Biosynthesis of serotonin in the CNS is initiated by tryptophan hydroxylase 2 (TPH2). In this study, we used Tph2-deficient (Tph2−/−) mice to evaluate the impact of serotonin depletion in the brain on mouse behavior. Tph2−/− mice exhibited increased depression-like behavior in the forced swim test but not in the tail suspension test. In addition, they showed decreased anxiety-like behavior in three different paradigms: elevated plus maze, marble burying and novelty-suppressed feeding tests. These phenotypes were accompanied by strong aggressiveness observed in the resident–intruder paradigm. Despite carrying only one copy of the gene, heterozygous Tph2+/− mice showed only 10% reduction in brain serotonin, which was not sufficient to modulate behavior in the tested paradigms. Our findings provide unequivocal evidence on the pivotal role of central serotonin in anxiety and aggression.
Keywords: aggression, anxiety, depression, serotonin, tryptophan hydroxylase
Introduction
Serotonin (5-hydroxytryptamine, 5-HT) is a monoamine that has a dual role, working both as an autacoid in the periphery and as a neurotransmitter in the brain. Synthesis of 5-HT starts with the conversion of the essential amino acid tryptophan (Trp) to 5-hydroxytryptophan (5-HTP) by tryptophan hydroxylase (TPH). In the second step, 5-HTP is decarboxylated to 5-HT by the aromatic amino acid decarboxylase. The discovery of a second Tph gene unraveled the existence of two independent 5-HT systems in vertebrates — peripheral and central — controlled by TPH1 and TPH2, respectively.1, 2 Although TPH1 is mainly expressed in the gut and is responsible for the synthesis of peripheral serotonin, TPH2 is expressed in the neurons of the raphé nuclei in the brainstem2, 3, 4 and in myenteric neurons in the gut,5 but not in other peripheral organs such as the lung, heart, kidney or liver.6, 7 Mice lacking TPH2 (Tph2−/−, Tph2-deficient mice) were recently generated by our group8 and others.3, 9, 10, 11, 12 Tph2−/− mice exhibit only minute amounts of brain serotonin, but normal formation and differentiation of serotonergic neurons.3, 8 The level of peripheral serotonin was unchanged in these mice, although it did not restore the brain 5-HT levels as serotonin cannot cross the blood brain barrier. Tph2−/− mice display normal size and no obvious abnormalities at birth, but exhibit growth retardation during early postnatal life. However, Tph2−/− mice catch up the body weight and at the age of 3 months are not any more distinguishable from wild-type mice. Moreover, these mice exhibit altered thermoregulation and respiratory control, and impaired maternal care.8
It has been postulated that reduction in brain serotonin leads to increased depressive and aggressive behaviors. In humans different polymorphisms of genes involved in the central serotonin synthesis and transmission are associated with various psychological abnormalities such as depression,13, 14, 15, 16, 17 anxiety disorders18 and aggression.19, 20 Moreover, differences in the level of serotonin or its metabolite, 5-hydroxyindoleacetic acid (5-HIAA), in the cerebrospinal fluid have been found in patients with depression21, 22, 23 and destructive behaviors, such as aggression, violence and impulsivity.22, 24, 25, 26 Further elucidation of mechanisms by which central serotonin is involved in depression and anxiety disorders is required for the improvement of existing medical treatment.
In this study, we evaluated the rate of serotonin synthesis in the central nervous system (CNS) of Tph2−/− and Tph2-heterozygous (Tph2+/−) mice, and investigated the consequence of complete and partial central serotonin depletion on anxiety, aggression and depression-like behavior.
Materials and methods
Animals
All animal procedures were in accordance with the ethical principles and guidelines for care and use of laboratory animals adopted by German local authorities corresponding to the standards prescribed by the American Physiological Society.
Mice were maintained in individually ventilated cages, 34 × 19 × 13 cm (Tecniplast Deutschland, Hohenpeissenberg, Germany) under a standard light/dark cycle from 7 am to 7 pm, with free access to standard chow (0.25% sodium, SSNIFF Spezialitäten, Soest, Germany) and drinking water ad libitum.
To obtain Tph2 gene-deleted mice on a pure genetic background, heterozygous Tph2-deficient animals on C57Bl/6 background (6th generation)8 were bred for further four generations to C57Bl/6 mice (Charles River, Sulzfeld, Germany). All experiments have been performed in adult (18–22 weeks old) F10 C57Bl/6 Tph2−/−, Tph2+/− and wild-type (Tph2+/+) male mice. To generate animals of the three above-mentioned genotypes Tph2+/− females were bred with Tph2−/− or Tph2+/+ male mice. Genotyping was performed using PCR with primer TPH34 (5′-AGC TGA GGC AGA CAG AAA GG-3′), TPH54 (5′-CCA AAG AGC TAC TCG ACC TAC G-3′) and Neo3 (5′-CTG CGC TGA CAG CCG GAA CAC-3′). Mice were single housed starting at 10–12 weeks of age as Tph2−/− males are highly aggressive and cannot be kept in groups. In order to avoid differences due to single-housing condition Tph2+/− and Tph2+/+ were single housed as well at least 4 weeks before experiments.
Group-housed (five mice per cage) 23–25 g male mice on FVB/N background were used as intruders in the resident–intruder paradigm.
Behavioral assessment
Behavioral testing was performed during the light cycle between 1 and 5 pm, with exception of tail suspension test (TST), which was performed during the dark cycle. In all experiments mice were habituated at least for one week to the experimental room. During this time handling of animals was done by the same experimenter who performed the tests. One batch of animals was used for the marble burying test (MBT) and was afterwards tested in the elevated plus maze (EPM) test with a 1 week interval between the two experiments. Another batch of animals was tested first in the novelty-suppressed feeding test (NSF), and 1 week later in the resident–intruder test (RI). Mice tested first in the open field were then examined in the forced swim test (FST). For the TST independent cohorts of naïve animals was used. Before, TST mice were kept 9 weeks under reversed dark–light cycle, with the light off at 10 am. Mouse behavior was video recorded (Panasonic camera HDC TM700, Hamburg, Germany) for subsequent offline analyses by the experimenter. For analysis of EPM and OF data Biobserve software (Viewer2 version 2.2.0.91, BIOBSERVE GmbH, Bonn, Germany) was used. In MBT, activity was measured by InfraMot (TSE systems, Bad Homburg Germany). In all experiments, the observer was blinded to the genotype.
Open field
A large arena (50 × 50 cm) under low illumination (30 Lux) was used as an OF to measure locomotor activity. Each mouse was placed into the arena facing the middle of the wall and its activity was measured during 10 min. The total distance traveled, time spent in the center and near the walls were calculated.
Elevated plus maze
The EPM test is based on the inborn aversion of rodents to open, bright illuminated spaces.27 The maze consisted of two open arms (30 × 5 cm) and two closed arms (30 × 5 cm) that were enclosed by a sidewall on all edges (height 15 cm). Mice were placed in the center of the maze (central platform) facing the closed arm. Total arm entries, percent of entries into the open arm ((open-arm enteries/total arm enteries) × 100) and time spent in open arms ((open arms/total session duration) × 100) were quantified during 10 min test. Arm entry was only defined when an animal (the mouse mass center) was at least 3 cm on an arm to differentiate entries from stretched attend postures into the arms.
Marble-burying test
Marble burying is a common test for validating anxiolytic effect of drugs.28 The test was conducted in a new cage (equally sized and illuminated as the home cage) with evenly spaced 15 clear glass marbles (20 mm diameter) in 5 cm of sawdust. During the test mice had access to food and water, and the test cage was covered with a metal grid. After 30 min the test was terminated by removing the mouse and the number of buried marbles was counted. A marble was scored as buried if more than two-thirds of it was covered with sawdust. During the test locomotor activity was evaluated by InfraMot (TSE systems).
Novelty-suppressed feeding
This test is based on a provoked conflict between the fear of mice to enter bright illuminated spaces and food seeking induced by hunger.29, 30 Animals were food deprived 23 h before testing. On the test day after placing mice into a novel home cage for 30 min, they were introduced into a new brightly illuminated test environment (cage 42 × 25 × 18 cm) where a single food pellet was centrally placed. After the first feeding event animals were returned to their home cages where they were allowed to eat pre-weighed food over a period of 5 min. Latency to the first eating episode (time between mouse introduction to arena with food pellet in the middle and the first feeding event) was used as an index of induced anxiety-like behavior. The amount of food consumed in the home cage provided a measure of appetitive drive.
Forced swim test
The FST, as originally described by Porsolt,31 assesses the tendency to give up attempting to escape from an unpleasant environment, whereby fewer attempts are interpreted as behavioral despair. The apparatus was a plastic beaker (17.5 cm diameter, 24 cm high), filled with water (24–26 °C) to a height of 18 cm. The time mice spent floating on the water (immobility time, sec) during 6 min as well as latency (sec) to the first immobility episode were manually observed. A mouse was judged to be immobile when it ceased struggling and remained floating motionless in water, making only those movements necessary to keep its head above water. Swimming was defined as vigorous movements with forepaws breaking the surface of the water.
Tail suspension test
The TST is another learned helplessness paradigm where animals cannot escape from an unpleasant situation. A reduction in struggling behavior (latency to the first immobile episode or increased total immobility) is interpreted as a reduction in intrinsic motivation to escape the situation. Mice were suspended by the tail using an adhesive tape to a platform. The latency to the first immobility episode and the duration of immobility over a 6 min period were continually manually measured. An animal was rated as immobile when there was no movement of the head, extremities or the torso.
Resident–intruder test
The RI test is based on the territory defensive behavior against unfamiliar intruding conspecifics.32 Each single-housed resident male was confronted in its home cage by a group-housed (five mice per cage) intruder male FVB/N mouse for 10 min. Each intruder mouse was used only once to avoid submissive/dominance effects after first interaction. Behavioral interactions during each confrontation were recorded and subsequently scored by an observer. Latency to the first attack, total amount of attacks and cumulative duration of attacks were analyzed.
Neurochemical assessments
To prepare brains for high-performance liquid chromatography (HPLC) analysis, animals were anesthetized by intraperitoneal ketamine (100 mg kg–1) and xylazine (10 mg kg–1) injection. Animals were transcardially perfused with phosphate-buffered saline containing 300 U ml–1 heparin (Braun, Melsungen, Germany) to remove the blood, containing peripheral 5-HT. Brains were removed, weighed and snap-frozen on dry ice. For the determination of serotonin and its metabolites, frozen tissues were homogenized in lysis buffer containing 10 μM ascorbic acid and 1.8% perchloric acid, centrifuged for 30 min at 20 000 g, 4 °C, and the supernatant was used for HPLC measurement. Tissue levels of 5-HTP, 5-HT and its metabolite 5-HIAA were analyzed using high sensitive HPLC with fluorometric detection (Shimadzu, Tokyo, Japan).33 Sample separation takes place at 20 °C on a C18 reversed-phase column (OTU LipoMareC18, AppliChrom Application & Chromatography, Oranienburg, Germany) using a 10 mM potassium phosphate buffer, pH 5.0, containing 5% methanol with a flow rate of 2 ml min–1. Fluorescence of 5-HTP and 5-HT is excited at 295 nm and measured at 345 nm. For the evaluation of serotonin synthesis in vivo animals were injected intraperitoneally with 100 mg kg–1 of aromatic amino acid decarboxylase inhibitor 3-hydroxybenzylhydrazine dihydrochloride (NSD-1015, CatNr. 54880, Sigma-Aldrich, Munich, Germany) 1 h before brain dissection. Amounts of 5-HT, 5-HTP and 5-HIAA were normalized to the wet tissue weight for statistical analysis. Calculation of substance levels was based on external standard values.
Real-time–PCR analysis
For real-time–PCR (RT–PCR) analysis animals were first decapitated, and brains were promptly removed and snap-frozen on dry ice. RNA from the whole brain was extracted with Trizol reagent (15596-018 Invitrogen, Darmstadt, Germany), and residual genomic DNA was removed by DNase I treatment (DNA amplification grade, Sigma-Aldrich). RNA was reverse transcribed using random hexamers and modified Moloney murine leukemia virus reverse transcriptase (Superscript II, Invitrogen) according to the manufacturer's protocol. RT–PCR was run in a technical triplicate using SYBR green reagent (Qiagen, Hilden, Germany) in a 384-well plate format (fast RT–PCR system 7900HT, Applied Biosystems, Darmstadt, Germany). The expression of the Tph2 gene was quantified using RT2 quantitative PCR primer assay (PPM27894A-200 SABioscience, Hilden, Germany). Tph2 expression was normalized to TATA-binding protein (TBP) mRNA expression (primers: forward 5′-CCC TAT CAC TCC TGC CAC ACC-3′, reverse 5′-CGA AGT GCA ATG GTCTTT AGG TC-3′). The method of Livak and Schmittgen34 was applied to compare gene expression levels between groups, using the equation 2−ΔΔCT.
Statistics
Results are expressed as mean±s.e.m. Statistical analysis was performed by unpaired Student's t-test and by one way ANOVA with Bonferroni's correction as a post-hoc test for multiple comparisons (PRISM, GraphPad, San Diego, CA, USA). P<0.05 was considered to be significant.
Results
Tph2 expression and serotonin levels in Tph2−/− mice
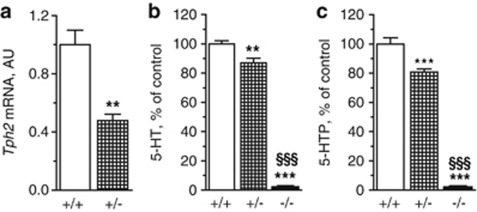
We first evaluated the amount of Tph2 transcripts in the brains of Tph2+/− and Tph2+/+ mice, containing one and two copies of the Tph2 gene, respectively. RT–PCR showed a 50% reduction in Tph2 gene expression in the whole brain of Tph2+/− mice in comparison with Tph2+/+ mice (Figure 1a). We next measured the amount of serotonin and its degradation product, 5-HIAA, in the whole brain of Tph2−/−, Tph2+/− and Tph2+/+ mice by HPLC. Tph2−/− mice contained <2% of Tph2+/+ 5-HT level and no detectable 5-HIAA in the brain (Figure 1b, Table 1), confirming previous results.8 However, only around 10% reduction in brain serotonin levels was observed in Tph2+/− in comparison with Tph2+/+ mice, whereas the level of 5-HT degradation product, 5-HIAA, was reduced nearly by half in Tph2+/− (Figure 1b, Table 1). We further evaluated the 5-HT synthesis rate in Tph2−/−, Tph2+/− and Tph2+/+ mice by blocking conversion of the 5-HTP to 5-HT by the aromatic amino acid decarboxylase inhibitor NSD. An around 20% decrease in accumulation of 5-HTP was observed in Tph2+/− in comparison with Tph2+/+ mice (Figure 1c, Table 1). As expected, Tph2−/− mice accumulated <2% of 5-HTP compared with Tph2+/+ (Figure 1c, Table 1).
Figure 1.
Tph2 expression and serotonin synthesis in Tph2−/− mice. (a) RT–PCR analysis of Tph2 expression in the brain. AU, arbitrary units. **P<0.01, Student's t-test. (b) 5-HT level in the whole brain (HPLC measurement). Tph2+/+ 5-HT level is taken as 100%. (c) 5-HTP level in the whole brain 1 h after NSD administration (100 mg kg–1, intraperitoneally) (HPLC measurement). Tph2+/+ 5-HTP level is taken as 100%. Data are shown as means±s.e.m., n=6. ***P<0.001, **P<0.01 vs Tph2+/+; §§§P<0.001 vs Tph2+/−, one-way ANOVA with Bonferroni correction. ANOVA, analysis of variance; HPLC, high-performance liquid chromatography; 5-HT, 5-hydroxytryptamine; 5-HTP, 5-hydroxytryptophan.
Table 1. Serotonin, 5-HIAA and 5-HTP in the brains of Tph2-deficient mice.
| 5-HT (pg mg–1) | 5-HIAA (pg mg–1) | 5-HTP (pg mg–1) | 5-HTP/NSD (pg mg–1) | |
|---|---|---|---|---|
| Tph2+/+ | 753.0±16.3 | 366.3±34.9 | 3.7±0.3 | 329.1±14.0 |
| Tph2+/− | 655.6±24.1** | 217.0±11.7* | 2.0±0.2* | 266.7±7.1* |
| Tph2−/− | 13.6±1.0*,*** | 0.0±0.0*,*** | 0.0±0.0*,*** | 5.7±0.3*,*** |
Abbreviations: 5-HT, 5-hydroxytryptamine; 5-HTP, 5-hydroxytryptophan; 5-HIAA, 5-hydroxyindoleacetic acid; NSD, 3-hydroxybenzylhydrazine dihydrochloride.
Serotonin (5-HT), 5-HTP and 5-HIAA levels were measured by HPLC in whole brain lysates. Serotonin synthesis rate was evaluated by the accumulation of 5-HTP in the brain during 1 h after administration of the AADC inhibitor, NSD (5-HTP/NSD column). Values are normalized to mg of wet brain tissue. Data are presented as means±s.e.m., n=6.
*P<0.001;
**P<0.01 vs Tph2+/+;
***P<0.001 vs Tph2+/− (one-way ANOVA with Bonferroni's correction).
Anxiety-like behavior in Tph2−/− mice
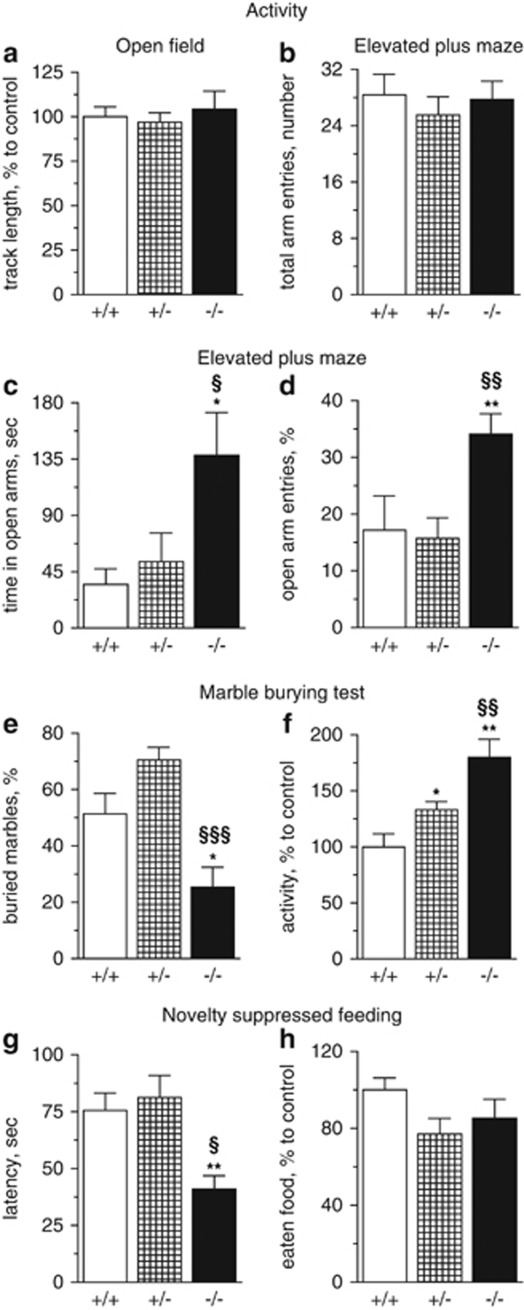
We first evaluated overall activity of Tph2−/− mice in the OF under low illumination conditions. Tph2−/− mice did not show any difference in locomotor activity in comparison with Tph2+/− and Tph2+/+ mice (Figure 2a).
Figure 2.
Locomotor activity and anxiety-like behavior in Tph2−/− mice. Open field: (a) Total distance traveled. Elevated plus maze: (b) Activity: total number of entries to closed and open arms. (c) Percentage of entries to open arms: entries to open arm/total arm entries. (d) Total time spent in open arms. Marble burying test: (e) Percentage of buried marbles: buried/introduced amount of marbles. (f) Locomotor activity during MBT. Novelty supressed feeding: (g) Latency to feed. (h) Consumed amount of food during 5 min after reaching the food pellet. (a–f): Tph2−/− n=10, Tph2+/− and Tph2+/+ n=12; (g, h): Tph2−/− n=11, Tph2+/− n=13, Tph2+/+ n=10. Data are shown as means±s.e.m. **P<0.01, *P<0.05 vs Tph2+/+; §§§P<0.001, §§P<0.01, §P<0.05 vs Tph2+/−, one-way ANOVA with Bonferroni correction. ANOVA, analysis of variance.
In EPM, Tph2−/− mice spent significantly more time in the open arms than Tph2+/+ and Tph2+/− (P=0.0161 and P=0.0133, respectively) (Figure 2c). Tph2−/− mice also exhibited twice the amount of open-arm entries compared with Tph2+/+ (P=0.0026 vs Tph2+/+, P=0.0054 vs Tph2+/−) (Figure 2d). However, total arm entries and total distance traveled were comparable between mice of all three genotypes (Figure 2b). Analysis of locomotion in the EPM over time showed that Tph2−/− mice extensively explored the brightest illuminated part of the open arms already during the first 5 min of testing, whereas Tph2+/+ animals did not enter the distal parts of the open arms during the whole 10 min of the test. Tph2+/− mice did not show any significant difference compared with Tph2+/+ mice neither in the total time spent in open arms nor in the open-arm entries (Figure 2c and d).
The amount of marbles buried by Tph2−/− mice in the MBT was significantly lower than that of Tph2+/+ and Tph2+/− animals (P=0.0199 and P<0.0001, respectively) (Figure 2e). Interestingly, the general activity of Tph2−/− animals during this test was almost twofold higher than that of Tph2+/+ (P=0.0046) (Figure 2f). There was no significant difference in the percentage of marbles buried by Tph2+/− mice compared with Tph2+/+ mice (Figure 2e). However, Tph2+/− showed an intermediate activity, significantly different from both Tph2−/− and Tph2+/+ mice (P=0.009 and P=0.023, respectively) (Figure 2f).
In the NSF task, Tph2−/− mice needed less time to reach and start eating the food pellet in the center of the arena compared with Tph2+/+ and Tph2+/− (P=0.002 and P=0.017, respectively) (Figure 2g). Food consumption, evaluated during 5 min following the test did not differ between the genotypes (Figure 2h). Tph2+/− mice did not show a significant difference in the latency to reach and start eating the food in comparison with both, Tph2+/+ and Tph2−/− (Figure 2g and h).
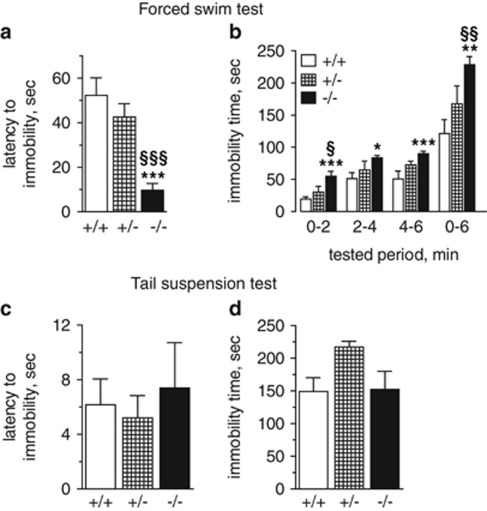
Depression-like behavior in Tph2−/− mice
In the FST, Tph2+/− mice did not show any significant difference in comparison with Tph2+/+ in the total immobility time or the latency to the first immobility episode, whereas Tph2−/− mice demonstrated reduced struggling behavior (Figure 3). They spent less time swimming until the first immobility episode (P=0.0001) (Figure 3a) and stayed longer immobile compared with Tph2+/−and Tph2+/+ littermates (P=0.005, in comparison with both genotypes) (Figure 3b). Moreover, Tph2−/− mice showed an increase in immobility time during each single 2 min episode compared with Tph2+/+mice (Figure 3b). In the TST no significant differences between genotypes could be found neither in the latency to immobility, nor in the struggling time (Figure 3c and d).
Figure 3.
Depression-like behavior in Tph2−/− mice. Forced swim test: (a) Latency to the first immobility episode. (b) Immobility time during 2 min intervals and the whole 6 min of tested period. Tail suspension test: (c) Latency to the first immobility episode. (d) Immobility time during 6 min of tested period. Data are shown as means±s.e.m., (a, b): n=10, (c, d): n=9. ***P<0.001, **P<0.01, *P<0.05 vs Tph2+/+; §§§P<0.001, §§P<0.01, §P<0.05 vs Tph2+/−, one-way ANOVA with Bonferroni correction. ANOVA, analysis of variance.
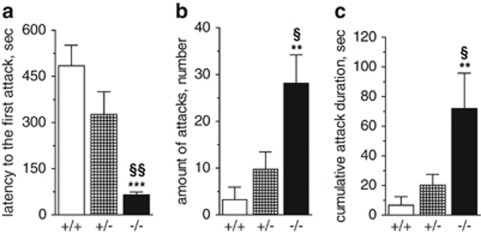
Aggressive behavior in Tph2−/− mice
In the RI test, Tph2−/− mice attacked the intruder almost six times faster than Tph2+/+ mice (P=0.0002) (Figure 4a). Furthermore, the number of attacks and the cumulative attack duration in the Tph2−/− vs Tph2+/+ group were elevated sevenfold (P=0.0014 and P=0.01, respectively) (Figure 4b and c). A qualitative analysis of attacks revealed a striking difference between Tph2−/− and Tph2+/+ mice: within 5 min of the test all mutant animals displayed aggressive bouts, while only 22% of Tph2+/+ mice showed such behavior. Though Tph2+/− mice tended to show an intermediate state of aggressive behavior between Tph2−/− and Tph2+/+ mice, neither the differences in the first attack latency nor the number of attacks between Tph2+/− and Tph2+/+ mice were significantly different (Figure 4a and b).
Figure 4.
Aggressive behavior in Tph2−/− mice. Resident–intruder test: (a) Attack latency: time between the introduction of intruder and the first attack of the resident. (b) Total amount of attacks by the resident. (c) Cumulative attack duration during 10 min tested time. Residents were Tph2−/− (n=9), Tph2+/− (n=8), Tph2+/+ (n=7). Data are shown as means±s.e.m., n=8. ***P<0.001, **P<0.01 vs Tph2+/+; §P<0.05, §§P<0.01 vs Tph2+/−, one-way ANOVA with Bonferroni correction. ANOVA, analysis of variance.
Discussion
Although the implication of brain serotonin in animal behavior has been recognized already in the last century, most of the studies were conducted using pharmacological or genetic inhibition of serotonin reuptake and 5-HT receptors. However, the role of serotonin per se in these studies was not completely clarified, because no suitable animal model was available yet. In this study, we used mice deficient in brain serotonin synthesis on a pure genetic background to evaluate the consequences of complete absence of this neurotransmitter in the CNS on aggression-, depression- and anxiety-like behavior.
A role of serotonin in the etiology of depressive disorders was suggested more than 50 years ago.35 Later on, formulation of the monoaminergic theory of depression led to the development of antidepressive drugs, which increase the monoaminergic activity.36 Moreover, severely depressed patients treated with Trp or 5-HTP show symptomatic improvement,37, 38 whereas, giving Trp-free diet to depressed individuals elicits a relapse in patients getting treatment with antidepressants.39, 40
In our experiments, mice depleted in brain serotonin exhibited a lack of motivation to struggle in the FST that can be interpreted as a depression-like phenotype, supporting the monoaminergic theory of depression. There is some discrepancy regarding this phenotype between our results and recent data showing a slight antidepressive effect in Tph2−/− mice on the second day of FST.10 These conflicting findings could be due to several reasons, such as different analysis methods — automated10 vs manual (our study)41 — or the 2-day FST protocol,10 which is commonly used for identifying a depressive state in rats vs the 1-day protocol usually performed for mice.42, 43 Moreover, the study of depression-like behavior by Savelieva et al.10 was performed on a mixed genetic background (C57BL/6Jx129S5/S), that may have masked the behavioral effect of Tph2 gene ablation.
Evaluation of mouse behavior in another widely used paradigm, the TST, did not reveal a depression-like phenotype in Tph2−/− mice. This finding is consistent with previous studies showing that depletion of serotonin using p-chlorophenylalanine (PCPA) does not change the outcome of the TST, whereas inhibition of catecholamine synthesis has a prodepressive effect in this test.44 Also in the first description of Tph2−/− mice10 no differences in the TST were observed at the first day of experiment. Interestingly, there are reports that show an increased immobility time in the TST in another genetic model of central serotonin depletion — heterozygous VMAT2-knockout mice.45 However, in these mice levels of other neurotransmitters are also changed and, therefore, the altered behavior in TST could not be interpreted as only due to the depletion of central serotonin. Surprisingly, when VMAT2 was ablated only in SERT-positive neurons, the behavior in TST was reversed: VMAT2SERT-Cre mice showed a clear antidepressive phenotype in the TST.46 However, these animals were on a mixed genetic background that may have veiled the effect of central serotonin ablation.
Although both tests, TST and FST, are widely used for the screening of antidepressants, the validity of these tasks to evaluate symptoms of intrinsic depressive behavior is not so clear.47 Moreover, the sensitivity of these two tests to pharmacological drugs is not identical, indicating that different neurochemical pathways may mediate the performance in these tasks.48 Additionally, mice being in two different inescapable situations (wet conditions in FST and dry in TST) could use different strategies to struggle. Accordingly, the direction of alterations in depression-like behavior observed in several hyposerotonergic models was not consistent across the studies and even controversial between the two tests (TST and FST) in frames of the same study.10, 46, 49 We observed a clear depression-like phenotype in FST, which was also highly reproducible in our hands—we obtained the same results in two independent experiments (data not shown). However, we could not confirm the depression-like phenotype of Tph2−/− mice in the TST. We cannot exclude that this phenotype was masked due to the performance of the test during the dark cycle. Further studies are required to clarify the impact of the dark–light cycle on the depression-like behavior in Tph2−/− animals.
Several previous studies failed to detect any drastic alteration in depression-like behavior in models of serotonin depletion after PCPA treatment.50, 51, 52 The clear depression-like phenotype observed in the FST in Tph2−/− mice can be a consequence of a life-long depletion in serotonergic transduction vs short-term effects of PCPA. In this respect, it is interesting to note, that mice prenatally exposed to PCPA show increased depression-related behavior in FST and TST and decreased anxiety.53, 54 Due to the extreme aggressiveness of Tph2−/− mice, animals used in our study could not be kept in groups and were single housed starting 10–12 weeks of age. We can also not exclude that alterations observed in the FST were primed by the prolonged single housing of animals, which may have had more pronounced consequences in Tph2−/− animals owing to their higher sensibility to isolation. In addition, hormonal changes resulting from exaggerated aggressiveness or higher sensitivity to stress, as well as the reduced fat content in Tph2−/− animals 10, 12 (our unpublished data) may have had an impact on the outcome of the FST.
As any of the behavior tests used in this study could be influenced by changed activity, we examined whether Tph2−/− mice have any alterations in locomotion. Neither activity in the OF (new environment), nor home cage activity measured by telemetry recording8 or InfraMot system (TSE Systems GmbH, data not shown) were different between Tph2−/− mice and control animals.
Serotonin has been postulated to have a role in aggression.55, 56 Low cerebrospinal 5-HIAA was correlated with elevated aggression in humans26, 57, 58 and monkeys.59 Furthermore, low-Trp diet resulted in increased aggressive behavior in humans,60 whereas Trp-enriched diet initiated a reduction of physical aggression in subjects that had a history of elevated aggression.61 Several genetic variations in serotonergic genes have been linked to impulsive aggression in humans.62, 63 Moreover, a positive correlation between low serotonin release and increased aggression was confirmed by microdialysis in freely moving animals during the RI test.64, 65 Inhibition of serotonin synthesis in rats led to increased aggressiveness, whereas enhancement of serotonin transmission suppressed aggressive behavior.66 Our study revealed that central serotonin deficiency led to highly increased aggressive behavior in mice. Interestingly, this phenotype was observed not only in males, but also in Tph2−/− females.8 Thus, our model provides strong evidence for increased aggression as a consequence of complete serotonin deficiency in the CNS being in line with two recently published hyposerotonergic animal models, TPH2 R439H knockin mice, bearing a single-nucleotide mutation, equivalent to a rare human variant (R441H) identified in depressed patients,49 and Pet-1 deficient animals, which lack most serotonergic neurons.62, 67 Altogether, these data argue for a direct correlation between the serotonin content in the brain and the level of aggression.
It was recently reported that the absence of brain serotonin leads to increased male–male mounting behavior in a 30 min social interactions task.9 This phenotype was not prominent during 10 min of the resident–intruder test performed in our study. Moreover, in several cases we had to interrupt the test due to the extreme aggressiveness of Tph2−/− animals. It can not be excluded that defensive behavior of serotonin-deficient animals was misinterpreted in the study of Liu et al.9
The behavioral evaluation performed in this study showed that Tph2−/− mice have decreased levels of aversive behavior in approach-avoidance-conflict tests, correlating with the hypothesis that enhanced serotonergic transmission in the brain facilitates anxiety, whereas a decrease in extracellular 5-HT leads to reduced anxious behavior. This hypothesis, formulated in early 1970s68 was further refined using animal models with 5-HT depletion by serotonin synthesis inhibition or lesions of serotonergic neurons.69, 70, 71, 72 Furthermore, studies in SERT overexpressing and SERT-deficient mice,73, 74, 75 in 5-HT1a-deficient animals,76 as well as in very recently published hyposerotonergic mouse models including Lmx1b-, Pet1- or VMAT2-deficient animals46, 77, 78 correlate with this hypothesis. Despite being in line with the low-anxiety phenotype, observed in the EPM and NSF tests, the results of the MBT poorly correlate with literature data from other genetic models affecting the serotonergic system.10, 62, 79 We suppose that opposite effects observed in our study originate mostly from the differences in the genetic background (pure C57Bl/6 used by us vs mixed in other studies)—a factor which may strongly affect serotonin-related behavior, as already shown in SERT-knockout mice.74 On the other hand, the experimental setup used by us was not identical to the one of other studies: the protocols differ in several aspects, such as amount of marbles, cage parameters and test conditions. Moreover, we cannot exclude that increased locomotion, unexpectedly observed during MBT and not reported in other studies, had an impact on results of this test in our experiments.
There is a vast amount of data about the contribution of molecular variants of TPH2 to psychiatric disorders in humans.15 Interestingly, one single-point mutation (R441H) found in a human cohort of late-onset depression was shown to markedly decrease activity of TPH2 and central serotonin level.80 A genetic mouse model carrying a single-point mutation (R439H) in Tph2, analogous to this human mutation exhibit significantly decreased tissue levels and synthesis rates of 5-HT in the brain, and shows pronounced depression-like behavior in TST, as well as increased aggression.49 To check whether reduction in Tph2 gene copy number may also significantly influence behavior in mice, we evaluated the phenotype of Tph2+/− animals. Quantification of Tph2 mRNA level revealed a decrease in Tph2 expression by half, suggesting that in wild-type animals both Tph2 alleles are functional and do not undergo epigenetic modification. Regardless, the 50% decrease in Tph2 transcriptional activity, only a 10% reduction in 5-HT level was observed. We missed this difference in our previous study,8 probably because it was masked by the more heterogeneous background of these animals. Such a slight decrease can be partially explained by a reduced turnover of serotonin by MAO in Tph2+/− mice, that is evident from the reduced level of the serotonin degradation product 5-HIAA (Table 1). However surprisingly, evaluation of 5-HT synthesis rate also revealed only a 20% decrease in Tph2+/− animals, which probably reflects the limited availability of Trp in the brain.81, 82 Nevertheless, the 10% decrease in brain 5-HT was not sufficient to alter mouse behavior: Tph2+/− were not different from Tph2+/+ mice in aggression, anxiety or depression-like behavior. Similar effects were recently observed in mice carrying the C1473G mutation in the Tph2 gene. This mutation resulted in a decreased 5-HT synthesis rate, but hardly changed serotonin content in the brain, and did not affect the behavior in depression and anxiety paradigms.83, 84 These findings suggest that a lack of one Tph2 allele alone is not sufficient to modulate aggression and depression-like behavior and therefore is unlikely to be of physiological significance. However, it cannot be excluded that genetic variation in other serotonin-related genes, restriction or alterations in nutrition, medical treatment and epigenetic modifications acquired during lifespan, may unmask a critical role of TPH2 hypo-expression in the development of pathological symptoms in human.
Taken together, using Tph2−/− mice on a pure genetic background, we provide strong evidence that central serotonin deficiency leads to exaggerated aggression and decreased anxiety and confirm that our animal model is useful to draw unequivocal conclusions about the physiological significance of this neurotransmitter.
Acknowledgments
This work was supported by a fellowship of the German Academic Exchange Service (DAAD) to VM (A07/99669). We thank Susanne da Costa Goncalves, Sabine Grüger, Manfred Ströhmann and Alexandra Wistel-Wozniak for the excellent technical assistance, Catherine Schweppe for the critical reading of the manuscript, Babila Tachu and Silke Frahm for the helpful suggestions in experiment design.
The authors declare no conflict of interest.
References
- Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Gutknecht L, Waider J, Kraft S, Kriegebaum C, Holtmann B, Reif A, et al. Deficiency of brain 5-HT synthesis but serotonergic neuron formation in Tph2 knockout mice. J Neural Transm. 2008;115:1127–1132. doi: 10.1007/s00702-008-0096-6. [DOI] [PubMed] [Google Scholar]
- Patel PD, Pontrello C, Burke S. Robust and tissue-specific expression of TPH2 versus TPH1 in rat raphe and pineal gland. Biol Psychiatry. 2004;55:428–433. doi: 10.1016/j.biopsych.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Neal KB, Parry LJ, Bornstein JC. Strain-specific genetics, anatomy and function of enteric neural serotonergic pathways in inbred mice. J Physiol. 2009;587:567–586. doi: 10.1113/jphysiol.2008.160416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zill P, Buttner A, Eisenmenger W, Bondy B, Ackenheil M. Regional mRNA expression of a second tryptophan hydroxylase isoform in postmortem tissue samples of two human brains. Eur Neuropsychopharmacol. 2004;14:282–284. doi: 10.1016/j.euroneuro.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Sakowski SA, Geddes TJ, Thomas DM, Levi E, Hatfield JS, Kuhn DM. Differential tissue distribution of tryptophan hydroxylase isoforms 1 and 2 as revealed with monospecific antibodies. Brain Res. 2006;1085:11–18. doi: 10.1016/j.brainres.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, et al. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci USA. 2009;106:10332–10337. doi: 10.1073/pnas.0810793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jiang Y, Si Y, Kim JY, Chen ZF, Rao Y. Molecular regulation of sexual preference revealed by genetic studies of 5-HT in the brains of male mice. Nature. 2011;472:95–99. doi: 10.1038/nature09822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savelieva KV, Zhao S, Pogorelov VM, Rajan I, Yang Q, Cullinan E, et al. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS One. 2008;3:e3301. doi: 10.1371/journal.pone.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Angoa Perez M, Francescutti-Verbeem DM, Shah MM, Kuhn DM. The role of endogenous serotonin in methamphetamine-induced neurotoxicity to dopamine nerve endings of the striatum. J Neurochem. 2010;115:595–605. doi: 10.1111/j.1471-4159.2010.06950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson DF. The genetics of depression: a review. Biol Psychiatry. 2006;60:84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Lohoff FW. Overview of the genetics of major depressive disorder. Curr Psychiatry Rep. 2010;12:539–546. doi: 10.1007/s11920-010-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes S, Mosienko V, Bashammakh S, Alenina N, Bader M. Tryptophan hydroxylase as novel target for the treatment of depressive disorders. Pharmacology. 2010;85:95–109. doi: 10.1159/000279322. [DOI] [PubMed] [Google Scholar]
- Van Den Bogaert A, Sleegers K, De Zutter S, Heyrman L, Norrback KF, Adolfsson R, et al. Association of brain-specific tryptophan hydroxylase, TPH2, with unipolar and bipolar disorder in a Northern Swedish, isolated population. Arch Gen Psychiatry. 2006;63:1103–1110. doi: 10.1001/archpsyc.63.10.1103. [DOI] [PubMed] [Google Scholar]
- Zill P, Baghai TC, Zwanzger P, Schule C, Eser D, Rupprecht R, et al. SNP and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene provide evidence for association with major depression. Mol Psychiatry. 2004;9:1030–1036. doi: 10.1038/sj.mp.4001525. [DOI] [PubMed] [Google Scholar]
- Hovatta I, Barlow C. Molecular genetics of anxiety in mice and men. Ann Med. 2008;40:92–109. doi: 10.1080/07853890701747096. [DOI] [PubMed] [Google Scholar]
- Craig IW, Halton KE. Genetics of human aggressive behaviour. Hum Genet. 2009;126:101–113. doi: 10.1007/s00439-009-0695-9. [DOI] [PubMed] [Google Scholar]
- Zalsman G, Patya M, Frisch A, Ofek H, Schapir L, Blum I, et al. Association of polymorphisms of the serotonergic pathways with clinical traits of impulsive-aggression and suicidality in adolescents: a multi-center study. World J Biol Psychiatry. 2011;12:33–41. doi: 10.3109/15622975.2010.518628. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Davis JM. Consistent evidence for a biological subtype of depression characterized by low CSF monoamine levels. Acta Psychiatr Scand. 1986;74:8–12. doi: 10.1111/j.1600-0447.1986.tb06219.x. [DOI] [PubMed] [Google Scholar]
- Placidi GP, Oquendo MA, Malone KM, Huang YY, Ellis SP, Mann JJ. Aggressivity, suicide attempts, and depression: relationship to cerebrospinal fluid monoamine metabolite levels. Biol Psychiatry. 2001;50:783–791. doi: 10.1016/s0006-3223(01)01170-2. [DOI] [PubMed] [Google Scholar]
- Stanley M, Traskman-Bendz L, Dorovini-Zis K. Correlations between aminergic metabolites simultaneously obtained from human CSF and brain. Life Sci. 1985;37:1279–1286. doi: 10.1016/0024-3205(85)90242-5. [DOI] [PubMed] [Google Scholar]
- Birger M, Swartz M, Cohen D, Alesh Y, Grishpan C, Kotelr M. Aggression: the testosterone-serotonin link. Isr Med Assoc J. 2003;5:653–658. [PubMed] [Google Scholar]
- Brown GL, Linnoila MI.CSF serotonin metabolite (5-HIAA) studies in depression, impulsivity, and violence J Clin Psychiatry 199051Suppl31–41.discussion 42–33. [PubMed] [Google Scholar]
- Stanley B, Molcho A, Stanley M, Winchel R, Gameroff MJ, Parsons B, et al. Association of aggressive behavior with altered serotonergic function in patients who are not suicidal. Am J Psychiatry. 2000;157:609–614. doi: 10.1176/appi.ajp.157.4.609. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Njung'e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol Biochem Behav. 1991;38:63–67. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, et al. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54:960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- Bessa JM, Mesquita AR, Oliveira M, Pego JM, Cerqueira JJ, Palha JA, et al. A trans-dimensional approach to the behavioral aspects of depression. Front Behav Neurosci. 2009;3:1. doi: 10.3389/neuro.08.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- Kulikov AV, Osipova DV, Naumenko VS, Popova NK. Association between Tph2 gene polymorphism, brain tryptophan hydroxylase activity and aggressiveness in mouse strains. Genes Brain Behav. 2005;4:482–485. doi: 10.1111/j.1601-183X.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sawada M, Kato T, Nagatsu T. Demonstration of tryptophan 5-monooxygenase activity in human brain by high sensitive high-performance liquid chromatography with fluorometric detection. Biochem Int. 1981;2:295–303. [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Brodie BB, Pletscher A, Shore PA. Evidence that serotonin has a role in brain function. Science. 1955;122:968. doi: 10.1126/science.122.3177.968. [DOI] [PubMed] [Google Scholar]
- Lopez-Munoz F, Alamo C. Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today. Curr Pharm Des. 2009;15:1563–1586. doi: 10.2174/138161209788168001. [DOI] [PubMed] [Google Scholar]
- Price LH, Malison RT, McDougle CJ, Pelton GH, Heninger GR. The neurobiology of tryptophan depletion in depression: effects of intravenous tryptophan infusion. Biol Psychiatry. 1998;43:339–347. doi: 10.1016/s0006-3223(97)00284-9. [DOI] [PubMed] [Google Scholar]
- van Praag HM. In search of the mode of action of antidepressants. 5-HTP/tyrosine mixtures in depressions. Neuropharmacology. 1983;22:433–440. doi: 10.1016/0028-3908(83)90193-4. [DOI] [PubMed] [Google Scholar]
- Miller HL, Delgado PL, Salomon RM, Licinio J, Barr LC, Charney DS. Acute tryptophan depletion: a method of studying antidepressant action. J Clin Psychiatry. 1992;53 Suppl:28–35. [PubMed] [Google Scholar]
- Schruers K, Griez E. The effects of tryptophan depletion on mood and psychiatric symptoms. J Affect Disord. 2003;74:305. doi: 10.1016/s0165-0327(02)00006-x. [DOI] [PubMed] [Google Scholar]
- Baker M. Animal models: inside the minds of mice and men. Nature. 2011;475:123–128. doi: 10.1038/475123a. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- Drugan RC, Morrow AL, Weizman R, Weizman A, Deutsch SI, Crawley JN, et al. Stress-induced behavioral depression in the rat is associated with a decrease in GABA receptor-mediated chloride ion flux and brain benzodiazepine receptor occupancy. Brain Res. 1989;487:45–51. doi: 10.1016/0006-8993(89)90938-4. [DOI] [PubMed] [Google Scholar]
- O'Leary OF, Bechtholt AJ, Crowley JJ, Valentino RJ, Lucki I. The role of noradrenergic tone in the dorsal raphe nucleus of the mouse in the acute behavioral effects of antidepressant drugs. Eur Neuropsychopharmacol. 2007;17:215–226. doi: 10.1016/j.euroneuro.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Fukui M, Rodriguiz RM, Zhou J, Jiang SX, Phillips LE, Caron MG, et al. Vmat2 heterozygous mutant mice display a depressive-like phenotype. J Neurosci. 2007;27:10520–10529. doi: 10.1523/JNEUROSCI.4388-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narboux-Neme N, Sagne C, Doly S, Diaz SL, Martin CB, Angenard G, et al. Severe serotonin depletion after conditional deletion of the vesicular monoamine transporter 2 gene in serotonin neurons: neural and behavioral consequences. Neuropsychopharmacology. 2011. [DOI] [PMC free article] [PubMed]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Bai F, Li X, Clay M, Lindstrom T, Skolnick P. Intra- and interstrain differences in models of “behavioral despair”. Pharmacol Biochem Behav. 2001;70:187–192. doi: 10.1016/s0091-3057(01)00599-8. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, et al. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci USA. 2008;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavioli EC, Vaughan CW, Marzola G, Guerrini R, Mitchell VA, Zucchini S, et al. Antidepressant-like effects of the nociceptin/orphanin FQ receptor antagonist UFP-101: new evidence from rats and mice. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:547–553. doi: 10.1007/s00210-004-0939-0. [DOI] [PubMed] [Google Scholar]
- O'Leary OF, Bechtholt AJ, Crowley JJ, Hill TE, Page ME, Lucki I. Depletion of serotonin and catecholamines block the acute behavioral response to different classes of antidepressant drugs in the mouse tail suspension test. Psychopharmacology. 2007;192:357–371. doi: 10.1007/s00213-007-0728-9. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Bourin M. Dose-dependent influence of buspirone on the activities of selective serotonin reuptake inhibitors in the mouse forced swimming test. Psychopharmacology. 1998;138:198–206. doi: 10.1007/s002130050663. [DOI] [PubMed] [Google Scholar]
- Vataeva LA, Kudrin VS, Vershinina EA, Mosin VM, Tiul'kova EI, Otellin VA. Behavioral alteration in the adult rats prenatally exposed to para-chlorophenylalanine. Brain Res. 2007;1169:9–16. doi: 10.1016/j.brainres.2007.06.056. [DOI] [PubMed] [Google Scholar]
- Vataeva LA, Kudrin VS, Vershinina EA, Mosin VM, Tiul'kova EI, Otellin VA. Maternal para-chlorophenylalanine exposure modifies central monoamines and behaviors in the adult offspring. Brain Res. 2008;1234:1–7. doi: 10.1016/j.brainres.2008.07.064. [DOI] [PubMed] [Google Scholar]
- Maas JW. Neurochemical differences between two strains of mice. Science. 1962;137:621–622. doi: 10.1126/science.137.3530.621. [DOI] [PubMed] [Google Scholar]
- Valzelli L, Giacalone E, Garattini S. Pharmacological control of aggressive behavior in mice. Eur J Pharmacol. 1967;2:144–146. doi: 10.1016/0014-2999(67)90040-4. [DOI] [PubMed] [Google Scholar]
- Coccaro EF. Impulsive aggression and central serotonergic system function in humans: an example of a dimensional brain-behavior relationship. Int Clin Psychopharmacol. 1992;7:3–12. doi: 10.1097/00004850-199200710-00001. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Hauger RL. Serotonin function and antiaggressive response to fluoxetine: a pilot study. Biol Psychiatry. 1997;42:546–552. doi: 10.1016/S0006-3223(97)00309-0. [DOI] [PubMed] [Google Scholar]
- Zajicek KB, Price CS, Shoaf SE, Mehlman PT, Suomi SJ, Linnoila M, et al. Seasonal variation in CSF 5-HIAA concentrations in male rhesus macaques. Neuropsychopharmacology. 2000;22:240–250. doi: 10.1016/S0893-133X(99)00097-4. [DOI] [PubMed] [Google Scholar]
- Zepf FD, Stadler C, Demisch L, Schmitt M, Landgraf M, Poustka F. Serotonergic functioning and trait-impulsivity in attention-deficit/hyperactivity-disordered boys (ADHD): influence of rapid tryptophan depletion. Hum Psychopharmacol. 2008;23:43–51. doi: 10.1002/hup.896. [DOI] [PubMed] [Google Scholar]
- Nantel-Vivier A, Pihl RO, Young SN, Parent S, Belanger SA, Sutton R, et al. Serotonergic contribution to boys' behavioral regulation. PLoS One. 2011;6:e20304. doi: 10.1371/journal.pone.0020304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TL, Vorhees CV, Williams MT. Mouse plasmacytoma-expressed transcript 1 knock out induced 5-HT disruption results in a lack of cognitive deficits and an anxiety phenotype complicated by hypoactivity and defensiveness. Neuroscience. 2009;164:1431–1443. doi: 10.1016/j.neuroscience.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz AJ, Beck A, Meyer-Lindenberg A, Sterzer P, Heinz A. Cognitive and neurobiological mechanisms of alcohol-related aggression. Nat Rev Neurosci. 2011;12:400–413. doi: 10.1038/nrn3042. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, van Erp AM, Tornatzky W, Miczek KA. Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur J Neurosci. 2003;17:371–378. doi: 10.1046/j.1460-9568.2003.02447.x. [DOI] [PubMed] [Google Scholar]
- van Erp AM, Miczek KA. Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J Neurosci. 2000;20:9320–9325. doi: 10.1523/JNEUROSCI.20-24-09320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, et al. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Wise CD, Berger BD, Stein L. Benzodiazepines: anxiety-reducing activity by reduction of serotonin turnover in the brain. Science. 1972;177:180–183. doi: 10.1126/science.177.4044.180. [DOI] [PubMed] [Google Scholar]
- Andrade TG, Macedo CE, Zangrossi H, Jr, Graeff FG. Anxiolytic-like effects of median raphe nucleus lesion in the elevated T-maze. Behav Brain Res. 2004;153:55–60. doi: 10.1016/j.bbr.2003.10.036. [DOI] [PubMed] [Google Scholar]
- Briley M, Chopin P, Moret C. Effect of serotonergic lesion on “anxious” behaviour measured in the elevated plus-maze test in the rat. Psychopharmacology. 1990;101:187–189. doi: 10.1007/BF02244124. [DOI] [PubMed] [Google Scholar]
- Critchley MA, Njung'e K, Handley SL. Actions and some interactions of 5-HT1A ligands in the elevated X-maze and effects of dorsal raphe lesions. Psychopharmacology. 1992;106:484–490. doi: 10.1007/BF02244819. [DOI] [PubMed] [Google Scholar]
- Soderpalm B, Engel JA. Serotonergic involvement in conflict behaviour. Eur Neuropsychopharmacol. 1990;1:7–13. doi: 10.1016/0924-977x(90)90004-t. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Holmes A, Lit Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2003;2:365–380. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Jennings KA, Loder MK, Sheward WJ, Pei Q, Deacon RM, Benson MA, et al. Increased expression of the 5-HT transporter confers a low-anxiety phenotype linked to decreased 5-HT transmission. J Neurosci. 2006;26:8955–8964. doi: 10.1523/JNEUROSCI.5356-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, et al. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Dai JX, Han HL, Tian M, Cao J, Xiu JB, Song NN, et al. Enhanced contextual fear memory in central serotonin-deficient mice. Proc Natl Acad Sci USA. 2008;105:11981–11986. doi: 10.1073/pnas.0801329105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyasova V, Fernandez SP, Laine J, Stankovski L, Muzerelle A, Doly S, et al. A genetically defined morphologically and functionally unique subset of 5-HT neurons in the mouse raphe nuclei. J Neurosci. 2011;31:2756–2768. doi: 10.1523/JNEUROSCI.4080-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Edwards J, Carroll J, Wiedholz L, Millstein RA, Jaing C, et al. Insertion mutation at the C-terminus of the serotonin transporter disrupts brain serotonin function and emotion-related behaviors in mice. Neuroscience. 2006;140:321–334. doi: 10.1016/j.neuroscience.2006.01.049. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gainetdinov RR, Beaulieu JM, Sotnikova TD, Burch LH, Williams RB, et al. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Fernstrom JD, Wurtman RJ. Brain serotonin content: physiological dependence on plasma tryptophan levels. Science. 1971;173:149–152. doi: 10.1126/science.173.3992.149. [DOI] [PubMed] [Google Scholar]
- Markus CR. Dietary amino acids and brain serotonin function; implications for stress-related affective changes. Neuromolecular Med. 2008;10:247–258. doi: 10.1007/s12017-008-8039-9. [DOI] [PubMed] [Google Scholar]
- Siesser WB, Zhang X, Jacobsen JP, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase 2 genotype determines brain serotonin synthesis but not tissue content in C57Bl/6 and BALB/c congenic mice. Neurosci Lett. 2010;481:6–11. doi: 10.1016/j.neulet.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenner K, Qadri F, Bert B, Voigt JP, Bader M. The mTPH2 C1473G single nucleotide polymorphism is not responsible for behavioural differences between mouse strains. Neurosci Lett. 2008;431:21–25. doi: 10.1016/j.neulet.2007.11.012. [DOI] [PubMed] [Google Scholar]