Abstract
Aims
Statins protect against cardiovascular-related mortality but induce skeletal muscle toxicity. To investigate mechanisms of statins, we tested the hypothesis that statins optimized cardiac mitochondrial function but impaired vulnerable skeletal muscle by inducing different level of reactive oxygen species (ROS).
Methods and results
In atrium of patients treated with statins, ROS production was decreased and oxidative capacities were enhanced together with an extensive augmentation of mRNAs expression of peroxisome proliferator-activated receptor gamma co-activator (PGC-1) family. However, in deltoid biopsies from patients with statin-induced muscular myopathy, oxidative capacities were decreased together with ROS increase and a collapse of PGC-1 mRNA expression. Several animal and cell culture experiments were conducted and showed by using ROS scavengers that ROS production was the triggering factor responsible of atorvastatin-induced activation of mitochondrial biogenesis pathway and improvement of antioxidant capacities in heart. Conversely, in skeletal muscle, the large augmentation of ROS production following treatment induced mitochondrial impairments, and reduced mitochondrial biogenesis mechanisms. Quercetin, an antioxidant molecule, was able to counteract skeletal muscle deleterious effects of atorvastatin in rat.
Conclusion
Our findings identify statins as a new activating factor of cardiac mitochondrial biogenesis and antioxidant capacities, and suggest the importance of ROS/PGC-1 signalling pathway as a key element in regulation of mitochondrial function in cardiac as well as skeletal muscles.
Keywords: Mitohormesis, Statin, ROS, Heart, Mitochondrial biogenesis
See page 1299 for the editorial comment on this article (doi:10.1093/eurheartj/ehr287)
Introduction
Statins (HMG-CoA reductase inhibitors) are the most frequently prescribed medication in developed countries. They impair cholesterol synthesis by inhibiting the synthesis of mevalonate, the rate-limiting step in cholesterol biosynthetic pathway.1 Statins reduce cardiovascular-related morbidity and mortality in patients with or without coronary artery disease.2 It is widely accepted that statins have pleiotropic beneficial effects at the cardiovascular level,3,4 particularly by enhancing the bioavailability of vascular NOS-derived NO. Statins are generally well tolerated but can provoke a variety of skeletal muscle associated, dose-dependant adverse reactions, ranging from muscle pain to rhabdomyolysis that sometimes persist several months after treatment withdrawal.5
Little is known about the molecular mechanisms by which statins protect against cardiovascular-related morbidity but lead to skeletal muscle injury. In vivo studies documenting the effects of statins on cardiac mitochondria are rare. In cardiomyocytes, Jones et al.6 showed that simvastatin protected mitochondria from oxidative stress. At the skeletal muscle level, in vitro investigations have shown that simvastatin inhibited complex I of the mitochondrial electron transport chain.7 Moreover, several studies have shown that mitochondrial impairments could be largely implicated in the deleterious effects of statins.8,9
The role of mitochondria also extends far beyond energy production,10 as they are important generators of reactive oxygen species (ROS), which can act either as second messengers or as a source of cellular damage, depending of the ROS production level.11–14 As a vital organ rich in mitochondria and with high oxygen use, the heart is prone to oxidative damage. Furthermore, metabolic and apoptotic pathway converge on mitochondria and are both recognized as playing a major role in disease.15,16 It is thus crucial to clearly characterize the mechanisms of statin-associated mitochondrial modifications in different muscle phenotypes.7,9,17,18
Advances in molecular biology have started to elucidate the transcriptional events governing mitochondrial biogenesis. Peroxisome proliferator-activated receptor gamma co-activator (PGC-1α) is considered to be the major regulator of mitochondrial biogenesis.19 Interestingly, a new concept proposed that mitochondrial biogenesis could be triggered by low doses of mitochondrial ROS to counteract stressor challenges and to re-establish homeostasis. This concept called mitochondrial hormesis or ‘mitohormesis’.13,20,21
However, the effects of statin treatment on the molecular events controlling mitochondrial biogenesis have never been investigated according to the muscular metabolic phenotype, although this may be a potential mechanism to control muscular mitochondrial function.
In the present study, we investigated the effects of statin treatment on ROS production-induced mitochondrial biogenesis in cardiac and skeletal muscles in humans, animals and cell culture models. We demonstrated that statin treatment produced a low amount of ROS in cardiac muscle, which activated mechanisms of mitochondrial biogenesis and the antioxidant system via the PGC-1 signalling pathway. In contrast, in patients with statin-induced myopathy as well as in skeletal muscle of rats, a large amount of ROS following statin treatment down-regulated mitochondrial biogenesis, and triggered deleterious effects on mitochondrial function. Altogether, our results demonstrated that statins acted via a ‘mitohormesis’ mechanism in order to activate mitochondrial biogenesis and protect cardiac metabolism.
Methods
Surgical procedure and human atrial appendage sampling
All surgeries were performed through a full median sternotomy incision and under cardiopulmonary bypass. Heparin (300U/kg, i.v.) was given. Arterial cannulation was performed via the distal ascending aorta. Single venous cannulation with a cavoatrial cannula was introduced via the right atrial appendage. The right atrial appendage was systematically harvested allowing the venous cannulation before starting cardiopulmonary bypass and was immediately rinsed in ice-cold Buffer S solution.22 Excess buffer was removed and subendocardial myocardial layer was harvested. A sample was immediately frozen in liquid nitrogen. Right atrial biopsies were obtained from six patients treated with statins and eight patients not treated with statins (Supplementary material online, Table S1). We excluded patients who had coronary artery disease. Informed consent was obtained from all patients and the study was approved by the Institutional Ethical Review Board of Strasbourg University.
Human deltoid biopsies
We studied biopsies from deltoid muscles in five patients with statin-induced myopathy, i.e. patients who developed muscle weakness and/or rhabdomyolysis while treated with a statin, in whom muscle weakness and rhabdomyolysis regressed after statin treatment discontinuation, and in whom other causes of myopathy were excluded. Muscle biopsy parameters were compared with those of five untreated normal subjects who volunteered for the study (Supplementary material online, Table S2). All subjects signed a consent form approved by the Institutional Review Board of New Civil Hospital of Strasbourg.
Animal experiments
Experiments were performed on adult male Wistar rats (Depré, France) weighing ∼250–300 g. They housed in neutral temperature environment (22° ± 2°C) on a 12:12h photoperiod and were provided food and water ad libitum. Animal procedures were conducted in accordance with the Declaration of Helsinki and were approved by our local Ethic Committee.
Atorvastatin (Tahor®) was generously provided by Pfizer and quercetin was kindly provided by C. Damgé and D. Betbeder (Lille, France). After 1 week of acclimation, 32 rats were randomly divided into four groups as followed: (i) animals treated with water (CONT, n = 8); (ii) animals treated with atorvastatin (ATO, n = 8, 10 mg/kg/day); (iii) animals treated with quercetin (QUERC, n = 8, 25 mg/kg/day); and (iv) animals treated with atorvastatin and quercetin (QUERC + ATO; n = 8; 10 mg/kg/day and 25 mg/kg/day, respectively). Atorvastatin animals were treated with atorvastatin during 2 weeks by oral gavage and animals treated with quercetin began the treatment 5 days before atorvastatin treatment.
Statistical analysis
Data are presented as mean ± SEM. Statistical analyses were performed using Student's t-test, one-way repeated measures or two-way ANOVA followed by a Tukey post test in animal study (GraphPad Prism 5, Graph Pad Software, Inc., San Diego, CA, USA). Statistical significance was displayed as *P < 0.05 or **P< 0.01 or ***P < 0.001.
Results
Statins promoted mitochondrial function and mitochondrial biogenesis in human cardiac muscle
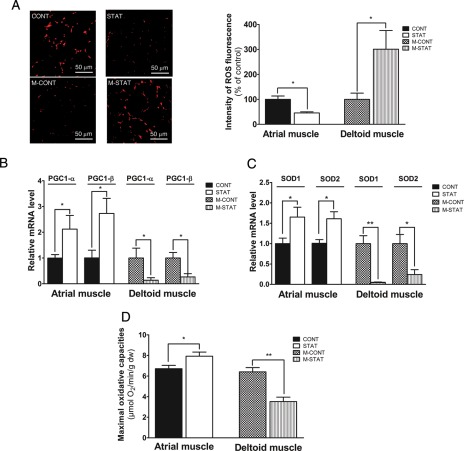
The patient's population underwent surgery for valvular aortic stenosis. The clinical characteristics are similar in both groups (Supplementary material online, Table S1). We studied different parameters in atrial muscles of 14 patients treated (n = 6, STAT group) or not (n = 8, CONT group) with statins. Dihydroethidium staining showed that ROS content was reduced in the STAT group (−51%, Figure 1A). Peroxisome proliferator-activated receptor gamma co-activator-1α and PGC-1β mRNA expression levels, both crucial transcriptional co-activators of numerous genes coding for mitochondrial proteins, were higher in the STAT group compared with the CONT group (Figure 1B). Cytosolic SOD1 and mitochondrial SOD2 mRNA levels were increased in cardiac muscle of patients treated with statins (Figure 1C). Maximal oxidative capacities (Vmax) were also increased in these patients (Figure 1D).
Figure 1.
Exploration of cardiac atrial muscle biopsies from patients treated (STAT group) or not (CONT group) with statins and of human muscle biopsies from patients with statin myopathy (M-STAT group) compared with control subjects (M-CONT group). (A) Representative pictures and quantification of reactive oxygen species fluorescence labelled with dihydroethidium. (B) PGC-1α and PGC-1β mRNA expression levels. (C) SOD1 and SOD2 mRNA expression levels. (D) Maximal mitochondrial respiration measured in the presence of ADP and glutamate/malate as substrates (Vmax). PGC-1α and -1β, peroxisome proliferator-activated receptor gamma co-activator 1 alpha and beta; SOD1, superoxide dismutase 1; SOD2, superoxide dismutase 2. Values were represented as mean ± SEM; n = 5–8 patients in each group, *P< 0.05; **P< 0.01 with unpaired t-test.
Mitochondrial function and biogenesis were impaired and reactive oxygen species production increased in muscle biopsies of patients with statin-induced myopathy
We analysed different parameters in deltoid muscles of patients (n = 5, M-STAT group) suffering from statin-induced myopathy as well as in age-matched control subjects (n = 5, M-CONT group). The plasmatic creatine kinase level was significantly higher in the M-STAT group compared with the M-CONT group (Supplementary material online, Table S2). Reactive oxygen species production measured with DHE staining was highly enhanced in M-STAT patients (Figure 1A). Peroxisome proliferator-activated receptor gamma co-activator-1α and PGC-1β (Figure 1B) as well as SOD1 and SOD2 mRNA levels (Figure 1C) were reduced in biopsies of these patients. Concerning muscular oxidative capacities, Vmax was largely lower in M-STAT patients (Figure 1D). Nevertheless, patient's population differs in term of statin treatment (Supplementary material online, Table S2). Then, in order to well characterize and to explore the mechanisms responsible of these alterations, we studied the effects of 2-week atorvastatin treatment with or without antioxidant treatment in rats.
Atorvastatin decreased total cholesterol in rats
Total cholesterol decreased by 20% in ATO and QUERC + ATO groups compared with CONT and QUERC groups (Supplementary material online, Figure S1A), whereas HDL-cholesterol level was not significantly different between groups (Supplementary material online, Figure S1B).
Atorvastatin treatment altered molecular pathways controlling mitochondrial biogenesis in muscles and antioxidant treatment abolished it
We explored the principal factors governing mitochondrial biogenesis mechanisms in two muscle types (oxidative cardiac and glycolytic skeletal muscles) after 2-week treatment with atorvastatin. A co-treatment with antioxidant quercetin was used in order to demonstrate the role played by ROS production. Expression of several genes implicated in mitochondrial biogenesis was analysed by q-RT-PCR (Supplementary material online, Table S3).
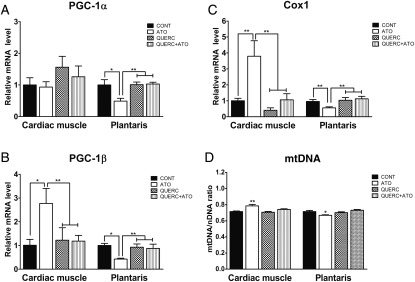
The peroxisome proliferator-activated receptor gamma co-activator-1α mRNA level was not modified in cardiac muscle but largely decreased in skeletal muscle after treatment with atorvastatin (Figure 2A). Expression of the co-activator PGC-1β mRNA which is a central factor controlling mitochondrial biogenesis was largely increased in cardiac muscle but significantly reduced in skeletal muscle after atorvastatin treatment (Figure 2B).
Figure 2.
Atorvastatin treatment altered molecular pathway of mitochondrial biogenesis in muscles and antioxidant treatment abolished it in rats. Peroxisome proliferator-activated receptor gamma co-activator-1α (A), peroxisome proliferator-activated receptor gamma co-activator-1β (B), cytochrome oxidase 1 mRNA expression levels (C) and relative amount of mtDNA determined by real-time PCR (D) in cardiac and plantaris muscles. Values were represented as mean ± SEM; n = 8; *P< 0.05; **P< 0.01; ***P< 0.001 between groups with a two-way ANOVA followed by a Tukey post test.
Cytochrome oxidase 1 (Cox1) mRNA expression implicated in mitochondrial metabolism and the nuclear respiratory factor 1 (NRF1) mRNA expression, which is implicated in mitochondrial biogenesis downstream from PGC-1α and PGC-1β, were both increased in cardiac muscle but were largely decreased in skeletal muscle after atorvastatin treatment (Figure 2C and Supplementary material online, Figure S2). Interestingly, when quercetin treatment was added to atorvastatin treatment, alterations of all these mRNA expression levels were abolished.
The relative amount of mitochondria in left ventricle and plantaris was determined by real-time PCR. Using Cytochrome b as the target gene for mtDNA and pyruvate kinase as the internal control for nuclear DNA, we showed that mtDNA content increased in cardiac muscle but was decreased in skeletal muscle after atorvastatin treatment (Figure 2D). Interestingly, when quercetin treatment was added to the atorvastatin treatment, alterations of mtDNA content were abolished.
These data suggested that atorvastatin activated the mechanisms of mitochondrial biogenesis and increased the mitochondrial mass in cardiac muscle but reduced mitochondrial biogenesis as well as mitochondrial mass in glycolytic skeletal muscle. These alterations were largely prevented by antioxidant treatment suggesting that modifications of redox potential could be responsible of the beneficial as well as deleterious effects of statins. In order to verify this hypothesis, we first explored the effects of statins as well as antioxidant treatment on ROS concentration in different muscle types.
Reactive oxygen species were reduced in myocardium, but largely increased in glycolytic skeletal muscle after chronic atorvastatin treatment
Total ROS production was measured in muscles by electron paramagnetic resonance (EPR). Interestingly, total ROS production was reduced in cardiac muscle of rats treated with atorvastatin and/or quercetin (Figure 3A). On the other hand, in glycolytic plantaris muscle treated with statin, total ROS production was increased and co-treatment with quercetin abolished this effect (Figure 3A).
Figure 3.
Reactive oxygen species were reduced in cardiac muscle of rats after chronic atorvastatin treatment, but were largely increased in glycolytic one. (A) Total reactive oxygen species production measured by electron paramagnetic resonance in cardiac and plantaris muscles. (B) Representative pictures and quantification of reactive oxygen species fluorescence labelled with dihydroethidium in cardiac and plantaris muscles. (C) GSH measurement in cardiac and plantaris muscles. (D) The relative mRNA level of mitochondrial superoxide dismutase2 in cardiac and plantaris muscles. Values were represented as mean ± SEM; n = 8; *P< 0.05; **P< 0.01; ***P< 0.001 between groups with a two-way ANOVA followed by a Tukey post test.
The dihydroethidium staining indicated that ROS concentration was decreased in cardiac muscle treated with atorvastatin and/or quercetin (Figure 3B). In plantaris muscle, ROS concentration was increased after statin treatment and this effect was abolished by antioxidant treatment (Figure 3B).
Antioxidant capacities were improved in cardiac muscle but importantly reduced in glycolytic skeletal muscle following atorvastatin treatment and antioxidant treatment prevented this reduction
GSH (reduced glutathione) was three-fold higher in cardiac muscle compared with glycolytic skeletal muscle (Figure 3C). In this skeletal muscle, atorvastatin reduced GSH content, but co-treatment with quercetin prevented this deleterious atorvastatin effect (Figure 3C). The superoxide dismutase (SOD2) mRNA level was increased in cardiac muscle after both treatments (Figure 3D) but in plantaris muscle, it decreased by approximately two-fold after atorvastatin treatment and co-treatment with quercetin prevented these alterations (Figure 3D).
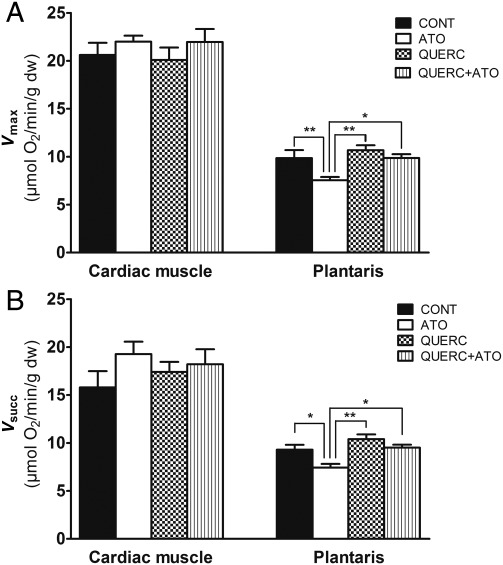
Quercetin protected mitochondrial respiration of glycolytic muscle against deleterious effects of atorvastatin in rats
Vmax and Vsucc were neither modified nor by statins nor by quercetin treatment or both in cardiac muscle (Figure 4A and B, respectively). In plantaris muscle, Vmax and Vsucc were lower in rats treated with atorvastatin, and co-treatment with quercetin prevented these deleterious effects (Figure 4A and B, respectively).
Figure 4.
Quercetin protected mitochondrial respiration of glycolytic muscle against deleterious effects of atorvastatin treatment in rats. Maximal mitochondrial respiration measured in the presence of ADP and glutamate/malate (Vmax; A) and succinate (Vsucc; B) as substrates in cardiac muscle and plantaris, respectively. Results were expressed as mean ± SEM; n = 8; *P< 0.05; **P< 0.01 between groups with a two-way ANOVA followed by a Tukey post test.
Atorvastatin-activated mitochondrial biogenesis and protected mitochondria by a reactive oxygen species-mechanism in H9C2 cardiomyocytes
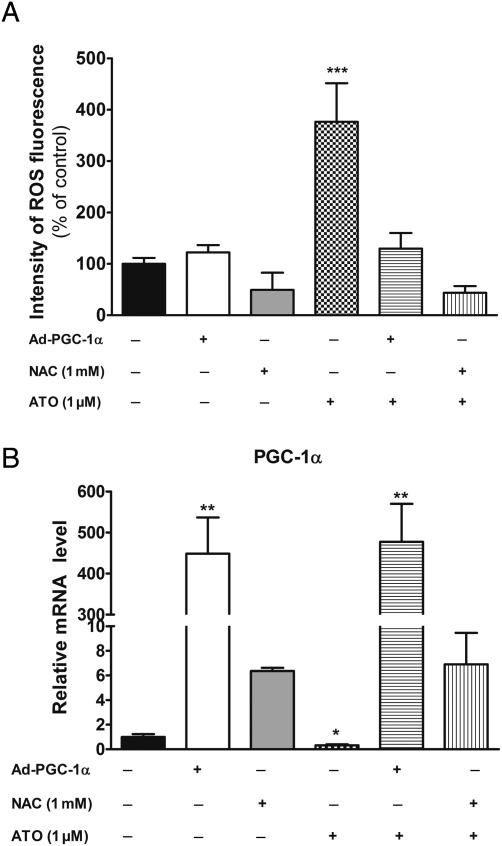
To further understand the mechanisms explaining cardiac beneficial effects of atorvastatin, we used a cell culture model. First, ROS production was evaluated with dihydroethidium staining method in H9C2 cardiomyocytes after acute exposure with atorvastatin (1 µmol/L) during 48 and 72h. Concerning short-term exposure (48h) with atorvastatin, ROS production was enhanced and when N-AcetylCysteine (NAC) (1 mmol/L), a ROS scavenger, was added it largely prevented this augmentation (Figure 5A). Peroxisome proliferator-activated receptor gamma co-activator-1α, NRF1, TFAm, and citrate synthase mRNA expression levels were significantly increased after atorvastatin incubation, and NAC abolished these effects (Figure 5B and Supplementary material online, Figure S2A–C, respectively).
Figure 5.
Atorvastatin induced expression of peroxisome proliferator-activated receptor gamma co-activator-1α mRNA expression levels by a mechanism implicating reactive oxygen species production in H9C2 cardiomyocytes. (A) Dihydroethidium staining in H9C2 cardiomyocytes treated with atorvastatin (1 µmol/L) during 48 and 72h in the presence or not of antioxidant molecule N-AcetylCysteine (1 mmol/L). (B) The mRNA expression level of peroxisome proliferator-activated receptor gamma co-activator-1α in different conditions. (C) The SOD2 mRNA level and protein concentration in different groups. (D) The TMRE-fluorescence intensity level in H9C2 cardiomyocytes exposed to doxorubicin (0.5 µmol/L) pre-treated or not with atorvastatin at different concentrations (0.5, 1, and 10 µmol/L). Values were represented as mean ± SEM; n = 4; *P < 0.05; **P < 0.01 atorvastatin condition vs. others groups in the same time incubation with a two-way ANOVA followed by a Tukey post test.
After 72h of exposure to atorvastatin (one day later), ROS concentration as well as mRNA levels of transcription factors implicated in mitochondrial biogenesis (PGC-1α, NRF1, TFAm) returned to their normal values. On the other hand, mRNA expression and protein concentration of SOD2, a key mitochondrial antioxidant enzyme regulated by PGC-1α, was largely increased and co-incubation with NAC abolished these effects. Altogether, our results suggested that statins initially increased ROS production, triggering activation of mitochondrial biogenesis pathways leading to mitochondrial adaptations (i.e. increased of antioxidant capacities), allowing to reduce the net ROS production.
Interestingly, we showed that these cellular adaptations following atorvastatin exposure at different concentrations (0.5, 1, and 10 µmol/L) protected H9C2 cardiomyocytes from doxorubicin treatment (Dox 0.5 µmol/L; Figure 5D). Indeed, Dox toxicity was characterized by a decrease of TMRE fluorescence intensity, suggesting a loss of mitochondrial membrane potential, and 48h of atorvastatin pre-treatment at different concentrations prevented this deleterious effect.
Statin impaired mitochondria by a reactive oxygen species mechanism in L6 myotubes
The same protocol was realized with skeletal myotubes differentiated from L6 myoblasts and divided in six groups. Control myotubes, myotubes incubated with NAC (1 mmol/L), or atorvastatin or both, myotubes infected with adenovirus encoding PGC-1α incubated or not with atorvastatin (1 µmol/L) during 48h. Acute atorvastatin treatment largely enhanced ROS production and when NAC was added to the medium, it largely decreased this production. Overexpression of PGC-1α in myotubes also abolished ROS augmentation following acute treatment with atorvastatin (Figure 6A). Interestingly, PGC-1α mRNA expression decreased after atorvastatin incubation, but NAC abolished these alterations (Figure 6B). As expected, PGC-1α overexpression efficiently increased PGC-1α mRNA expression (Figure 6B).
Figure 6.
Atorvastatin decreased peroxisome proliferator-activated receptor gamma co-activator-1α mRNA expression levels due to the increase of reactive oxygen species level in L6 Woody myotubes. L6 myotubes were infected or not with adenovirus encoding peroxisome proliferator-activated receptor gamma co-activator-1α and were treated or not with atorvastatin (1 µmol/L) as well as of N-AcetylCysteine (1 mmol/L). We measured (A) Dihydroethidium staining, and (B) peroxisome proliferator-activated receptor gamma co-activator-1α mRNA expression in the different groups. Values were represented as mean ± SEM; n = 3; *P < 0.05 compared with all others conditions; **P < 0.01 compared with all other conditions with a one-way ANOVA followed by a Dunett post test.
In vitro exposure to atorvastatin increased mitochondrial H2O2 and reactive oxygen species production lightly in cardiac but largely in glycolytic fibres of rats
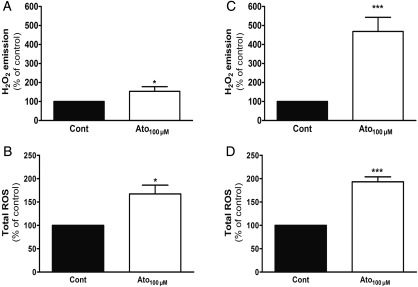
We measured the in vitro capacity of atorvastatin to directly induce mitochondrial ROS in function of muscle phenotype. During the H202 measurement, atorvastatin (100 µmol/L) was added in the presence of muscle fibres from rat cardiac and plantaris muscles. Interestingly, atorvastatin enhanced mitochondrial H2O2 production in cardiac muscle (+53%, Figure 7A), whereas this production increased robustly in plantaris (+368%, Figure 7C) with an increase significantly higher in skeletal muscle compared with myocardium.
Figure 7.
In vitro atorvastatin increased slightly reactive oxygen species production in cardiac muscle, whereas it highly increased in plantaris muscle. Mitochondrial H2O2 emission in permeabilized fibres and total reactive oxygen species production measured by electron paramagnetic resonance were evaluated in cardiac (A and B, respectively) and plantaris muscles (C and D, respectively) after addition of 100µmol/L atorvastatin. Values represented in percentage of control; n = 7–10, *P< 0.05, and ***P< 0.001 vs. control with paired t-test.
By EPR, we also showed that in vitro atorvastatin (100µmol/L) enhanced total ROS production more in plantaris (+93%; Figure 7D) than in cardiac muscle (+67%; Figure 7B).
Discussion
This study provides two major findings: (i) in human as well as in rat, statins triggered transcriptional activation of mitochondrial biogenesis in myocardium (increase of PGC-1α and -β expression levels) as well as antioxidant capacities via a mechanism implicating ROS signalling pathway, (ii) in skeletal muscle of patients with statin-induced myopathy as well as in rat skeletal muscle with low antioxidant capacities, these molecules induced high-oxidative stress, responsible of transcriptional deactivation of mitochondrial biogenesis as well as mitochondrial dysfunctions. Altogether, we showed that statins increased ROS production differently in heart and skeletal muscle and could be represent as a double-edged sword: they are beneficial by playing an important role in cell signalling involved in antioxidant defence network in cardiac muscle, but could be harmful by inducing excessive oxidative stress in vulnerable skeletal muscle.21 We proposed that activation of PGC-1 expression could be triggered by low doses of mitochondrial ROS generation following atorvastatin treatment, in order to counteract stressor challenges and hence re-establish homeostasis. This phenomenon was called mitochondrial hormesis or ‘mitohormesis’.13,20 Our data suggested that statins could act through this mitohormesis mechanism.
With atrial tissues obtained during coronary bypass surgery of patients treated or not with statins, we studied the mitochondrial respiration in skinned atrial fibres.22 Interestingly, maximal mitochondrial respiration as well as genes expression of antioxidant enzymes and factors controlling mitochondrial biogenesis was higher in myocardium of treated patients. In particular, our results showed in human cardiac muscle that statins treatment activated PGC1-α and -β which were considered as the major regulators of several crucial aspects of energy metabolism.23,24 By increasing these central factors, statins could strengthen human cardiac muscle defence against metabolic stress by improving antioxidant defence, which seems to be the case since expression of SOD1 and SOD2 were higher in atrium of patients treated with statins. However, in deltoid biopsies of patients with statin-induced myopathy, mitochondrial function was impaired together with an abnormally high induction of ROS production. In addition, PGC-1α and PGC-1β mRNA expressions were down-regulated suggesting deactivation of mitochondrial biogenesis signalling pathway. Nevertheless, patient's population differs in term of statin treatment and treatment associated with statins (Supplementary material online, Table S1).These results are in accordance with some studies showing that skeletal muscle mitochondria are impaired after statins treatment or in acute conditions7,9 and give a molecular details of these muscular abnormalities. Sirvent et al.7 showed that simvastatin-induced mitochondrial impairment resulting from inhibition of the respiratory chain complex I in human skeletal muscle. Concerning genes expression, a recent study showed in skeletal muscle from statin-treated but non-myopathic patients, that expression of genes responsible for mitochondrial function was unaffected, suggesting that regulation of mitochondrial biogenesis was specifically impaired in skeletal muscle of patients with statin-induced myopathy.25
In order to clearly demonstrate and explain why and how statins could induce opposite effects in muscles with different phenotypes (i.e. cardiac vs. skeletal muscle), we carried out several experiments with animal and cell models. In particular, we studied the role played by muscular ROS production as well as the role played by co-factors PGC-1α and PGC-1β in the mechanisms of muscular mitochondrial adaptations and impairments following statin treatment.
In cardiac muscle of rats, chronic treatment with atorvastatin activated PGC-1β expression more that PGC-1α, improved antioxidant capacities and decreased cellular ROS concentration, confirming the effects observed in human patients treated with statins. Our results suggested that in rat, statins changed the constitutive mitochondrial biogenesis level in order to change qualitatively more than quantitatively the mitochondrial properties, particularly for improving mitochondrial ROS-detoxifying capacities. Because of the volume occupied by mitochondria in rat cardiomyocytes (∼30% in adult) and the extremely low cytosolic volume (∼4–7%), it is clear that the increase of mitochondrial mass will automatically be at the expense of myofibrillar volume, thus compromising contractile function.19 Consequently, increase of mitochondrial mass will be low and strictly controlled in adult heart of rats. On the other hand, in human, the metabolic rate was considerably lower than in rat and it seems to be possible to enlarge the mitochondrial mass. Then, we can speculate that in human, an increase of both PGC-1α and β was necessary in order to highly improve quantitatively as well as qualitatively the mitochondrial function in myocardium (i.e. an increase of mitochondrial volume as well as antioxidant capacities). In rats, we can postulate that activation of PGC-1β expression, which is known to preferentially induce genes involved in the removal of ROS,26,27 is essential in order to improve mitochondrial ROS-detoxifying capacities of the myocardium and to slightly increase mitochondrial mass.
Interestingly, when cardiomyocytes in culture or permeabilized cardiac fibres were acutely exposed to atorvastatin, it induced an increase of mitochondrial ROS production, whereas after 3 days in culture or 2-week treatment in rats, statins increased SOD2 protein concentration and reduced basal ROS concentration in cardiac muscle. These results suggest that it's the initial augmentation of ROS production following statins treatment which could trigger the improvement of antioxidant capacities and mitochondrial function allowing to reduced the basal ROS concentration in the following weeks. In order to verify these hypothesize, we used an antioxidant molecule in addition to atorvastatin treatment and tested it in our in vivo and in vitro models (quercetin and NAC treatments, respectively). We found that these treatments, by reducing the initial augmentation of ROS, prevented the enhancement of PGC1 expression as well as mRNA expression of antioxidant enzymes in cardiac cells. Altogether, we found that atorvastatin increased ROS production which initiated expression of PGC-1α and -β, leading to mitochondrial biogenesis and the augmentation of oxidative capacities in cardiac muscle. We can speculate that statins, by a PGC-1s dependant-mechanism, could play a key role in the ROS homeostatic cycle in cardiac muscle.
Next step was to determine whether atorvastatin treatment were able to protect cardiomyocytes from a chemical stress. Toxic effects of doxorubicin were explored in cardiomyocytes. Our results showed that atorvastatin incubation prevented mitochondrial cardiotoxicity by reducing the loss of mitochondrial membrane potential and by inducing mitochondrial biogenesis pathways. This is in accordance with studies showing that statins have antioxidant properties28 and attenuated mitochondrial membrane depolarization after exposure to oxidative stress in cardiac myocytes.6 Then, statins treatment, by inducing mitochondrial adaptations, reduced metabolic toxic effects of this molecule frequently used in clinical setting.
In glycolytic skeletal muscle with low glutathione content, statins robustly increased production of mitochondrial ROS in vitro as well as in vivo confirming the effects observed in deltoid biopsies of patients with statin-induced myopathy. These results and the fact that antioxidant treatments prevented skeletal muscle from deleterious effects of statins, suggested that high-oxidative stress could be the triggering factor inducing mitochondrial dysfunction and down-regulation of mitochondrial biogenesis mechanisms. Moreover, when PGC-1α was overexpressed in L6 myotubes, ROS production was decreased and deleterious effects were prevented, showing the central role played by this transcriptional co-factor in order to prevent deleterious effect of statins. Interestingly, the fact that quercetin treatment which is a powerful and natural antioxidant molecule,29 protect mitochondria of skeletal muscle in animals, suggest that it is possible to find therapeutical strategies consisting of reducing oxidative stress in skeletal muscle of patients with statin-associated myopathy.
In conclusion, our study clarifies contradictory studies from the literature showing beneficial effects of statins in cardiac muscle while several studies demonstrated deleterious effects in skeletal muscle.8 Our results suggest that statins acted through a ‘mitohormesis mechanism’ and protected cardiac muscles, by stimulating mitochondrial biogenesis and antioxidant defence through ROS-induced PGC-1 family expression (Figure 8). Conversely, when ROS-detoxifying constituents were not sufficient to decrease the initial statin induced ROS production, as in skeletal muscle of rat and in deltoid muscle of some patients, statins could be toxic for mitochondria but an adequate antioxidant therapy should prevent these muscular impairments.
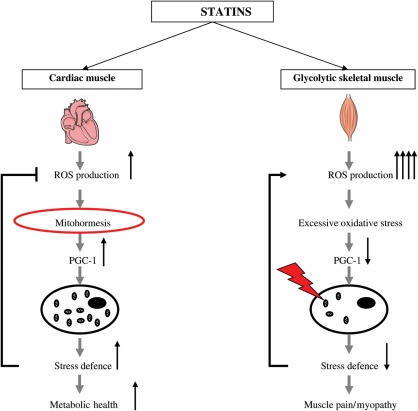
Figure 8.
Scheme illustrating the action of statins on mitochondrial function according to muscular phenotype. Statins acted through a ‘mitohormesis mechanism’ and protected oxidative cardiac muscle, by stimulating the mitochondrial biogenesis through mild oxidative stress and improved metabolic health. Conversely, when reactive oxygen species-detoxifying constituents were not sufficient to decrease the initial statin induced high-oxidative stress in glycolytic skeletal muscle, they induced mitochondrial dysfunctions, down-regulation of mitochondrial biogenesis and muscular pains or myopathy.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by grants of the Ecole Polytechnique Fédérale de Lausanne, the Swiss National Science Foundation, Université de Strasbourg, NIH (DK59820), and EU Ideas programme (Sirtuins; ERC-2008-AdG-23118).
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
We thank and extend sincere appreciation to patients for participation in this study. We thank Auwerx laboratory including M. Lagouge and J. Feige, and Metzger laboratory (IGBMC, Illkirch, France), including D. Duteil and C. Chambon for technical help for q-RT-PCR. A.-M. Roussel and I. Hininger (INSERM U884, Grenoble, France) are acknowledged for technical assistance concerning glutathione measurement. We are grateful to Fabrice Favret, I. Bentz, F. Goupilleau, S. Gaouar and D. Fumagalli for technical assistance. We thank C. Damgé and D. Betbeder (EA 2689, Lille, France) for providing quercetin. We are grateful to the Cardiovascular Operated Patients Association (ONCOVAS) for its help in funding the study.
References
- 1.Staffa JA, Chang J, Green L. Cerivastatin and reports of fatal rhabdomyolysis. N Engl J Med. 2002;346:539–540. doi: 10.1056/NEJM200202143460721. doi:10.1056/NEJM200202143460721. [DOI] [PubMed] [Google Scholar]
- 2.Jasinska M, Owczarek J, Orszulak-Michalak D. Statins: a new insight into their mechanisms of action and consequent pleiotropic effects. Pharmacol Rep. 2007;59:483–499. [PubMed] [Google Scholar]
- 3.Giordano N, Senesi M, Mattii G, Battisti E, Villanova M, Gennari C. Polymyositis associated with simvastatin. Lancet. 1997;349:1600–1601. doi: 10.1016/S0140-6736(05)61628-5. doi:10.1016/S0140-6736(05)61628-5. [DOI] [PubMed] [Google Scholar]
- 4.Bonetti PO, Lerman LO, Napoli C, Lerman A. Statin effects beyond lipid lowering—are they clinically relevant? Eur Heart J. 2003;24:225–248. doi: 10.1016/s0195-668x(02)00419-0. doi:10.1016/S0195-668X(02)00419-0. [DOI] [PubMed] [Google Scholar]
- 5.Echaniz-Laguna A, Mohr M, Tranchant C. Neuromuscular symptoms and elevated creatine kinase after statin withdrawal. N Engl J Med. 2010;362:564–565. doi: 10.1056/NEJMc0908215. doi:10.1056/NEJMc0908215. [DOI] [PubMed] [Google Scholar]
- 6.Jones SP, Teshima Y, Akao M, Marban E. Simvastatin attenuates oxidant-induced mitochondrial dysfunction in cardiac myocytes. Circ Res. 2003;93:697–699. doi: 10.1161/01.RES.0000097262.21507.DF. doi:10.1161/01.RES.0000097262.21507.DF. [DOI] [PubMed] [Google Scholar]
- 7.Sirvent P, Bordenave S, Vermaelen M, Roels B, Vassort G, Mercier J, Raynaud E, Lacampagne A. Simvastatin induces impairment in skeletal muscle while heart is protected. Biochem Biophys Res Commun. 2005;338:1426–1434. doi: 10.1016/j.bbrc.2005.10.108. doi:10.1016/j.bbrc.2005.10.108. [DOI] [PubMed] [Google Scholar]
- 8.Sirvent P, Mercier J, Lacampagne A. New insights into mechanisms of statin-associated myotoxicity. Curr Opin Pharmacol. 2008;8:333–338. doi: 10.1016/j.coph.2007.12.010. doi:10.1016/j.coph.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Kaufmann P, Torok M, Zahno A, Waldhauser KM, Brecht K, Krahenbuhl S. Toxicity of statins on rat skeletal muscle mitochondria. Cell Mol Life Sci. 2006;63:2415–2425. doi: 10.1007/s00018-006-6235-z. doi:10.1007/s00018-006-6235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bindoff L. Mitochondria and the heart. Eur Heart J. 2003;24:221–224. doi: 10.1016/s0195-668x(02)00694-2. doi:10.1016/S0195-668X(02)00694-2. [DOI] [PubMed] [Google Scholar]
- 11.Brookes PS. Mitochondrial H(+) leak and ROS generation: an odd couple. Free Radic Biol Med. 2005;38:12–23. doi: 10.1016/j.freeradbiomed.2004.10.016. doi:10.1016/j.freeradbiomed.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Lecarpentier Y. Physiological role of free radicals in skeletal muscles. J Appl Physiol. 2007;103:1917–1918. doi: 10.1152/japplphysiol.01047.2007. doi:10.1152/japplphysiol.01047.2007. [DOI] [PubMed] [Google Scholar]
- 13.Sano M, Fukuda K. Activation of mitochondrial biogenesis by hormesis. Circ Res. 2008;103:1191–1193. doi: 10.1161/CIRCRESAHA.108.189092. doi:10.1161/CIRCRESAHA.108.189092. [DOI] [PubMed] [Google Scholar]
- 14.Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res. 2008;103:1232–1240. doi: 10.1161/01.RES.0000338597.71702.ad. doi:10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michelakis ED, Wilkins MR, Rabinovitch M. Emerging concepts and translational priorities in pulmonary arterial hypertension. Circulation. 2008;118:1486–1495. doi: 10.1161/CIRCULATIONAHA.106.673988. doi:10.1161/CIRCULATIONAHA.106.673988. [DOI] [PubMed] [Google Scholar]
- 16.Mettauer B, Zoll J, Sanchez H, Lampert E, Ribera F, Veksler V, Bigard X, Mateo P, Epailly E, Lonsdorfer J, Ventura-Clapier R. Oxidative capacity of skeletal muscle in heart failure patients versus sedentary or active control subjects. J Am Coll Cardiol. 2001;38:947–954. doi: 10.1016/s0735-1097(01)01460-7. doi:10.1016/S0735-1097(01)01460-7. [DOI] [PubMed] [Google Scholar]
- 17.Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J Biol Chem. 2007;282:194–199. doi: 10.1074/jbc.M606116200. doi:10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- 18.Seachrist JL, Loi CM, Evans MG, Criswell KA, Rothwell CE. Roles of exercise and pharmacokinetics in cerivastatin-induced skeletal muscle toxicity. Toxicol Sci. 2005;88:551–561. doi: 10.1093/toxsci/kfi305. doi:10.1093/toxsci/kfi305. [DOI] [PubMed] [Google Scholar]
- 19.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. doi:10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 20.Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. doi:10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Gems D, Partridge L. Stress-response hormesis and aging: “that which does not kill us makes us stronger”. Cell Metab. 2008;7:200–203. doi: 10.1016/j.cmet.2008.01.001. doi:10.1016/j.cmet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Seppet E, Eimre M, Peet N, Paju K, Orlova E, Ress M, Kovask S, Piirsoo A, Saks VA, Gellerich FN, Zierz S, Seppet EK. Compartmentation of energy metabolism in atrial myocardium of patients undergoing cardiac surgery. Mol Cell Biochem. 2005;270:49–61. doi: 10.1007/s11010-005-3780-y. doi:10.1007/s11010-005-3780-y. [DOI] [PubMed] [Google Scholar]
- 23.Zheng Z, Chen H, Ke G, Fan Y, Zou H, Sun X, Gu Q, Xu X, Ho PC. Protective effect of perindopril on diabetic retinopathy is associated with decreased vascular endothelial growth factor-to-pigment epithelium-derived factor ratio: involvement of a mitochondria-reactive oxygen species pathway. Diabetes. 2009;58:954–964. doi: 10.2337/db07-1524. doi:10.2337/db07-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Wong CW. Statins enhance peroxisome proliferator-activated receptor gamma coactivator-1alpha activity to regulate energy metabolism. J Mol Med (Berlin, Germany) 2010;88:309–317. doi: 10.1007/s00109-009-0561-1. [DOI] [PubMed] [Google Scholar]
- 25.Draeger A, Sanchez-Freire V, Monastyrskaya K, Hoppeler H, Mueller M, Breil F, Mohaupt MG, Babiychuk EB. Statin therapy and the expression of genes that regulate calcium homeostasis and membrane repair in skeletal muscle. Am J Pathol. 2010;177:291–299. doi: 10.2353/ajpath.2010.091140. doi:10.2353/ajpath.2010.091140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lelliott CJ, Medina-Gomez G, Petrovic N, Kis A, Feldmann HM, Bjursell M, Parker N, Curtis K, Campbell M, Hu P, Zhang D, Litwin SE, Zaha VG, Fountain KT, Boudina S, Jimenez-Linan M, Blount M, Lopez M, Meirhaeghe A, Bohlooly YM, Storlien L, Stromstedt M, Snaith M, Oresic M, Abel ED, Cannon B, Vidal-Puig A. Ablation of PGC-1beta results in defective mitochondrial activity, thermogenesis, hepatic function, and cardiac performance. PLoS Biol. 2006;4:e369. doi: 10.1371/journal.pbio.0040369. doi:10.1371/journal.pbio.0040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. doi:10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 28.Davignon J, Jacob RF, Mason RP. The antioxidant effects of statins. Coron Artery Dis. 2004;15:251–258. doi: 10.1097/01.mca.0000131573.31966.34. doi:10.1097/01.mca.0000131573.31966.34. [DOI] [PubMed] [Google Scholar]
- 29.Bors W, Michel C, Saran M. Flavonoid antioxidants: rate constants for reactions with oxygen radicals. Methods Enzymol. 1994;234:420–429. doi: 10.1016/0076-6879(94)34112-5. doi:10.1016/0076-6879(94)34112-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.