Abstract
Abrupt osmotic changes during rapid correction of chronic hyponatremia result in demyelinative brain lesions, but the sequence of events linking rapid osmotic changes to myelin loss is not yet understood. Here, in a rat model of osmotic demyelination syndrome, we found that massive astrocyte death occurred after rapid correction of hyponatremia, delineating the regions of future myelin loss. Astrocyte death caused a disruption of the astrocyte-oligodendrocyte network, rapidly upregulated inflammatory cytokines genes, and increased serum S100B, which predicted clinical manifestations and outcome of osmotic demyelination. These results support a model for the pathophysiology of osmotic brain injury in which rapid correction of hyponatremia triggers apoptosis in astrocytes followed by a loss of trophic communication between astrocytes and oligodendrocytes, secondary inflammation, microglial activation, and finally demyelination.
Adapting to anisosmolarity and changes in osmolarity is essential for cell survival.1 In humans, hyponatremia is the most common electrolyte disorder and is associated with high morbidity and mortality.2,3 Interestingly, if chronic hyponatremia is corrected too quickly, there is a risk of osmotic demyelination syndrome (ODS), which can be fatal.4,5 Osmotic demyelination syndrome is a severe neurologic disorder that is characterized by discrete demyelinative lesions in the brain. The occurrence of these lesions in the pons is called central pontine myelinolysis, and the development of lesions outside the pons is known as extrapontine myelinolysis. Although the mechanisms that link rapid correction of chronic hyponatremia to ODS are poorly understood, several lines of evidence have suggested that the regulation of brain ionic strength may be defective or inefficient during rapid correction of chronic hyponatremia.6,7 During chronic hyponatremia, the brain is maintained at a nearly constant volume at the expense of potassium and organic osmolytes (osmotically active small molecules). When chronic hyponatremia is corrected too rapidly, the concentration of brain sodium increases beyond normal levels. In addition, the reaccumulation of organic osmolytes occurs at a much slower rate than for sodium. Therefore, the overshooting of the optimal sodium concentration in the brain occurs in the context of depleted organic osmolytes.7 Interestingly, regional differences in organic osmolyte reaccumulation have been associated with particular patterns of demyelination in ODS,8 but the mechanisms connecting impaired osmolyte reaccumulation, myelin loss, and the cells involved in this process remain elusive.
Studies have suggested that astrocytes are the primary cells that are responsible for regulating brain water content and organic osmolyte homeostasis. Astrocytes are in close contact with blood vessels, and pure astroglial cultures have shown that these cells are implicated in the regulation of organic osmolyte fluxes during anisosmotic conditions.9,10
A wealth of evidence has established the central role of astrocytes in the development and maintenance of normal myelin in the central nervous system (CNS),11–13 and astrocyte pathology is well recognized in many demyelinating diseases.14–16 Because astrocytes can modulate the concentration of organic osmolytes in the brain and regulate myelin integrity, we hypothesized that these cells might contribute to the pathophysiology of osmotic-induced demyelination. These experiments were designed to test this hypothesis in a rat model of demyelination induced by rapid correction of chronic hyponatremia.
RESULTS
Serum Sodium Values before and after Hyponatremia Correction
Administration of DDAVP (1-desamino-8-D-arginine vasopressin) and a liquid diet resulted in severe hyponatremia in all experimental groups (Supplemental Tables S1 through S5). Correction of hyponatremia with intraperitoneal hypertonic saline resulted in a significant increase in serum sodium (SNa) at 24 hours postinjection.
Region-specific Astrocyte Death Occurs Early after Rapid Correction of Hyponatremia
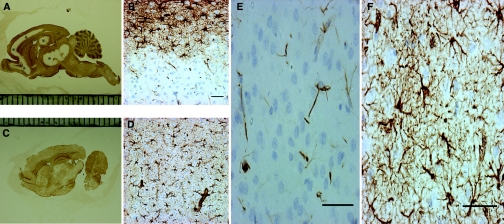
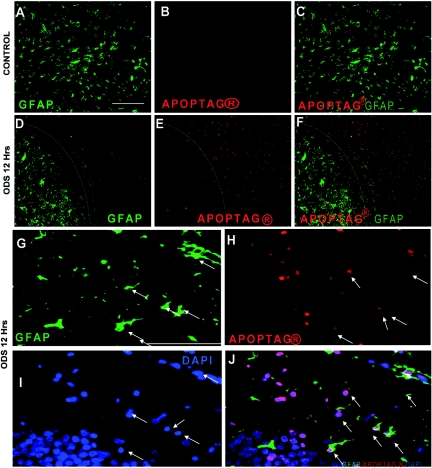
We studied the distribution of the astrocyte marker glial fibrillary acidic protein (GFAP) using immunohistochemistry (IHC) in the brains of rats after rapid correction of chronic hyponatremia, and we observed a profound postcorrection loss of GFAP. Areas of GFAP loss were sharply demarcated from normal brain regions, and no GFAP loss was observed in uncorrected rats (Figure 1, A through E). Because dehydration can induce a selective and reversible loss of GFAP in the rat hypothalamus,17 there was a possibility that the changes observed in the GFAP immunostaining reflected the plasticity of GFAP instead of cell death. To resolve this issue, we performed IHC for two other astrocytic markers, aquaporin 4 and serum calcium binding protein 100 B (S100B), and we observed the same pattern of antigen reactivity loss (Supplemental Figure S1). These data suggest the presence of extensive astrocyte damage as opposed to plastic adaptation of viable astrocytes during hyponatremia correction. To prove this hypothesis, we analyzed apoptosis using DNA fragmentation IHC (Apoptag®) and found intense apoptotic signals in the region of astrocyte loss 12 hours after ODS induction (Figure 2, E, F, and H). Interestingly, there was no evidence of apoptosis in uncorrected rats (Figure 2, B and C). In corrected rats, apoptotic signals were observed in all areas of GFAP loss, and Apoptag® colocalized with dying GFAP-positive astrocytes (Figure 2J). Thus, the disappearance of astrocyte-specific proteins (i.e. GFAP, aquaporin 4, and S100B) and the increase in DNA fragmentation in GFAP-positive cells indicate that GFAP loss reflected apoptotic astrocyte death after rapid correction of chronic hyponatremia.
Figure 1.
Rapid correction of chronic hyponatremia induces glial fibrillary acidic protein (GFAP) loss. In (A), immunohistochemistry for GFAP in rats 2 days after hyponatremia correction reveals a salient lack of immunoreactivity in the cortical regions, hippocampus, and basal ganglia (marked with *). At a higher magnification, in (B) the cortex of corrected rats shows a crisp demarcation between the area of GFAP reactivity and the region negative for GFAP. This contrasts with the normal GFAP (C and D) staining that was observed in uncorrected rats. In corrected rats, regions lacking macroscopic GFAP staining did not display any GFAP-positive cells (E) and only a limited amount of immunoreactive cellular debris. In contrast, as seen in (F), uncorrected control rats show a homogenous, star-shaped cellular GFAP staining pattern. The scale bar is 50 μm.
Figure 2.
Astrocyte apoptosis occurs early in osmotic demyelination syndrome (ODS). Double immunofluorescence for glial fibrillary acidic protein (GFAP) (green) and DNA damage (Apoptag® in red) showing homogenous GFAP staining in uncorrected control rats with no apoptotic cells (A through C). In contrast, 12 hours after correction of hyponatremia (D), a crisp demarcation (white line) distinguishes the astrocyte-depleted region from the adjacent regions containing undisturbed astrocytes. As shown in (E) and (F), the astrocyte-depleted region is strongly positive for DNA damage and also displays a clear delineation from the normal, nonapoptotic region (white line). At higher magnification, degenerating astrocytes stain positive for GFAP (G) but have lost their characteristic shape. Many apoptotic nuclei (H) also show bright staining for 4′,6′-diamino-2-phenylindole (DAPI) (I), and in (J) these cells colocalize with GFAP-positive cells (arrows). The scale bar is 100 μm.
Reactive Astrocyte Hypertrophy Occurs around Regions of Astrocyte Death in ODS
Astrocyte hypertrophy and proliferation, which is termed gliosis, have been associated with several models of CNS injury. Gliosis increases the production of GFAP and can easily be experimentally detected using IHC or quantitative reverse transcription (RT)-PCR.18 After rapid correction of hyponatremia, we observed that GFAP-positive astrocytes surrounding the lesions appeared hypertrophic and had thicker extensions compared with astrocytes in hyponatremic or normonatremic rats (Supplemental Figure S2, A through C). These changes were observed as early as 12 hours but were more prominent 24 hours to 6 days postcorrection. To quantify the gliosis detected by IHC after hyponatremia correction, we utilized the recently described astrocyte transcriptome, which demonstrated the high sensitivity and specificity of the aldehyde dehydrogenase 1 family, member L1 (Aldh1L1) for astrocytic lineage.19 We studied the expression of GFAP and Aldh1L1 in hyponatremic rats and corrected rats 12 and 24 hours after ODS induction. In accordance with the increased GFAP expression observed in the surrounding gliotic astrocytes, we found that GFAP mRNA levels remained stable at 12 hours and increased at 24 hours posthyponatremia correction (Supplemental Figure S2D). In contrast, Aldh1L1 transcript levels decreased significantly at 12 and 24 hours after ODS induction (Supplemental Figure S2E). Because all GFAP-positive astrocytes also express Aldh1L1, the ratio between the transcript levels of GFAP and Aldh1L1 should indicate the extent to which GFAP mRNA is upregulated in viable astrocytes of normonatremic, hyponatremic, and injured rats. Compared with normonatremic rats, we found that the GFAP/Aldh1L1 mRNA ratio was 1.5 and 3 times higher at 12 and 24 hours postcorrection, respectively (P < 0.05 and P < 0.001), whereas no changes were observed in uncorrected controls (Supplemental Figure S2F). These results indicate that first, there is no change in GFAP expression during chronic hyponatremia and, second, consistent with previous observations,20 they demonstrate that after rapid correction of hyponatremia, the remaining viable astrocytes underwent reactive gliosis, which was indicated by GFAP induction.
Astrocyte Death Precedes Myelin Loss in a Rat Model of ODS
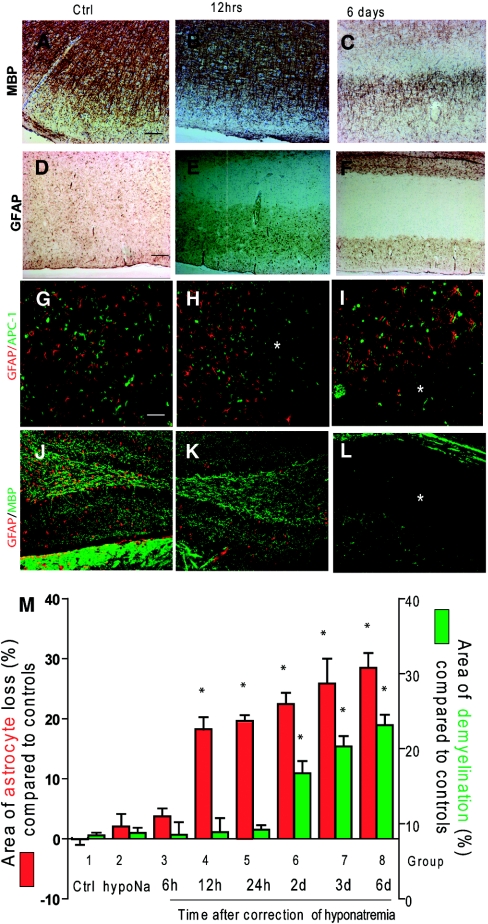
Because we observed that astrocyte death occurred in regions reported to be preferentially affected by demyelination in ODS, we wanted to investigate the temporal relationship between astrocyte death and myelin damage after rapid correction of hyponatremia. Therefore, we analyzed and quantified astrocyte death and myelin loss from 12 hours to 6 days postcorrection. As previously reported,4,21,22 the first indications of demyelination were observed 48 to 72 hours after hyponatremia correction (Figure 3, A through C and M). Conversely, GFAP staining revealed a gradual pattern of astrocyte loss starting at 12 hours and extending to 6 days postcorrection (Figure 3, D through F and M). At the earliest indication of astrocyte death (12 and 24 hours postcorrection), no myelin or oligodendrocyte loss was detected, which suggests that astrocyte loss precedes demyelination (Figure 3, G through L and M). Within 6 days after hyponatremia correction, all demyelinated regions in rapidly corrected rats displayed a striking loss of astrocytes; however, no demyelination was found in areas with normal-looking astrocytes. This supports the hypothesis that myelin loss only occurs in regions where astrocyte loss has already occurred (Figure 3, I and L).
Figure 3.
Loss of astrocytes occurs before demyelination after excessive correction of chronic hyponatremia. Myelin labeling (anti-MBP) in the cortex of uncorrected hyponatremic rats (A) and 12 hours (B) postcorrection did not show any reduction of MBP staining in uncorrected rats or 12 hours after hyponatremia correction. However, at 6 days postcorrection (C), myelin staining was reduced in the subcortical regions. Homogenous GFAP staining was found in uncorrected controls (D), but there was a marked loss of subcortical GFAP staining as early as 12 hours after the correction (E) that persisted for up to 6 days postcorrection (F). Double immunofluorescence detection of astrocytes (GFAP in red) and oligodendrocytes (adenomatous polyposis coli (APC) clone CC1 in green) shows a normal density of astrocytes and oligodendrocytes in uncorrected control rats (G). Astrocyte loss occurred 12 hours after the correction of hyponatremia (*) without evident oligodendrocyte death (H), whereas both astrocytes and oligodendrocytes were lost 6 days postcorrection, which is shown in the lower half of the image (*) (I). In (J) and (K), double immunofluorescence showing the preservation of myelin (MBP in red) in the hippocampus at 12 hours postcorrection despite astrocyte death (GFAP in green). Six days after correction (L), myelin loss had occurred in the astrocyte-depleted regions (*). The scale bar is 50 μm. In the graph (M), astrocyte loss (GFAP-negative brain surface area in green) and demyelination (MBP-negative brain surface area in red) were quantified after hyponatremia correction by computer-assisted image analysis, showing the progression of astrocyte and myelin damage over the 6 days after correction of serum sodium. Each group represents a different time point postcorrection. (*, P < 0.001 compared with controls, ANOVA test, n = 6 for each group). Ctrl, control; GFAP, glial fibrillary acidic protein; MBP, myelin basic protein; hypoNa, hyponatremia.
Astrocyte Death Does Not Involve Activated Microglia, Lymphocyte, or Neutrophil Invasion
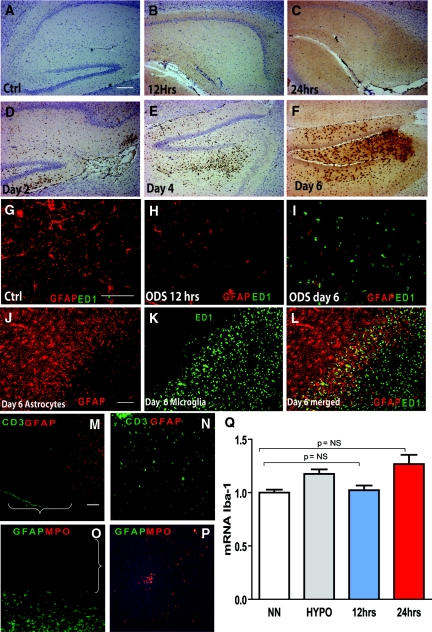
Microglial activation occurs during ODS, but the contribution of microglia in the genesis of ODS lesions is unclear.23 Once activated, microglia release proinflammatory cytokines and free radicals that can contribute to astrocyte death;24 therefore, astrocyte death in ODS may occur after microglial activation. We reasoned that if activated microglia played a role in astrocyte loss, microglial cells should be present at the earliest indication of astrocyte death. To test this hypothesis, we used IHC to detect ectodermal dysplasia 1 (ED1) expression, which is a marker of activated microglia/macrophages. Interestingly, no ED1-positive cells were detected 12 hours after the correction of hyponatremia (Figure 4, B and H). We found that microglial activation did not occur until 2 days postcorrection (Figure 4, A through F and I), which was confirmed by analyzing the expression of another well-described marker of microglial activation, ionizing calcium-binding adaptor molecule (Iba1). Compared with normonatremic controls, RT-PCR did not reveal any difference in Iba1 gene expression 12 hours after hyponatremia correction (Figure 4Q). After correction, infiltrating microglia was primarily observed in the regions of GFAP loss (Figure 4, I through L). Using the same techniques, we did not find any evidence of lymphocytes or neutrophils invasion during ODS at any of the time points assayed after hyponatremia correction (Figure 4, M through P).
Figure 4.
Loss of astrocytes is independent of microglial activation and lymphocytes/neutrophils infiltration. No activated microglial cells (anti-CD68, clone ED1) were present in controls (A) or in rats at 12 hours (B) or 24 hours (C) postcorrection. Microglial cells were detected at 2 (D), 4 (E), and 6 days after the correction (F). The scale bar is 100 μm. Double immunofluorescence for activated microglia (CD68, clone ED1 in green) and astrocytes (GFAP in red) in uncorrected controls (G) confirmed the homogenous distribution of astrocytes and the absence of microglia. Astrocytes were scarce 12 hours postcorrection, and no microglial cells were detected (H). (I) Massive microglial activation and complete astrocyte loss were found 6 days postcorrection. At that time, numerous hypertrophic astrocytes were seen at the lesion border (J), and activated microglial cells were only present at the center and within the borders of the lesions (K through L). The scale bar is 100 μm. In (M through P), double immunofluorescence for astrocytes (GFAP in red) and lymphocytes (CD3 in green) in the brain (M) with spleen tissue as a positive control (N) and in (O), double immunofluorescence for neutrophils (myeloperoxidase (MPO) in red) and astrocytes (GFAP in green) with thymus tissue as a positive control (P) demonstrates that no MPO- or CD3-positive cells could be detected either within or around the regions of astrocyte loss (brackets). The scale bar is 100 μm. The graph in (Q) shows that no significant differences in the gene expression of Iba1 mRNA, a marker of microglial activation, in normonatremic controls (NN) or hyponatremic rats (HYPO) and 12 and 24 hours after correction (n = 5 to 7, P = NS by ANOVA). Ctrl, control; GFAP, glial fibrillary acidic protein; ODS, osmotic demyelination syndrome; ED1, ectodermal dysplasia 1.
Early Downregulation of Astrocyte-Oligodendrocyte Gap Junction Proteins after ODS Induction
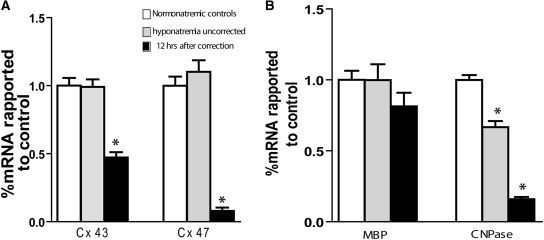
Astrocytes and oligodendrocytes are connected by complex gap junctions composed of connexins (Cx), and astrocytes provide trophic support to oligodendrocytes, which is important for myelination.13,25 In the CNS, Cx43 expression is restricted to astrocytes and couples with Cx47, which is only present in oligodendrocytes.26 Impairment of heterotypic Cx43:Cx47 gap junctions by deleting one of the partners results in myelin pathology22. We studied Cx43 gene expression and found that it was substantially downregulated 12 hours after hyponatremia correction (P < 0.001 compared with controls). This was particularly notable given the high incidence of concomitant astrocyte death. We then studied the expression of Cx47, which is the oligodendroglial partner of Cx43 and found a significant reduction of Cx47 mRNA 12h after the correction of hyponatremia. (P < 0.0001 compared with controls; Figure 5A). Interestingly, no alteration in the expression of Cx43 or Cx47 was found in hyponatremic rats. These results provide evidence that astrocyte loss after the correction of chronic hyponatremia leads to an anatomic disruption of the astrocyte-oligodendrocyte gap junction unit.
Figure 5.
Rapid correction of hyponatremia induces dysregulation of gap junction proteins and myelin-related genes. (A) Quantification of for connexin genes expression by RT-PCR shows that astrocytic connexin 43 and oligodendroglial connexin 47 are substantially downregulated 12 hours postcorrection. Chronic hyponatremia did not cause any changes in connexin expression (P < 0.001 for both genes, ANOVA, n = 5 for all groups). (B) Downregulation of CNPase gene expression was observed during hyponatremia and at 12 hours postcorrection, but we did not observe changes in myelin basic protein (MBP) mRNA levels in either conditions (n = 5 for each group, * P < 0.001 by ANOVA for CNPase and NS for MBP). Cx, connexin; MBP, myelin basic protein; CNPase, 2′,3′-cyclic nucleotide 3′-phosphodiesterase.
The mRNA level of myelin basic protein (MBP), which is an oligodendrocyte-specific protein, is a strong indicator of myelination and oligodendrocyte integrity.27,28 Conversely, oligodendrocyte gene expression of the oligodendrocyte-specific protein 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) is not necessary for normal myelination, but CNPase mRNA is rapidly downregulated during inflammation despite cell viability.29,30 To gain additional insight into the temporal relationship between astrocyte damage and myelin loss, we studied the expression of these two genes 12 hours after hyponatremia correction. No changes in MBP gene expression were found, but we did find a significant downregulation of CNPase mRNA during chronic hyponatremia and a further decrease after correction (Figure 5B).
S100B Is Released, and Inflammatory Genes Are Robustly Induced upon Astrocyte Death in Early ODS
S100B, which is a calcium-binding protein that is released by astrocytes during metabolic stress, can act as a damage-associated molecular pattern. Serum and cerebral spinal fluid levels of S100B have been used as markers of CNS injury.31 In neuromyelitis optica, astrocytes die before demyelination occurs, and cerebral spinal fluid or serum levels of S100B correlate with the clinical manifestations of the disease.32 We explored the possibility that astrocyte death in ODS induces S100B release by evaluating serum S100B levels after hyponatremia correction. We observed that there was a significant increase in serum S100B at 12 hours postcorrection that continued until 3 days postcorrection (P < 0.001 compared with S100B levels before the correction; Figure 6A). Immunofluorescence detected S100B in astrocytes and in the extracellular matrix (Supplemental Figure S3, B and C).
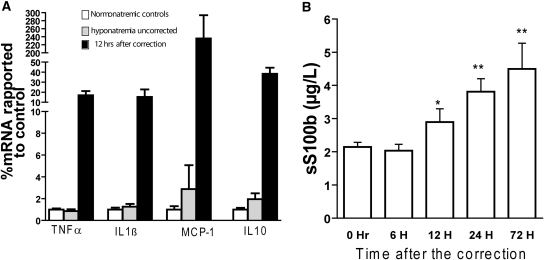
Figure 6.
Astrocyte death is accompanied by a release of S100B and changes in inflammatory marker gene expression. (A) quantitative RT-PCR for IL1β, TNFα, macrophage chemotactic protein 1 (MCP-1), and IL10 in normonatremic rats, hyponatremic controls, and 12 hours postcorrection revealed upregulation of these genes. (P < 0.01 for all four genes, ANOVA, n = 5 for each group). (B) A gradual increase in serum S100B occurred after rapid hyponatremia correction with a significant increase beginning 12 hours postcorrection (*P < 0.05, **P < 0.001, ANOVA, n = 5 to 9).
S100B acts on astrocytes in an autocrine and paracrine fashion to stimulate the production of several inflammatory cytokines including IL1β, TNFα, and macrophage chemotactic protein 1 (MCP-1).33,34 These cytokines have been associated with some of the relevant pathologic observations that occur after rapid correction of hyponatremia. For example, they can potentiate a persistent opening of the blood-brain barrier (BBB), activate microglia, and cause direct oligodendrocyte death and demyelination.35–37 We investigated whether changes in the transcription of inflammatory genes in rat brain occur during normonatremia, chronic hyponatremia and/or 12 hours after the correction of chronic hyponatremia. We found a strong upregulation of TNFα, IL1β, MCP-1, and IL10 at 12 hours posthyponatremia correction (Figure 6B). These results are particularly intriguing, because no peripheral immune cells or microglia could be detected at this time point.
Serum Levels of the Astrocytic Protein S100B Predict Inadvertent Correction of Hyponatremia and Correlate with the Neurologic Manifestation of ODS
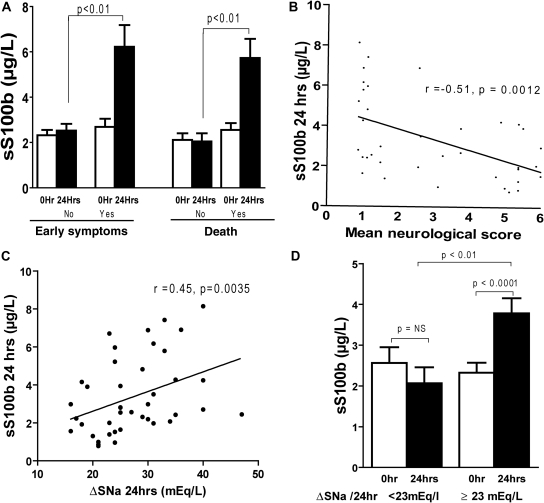
In humans, ODS is associated with a variable prognosis ranging from full recovery to death. The onset of ODS is insidious, and the magnitude of the disease course is delayed by 1 to 3 days after the correction of hyponatremia.38 Rapid recognition of ODS could aid in treatment because prompt reduction in serum sodium levels can benefit patients in the early stages of the disease.39,40 Therefore, we investigated whether the early neurologic manifestations and fatal outcome in ODS could be predicted on the basis of serum S100B levels. Compared with asymptomatic rats, we showed that 24 hours after serum sodium correction, S100B was significantly higher in rats undergoing early neurologic manifestations (within 2 days postcorrection). In addition, S100B levels were elevated in rats that died within 2 days or after 6 days postcorrection. Furthermore, S100B levels correlated with the gravity of the neurologic damage (Figure 7, A and B).
Figure 7.
Serum S100B predicts neurologic deficit and correlates with the correction gradient of serum sodium in osmotic demyelination syndrome. (A) shows that early neuropathological manifestations and death are associated with higher S100B concentrations after correction with hypertonic saline (P < 0.001 by Wilcoxon signed-rank test for matched pairs). (B) S100B levels correlated with the neurologic manifestations (n = 42, r = −0.51, P = 0. 0012) and the gradient of serum sodium. (C) n = 42, r = 0.45, P = 0.0035 by Spearman correlation. (D) Serum S100B levels were elevated 24 hours postcorrection in rats that achieved a gradient greater than 23 mEq/L/24 hours (P < 0.0001 by Wilcoxon signed-rank test for matched pairs), whereas no changes were seen in rats with a gradient less than 23 mEq/L/24 hours (n = 11 for the low gradient group and n = 31 for the high gradient group).
The correction gradient present 24 hours after the correction of hyponatremia is a major determinant of subsequent brain demyelination. Previous findings have shown that pathologic lesions occur in the majority of rats that achieve an SNa gradient greater than 23 mEq/L/24 hours.41 We investigated whether there was any difference in S100B serum levels in rats with respect to the 23 mEq/L per 24-hour threshold. To accomplish this, we separated the rats into two groups on the basis of SNa gradient achieved at 24 hours postcorrection. We found that rats with a gradient less than 23mEq/L showed no increase in S100B levels compared with controls, whereas rats with a gradient greater than 23mEq/L displayed a significant increase in serum S100B (Figure 7C). Importantly, the magnitude of the increase in S100B levels directly correlated with the serum sodium gradient achieved during the correction (Figure 7D). Taken together, these data suggest that S100B could serve as a marker of neurologic damage in patients after rapid correction of chronic hyponatremia.
Astrocyte Death Occurs without Neuronal Damage in ODS
After brain injury, neuron specific enolase (NSE) levels in the serum have been shown to increase in correlation with neuronal damage.42 We investigated whether neuronal damage occurs concomitantly with astrocyte loss in ODS and whether NSE could serve as a marker of injury. We performed IHC for the neuronal marker microtubule-associated protein 2, and we did not find any apparent neuronal loss in the region of astrocyte death (Supplemental Figure S4A). We also assayed serum NSE mRNA levels at 12 and 24 hours after hyponatremia correction, but we did not find a significant increase in NSE at either time point (Supplemental Figure S4, B and C).
Astrocyte Death Is Concomitant with BBB Damage in ODS
Astrocytes are a physical and functional component of the BBB. Astrocyte damage induces BBB dysfunction, and BBB abnormalities can theoretically also result in astrocyte damage. To investigate the relationship between astrocyte damage and BBB dysfunction, we studied BBB permeability at different time points after hyponatremia correction using IHC for IgG. We found that the first evidence of BBB damage occurred 12 hours postcorrection, which coincides with the time of astrocyte death (Figure 8, A through I). Six hours after hyponatremia correction, no evidence of BBB dysfunction was detected.
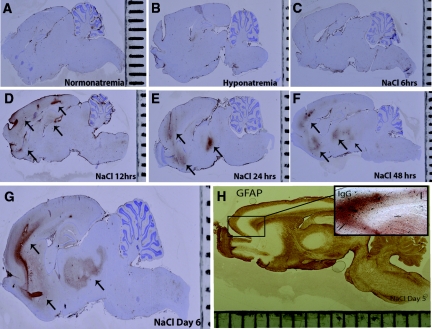
Figure 8.
BBB is disrupted in astrocyte-depleted regions in osmotic demyelination syndrome. The kinetics of BBB permeability after hyponatremia correction were evaluated using IHC for IgG. No extravated IgG was found in normonatremic rats (A), uncorrected hyponatremic controls (B), and 6 hours postcorrection (C), which suggests that BBB damage did not occur in these groups. In contrast, at 12 hours (D), 24 hours (E), and 48 hours (F) postcorrection, significant IgG immunoreactivity was observed in the subcortical regions and basal ganglia (arrows). This pattern persisted until 6 days postcorrection (G). In rats treated with hypertonic saline, IgG deposition was preferentially seen in astrocyte-depleted regions (H and I).
DISCUSSION
The ability of a cell to cope with changes in extracellular osmolarity is essential for survival, and abrupt changes in the tonicity of extracellular fluid can result in cell death.1 Aggressive correction of chronic hyponatremia in patients can induce fatal CNS demyelination.4,5,43 During chronic hyponatremia, organic osmolyte stores are depleted, and the reaccumulation of these osmolytes is impaired upon rapid hyponatremia correction.6–8
Recent evidence suggests that insufficient organic osmolytes during correction of chronic hyponatremia is involved in astrocyte death in ODS. One important clue is that the distribution of astrocyte loss after ODS induction in this study seems to correlate with both the distribution of the main organic osmolyte transporter in the rat brain and the gradient of osmolyte depletion during chronic hyponatremia.8,44 Another key observation was that supplementation with the osmolyte myo-inositol prevented experimental ODS45 and astrocyte death upon hyperosmotic challenge in vitro.46
By studying the temporal relationship between astrocyte and myelin loss, we showed that astrocyte death precedes demyelination. Connexins connect astrocytes to each other and to oligodendrocytes in a network termed the “glial syncytium,” and the integrity of this network is crucial for normal CNS functions,26,47–49 such as myelination and remyelination upon demyelinative injury.11,13,25 The importance of the glial syncytium for myelination has been shown in studies utilizing genetic deletions of connexins 43 and 47.12,50 In this study, we provided conclusive evidence that rapid correction of chronic hyponatremia results in early changes to the astrocyte-oligodendrocyte gap junction complex, which may contribute to demyelination in ODS.
In addition, these data suggest an interesting conclusion regarding the S100B protein. Autocrine and paracrine interactions between stress-induced astrocyte production of S100B and receptor-advanced glycosylated end products after injury contributes to cytokines production and the inflammatory response in the CNS.33,34 We found that increased production of S100B in ODS correlated with neurologic manifestations and death. One possible explanation for this result is that the increase in S100B may reflect damage to the BBB during rapid correction of chronic hyponatremia. Alternatively, S100B induction may simply be a direct marker of astrocyte demise, which results in increased production of inflammatory cytokines.30,35–37 Although astrocytes can potentially produce all of the cytokines that were upregulated in our experiments, further studies, including cell culture models, are needed to fully characterize the changes in astrocyte phenotype and gene expression during ODS. Astrocyte death was also accompanied by a significant and early downregulation of CNPase; however, the expression of CNPase is not necessary for proper myelination.29 The striking differential regulation of MBP and CNPase during hyponatremia and its correction suggests the presence of early oligodendrocyte disturbance accompanying astrocyte death.
These data also suggest that astrocyte death is associated with local disruption of the BBB, which is known to affect the ODS lesions.39,51 Together, these observations provide several mechanistic links between astrocyte death and the ensuing demyelination seen in ODS.
Consistent with a previous study,20 we found evidence of gliosis in nonlesioned rat brain areas surrounding lesioned regions. The full significance of these findings remains unknown, but one might hypothesize that gliosis prevents astrocyte death and limits the extent of demyelination. Another explanation could be that gliosis act in combination with astrocyte death to promote late demyelination in ODS.
We demonstrated that activated microglia appear after astrocyte loss and concomitantly with the earliest demyelinative lesions, which strongly suggests that microglia are not directly responsible for the initial pathogenesis of ODS. An attractive possibility is that microglia are activated by the inflammatory mediators produced during astrocyte death and appear late in ODS to scavenge myelin debris.
Astrocytes are not only a structural component of the BBB, but they are also necessary for the induction of many important BBB basal lamina proteins, which regulate the permeability of the BBB. In these experiments, we also showed that the BBB dysfunction occurred concurrently with the earliest morphologic evidence of astrocyte death. Two potential explanations could account for these findings: (1) astrocyte death could induce dysfunction of the BBB, which has been shown in other models11,52 or (2) the BBB dysfunction itself could induce damage to astrocytes. The second hypothesis is not supported by other models of BBB damage including osmotic disruption by mannitol infusion or stroke, where no astrocyte death occurs despite a dramatic BBB rupture.53,54 The results presented here cannot unequivocally prove whether astrocyte death in ODS is the consequence of BBB dysfunction or vice versa.
The clinical outcome of ODS is unpredictable and may have devastating consequences that could be avoided if the patients who could benefit from hyponatremia reintroduction were identified. These results suggest that serum S100B could be used to identify these patients before their symptoms manifest, but additional studies are needed to fully characterize the usefulness of this marker in humans.
Differences in astrocyte populations within the same species and between different species55 have been described on the basis of morphology, cell surface channels, and location. In addition, specific risk factors, such as malnutrition and alcoholism, have been associated with ODS in humans. Therefore, any speculation on the role of astrocyte pathology in human ODS should be cautious and cognizant of these points.
In summary, these results suggest that astrocytes are a key target in ODS. In addition, these data support a novel mechanism for ODS in which rapid correction of hyponatremia causes astrocyte death and a loss of cell-to-cell communication between astrocytes and oligodendrocytes. Along with the inflammatory microenvironment created by dying astrocytes, this could explain the salient pathologic features of ODS, including increased BBB permeability, microglial activation, and demyelination.
CONCISE METHODS
Rats and Hyponatremia Induction and Correction
Male Wistar rats (200 to 300 g) were used for all experiments. Chronic hyponatremia was induced by a combination of continuous DDAVP treatment (Minirin® Ferring. Sweden) through osmotic minipump (Alzet®) and a liquid diet (AIN 76; Technilab BMI Sommeren Netherlands).43 Hyponatremia was maintained for 4 days and was rapidly corrected by a single intraperitoneal injection of 1 M NaCl. To obtain a wide range of SNa levels for S100B analysis, rats were administered two different doses of hypertonic saline (1 ml/100 g of body weight or 2 ml/100 g of body weight). A high correction gradient after hypertonic saline injection results in a high rate of early mortality, which precluded analysis of rats during late ODS. To circumvent this problem, the experiments were repeated to obtain enough rats for histologic studies, RT-PCR analysis, and S100B experiments. Rats were allowed to eat 1 day after the correction and drink 2 days after the correction to avoid a secondary decrease in serum sodium levels.39 Procedures were performed in accordance with the guidelines for animal care at Université Libre de Bruxelles.
Quantification of Astrocyte Death and Myelin Loss
For histologic quantification of astrocyte and myelin loss, the rats were euthanized at various time points depending on their group allocation, and rat brains were processed for immunohistochemistry (GFAP and MBP) as described later. Areas of astrocytes and myelin loss were quantified using computer assisted image analysis.56
The rats were allocated into different groups. Group 1 consisted of normonatremic rats, group 2 comprised uncorrected hyponatremic rats (electrolyte levels were measured 4 days after the insertion of the minipump), and groups 3 to 5 and 6 to 8 consisted of corrected rats that were processed for IHC 6, 12, and 24 hours and 2, 3, and 6 days after SNa correction, respectively. The serum SNa concentration was analyzed at 6 and 12 hours postcorrection for groups 3 and 4 and 24 hours after the correction for groups 5 to 8.
Immunohistochemistry, Double Immunofluorescence, and Apoptosis Detection
Immunohistochemistry was used to detect astrocytes, myelin, oligodendrocytes, neutrophils, microglia and lymphocytes as described previously.56 The antibodies used are listed in Supplemental Table S4. Double immunofluorescence was performed using a tyramide amplification kit and commercially available fluorescence secondary antibodies. Apoptosis detection was performed using the commercial kit Apoptag®, which detects nick-ended DNA fragments present in apoptotic cells.
Blood-Brain Barrier Integrity Analysis
The localization of the BBB rupture was performed by one-step IHC to detect rat IgG in brain slices. Detailed procedures have been previously described.56
S100B Measurement and Kinetics
To determine whether the S100B serum concentration increased after hyponatremia correction, we assayed S100B levels in living rats at different time points after being treated with hypertonic saline. To investigate the correlation between the correction gradient and S100B, statistical analysis required numerous data points at various gradients. Because the initial saline injection (2 ml/100 g of body weight) resulted in high SNa gradients, we used a half-dose of hypertonic saline to obtain more rats with lower serum sodium gradients. For correlation studies, S100B was analyzed along with the SNa gradient in all of the rats that received hypertonic saline and manifested neurologic symptoms. Neurologic symptoms were recorded daily and graded as described elsewhere.57
Blood Measurements
Blood samples were collected from the tail vein, and electrolytes were analyzed by MODULAR p800 (Roche). S100B (S100 A1B and S100 BB) and NSE were measured with an automated, noncompetitive, electrochemiluminescent immunoassay on a Cobas e411 (Roche Diagnostics) following the manufacturer's instructions.42
RNA Isolation and RT-PCR
Preliminary experiments as well as the IHC experiments described above showed that the cortex was invariably affected during rapid correction of hyponatremia, and therefore that region was chosen for all of the PCR studies. In a separate set of rats (Supplemental Table S3), total RNA was extracted from the rat cortices using the TripureTM reagent and a High Pure RNA Isolation Kit TM (both from Roche) according to the manufacturer's instructions. After quantification of extracted RNA, reverse transcription, and amplification of the subsequent cDNA, the PCR product was quantified using the ΔCt method with the glyceraldehyde-3-phosphate dehydrogenase gene as an internal control. The primer sequences used for amplification are described in Supplemental Table S5.
Statistical Analyses
All of the data were analyzed using Statdirect softwareTM (StatsDirect Ltd.). A Shapiro-Wilk's test was conducted for all variables to assess normality. A paired or unpaired t test (or their nonparametric alternatives) and ANOVA or a Kruskal Wallis test were used to assess variance between the groups and were followed by the appropriate least statistical significance post hoc tests. Correlation was analyzed by a Spearman rank test. All of the data are presented as the means ± SEM, and statistical significance was accepted when P < 0.05.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
The authors are indebted to Michelle Authelet for her helpful technical assistance and to Drs. Will Renthal and Nishi Mehta for their help in the preparation of the manuscript. Part of this manuscript was presented as abstract at Renal Week 2009 (October 27 to November 1, 2009; San Diego, CA) This study was supported by grants from the Fonds National de la Recherche Scientifique and the Fonds de la Recherche Scientifique Médicale, conventions 3.4509.03, 3.4656.09, and 3.4504.10; by a grant from the Belgian PAI/IAP P6/43; by Diane Program 816856; and by the Fondation Erasme.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Burg MB, Ferraris JD, Dmitrieva NI: Cellular response to hyperosmotic stresses. Physiol Rev 87: 1441–1474, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Adrogue HJ, Madias NE: Hyponatremia. N Engl J Med 342: 1581–1589, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Wald R, Jaber BL, Price LL, Upadhyay A, Madias NE: Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med 170: 294–302, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Kleinschmidt-DeMasters BK, Norenberg MD: Rapid correction of hyponatremia causes demyelination: Relation to central pontine myelinolysis. Science 211: 1068–1070, 1981 [DOI] [PubMed] [Google Scholar]
- 5. Sterns RH, Riggs JE, Schochet SS, Jr: Osmotic demyelination syndrome following correction of hyponatremia. N Engl J Med 314: 1535–1542, 1986 [DOI] [PubMed] [Google Scholar]
- 6. Sterns RH, Silver SM: Brain volume regulation in response to hypo-osmolality and its correction. Am J Med 119: S12–S16, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Verbalis JG, Gullans SR: Rapid correction of hyponatremia produces differential effects on brain osmolyte and electrolyte reaccumulation in rats. Brain Res 606: 19–27, 1993 [DOI] [PubMed] [Google Scholar]
- 8. Lien YHH: Role of organic osmolytes in myelinolysis: A topographic study in rats after rapid correction of hyponatremia. J Clin Invest 95: 1579–1586, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Olson JE: Osmolyte contents of cultured astrocytes grown in hypoosmotic medium. Biochim Biophys Acta 1453: 175–179, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Pasantes-Morales H, Alavez S, Sanchez Olea R, Moran J: Contribution of organic and inorganic osmolytes to volume regulation in rat brain cells in culture. Neurochem Res 18: 445–452, 1993 [DOI] [PubMed] [Google Scholar]
- 11. Liedtke W, Edelmann W, Bieri PL, Chiu FC, Cowan NJ, Kucherlapati R, Raine CS: GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination. Neuron 17: 607–615, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Lutz SE, Zhao Y, Gulinello M, Lee SC, Raine CS, Brosnan CF: Deletion of astrocyte connexins 43 and 30 leads to a dysmyelinating phenotype and hippocampal CA1 vacuolation. J Neurosci 29: 7743–7752, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD: Astrocytes promote myelination in response to electrical impulses. Neuron 49: 823–832, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dietrich J, Lacagnina M, Gass D, Richfield E, Mayer-Proschel M, Noble M, Torres C, Proschel C: EIF2B5 mutations compromise GFAP+ astrocyte generation in vanishing white matter leukodystrophy. Nat Med 11: 277–283, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Borrett D, Becker LE: Alexander's disease: A disease of astrocytes. Brain 108: 367–385, 1985 [DOI] [PubMed] [Google Scholar]
- 16. Itoyama Y, Sekizawa T, Openshaw H, Kogure K, Goto I: Early loss of astrocytes in herpes simplex virus-induced central nervous system demyelination. Ann Neurol 29: 285–292, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hawrylak N, Fleming JC, Salm AK: Dehydration and rehydration selectively and reversibly alter glial fibrillary acidic protein immunoreactivity in the rat supraoptic nucleus and subjacent glial limitans. Glia 22: 260–271, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Pekny M, Nilsson M: Astrocyte activation and reactive gliosis. Glia 50: 427–434, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA: A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J Neurosci 28: 264–278, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iwama S, Sugimura Y, Suzuki H, Murase T, Ozaki N, Nagasaki H, Arima H, Murata Y, Sawada M, Oiso Y: Time-dependent changes in proinflammatory and neurotrophic responses of microglia and astrocytes in a rat model of osmotic demyelination syndrome. GLIA 59: 452–462, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Kleinschmidt-DeMasters BK, Norenberg MD: Neuropathologic observations in electrolyte-induced myelinolysis in the rat. J Neuropathol Exp Neurol 41: 67–80, 1982 [DOI] [PubMed] [Google Scholar]
- 22. Rojiani AM, Cho ES, Sharer L, Prineas JW: Electrolyte-induced demyelination in rats- 2: Ultrastructural evolution. Acta Neuropathologica 88: 293–299, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Takefuji S, Murase T, Sugimura Y, Takagishi Y, Hayasaka S, Oiso Y, Murata Y: Role of microglia in the pathogenesis of osmotic-induced demyelination. Exp Neurol 204: 88–94, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Suk K, Lee J, Hur J, Kim YS, Lee MS, Cha SH, Yeou Kim S, Kim H: Activation-induced cell death of rat astrocytes. Brain Res 900: 342–347, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Watkins TA, Emery B, Mulinyawe S, Barres BA: Distinct stages of myelination regulated by γ-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron 60: 555–569, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Orthmann-Murphy JL, Freidin M, Fischer E, Scherer SS, Abrams CK: Two distinct heterotypic channels mediate gap junction coupling between astrocyte and oligodendrocyte connexins. J Neurosci 27: 13949–13957, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shine HD, Readhead C, Popko B, Hood L, Sidman RL: Morphometric analysis of normal, mutant, and transgenic CNS: Correlation of myelin basic protein expression to myelinogenesis. J Neurochem 58: 342–349, 1992 [DOI] [PubMed] [Google Scholar]
- 28. Woodruff RH, Franklin RJ: The expression of myelin protein mRNAs during remyelination of lysolecithin-induced demyelination. Neuropathol Appli Neurobiol 25, 226–235, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA: Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet 33: 366–374, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Jana M, Pahan K: Redox regulation of cytokine-mediated inhibition of myelin gene expression in human primary oligodendrocytes. Free Radic Biol Med 39: 823–831, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sen J, Belli A: S100B in neuropathologic states: The CRP of the brain? J Neurosci Res 85: 1373–1380, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Takano R, Misu T, Takahashi T, Sato S, Fujihara K, Itoyama Y: Astrocytic damage is far more severe than demyelination in NMO: A clinical CSF biomarker study. Neurology 75: 208–216, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Gerald P, Christiane S, Florian K, Björn V, Dennis W, Volker A, Matthias R: Autocrine S100B effects on astrocytes are mediated via RAGE. J Neuroimmunol 184: 214–222, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Saha A, Kim SJ, Zhang Z, Lee YC, Sarkar C, Tsai PC, Mukherjee AB: RAGE signaling contributes to neuroinflammation in infantile neuronal ceroid lipofuscinosis. FEBS Lett 582: 3823–3831, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Benn T, Halfpenny C, Scolding N: Glial cells as targets for cytotoxic immune mediators. Glia 36: 200–211, 2001 [DOI] [PubMed] [Google Scholar]
- 36. de Boer AG, Breimer DD: Cytokines and blood-brain barrier permeability. In: Progress in Brain Research, Volume 115, edited by Sharma HS, Westman J. New York, Elsevier, 1998, pp 425–451 [DOI] [PubMed] [Google Scholar]
- 37. Ousman SS, David S: MIP-1α, MCP-1, GM-CSF, and TNF-α control the immune cell response that mediates rapid phagocytosis of myelin from the adult mouse spinal cord. J Neurosci 21: 4649–4656, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sterns RH, Cappuccio JD, Silver SM, Cohen EP: Neurologic sequelae after treatment of severe hyponatremia: A multicenter perspective. J Am Soc Nephrol 4: 1522–1530, 1993 [DOI] [PubMed] [Google Scholar]
- 39. Gankam Kengne F, Soupart A, Pochet R, Brion JP, Decaux G: Re-induction of hyponatremia after rapid overcorrection of hyponatremia reduces mortality in rats. Kidney Int 76: 614–621, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Perianayagam A, Sterns RH, Silver SM, Grieff M, Mayo R, Hix J, Kouides R: DDAVP is effective in preventing and reversing inadvertent overcorrection of hyponatremia. Clinical J Am Soc Nephrol 3: 331–336, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Soupart A, Stenuit A, Perier O, Decaux G: Limits of brain tolerance to daily increments in serum sodium in chronically hyponatraemic rats treated with hypertonic saline or urea: Advantages of urea. Clin Sci 80: 77–84, 1991 [DOI] [PubMed] [Google Scholar]
- 42. Nguyen DN, Spapen H, Su F, Schiettecatte J, Shi L, Hachimi-Idrissi S, Huyghens L: Elevated serum levels of S-100beta protein and neuron-specific enolase are associated with brain injury in patients with severe sepsis and septic shock. Crit Care Med 34: 1967–1974, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Verbalis JG, Martinez AJ: Neurological and neuropathological sequelae of correction of chronic hyponatremia. Kidney Int 39: 1274–1282, 1991 [DOI] [PubMed] [Google Scholar]
- 44. Ibsen L, Strange K: In situ localization and osmotic regulation of the Na(+)-myo-inositol cotransporter in rat brain. Am J Physiol 271: F877–F885, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Silver SM, Schroeder BM, Sterns RH, Rojiani AM: Myoinositol administration improves survival and reduces myelinolysis after rapid correction of chronic hyponatremia in rats. J Neuropathol Exp Neurol 65: 37–44, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Hijab S, Havalad S, Snyder AK: The role of organic osmolytes in the response of cultured astrocytes to hyperosmolarity. Am J Ther, 2010. doi: 10.1097/MJT.0b013e3181cd816f [DOI] [PubMed] [Google Scholar]
- 47. Nagy JI, Rash JE: Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain Res Brain Res Rev 32: 29–44, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Giaume C: Astroglial wiring is adding complexity to neuroglial networking. Front Neuroenergetics 2: 129, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C: Astroglial metabolic networks sustain hippocampal synaptic transmission. Science 322: 1551–1555, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Odermatt B, Wellershaus K, Wallraff A, Seifert G, Degen J, Euwens C, Fuss B, Bussow H, Schilling K, Steinhauser C, Willecke K: Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. J Neurosci 23: 4549–4559, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Murase T, Sugimura Y, Takefuji S, Oiso Y, Murata Y: Mechanisms and therapy of osmotic demyelination. Am J Med 119: S69–S73, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Willis CL, Nolan CC, Reith SN, Lister T, Prior MJ, Guerin CJ, Mavroudis G, Ray DE: Focal astrocyte loss is followed by microvascular damage, with subsequent repair of the blood-brain barrier in the apparent absence of direct astrocytic contact. Glia 45: 325–337, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Friedman B, Schachtrup C, Tsai PS, Shih AY, Akassoglou K, Kleinfeld D, Lyden PD: Acute vascular disruption and aquaporin 4 loss after stroke. Stroke 40: 2182–2190, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Suzuki M, Iwasaki Y, Yamamoto T, Konno H, Kudo H: Sequelae of the osmotic blood-brain barrier opening in rats. J Neurosurg 69: 421–428, 1988 [DOI] [PubMed] [Google Scholar]
- 55. Zhang Y, Barres BA: Astrocyte heterogeneity: An underappreciated topic in neurobiology. Curr Opin Neurobiol 20: 588–594, 2010 [DOI] [PubMed] [Google Scholar]
- 56. Gankam-Kengne F, Soupart A, Pochet R, Brion JP, Decaux G: Minocycline protects against neurologic complications of rapid correction of hyponatremia. J Am Soc Nephrol 21: 2099–2108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sugimura Y, Murase T, Takefuji S, Hayasaka S, Takagishi Y, Oiso Y, Murata Y: Protective effect of dexamethasone on osmotic-induced demyelination in rats. Exp Neurol 192: 178–183, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.