Abstract
Objective:
The purposes of this study were to determine the normal retinal microvasculature measurements in human infants who are born at term and to determine whether birth weight influences measurements of retinal microvasculature.
Study Design:
Retinal arteriole and venule measurements were obtained in a cohort of 24 infants who were born at term. Digital images of both the retinas were obtained using a digital retinal camera after pupillary dilation.
Result:
In all, 24 newborn infants born at term (12 females and 12 males) were analyzed in this study. The measured retinal arteriole diameters were from 66.8 to 147.8 μm (mean, 94.2±19.6 μm), and the venule diameters were from 102.0 to 167.8 μm (mean, 135.2±19.1 μm). Seven babies in the sample had low birth weight (LBW), while 17 babies were born with normal weight. Babies with lower birth weights had larger arteriole (113.1±17.9 μm vs 86.4±14.4 μm; P=0.0009) and venule diameters (151.7±14.9 μm vs 128.4±16.9 μm; P=0.0040).
Conclusion:
Retinal venules and arterioles in LBW babies are larger compared with those of normal-birth-weight babies. We postulate that the difference observed in our study was due to in utero pathophysiological changes that occurred in the cerebral circulation of growth-restricted fetuses.
Keywords: retinal arterioles, retinal venules, middle cerebral artery, intrauterine growth restriction
Introduction
In utero insults that result in low-birth-weight (LBW) infants (birth weight <2500 g)1 are now well recognized as risk factors contributing to the development of vascular-related diseases in adulthood.2, 3, 4, 5, 6 LBW infants are a heterogeneous group of infants, comprising infants who are premature (<37 completed weeks of gestation),1 growth-restricted (weight below the 10th percentile for their gestational age)1 or a combination of both. The exact mechanism of this phenomenon has yet to be fully understood, but there is increasing evidence to suggest that microcirculatory pathology forms the mechanistic link between fetal insult and the adult manifestation of illness.7, 8, 9, 10, 11 The challenge has been to investigate microcirculatory changes in vivo. The retina provides an opportunity for in vivo investigation of human microcirculation, and changes in the retinal vessels have been identified in some individuals who had LBW as infants and later developed hypertension, ischemic heart disease, stroke and renal disease.9, 12, 13, 14, 15 The ability of retinal-imaging technology to assess and measure the retinal microvasculature makes this a very valuable assessment tool.16, 17, 18 Studies involving young children, adolescents and adults who were born small have shown abnormalities in the retinal vasculature.9, 12, 15, 19, 20, 21, 22, 23
Although the retinal microvascular of premature infants is routinely assessed to detect and treat retinopathy of prematurity,24 there are no published studies regarding the use of retinal-imaging technology to assess the retinal microvasculature of at-term, growth-restricted infants. There are also no published data concerning normal measurements of retinal microvasculature in infants. The purpose of the present study was to determine normal measurements of the retinal microvasculature in human infants who are born at term. This study also investigated whether birth weight influences measurements of retinal microvasculature.
Methods
This study was performed in the Department of Neonatology, The Townsville Hospital, Douglas, QLD, Australia. The Department of Neonatology is a tertiary perinatal center catering to more than 10 000 births each year. The study commenced in August 2010, and the data presented in this study are based on patients recruited until May 2011. This study was approved by the Townsville Health District Human Research Ethics Committee. Written parental consent was obtained, and babies with syndromes, prematurity and chromosomal abnormalities were excluded. All assessments were performed within the first 7 days of life. Babies with birth weights of ⩽2500 g were classified as LBW babies, and babies weighing from 2501 to 4500 g were classified as appropriate for gestational age babies. Only babies who were born at term (37 weeks of gestation completed) were included in this study.
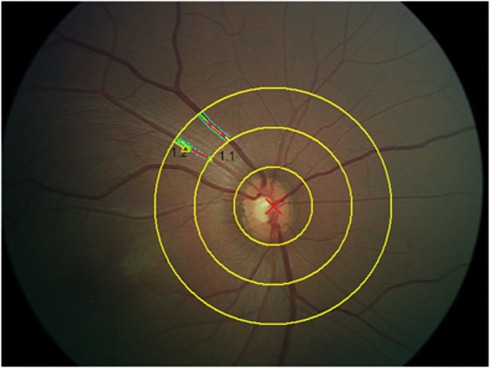
After pupillary dilation, digital images of both the retinas were obtained using a digital retinal camera (RetCam, Massie Laboratories, Dublin, CA, USA). Measurements of the diameters of retinal vessels were then obtained using a predetermined protocol that first involved the identification of retinal vessels located from 0.5 to 1 disc diameter from the margin of the optic disc (Figure 1). Measurements of vessel diameter were then obtained using semi-automated software (Vesselmap, IMEDOS GmbH, Jena, Germany).25, 26 Vascular diameter was computed as wall-to-wall distance within the vessel. The caliber of directly viewed vessels was determined by the size of the red-cell column, because the vessel walls and peripheral plasma layer are nearly transparent.27 Measurements of vessels from each eye were obtained, and the largest venule and arteriole for each patient was determined. These measurements were then used for analysis. An intra-class correlation coefficient was used to determine the reliability of this technique;28 this correlation coefficient was 0.90 (95% confidence interval of 0.75 to 0.96). A previously published study in infants has shown that the blood flow in the central retinal arteries is similar in both the eyes.29
Figure 1.
Retinal image from a newborn infant showing identification and measurement of retinal vessels from 0.5 to 1.0 disc diameters from the margin of the optic disc.
In adult eyes, correction can be applied to compensate for inaccuracies in the measurements of retinal structure that occur because of refractive error; this correction requires parameters such as axial length and keratometry (curvature of the anterior surface of the cornea) to be known.30 In infants, these calculations are more challenging because obtaining these measurements is difficult and the eye is continuing to grow.30 Statistical analysis was carried out using Stata ver. 11.0 (Stata, College Station, TX, USA). Using Student's t-test, P values <0.05 were considered significant.
Results
A total of 247 babies were admitted to the department during the study period. Of these, 99 were suitable for recruitment, and their parents were approached for participation. Written consent was obtained for 24. All 24 newborn infants born at term (12 females and 12 males) were analyzed in this study. Birth weights ranged from 1845 to 4310 g (mean, 3029±649 g) with gestational ages of 37 to 41.6 weeks (mean, 38.7±1.4 weeks). Retinal arteriole diameters were from 66.8 to 147.8 μm (mean, 94.2±19.6 μm), and venule diameters were from 102.0 to 167.8 μm (mean, 135.2±19.1 μm). Table 1 compares the differences in these measurements between male and female infants.
Table 1. Comparison of measurements between male and female infants.
| Male | Female | P-value | |
|---|---|---|---|
| Number | 12 | 12 | |
| Birth weight (g) | 3017±537 | 3041±770 | 0.9282 |
| Gestation (weeks) | 38.3±1.1 | 39.2±1.4 | 0.0994 |
| Venule diameter (μm) | 130.2±18.8 | 140.3±18.9 | 0.2029 |
| Arteriole diameter (μm) | 90.1±17.2 | 98.2±21.6 | 0.3206 |
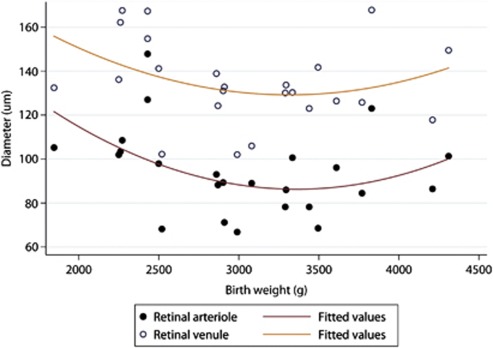
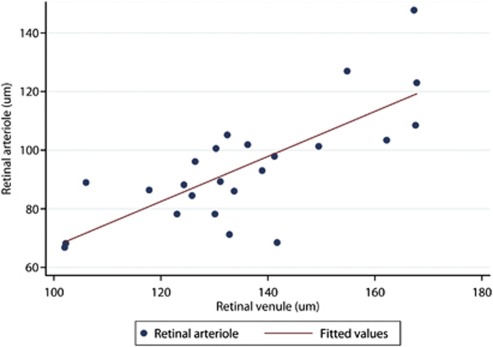
The infants were divided into two cohorts based on birth weight (LBW and appropriate for gestational age). There were 7 LBW babies and 17 appropriate for gestational age babies. Babies with LBW had larger arteriole (113.1±17.9 μm vs 86.4±14.4 μm; P=0.0009) and venule diameters (151.7±14.9 μm vs 128.4±16.9 μm; P=0.0040). Figures 2 and 3 show the relationship between birth weight and vessel diameters. Pearson's coefficient of correlation between retinal arteriole and venule diameter was 0.7522 (95% confidence interval 0.50 to 0.89; P<0.0001).
Figure 2.
Relationships between birth weight and the diameters of retinal arterioles, and venules in infants born at term.
Figure 3.
Increasing retinal arteriole diameter is closely correlated with increasing retinal venule diameter.
Discussion
To date, studies of retinal vasculature in infants have mainly focused on premature infants and retinopathy of prematurity.25, 26 To our knowledge, this is the first study to investigate measurements of retinal microvasculature using digital retinal imaging in infants born at term. These measurements could be used as a baseline for future studies that investigate the effects of birth weight on retinal microvasculature. Previous studies have shown a strong relationship between LBW and retinal vasculature size in older children,15, 19, 22, 23, 31 adolescents32 and adults.9, 12, 13 However, no published studies utilized baseline measurements of infant retinal vasculature for comparison. For the first time, we were able to measure retinal arteriole and venule sizes in LBW infants during infancy. The data from this study show significantly higher retinal vessel diameters in LBW babies. By contrast, previously published studies of young children15, 22 have shown that children who were born as LBW infants had narrower retinal arteriolar calibers. Narrowing of these vessels has been linked to the development of cardiovascular diseases in adults.7, 14, 33
Why are the diameters of retinal vessels significantly larger in LBW babies? Only infants born at term were reviewed in this study; thus, the cause of LBW in this cohort was intrauterine growth restriction. The retinal images in this study were all taken during the first week of life, so we propose that the differences observed in our study were due to pathophysiological changes that occurred in utero. There are many causes of intrauterine growth restriction, but the most common is uteroplacental insufficiency, which results in fetal hypoxia.34, 35
Fetal cerebrovascular responses to hypoxia are fundamentally different from those observed in the cerebral circulation of adults.36 The vasculature of the immature brain is highly plastic and can respond to hypoxia with robust increases in capillary density.36 Endothelial vasodilator capacity is typically depressed in fetal cerebral arteries, and the endothelium contributes relatively little to hypoxic vasodilatation in the fetus.36, 37 By contrast, the endothelial contribution to hypoxic vasodilatation increases throughout early postnatal life, becoming quite prominent in the cerebral arteries of adults.37, 38 Hypoxia exerts effects on vascular smooth muscle through various mechanisms.36 The smaller and more peripheral cerebral arteries relax quickly and completely in response to hypoxia, whereas the larger and more proximal arteries, including the common carotid, maintain muscle tone much better and have a more important role in the gradual adjustments of cerebrovascular tissue to resist hypoxia.39
Animal studies have provided insight into some aspects of the basic pathophysiology of intrauterine growth restriction, and studies using technologies such as Doppler ultrasound to investigate maternal and fetal vessels have added further information. Doppler ultrasound allows for the assessment of the vascular effects of placental dysfunction on the placental and fetal vasculature.40 In response to hypoxia, the fetus uses a compensatory mechanism to redistribute cardiac output and blood supply to the brain to maintain constant blood delivery to this organ (the head-sparing effect).41 The result is a decrease in cerebral blood-flow resistance and vasodilatation of the arteries. This effect, which can be measured using Doppler ultrasound, shows a decrease in resistance and an increase in blood flow in the middle cerebral artery.40 Studies of growth-restricted fetuses have confirmed dilatation of the middle cerebral artery and the resulting increase in blood flow to the brain compared with fetuses with normal growth.42, 43, 44
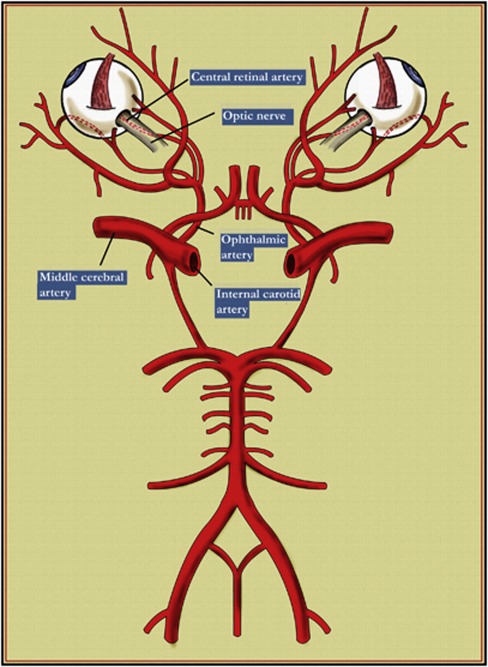
Figure 4 shows the close relationship between the retinal artery and the middle cerebral artery. The endothelia of the vessels in the brain and retina are lined with continuous endothelial cells, connected by tight junctions that help to maintain the blood–brain barrier.45 Doppler flowmetry data from newborn babies have shown that an increase in blood flow in the middle cerebral and ophthalmic artery is closely followed by an increase in blood flow in the central retinal artery.29 Blood flow in the middle cerebral artery and cerebral blood flow are spatially and temporally coupled to fetal brain function and metabolism, and we postulate that, in a growth-restricted fetus, the same neurovascular coupling extends to the retinal artery. Dilation of the middle cerebral artery possibly results in dilatation of retinal vessels in growth-restricted, LBW infants.
Figure 4.
Diagram showing the Circle of Willis, middle cerebral artery and origin of the ophthalmic and central retinal arteries.
The main limitation of our study was its relatively small sample size. It was also difficult to account for any refractive error that could have contributed to the results. We plan to follow this cohort over time to identify the changes in retinal vasculature as these infants grow.
Acknowledgments
This study was supported by The Royal Australasian College of Physicians Research and Education Grant.
The authors declare no conflict of interest.
References
- International Classification of Diseases and Related Health Problems 10th revision. World Health Organization: Geneva; 1992 [Google Scholar]
- Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2 (8663:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Birth weight and hypertension. Hypertension. 2006;48 (3:357–358. doi: 10.1161/01.HYP.0000236552.04251.42. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006;49 (2:270–283. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The developmental origins of chronic adult disease. Acta Paediatr Suppl. 2004;93 (446:26–33. doi: 10.1111/j.1651-2227.2004.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353 (17:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- Sasongko MB, Wong TY, Wang JJ. Retinal arteriolar changes: intermediate pathways linking early life exposures to cardiovascular disease. Microcirculation. 2010;17 (1:21–31. doi: 10.1111/j.1549-8719.2009.00007.x. [DOI] [PubMed] [Google Scholar]
- Struijker-Boudier HA, Rosei AE, Bruneval P, Camici PG, Christ F, Henrion D, et al. Evaluation of the microcirculation in hypertension and cardiovascular disease. Eur Heart J. 2007;28 (23:2834–2840. doi: 10.1093/eurheartj/ehm448. [DOI] [PubMed] [Google Scholar]
- Liew G, Wang JJ, Duncan BB, Klein R, Sharrett AR, Brancati F, et al. Low birthweight is associated with narrower arterioles in adults. Hypertension. 2008;51 (4:933–938. doi: 10.1161/HYPERTENSIONAHA.107.101584. [DOI] [PubMed] [Google Scholar]
- Martin H, Hu J, Gennser G, Norman M. Impaired endothelial function and increased carotid stiffness in 9-year-old children with low birthweight. Circulation. 2000;102 (22:2739–2744. doi: 10.1161/01.cir.102.22.2739. [DOI] [PubMed] [Google Scholar]
- Mimoun L, Massin P, Steg G. Retinal microvascularisation abnormalities and cardiovascular risk. Arch Cardiovasc Dis. 2009;102 (5:449–456. doi: 10.1016/j.acvd.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Chapman N, Mohamudally A, Cerutti A, Stanton A, Sayer AA, Cooper C, et al. Retinal vascular network architecture in low-birth-weight men. J Hypertens. 1997;15 (12 Part 1:1449–1453. doi: 10.1097/00004872-199715120-00012. [DOI] [PubMed] [Google Scholar]
- Hellstrom A, Dahlgren J, Marsal K, Ley D. Abnormal retinal vascular morphology in young adults following intrauterine growth restriction. Pediatrics. 2004;113 (2:e77–e80. doi: 10.1542/peds.113.2.e77. [DOI] [PubMed] [Google Scholar]
- McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Klein BE, et al. Meta-analysis: retinal vessel caliber and risk for coronary heart disease. Ann Intern Med. 2009;151 (6:404–413. doi: 10.7326/0003-4819-151-6-200909150-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Ponsonby AL, Wong TY, Brown SA, Kearns LS, Cochrane J, et al. Effect of birth parameters on retinal vascular caliber: the Twins Eye Study in Tasmania. Hypertension. 2009;53 (3:487–493. doi: 10.1161/HYPERTENSIONAHA.108.125914. [DOI] [PubMed] [Google Scholar]
- Wong TY, Knudtson MD, Klein R, Klein BE, Meuer SM, Hubbard LD. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology. 2004;111 (6:1183–1190. doi: 10.1016/j.ophtha.2003.09.039. [DOI] [PubMed] [Google Scholar]
- Liew G, Wang JJ, Mitchell P, Wong TY. Retinal vascular imaging: a new tool in microvascular disease research. Circ Cardiovasc Imaging. 2008;1 (2:156–161. doi: 10.1161/CIRCIMAGING.108.784876. [DOI] [PubMed] [Google Scholar]
- Chapman N, Witt N, Gao X, Bharath AA, Stanton AV, Thom SA, et al. Computer algorithms for the automated measurement of retinal arteriolar diameters. Br J Ophthalmol. 2001;85 (1:74–79. doi: 10.1136/bjo.85.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung N, Wong TY, Liew G, Saw SM.Low birth weight and retinal vascular caliber in young children Pediatrics 2008121(4862–863.author reply 863. [DOI] [PubMed] [Google Scholar]
- Cheung N, Islam FM, Saw SM, Shankar A, de Haseth K, Mitchell P, et al. Distribution and associations of retinal vascular caliber with ethnicity, gender, and birth parameters in young children. Invest Ophthalmol Vis Sci. 2007;48 (3:1018–1024. doi: 10.1167/iovs.06-0978. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Cheung N, de Haseth K, Taylor B, Rochtchina E, Islam FM, et al. Blood pressure and retinal arteriolar narrowing in children. Hypertension. 2007;49 (5:1156–1162. doi: 10.1161/HYPERTENSIONAHA.106.085910. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Liew G, Rochtchina E, Wang JJ, Robaei D, Cheung N, et al. Evidence of arteriolar narrowing in low-birth-weight children. Circulation. 2008;118 (5:518–524. doi: 10.1161/CIRCULATIONAHA.107.747329. [DOI] [PubMed] [Google Scholar]
- Tapp RJ, Williams C, Witt N, Chaturvedi N, Evans R, Thom SA, et al. Impact of size at birth on the microvasculature: the Avon Longitudinal Study of Parents and Children. Pediatrics. 2007;120 (5:e1225–e1228. doi: 10.1542/peds.2006-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Section on Ophthalmology, American Academy of P, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and S Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2006;117 (2:572–576. doi: 10.1542/peds.2005-2749. [DOI] [PubMed] [Google Scholar]
- Grunwald L, Mills MD, Johnson KS, Karp KA, Quinn GE, Ying GS, et al. The rate of retinal vessel dilation in severe retinopathy of prematurity requiring treatment Am J Ophthalmol 2009147(61086–1091.1091 e1081–1082. [DOI] [PubMed] [Google Scholar]
- Johnson KS, Mills MD, Karp KA, Grunwald JE. Quantitative analysis of retinal vessel diameter reduction after photocoagulation treatment for retinopathy of prematurity. Am J Ophthalmol. 2007;143 (6:1030–1032. doi: 10.1016/j.ajo.2007.01.058. [DOI] [PubMed] [Google Scholar]
- Archer DB, Gardiner TA, Stitt AW.Retinal Vascular Disease,1 edn.Springer: Heidelberg; 2010 [Google Scholar]
- Costa-Santos C, Bernardes J, Ayres-de-Campos D, Costa A, Costa C. The limits of agreement and the intraclass correlation coefficient may be inconsistent in the interpretation of agreement. J Clin Epidemiol. 2011;64 (3:264–269. doi: 10.1016/j.jclinepi.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Papacci P, Romagnoli C, Favuzzi A, Luciano R, Giannini R, De Carolis MP, et al. Doppler ultrasound of blood flow velocities in ophthalmic and central retinal arteries during the early neonatal period. Am J Ophthalmol. 1998;126 (5:691–697. doi: 10.1016/s0002-9394(98)00203-7. [DOI] [PubMed] [Google Scholar]
- De Silva DJ, Cocker KD, Lau G, Clay ST, Fielder AR, Moseley MJ. Optic disk size and optic disk-to-fovea distance in preterm and full-term infants. Invest Ophthalmol Vis Sci. 2006;47 (11:4683–4686. doi: 10.1167/iovs.06-0152. [DOI] [PubMed] [Google Scholar]
- Hellstrom A, Hard AL, Chen Y, Niklasson A, Albertsson-Wikland K. Ocular fundus morphology in preterm children. Influence of gestational age, birth size, perinatal morbidity, and postnatal growth. Invest Ophthalmol Vis Sci. 1997;38 (6:1184–1192. [PubMed] [Google Scholar]
- Gopinath B, Baur LA, Wang JJ, Teber E, Liew G, Cheung N, et al. Smaller birth size is associated with narrower retinal arterioles in early adolescence. Microcirculation. 2010;17 (8:660–668. doi: 10.1111/j.1549-8719.2010.00062.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Wong TY, Sharrett AR, Klein R, Folsom AR, Jerosch-Herold M. Relationship between retinal arteriolar narrowing and myocardial perfusion: multi-ethnic study of atherosclerosis. Hypertension. 2008;51 (1:119–126. doi: 10.1161/HYPERTENSIONAHA.107.098343. [DOI] [PubMed] [Google Scholar]
- Hendrix N, Berghella V. Non-placental causes of intrauterine growth restriction. Semin Perinatol. 2008;32 (3:161–165. doi: 10.1053/j.semperi.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Sankaran S, Kyle PM. Aetiology and pathogenesis of IUGR. Best Pract Res Clin Obstet Gynaecol. 2009;23 (6:765–777. doi: 10.1016/j.bpobgyn.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Pearce W. Hypoxic regulation of the fetal cerebral circulation. J Appl Physiol. 2006;100 (2:731–738. doi: 10.1152/japplphysiol.00990.2005. [DOI] [PubMed] [Google Scholar]
- Zurcher SD, Ong-Veloso GL, Akopov SE, Pearce WJ. Maturational modification of hypoxic relaxation in ovine carotid and cerebral arteries: role of endothelium. Biol Neonate. 1998;74 (3:222–232. doi: 10.1159/000014028. [DOI] [PubMed] [Google Scholar]
- Pearce WJ, Ashwal S, Cuevas J. Direct effects of graded hypoxia on intact and denuded rabbit cranial arteries. Am J Physiol. 1989;257 (3 Part 2:H824–H833. doi: 10.1152/ajpheart.1989.257.3.H824. [DOI] [PubMed] [Google Scholar]
- Pearce WJ. Mechanisms of hypoxic cerebral vasodilatation. Pharmacol Ther. 1995;65 (1:75–91. doi: 10.1016/0163-7258(94)00058-b. [DOI] [PubMed] [Google Scholar]
- Baschat DAA. Fetal responses to placental insufficiency: an update. BJOG. 2004;111 (10:1031–1041. doi: 10.1111/j.1471-0528.2004.00273.x. [DOI] [PubMed] [Google Scholar]
- Peeters LL, Sheldon RE, Jones MD, Jr, Makowski EL, Meschia G. Blood flow to fetal organs as a function of arterial oxygen content. Am J Obstet Gynecol. 1979;135 (5:637–646. doi: 10.1016/s0002-9378(16)32989-1. [DOI] [PubMed] [Google Scholar]
- Vyas S, Nicolaides KH, Bower S, Campbell S. Middle cerebral artery flow velocity waveforms in fetal hypoxaemia. Br J Obstet Gynaecol. 1990;97 (9:797–803. doi: 10.1111/j.1471-0528.1990.tb02573.x. [DOI] [PubMed] [Google Scholar]
- Bartha JL, Moya EM, Hervias-Vivancos B. Three-dimensional power Doppler analysis of cerebral circulation in normal and growth-restricted fetuses. J Cereb Blood Flow Metab. 2009;29 (9:1609–1618. doi: 10.1038/jcbfm.2009.70. [DOI] [PubMed] [Google Scholar]
- Story L, Damodaram M, Paramasivam G, Rutherford MSK. Vessel Diameter in Growth Restricted Fetuses. Arch Dis Child Fetal Neonatal Ed. 2009;94 (3:Fa13. [Google Scholar]
- Invernici G, Ponti D, Corsini E, Cristini S, Frigerio S, Colombo A, et al. Human microvascular endothelial cells from different fetal organs demonstrate organ-specific CAM expression. Exp Cell Res. 2005;308 (2:273–282. doi: 10.1016/j.yexcr.2005.04.033. [DOI] [PubMed] [Google Scholar]