Abstract
Different viruses trigger pattern recognition receptor systems, such as Toll-like receptors or cytosolic RIG-I like helicases (RLH), and thus induce early type I interferon (IFN-I) responses. Such responses may confer protection until adaptive immunity is activated to an extent that the pathogen can be eradicated. Interestingly, the same innate immune mechanisms that are relevant for early pathogen defense have a role in ameliorating experimental autoimmune encephalomyelitis (EAE), a rodent model of human multiple sclerosis. We and others found that mice devoid of a component of the IFN-I receptor (Ifnar1−/−) showed significantly enhanced autoimmune disease of the central nervous system (CNS). A detailed analysis revealed that in wild-type mice IFN-I triggering of myeloid cells was instrumental in reducing brain damage. A more recent study indicated that similar to Ifnar1−/− mice, RLH-signaling-deficient mice showed enhanced autoimmune disease of the CNS as well. Moreover, when peripherally treated with synthetic RLH ligands wild-type animals with EAE disease showed reduced clinical scores. Under such conditions, IFN-I receptor triggering of dendritic cells had a crucial role. The therapeutic effect of treatment with RLH ligands was associated with negative regulation of Th1 and Th17 T-cell responses within the CNS. These experiments are consistent with the hypothesis that spatiotemporal conditions of, and cell types involved in, disease-ameliorating IFN-I responses differ significantly, depending on whether they were endogenously induced in the context of EAE pathogenesis within the CNS or upon therapeutic RLH triggering in the periphery. It is attractive to speculate that RLH triggering represents a new strategy to treat multiple sclerosis by stimulating endogenous immunoregulatory IFN-I responses.
Keywords: experimental autoimmune encephalomyelitis, interferon-beta, new therapy approach
During the last decade a detailed understanding of molecular mechanisms associated with the recognition of pathogens has been achieved. In addition to Toll-like receptors, retinoic acid-inducible gene I (RIG I)-like helicases (RLH), nucleotide-binding oligemerization domain-like receptors, C type lectin-like receptors and intracellular DNA receptors, probably other, not-yet-identified mechanisms exist that have a role in pathogen recognition.1 Many signaling pathways of the innate immune system result in the induction of type I interferons (IFN-I) and other cytokines. IFN-I is of particular interest because it is induced within hours after infection, often at high quantities, and it can induce an antiviral state of cells. Virtually all cells of the body express the IFN-I receptor. Among IFN-I, 1 IFN-beta, more than 10 IFN-alphas and a number of less-dominant subtypes are found.2 In cell culture, upon infection basically any cell type mounts IFN-I responses, whereas in vivo certain immune-cell subsets such as dendritic cells (DCs) and in particular plasmacytoid DCs are important IFN-I producers.3
IFN-I responses have a role in pathogen defense on different levels. Besides IFN-I-stimulated cells being less readily infected, they typically show enhanced MHC-I expression levels. Especially antigen-presenting cells such as DCs show an improved maturation and induce optimal T-cell responses upon IFN-I receptor engagement.4, 5, 6 Furthermore, the functions of other immune cells may be affected in that they show modified homing properties7, 8 and enhanced or reduced effector function. It was reported that, upon various infections, antibody production by B cells,9 as well as T-cell expansion and cytokine expression critically requires direct IFN-I receptor triggering. In the context of T-cell stimulations, IFN-I may act as a third signal to further enhance T-cell expansion.10 Furthermore, IFN-I may exert anti-tumoral function.11
In addition to its protective effects in many different infectious diseases, IFN-I responses may also be detrimental12 and confer immunopathology. With regard to the latter aspect, IFN-I has also been shown to enhance inflammatory processes in different autoimmune diseases, such as systemic lupus erythematosus.13 Nevertheless, local IFN-I induction may as well induce immunoregulation and reduce inflammation, as shown in rheumatoid arthritis and multiple sclerosis. In this review, the current view of how IFN-beta treatment affects the disease severity of multiple sclerosis (MS) is summarized. Furthermore, new insights into the role of IFN-I in the rodent model of MS, the experimental autoimmune encephalomyelitis (EAE), are discussed. Finally, new directions of MS treatment strategies are highlighted.
IFN-beta treatment of relapsing–remitting multiple sclerosis
MS is an autoimmune demyelinating disease of the central nervous system (CNS). Disease onset typically occurs in young adults, with increased incidence in women.14 It is believed that long before clinical manifestation, inflammatory T cells specific for antigen structures similar to myelin are activated in the periphery.15 Such cells cross the blood–brain barrier and move into the CNS, where they induce inflammatory processes.16 Therapeutic approaches available today primarily aim at modulating or interfering with these immunological processes. For treatment of relapsing–remitting MS IFN-beta is licensed as a therapeutic. IFN-beta treatment reduces the frequency of clinical exacerbations by approximately 35% and delays the progression of disability.17 However, 30–50% of MS patients do not respond to IFN-beta treatment.18 This is either associated with aberrations in the IFN-I signaling cascade19, 20, 21, 22, 23, 24 or the presence and/or induction of IFN-beta-neuralizing antibody responses.25 In particular, induction of IFN-beta-neutralizing antibody responses constitute a problem that may turn responders into non-responders. The incidence of the induction of IFN-beta-specific antibody responses differs among marketed products and presumably is caused by aggregates, oxidated products, trace amounts of product-related impurities and to a lesser extent by differences in the amino-acid composition and post-translational modifications of the product.26 Considering that IFN-beta treatment may be associated with the induction of adverse effects such as flu-like symptoms, potential liver damage and psychiatric side effects,27 biomarkers that discriminate responders and non-responders even before initiation of the IFN-beta treatment would be helpful; however, these are not yet available.
Although Epstein–Barr virus infection together with a genetic predisposition may be a certain risk factor for disease development, so far the etiology of MS is largely unclear.28 As patients' materials, such as blood and brain samples, are not easily assessable, knowledge about how IFN-beta treatment affects autoimmune inflammation within the CNS is limited.29 In the human system, IFN-beta induces a Th2 shift. This notion is supported by experimental data indicating that upon IFN-beta treatment myelin basic protein-reactive human T-cell clones show reduced activity and a Th2 shift.30 Similarly, upon in vitro IFN-beta stimulation of peripheral blood mononuclear cells and after IFN-beta treatment of healthy volunteers or MS patients, enhanced IL-10 expression was detected.31 On the other hand, in 60% of MS patients treated with IFN-beta, enhanced percentages of proinflammatory Th1 cells were observed that produced IFN-gamma,32 indicating that IFN-beta may also induce proinflammatory pathways. In more recent studies it was verified that under IFN-beta treatment not only anti-inflammatory but also Th1-associated gene signatures were found.33, 34 With the discovery of a new inflammatory Th subset, Th17 cells, several IFN-beta-mediated effects also on Th17 have been found. Elevated levels of IL-17 have been reported in peripheral blood mononuclear cells, cerebrospinal fluid and active CNS lesions of MS patients.35, 36 IFN-beta enhances activation-induced apoptosis in Th17 but not in Th1 cells. This can be explained by the enhanced IFN-I receptor expression levels in Th17 cells compared with Th1 cells.37 A direct IFN-beta treatment of naive CD4+CD45RA+ T cells cultured under Th17-polarizing conditions resulted in downregulation of RORγc, IL-17A and IL-23R, but upregulation of IL-10 gene expression, and had no effects on the Th1 and Th2-associated transcription factors T-bet and GATA-3.38 Furthermore, IFN-beta-stimulated DC showed a reduced IL-23 and IL-1β production and an enhanced IL-27 secretion.38, 39 In remitting–relapsing MS patients IFN-beta non-responders showed higher IL-17F serum levels than responders.40 Collectively, data showing IFN-beta effects on Th1 as well as on Th2 and Th17 cells indicate that in humans the immunomodulatory function of IFN-beta treatment cannot simply be explained by the induction of a Th1/Th17 to Th2 shift, but that more complex mechanisms have a role.
Experimental autoimmune encephalomyelitis, a rodent model of multiple sclerosis
For analysis of MS-associated disease mechanisms the EAE in rodents is a broadly accepted animal model. In order to induce EAE, mice or rats are immunized subcutaneously with MOG35−55 peptide in Freund's complete adjuvant (CFA). Typically, 10–15 days later, clinical signs of CNS autoimmunity manifest that slightly improve with time. In sterile inflammation of the CNS it is generally believed that Th1 and Th17 cells confer disease, while Th2 cells are protective.41 More recent data suggest that Th1 and Th17 cells concomitantly induce autoimmunity and confer different pathogenic processes within the CNS.42, 43 Generally, Th17 cells seem to be more plastic compared with Th1 cells. Th17 cells pass through different phases of differentiation, including induction, amplification and stabilization. Induction is mediated by TGF-beta and IL-6, whereas amplification is mediated by IL-21, and terminal differentiation and stabilization is conferred by IL-23.44 Interestingly, none of the known Th17 signature cytokines including IL-17A, IL-17F, IL-21 and IL-22 have been found to be mandatory for the development of EAE. Instead, granulocyte macrophage colony-stimulating factor that is induced by IL-23 fulfills the criteria of an encephalitogenic cytokine.45
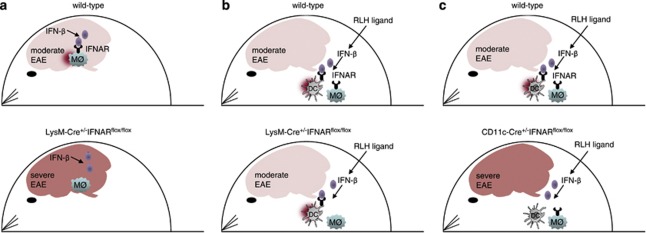
Upon EAE induction in Ifnar1−/− mice that are devoid of the IFN-I receptor, clinical signs of autoimmunity became apparent with similar kinetics as observed in wild-type controls.46 Of note, the overall disease score was significantly enhanced in IFN-I receptor-deficient mice in a manner that even occasional death was observed under conditions where wild-type mice developed only moderate symptoms. Furthermore, disease severity remained elevated also at later time points. These observations were in line with earlier experiments in which Ifnb−/− mice devoid of the IFN-beta gene were used for EAE studies. In these experiments also Ifnb−/− mice showed enhanced disease and an increased influx of mononuclear cells into the CNS.47 Interestingly, in diseased animals Prinz et al.46 detected enhanced IFN-beta levels exclusively within the CNS but not in the serum of diseased wild-type animals. These observations are compatible with the model that during the course of EAE an IFN-beta response is induced within the CNS that locally stimulates cells and thus modulates inflammatory processes within inflammatory foci. EAE studies in Ifnar1−/− mice are limited by the fact that the IFN-I receptor is expressed basically on all cells of the body. To study whether T cells, monocytes, neurons or some other cell type had to be IFN-I-stimulated in order to reduce disease, mice with a cell type-specific deletion of the Ifnar1 gene were generated. This was accomplished by flanking the transmembranic exon 10 of the Ifnar1 chain by loxP sites in a manner that upon Cre-mediated deletion of exon 10 a frame shift was induced that resulted in a truncated Ifnar1 chain devoid of the transmembranic and cytoplasmatic signaling domain.7 Such Ifnar1fl/fl mice carrying two conditional alleles showed a similar IFN-I signaling as compared with wild-type mice, whereas conditional mice carrying two alleles with a deleted exon 10 showed an defective IFN-I signaling as observed in Ifnar1−/− mice.7 Analysis of mice with a cell type-specific IFN-I receptor deletion revealed that IFN-I receptor engagement neither of T cells nor of neurons had a role. Instead, IFN-I receptor signaling of myeloid cells comprising monocytes, macrophages, microglia and granulocytes was critically involved in amelioration of the overall disease score46 (for a schematic depiction, see Figure 1a). A detailed analysis revealed that IFN-beta stimulation of monocytes reduced lipopolysaccharide (LPS)-induced chemokine production. Furthermore, in mice with a myeloid cell-specific Ifnar1 deletion, peripheral myeloid cells showed an overall activated phenotype as also demonstrated by enhanced MHC-II expression.
Figure 1.
Schematic depiction of the cellular mechanisms of endogenously induced versus therapeutically triggered type I interferon responses that modulate Th1/Th17-mediated autoimmunity in the CNS. (a) In EAE, IFN-I receptor engagement of myeloid cells (MQ) is critical to ameliorate disease burden. (b, c) Upon treatment with RLH ligands, therapeutic effects are (b) independent of IFN-I receptor triggering of myeloid cells (c) and dependent on IFN-I receptor triggering of DCs.
Similar to the observation in humans, exogenously administered IFN-beta can suppress EAE in wild-type mice.48, 49 In this context, it is interesting that IFN-beta treatment significantly attenuated the progression of EAE symptoms in Th1-induced EAE, whereas the symptoms of Th17-induced EAE were exacerbated.40 IFN-I can directly inhibit TGF-beta/IL-6-induced Th17 development. This is demonstrated by the fact that IFN-I receptor deficiency resulted in elevated numbers of encephalitogenic Th17 cells.50 The finding that LPS-stimulated bone marrow-derived macrophages or DC devoid of IFN-I receptor showed a defective IL-27 production51 suggested that upon EAE induction in Ifnar1−/− mice IL-27 responses also were flawed. As IL-27 suppresses differentiation of Th17 cells and promotes T cells to secrete IL-10, defective IL-27 responses further promote a Th2 shift. Additionally, IFN-I stimulation inhibits IL-23-dependent Th17 expansion.52 As pointed out above, the currently known Th17 signature cytokines IL-17A, IL-17F, IL-21 and IL-22 have not been found to have a critical role in promoting the development of EAE. Instead, IL-23-induced Th17 cells produce granulocyte macrophage colony-stimulating factor (GM-CSF) that seems to be important in conferring disease.45 This cytokine stimulates invading myeloid cells, which in turn promote and sustain inflammation within the CNS.53 For a better understanding of how the IFN-beta stimulation of myeloid cells affects their responsiveness to GM-CSF stimulation, additional studies are necessary.
Therapeutic potential of treatment with RLH ligands
Similar to Ifnar1−/− mice, Ips-1−/− mice (also referred to as Cardif−/−, Mavs−/− or Visa−/−)54 devoid of the RLH system showed enhanced autoimmune inflammatory disease of the CNS.55 By analogy to the above-discussed experiments with Ifnar1−/− mice, this observation argues for the possibility that IFN-I responses induced by peripheral RLH triggering might show some therapeutic effect. Indeed, disease severity was markedly reduced in RIG-I or MDA5-stimulated mice.55 This therapeutic effect was only observed in IFN-I receptor-competent mice, indicating that IFN-I is the key effector cytokine. Interestingly, engagement of the IFN-I receptor on myeloid cells including monocytes, macrophages, microglia or granulocytes did not account for the suppression of CNS autoimmunity upon treatment with RLH ligands. Instead, IFN-I receptor expression on DCs was essential under such conditions (for a schematic depiction, compare Figures 1b and c). Treatment with RLH ligands inhibited proliferation and induced apoptosis of MOG35−55-specific Th1 and Th17 cells. Of note, the experimental data obtained so far do not support the idea that RLH ligand treatment affects the frequency or the function of CD4+Foxp3+Treg cells. Currently, the hypothesis that soluble factors such as IFN-I and IL-27 secreted by RLH-stimulated DCs inhibit encephalitogenic T-cell responses is favored. This was demonstrated in mice with EAE disease showing a conditional deletion of the IFN-I receptor on DCs that were no longer responsive to RLH ligand treatment. On the contrary, mice with a conditional deletion of the IFN-I receptor on myeloid cells showed even enhanced disease, as observed earlier,46 and still responded to RLH ligand therapy. In conclusion, IFN-I receptor engagement of DCs is crucially required to confer therapeutic effects in the CNS, whereas IFN-I receptor engagement of myeloid cells is not required. These observations are in contrast to the disease-ameliorating effects of IFN-I responses induced in the context of EAE pathogenesis, where IFN-I receptor triggering of myeloid cells had a crucial role (for a schematic depiction, see Figure 1). This can probably be explained by the fact that therapeutic RLH ligand treatment elicits IFN-I responses primarily in peripheral lymph nodes, whereas in EAE pathogenesis IFN-beta responses are exclusively observed locally within the CNS. Of note, endogenous IFN-I responses do not induce antibodies against IFN-I, thus ensuring that the efficacy of therapeutic RLH ligand treatment is maintained even after repeated treatments.
Perspectives
Analysis of a rodent model of MS revealed the significance of IFN-I in ameliorating autoimmune inflammation within the CNS. Under such conditions IFN-I receptor engagement of myeloid cells had a critical role in improvement of the overall physical status. Future experiments will reveal which myeloid cell subset, and whether perhaps other professional antigen-presenting cells, has to be IFN-I receptor-triggered in order to confer disease amelioration. Interestingly, RLH-deficient mice showed an as much enhanced autoimmune disease as IFN-I receptor-deficient mice, suggesting that RLH triggering might improve disease. Indeed, peripheral treatment with complexed RNA of wild-type mice with EAE disease induced a significant overall improvement. This effect was dependent on IFN-I triggering of DCs. Considering that approximately 30% of MS patients under IFN-beta therapy develop IFN-beta neutralizing antibody responses that reduce efficacy of IFN-beta treatment, the above-summarized results suggest that RLH triggering might be a new strategy to induce IFN-I responses that have an impact on MS and would not induce auto-antibody responses. This is due to the fact that in vivo bioactive IFN-I is freshly produced by stimulated cells and upon secretion is transported by the lymph or the blood stream to the site of action. In the periphery, most of the recirculating IFN-I is absorbed within the liver, resulting in a rapid decay of bioactive IFN-I in the blood within 1–2 days. Before the RLH ligand treatment concept can be further evaluated in patients, it will be of interest to study whether RLH ligand treatment can be modified in a manner that IFN-I responses within the CNS are selectively triggered. New RLH ligand formulations might be helpful in reaching this objective. RLH ligand treatment of DCs induces IFN-beta as well as IFN-alpha responses. Therefore it might be interesting to study whether RLH ligands can be modified in such a way that only IFN-beta or IFN-alpha responses are elicited and whether both responses would show a similar impact on the disease course. Furthermore, specific targeting of relevant antigen-presenting cells by RLH ligand treatment might be attractive because such a strategy probably would induce less fulminant cytokine responses which would cause reduced overall adverse effects. A detailed analysis of DC-mediated effects that affect the balance between Th1/Th17 and Th2 responses and disease severity would help to further refine potential therapeutic strategies. In conclusion, the recent observation that RLH ligand treatment has an ameliorating effect on autoimmune inflammation of the CNS in a rodent model might pave the way for new therapeutic strategies of MS by inducing endogenous IFN-I responses.
Acknowledgments
We thank Theresa Frenz for critically reading the manuscript and for help with the artwork.
References
- Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Takaoka A, Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234:142–162. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft T, Pang KC, Thomas E, Hertzog P, Hart DN, Trapani J, et al. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–1953. [PubMed] [Google Scholar]
- Longhi MP, Trumpfheller C, Idoyaga J, Caskey M, Matos I, Kluger C, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med. 2009;206:1589–1602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlato S, Romagnoli G, Spadaro F, Canini I, Sirabella P, Borghi P, et al. LOX-1 as a natural IFN-alpha-mediated signal for apoptotic cell uptake and antigen presentation in dendritic cells. Blood. 2010;115:1554–1563. doi: 10.1182/blood-2009-07-234468. [DOI] [PubMed] [Google Scholar]
- Kamphuis E, Junt T, Waibler Z, Forster R, Kalinke U. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood. 2006;108:3253–3261. doi: 10.1182/blood-2006-06-027599. [DOI] [PubMed] [Google Scholar]
- Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- Coro ES, Chang WL, Baumgarth N. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J Immunol. 2006;176:4343–4351. doi: 10.4049/jimmunol.176.7.4343. [DOI] [PubMed] [Google Scholar]
- Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- Bracarda S, Eggermont AM, Samuelsson J. Redefining the role of interferon in the treatment of malignant diseases. Eur J Cancer. 2010;46:284–297. doi: 10.1016/j.ejca.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Carrero JA, Calderon B, Unanue ER. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J Exp Med. 2004;200:535–540. doi: 10.1084/jem.20040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akeno N, Smith EP, Stefan M, Huber AK, Zhang W, Keddache M, et al. IFN-alpha mediates the development of autoimmunity both by direct tissue toxicity and through immune cell recruitment mechanisms. J Immunol. 2011;186:4693–4706. doi: 10.4049/jimmunol.1002631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann P, Toyka KV, Bassetti C, Beer K, Beer S, Buettner U, et al. Escalating immunotherapy of multiple sclerosis—new aspects and practical application. J Neurol. 2004;251:1329–1339. doi: 10.1007/s00415-004-0537-6. [DOI] [PubMed] [Google Scholar]
- Chastain EM, Miller SD. Molecular mimicry as an inducing trigger for CNS autoimmune demyelinating disease. Immunol Rev. 2012;245:227–238. doi: 10.1111/j.1600-065X.2011.01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R, Steinman L. Innate and adaptive autoimmunity directed to the central nervous system. Neuron. 2009;64:123–132. doi: 10.1016/j.neuron.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Tourbah A, Lyon-Caen O. Interferons in multiple sclerosis: ten years' experience. Biochimie. 2007;89:899–902. doi: 10.1016/j.biochi.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Rio J, Nos C, Tintore M, Tellez N, Galan I, Pelayo R, et al. Defining the response to interferon-beta in relapsing-remitting multiple sclerosis patients. Ann Neurol. 2006;59:344–352. doi: 10.1002/ana.20740. [DOI] [PubMed] [Google Scholar]
- Comabella M, Craig DW, Morcillo-Suarez C, Rio J, Navarro A, Fernandez M, et al. Genome-wide scan of 500 000 single-nucleotide polymorphisms among responders and nonresponders to interferon beta therapy in multiple sclerosis. Arch Neurol. 2009;66:972–978. doi: 10.1001/archneurol.2009.150. [DOI] [PubMed] [Google Scholar]
- Vosslamber S, van der Voort LF, van den Elskamp IJ, Heijmans R, Aubin C, Uitdehaag BM, et al. Interferon regulatory factor 5 gene variants and pharmacological and clinical outcome of Interferonbeta therapy in multiple sclerosis. Genes Immun. 2011;12:466–472. doi: 10.1038/gene.2011.18. [DOI] [PubMed] [Google Scholar]
- Cunningham S, Graham C, Hutchinson M, Droogan A, O'Rourke K, Patterson C, et al. Pharmacogenomics of responsiveness to interferon IFN-beta treatment in multiple sclerosis: a genetic screen of 100 type I interferon-inducible genes. Clin Pharmacol Ther. 2005;78:635–646. doi: 10.1016/j.clpt.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Wergeland S, Beiske A, Nyland H, Hovdal H, Jensen D, Larsen JP, et al. IL-10 promoter haplotype influence on interferon treatment response in multiple sclerosis. Eur J Neurol. 2005;12:171–175. doi: 10.1111/j.1468-1331.2004.01102.x. [DOI] [PubMed] [Google Scholar]
- Byun E, Caillier SJ, Montalban X, Villoslada P, Fernandez O, Brassat D, et al. Genome-wide pharmacogenomic analysis of the response to interferon beta therapy in multiple sclerosis. Arch Neurol. 2008;65:337–344. doi: 10.1001/archneurol.2008.47. [DOI] [PubMed] [Google Scholar]
- Forster S. Interferon signatures and immune responses in disease. Immunol Cell Biol. 2012;90:520–527. doi: 10.1038/icb.2012.12. [DOI] [PubMed] [Google Scholar]
- Hesse D, Sorensen PS. Using measurements of neutralizing antibodies: the challenge of IFN-beta therapy. Eur J Neurol. 2007;14:850–859. doi: 10.1111/j.1468-1331.2007.01769.x. [DOI] [PubMed] [Google Scholar]
- van Beers MM, Jiskoot W, Schellekens H. On the role of aggregates in the immunogenicity of recombinant human interferon beta in patients with multiple sclerosis. J Interferon Cytokine Res. 2010;30:767–775. doi: 10.1089/jir.2010.0086. [DOI] [PubMed] [Google Scholar]
- Goeb JL, Even C, Nicolas G, Gohier B, Dubas F, Garre JB. Psychiatric side effects of interferon-beta in multiple sclerosis. Eur Psychiatry. 2006;21:186–193. doi: 10.1016/j.eurpsy.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Sundqvist E, Sundstrom P, Linden M, Hedstrom AK, Aloisi F, Hillert J, et al. Epstein-Barr virus and multiple sclerosis: interaction with HLA. Genes Immun. 2012;13:14–20. doi: 10.1038/gene.2011.42. [DOI] [PubMed] [Google Scholar]
- Axtell RC, Raman C, Steinman L. Interferon-beta exacerbates Th17-mediated inflammatory disease. Trends Immunol. 2011;32:272–277. doi: 10.1016/j.it.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozovska ME, Hong J, Zang YC, Li S, Rivera VM, Killian JM, et al. Interferon beta induces T-helper 2 immune deviation in MS. Neurology. 1999;53:1692–1697. doi: 10.1212/wnl.53.8.1692. [DOI] [PubMed] [Google Scholar]
- Rudick RA, Ransohoff RM, Peppler R, VanderBrug Medendorp S, Lehmann P, Alam J. Interferon beta induces interleukin-10 expression: relevance to multiple sclerosis. Ann Neurol. 1996;40:618–627. doi: 10.1002/ana.410400412. [DOI] [PubMed] [Google Scholar]
- Dayal AS, Jensen MA, Lledo A, Arnason BG. Interferon-gamma-secreting cells in multiple sclerosis patients treated with interferon beta-1b. Neurology. 1995;45:2173–2177. doi: 10.1212/wnl.45.12.2173. [DOI] [PubMed] [Google Scholar]
- Satoh J, Nanri Y, Tabunoki H, Yamamura T. Microarray analysis identifies a set of CXCR3 and CCR2 ligand chemokines as early IFNbeta-responsive genes in peripheral blood lymphocytes in vitro: an implication for IFNbeta-related adverse effects in multiple sclerosis. BMC Neurol. 2006;6:18. doi: 10.1186/1471-2377-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandinger KP, Sturzebecher CS, Bielekova B, Detore G, Rosenwald A, Staudt LM, et al. Complex immunomodulatory effects of interferon-beta in multiple sclerosis include the upregulation of T helper 1-associated marker genes. Ann Neurol. 2001;50:349–357. doi: 10.1002/ana.1096. [DOI] [PubMed] [Google Scholar]
- Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- Durelli L, Conti L, Clerico M, Boselli D, Contessa G, Ripellino P, et al. T-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-beta. Ann Neurol. 2009;65:499–509. doi: 10.1002/ana.21652. [DOI] [PubMed] [Google Scholar]
- Ramgolam VS, Sha Y, Jin J, Zhang X, Markovic-Plese S. IFN-beta inhibits human Th17 cell differentiation. J Immunol. 2009;183:5418–5427. doi: 10.4049/jimmunol.0803227. [DOI] [PubMed] [Google Scholar]
- Sweeney CM, Lonergan R, Basdeo SA, Kinsella K, Dungan LS, Higgins SC, et al. IL-27 mediates the response to IFN-beta therapy in multiple sclerosis patients by inhibiting Th17 cells. Brain Behav Immun. 2011;25:1170–1181. doi: 10.1016/j.bbi.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P, et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchroo VK, Anderson AC, Waldner H, Munder M, Bettelli E, Nicholson LB. T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire. Annu Rev Immunol. 2002;20:101–123. doi: 10.1146/annurev.immunol.20.081701.141316. [DOI] [PubMed] [Google Scholar]
- Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, et al. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- Prinz M, Schmidt H, Mildner A, Knobeloch KP, Hanisch UK, Raasch J, et al. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity. 2008;28:675–686. doi: 10.1016/j.immuni.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Teige I, Treschow A, Teige A, Mattsson R, Navikas V, Leanderson T, et al. IFN-beta gene deletion leads to augmented and chronic demyelinating experimental autoimmune encephalomyelitis. J Immunol. 2003;170:4776–4784. doi: 10.4049/jimmunol.170.9.4776. [DOI] [PubMed] [Google Scholar]
- Brod SA, Burns DK. Suppression of relapsing experimental autoimmune encephalomyelitis in the SJL/J mouse by oral administration of type I interferons. Neurology. 1994;44:1144–1148. doi: 10.1212/wnl.44.6.1144. [DOI] [PubMed] [Google Scholar]
- Brod SA, Khan M. Oral administration of IFN-alpha is superior to subcutaneous administration of IFN-alpha in the suppression of chronic relapsing experimental autoimmune encephalomyelitis. J Autoimmun. 1996;9:11–20. doi: 10.1006/jaut.1996.0003. [DOI] [PubMed] [Google Scholar]
- Guo B, Chang EY, Cheng G. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J Clin Invest. 2008;118:1680–1690. doi: 10.1172/JCI33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Mildner A, Mack M, Schmidt H, Bruck W, Djukic M, Zabel MD, et al. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain. 2009;132:2487–2500. doi: 10.1093/brain/awp144. [DOI] [PubMed] [Google Scholar]
- Michallet MC, Meylan E, Ermolaeva MA, Vazquez J, Rebsamen M, Curran J, et al. TRADD protein is an essential component of the RIG-like helicase antiviral pathway. Immunity. 2008;28:651–661. doi: 10.1016/j.immuni.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Dann A, Poeck H, Croxford AL, Gaupp S, Kierdorf K, Knust M, et al. Cytosolic RIG-I-like helicases act as negative regulators of sterile inflammation in the CNS. Nat Neurosci. 2011;15:98–106. doi: 10.1038/nn.2964. [DOI] [PubMed] [Google Scholar]