Abstract
Pre-renal acute kidney injury (AKI) is assumed to represent a physiological response to underperfusion. Its diagnosis is retrospective after a transient rise in plasma creatinine, usually associated with evidence of altered tubular transport, particularly that of sodium. In order to test whether pre-renal AKI is reversible because injury is less severe than that of sustained AKI, we measured urinary biomarkers of injury (cystatin C, neutrophil gelatinase-associated lipocalin (NGAL), γ-glutamyl transpeptidase, IL-18, and kidney injury molecule-1 (KIM-1)) at 0, 12, and 24 h following ICU admission. A total of 529 patients were stratified into groups having no AKI, AKI with recovery by 24 h, recovery by 48 h, or the composite of AKI greater than 48 h or dialysis. Pre-renal AKI was identified in 61 patients as acute injury with recovery within 48 h and a fractional sodium excretion <1%. Biomarker concentrations significantly and progressively increased with the duration of AKI. After restricting the AKI recovery within the 48 h cohort to pre-renal AKI, this increase remained significant. The median concentration of KIM-1, cystatin C, and IL-18 were significantly greater in pre-renal AKI compared with no-AKI, while NGAL and γ-glutamyl transpeptidase concentrations were not significant. The median concentration of at least one biomarker was increased in all but three patients with pre-renal AKI. Thus, the reason why some but not all biomarkers were increased requires further study. The results suggest that pre-renal AKI represents a milder form of injury.
Keywords: acute kidney injury, acute renal failure, creatinine
The concept of ‘pre-renal azotaemia' is ingrained into the clinical practice of nephrology.1 However, the distinction between pre-renal acute kidney injury (AKI) when used to classify a group of causes of AKI leading to renal underperfusion and pre-renal AKI as a distinct and uniquely reversible functional type of AKI, rather than AKI due to parenchymal injury, has often been a source of confusion and controversy.1, 2 The distinction is potentially important because it is argued that correction of ‘pre-renal factors' causing renal underperfusion may reverse pre-renal AKI, whereas neglecting these causes AKI to become irreversible. To some, the diagnosis automatically implies a need for intravenous fluids, although the cause may be right heart failure or cirrhosis. To others, the ‘pre-renal' state is not of particular concern, and yet there is evidence that, even if AKI is reversed, there remains an increased potential for a range of adverse events including renal toxicity, volume overload, acid–base and electrolyte imbalance, abnormal drug elimination, and mortality, particularly on the background of chronic kidney disease.3, 4 Further confusion arises from the name, ‘acute tubular necrosis', being used to describe ‘established' pre-renal AKI, implying severe structural injury, despite a lack of obvious necrosis in most human biopsies.
Reversal of AKI in less than 24–72 h after fluid replacement is the most common definition of pre-renal AKI.5, 6, 7, 8, 9, 10 From a clinical perspective, where the aim is to triage patients as soon as possible, waiting for reversal delays both diagnosis and appropriate intervention in those patients who require more than rehydration to beyond the treatment window of all potential renoprotective pharmaceutical therapies.11 We require clear criteria for the prospective diagnosis of AKI, rather than diagnosis by exclusion. Although reversibility could reflect structural integrity,1, 10 there is actually little evidence for or against renal structural integrity in pre-renal AKI.2, 5, 12 A low fractional excretion of sodium (FENa) and/or urea (FEurea)9, 13 are often used to define pre-renal AKI, although diuretics and sepsis make interpretation of the FENa difficult2, 14 and aging and sepsis alter FEurea.14, 15
We hypothesized that evidence of structural injury in patients classified as having pre-renal AKI would suggest that pre-renal AKI is simply part of the continuum of AKI. In that case, reversibility might be explained simply by less severe injury. We examined this by prospectively comparing urinary biomarkers of AKI reported to differentiate pre-renal and established AKI,8, 16, 17, 18 interleukin-18 (IL-18), neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), cystatin C, and γ-glutamyl transpeptidase (GGT), in a cohort of critically ill patients.
RESULTS
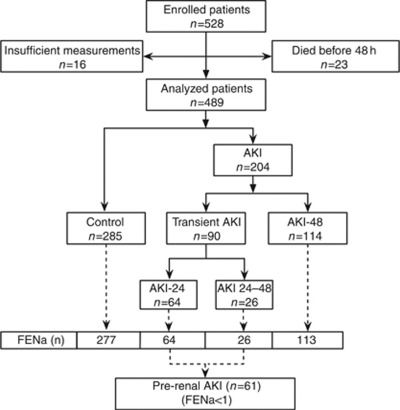
Of 528 patients enrolled in the study, 489 patients had consecutive plasma creatinine measurements (Figure 1). In all, 204 patients satisfied the Acute Kidney Injury Network plasma creatinine definition of AKI, whereas the remaining patients were classified as no-AKI (n=285). Ninety patients had transient AKI (TAKI), defined as reversal within 48 h. Sixty-one TAKI patients had FENa<1 and were therefore classified as pre-renal AKI. Among those not classified, 23 died before 48 h and 16 had insufficient measurements for classification. Clinical characteristics of the cohort as a whole have been given in detail elsewhere.19, 20 Briefly, patients were 61±17 years old, 40.3% women, 61.5% with FENa<1%, 18.8% with preexisting CKD, 90.2% on mechanical ventilation, 19.4% with sepsis, and 19% followed cardiac surgery. Pre-renal AKI and AKI-48 patients had progressively more severe illness, more CKD, and higher median baseline creatinine values than no-AKI patients (Table 1).
Figure 1.
Patient flow and numbers (n). Fractional excretion of sodium (FENa; n): patients for whom a fractional excretion of sodium was measured. Acute kidney injury (AKI) 24: recovery within 24 h. AKI 24–48: recovery 24–48 h. AKI-48: no recovery within 48 h or renal replacement therapy. Pre-renal AKI: transient AKI and FENa<1.
Table 1. Clinical characteristics and outcomes.
| Demographic | No-AKI | Pre-renal | AKI-48 | P |
|---|---|---|---|---|
| Age, mean (s.d.), years | 60 (17) | 58 (17) | 65 (16) | 0.011 |
| Female, % (no.) | 43.9 (125) | 37.7 (23) | 30.7 (35) | 0.061 |
| Weight, mean (s.d.), kg | 78 (19) | 83 (19) | 85 (21) | 0.0028 |
| APACHE II, mean (s.d.) | 16 (5) | 18 (6) | 21 (6) | <0.0001 |
| SOFA, mean (s.d.) | 5.4 (2.4) | 6.7 (2.7) | 8.1 (2.9) | <0.0001 |
| FENa, median (IQR), % | 0.68 (0.28–1.7) | 0.25 (0.13–0.50) | 0.76 (0.34–1.7) | |
| FEurea, median (IQR), %a | 45 (33–60) | 32 (17–42) | 29 (18–40) | <0.0001 |
| FENa<1, % (no.) | 58.6 (167) | 100 (61) | 58.8 (67) | |
| FEurea <35, % (no.)a | 16.1 (46) | 42.6 (26) | 43.9 (50) | <0.0001 |
| Baseline plasma creatinine, median (IQR), mg/dl | 0.79 (0.68–1.0) | 0.83 (0.68–1.0) | 0.99 (0.79–1.2) | <0.0001 |
| AKI on admission | — | 70.5 (43) | 70.2 (80) | |
| CKD on admission, % (no.), baseline eGFR<60 ml/min | 17.2 (49) | 13.1 (8) | 28.1 (32) | 0.026 |
| Mechanical ventilation, % (no.) | 90.9 (259) | 82 (50) | 91.2 (104) | 0.79 |
| Hypotension, % (no.) | 51 (144) | 51 (31) | 61 (70) | 0.062 |
| Diagnostic class | ||||
| Abdominal aortic aneurysm rupture and repair | 1.8 (5) | 3.3 (2) | 11 (13) | |
| Abdominal surgery or inflammation | 12 (33) | 12 (7) | 8.8 (10) | |
| Burns | 0.35 (1) | 3.3 (2) | 0 | |
| Cardiac arrest or failure | 11 (31) | 15 (9) | 6.1 (7) | |
| Cardiac surgery | 20 (57) | 8.2 (5) | 20 (23) | |
| Collapse, cause unknown | 0.35 (1) | 3.3 (2) | 0 | |
| Neurological surgery, injury, seizure, or hemorrhage | 15.8 (9) | 15 (9) | 4.4 (5) | |
| Other | 0.7 (2) | 0 | 0 | |
| Pulmonary or thoracic surgery or failure | 13 (37) | 9.8 (6) | 17 (19) | |
| Sepsis | 15 (43) | 26 (16) | 28 (32) | |
| Trauma | 11 (30) | 4.9 (3) | 4.4 (5) |
Abbreviations: AKI, acute kidney injury; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; FENa, fractional excretion of sodium; FEurea, fractional excretion of urea; IQR, interquartile range.
Available from one center only (no-AKI, n=162; pre-renal AKI, n=43; AKI-48, n=77).
P-values are one-way ANOVA or χ2 test for trend.
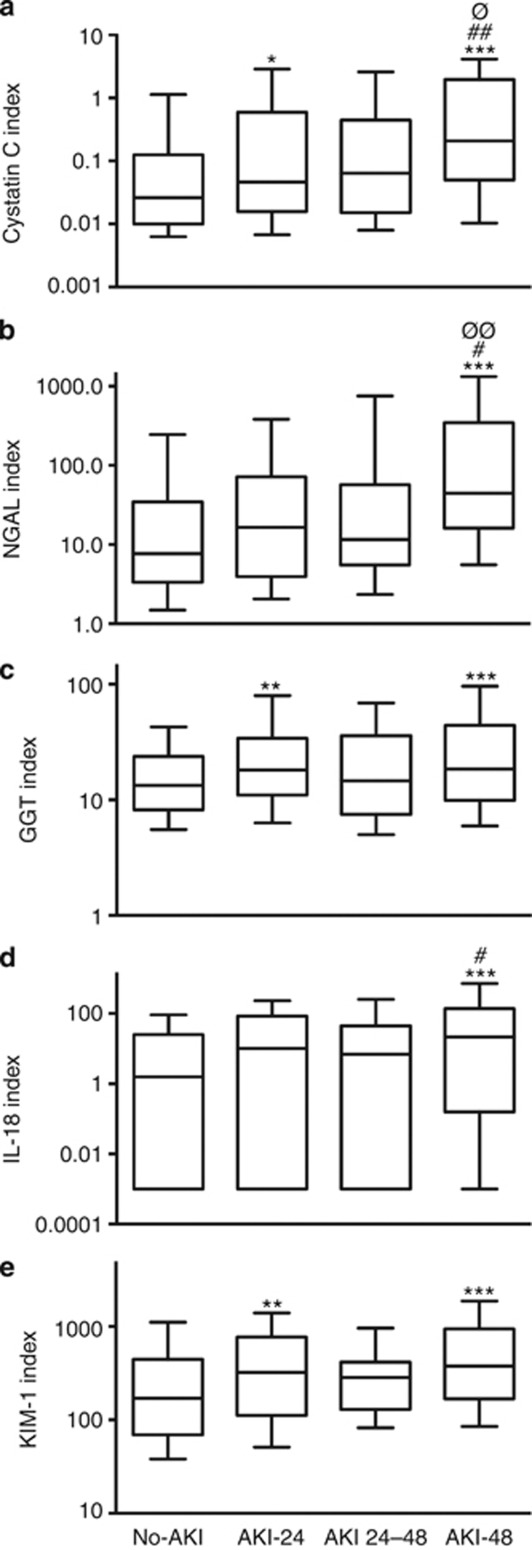
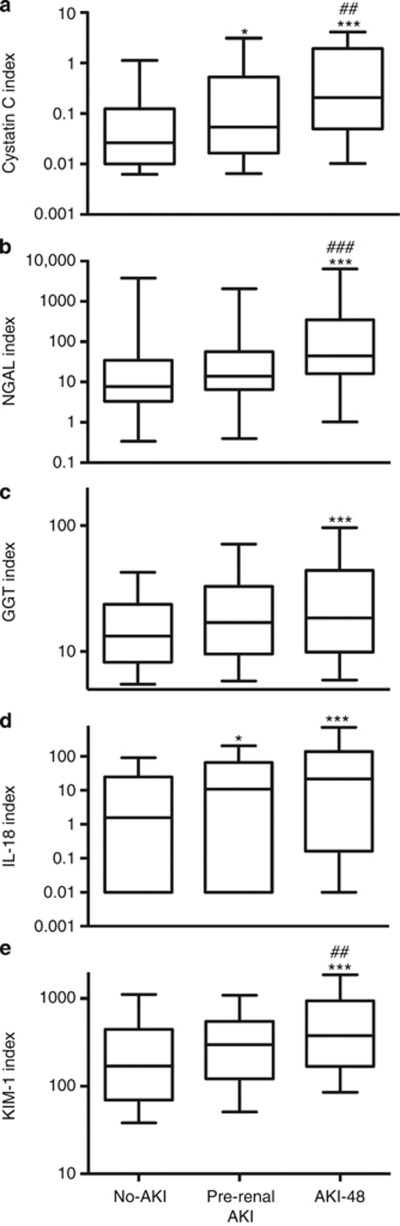
All biomarker concentrations increased progressively with increasing duration of AKI (analysis of variance, ANOVA, P<0.001, Figure 2). After restricting the TAKI cohort to patients with FENa<1% (pre-renal AKI), this increase remained (ANOVA, P<0.001 for cystatin C (CysC), NGAL, KIM-1, IL-18, and P<0.01 for GGT following adjustment for covariates, Figure 3). At least one biomarker concentration was increased in all but three patients with pre-renal AKI (see below). All biomarkers had greater median concentrations in pre-renal AKI than in no-AKI, and the KIM-1, CysC, and IL-18 distributions differed from no-AKI in post-hoc analysis (Mann–Whitney, P<0.05, Table 2).
Figure 2.
Effect of duration of acute kidney injury (AKI) on urinary biomarker concentration. Cystatin C (CysC) (a), neutrophil gelatinase-associated lipocalin (NGAL) (b), γ-glutamyl transpeptidase (GGT) (c), interleukin (IL)-18 (d), and kidney injury molecule (KIM)-1 (e). All biomarkers, P<0.001 (analysis of variance). Post-hoc difference from no-AKI*, AKI 24–48, or AKI-48 from AKI-24#, AKI 24–48 vs. AKI-48∅ (*,#,∅P<0.05, **,##,∅∅P<0.01, ***P<0.001). Whiskers 10th–90th percentile.
Figure 3.
Effect of pre-renal acute kidney injury (AKI) and AKI-48 on urinary biomarker concentration. Cystatin C (CysC) (a), neutrophil gelatinase-associated lipocalin (NGAL) (b), γ-glutamyl transpeptidase (GGT) (c), interleukin (IL)-18 (d), and kidney injury molecule (KIM)-1 (e). All biomarkers, P<0.001 (analysis of variance). Post-hoc least significant difference from no-AKI*, from pre-renal AKI# (*P<0.05, ##P<0.01, ***,###P<0.001). Whiskers 10th–90th percentile.
Table 2. Urinary biomarker concentrationsa.
| Biomarker | No-AKI (n=285)b | Pre-renal AKI (n=61)c | Pre-renal AKI vs. no-AKI P-value | AKI 48 (n=114)d | AKI 48 vs. no-AKI P-value | AKI 48 vs. pre-renal AKI P-value | Test for trend P-value (ANOVA)e |
|---|---|---|---|---|---|---|---|
| CysC, median (IQR) mg/mmolCr | 0.026 (0.010–0.12) | 0.054 (0.017–0.53) | 0.017 | 0.21 (0.05–1.9) | <0.001 | 0.0097 | <0.001 |
| NGAL, median (IQR) μg/mmolCr | 7.7 (3.3–35) | 14 (6.5–56) | 0.052 | 44 (16–345) | <0.001 | <0.001 | <0.001 |
| GGT, median (IQR) U/mmolCr | 13 (8.2–24) | 17 (9.6–33) | 0.097 | 18.5 (9.9–45) | <0.001 | 0.25 | <0.01 |
| IL-18, median (IQR) ng/mmolCr | 1.6 (0.001–25) | 10.9 (0.001–66) | 0.017 | 22 (0.16–137) | <0.001 | 0.053 | <0.001 |
| KIM-1, median (IQR) μg/mmolCr | 170 (69–445) | 297 (121–549) | 0.028 | 376 (169–943) | <0.001 | 0.034 | <0.001 |
Abbreviations: AKI, acute kidney injury; CysC, cystatin C; GGT, γ-glutamyl transpeptidase; IL, interleukin; IQR, interquartile range; KIM, kidney injury molecule; NGAL, neutrophil gelatinase-associated lipocalin.
Biomarker concentrations shown are the median (IQR) of the maximum concentration indexed to creatinine within 24 h of admission.
For all biomarkers except NGAL: n=280.
For all biomarkers except NGAL: n=57.
For all biomarkers except NGAL: n=103.
After adjustment for age, sex, APACHE II score, and estimated baseline glomerular filtration rate and sepsis.
Significance is vs. no-AKI in each case.
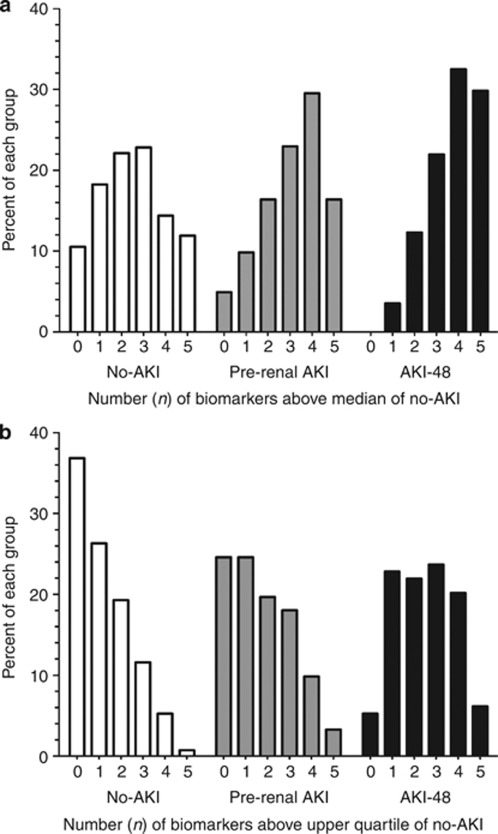
Pre-renal AKI and AKI-48 patients had progressively greater proportions of biomarkers above the median of the no-AKI group (Figure 4). We analyzed the pre-renal AKI and AKI-48 patients with no evidence of increased biomarker concentrations (no biomarker above the median of the no-AKI group) for factors such as reduced baseline estimated glomerular filtration rate (GFR) and very short or very long insult to admission time intervals, as these factors could have caused misclassification as AKI or reduced biomarker performance.20 There were only three patients with biomarker concentrations less than the median biomarker concentration for no-AKI, and these were in the pre-renal AKI group. In each of these cases, the diagnosis of AKI was based on a post-entry trough creatinine concentration between 50 and 60 μmol/l and a relatively low maximum creatinine concentration (80 or 90 μmol/l). None were oliguric. The clinical diagnosis was burns, trauma, or sub-arachnoid hemorrhage, and the time from onset to first creatinine measurement ranged from 7 to 17 h. A further five pre-renal AKI patients had only one biomarker greater than the median of the no-AKI group. The putative insult was more than 24 h before the first measurement in two patients. The short time course of GGT may explain why some biomarker concentrations were not increased.20 Two of the remaining five patients had underlying CKD; KIM-1 was increased in one and IL-18 in the other. The fifth and final patient samples were obtained 4 h after abdominal surgery, when only GGT was increased, which is consistent with the short time course of this biomarker.20
Figure 4.
Proportion of patients with n biomarkers above the median or upper quartile of the no-acute kidney injury (AKI) group. (a) No-AKI is normally distributed. A greater proportion of patients have multiple biomarkers elevated in the pre-renal AKI and AKI groups. (b) Progressively fewer biomarkers have no biomarker above the upper quartile of the no-AKI group, whereas progressively more have 3, 4, or 5 biomarkers above the upper quartile of the no-AKI group.
Urinary biomarker concentrations were compared with the absolute FENa. Within the total cohort only GGT was correlated, although poorly so (Spearman's rho=0.13, P=0.003). Within the cohort with FENa⩾1, only CysC and NGAL concentrations were correlated (also poorly) with increasing FENa. For CysC, Spearman's rho was 0.21 (P=0.0062); for NGAL rho was 0.17 (P=0.023). No corresponding correlations were seen with the FEUrea data (note this is a smaller data set).
No patient for whom AKI was transient (<48 h) needed dialysis, but 15.8% (n=19) of patients in AKI-48 group required renal replacement therapy, Table 3. Mortality increased with increasing duration of AKI (no-AKI, 9.1% (n=26); TAKI, 12.2% (11); AKI-48, 18.4% (21)), χ2 test for trend, P<0.01) and length of intensive care unit (ICU) stay was longer (P<0.0001).
Table 3. Outcomes according to AKI status.
| Outcome | No-AKI (n=285) | Pre-renal AKI (n=61) | AKI 48 (n=114) | Test for trend P-value |
|---|---|---|---|---|
| RRT, % (n) | 0 | 0 | 15.8 (19) | <0.0001 |
| Death in 30 days, % (n) | 9.1 (26) | 9.8 (6) | 18.4 (21) | 0.012 |
| Length of ICU stay (h) | 68 (41–160) | 103 (50–196) | 141 (66–270) | <0.0001 |
Abbreviations: AKI, acute kidney injury; ICU, intensive care unit; RRT, renal replacement therapy.
Subgroup analyses
The possibility that sepsis confounded the FENa measurement2, 15 was examined by analyzing the subgroup without sepsis (n=394). All biomarkers increased with increasing duration of AKI (Supplementary Figure 1 online), and GGT was increased in pre-renal AKI-FENa compared with no-AKI (P<0.05, Supplementary Tables 1 and 2 online).
As FEurea<35 is as an alternative to FENa<1 for defining pre-renal AKI, FEurea was measured in 313 patients in one of the two centers. Twenty-nine patients had TAKI plus FEurea<35. Urinary CysC, NGAL, and IL-18 were elevated in this group compared with the no-AKI group (Supplementary Table 3 online).
DISCUSSION
In this prospective study of critically ill ICU patients, we observed that urinary biomarker concentrations increased with increasing duration of AKI, and for urinary CysC, IL-18, and KIM-1 these were significantly increased in pre-renal AKI compared with patients with no-AKI.
In experimental nephrotoxic injury, the concentrations of renal injury biomarkers increase in proportion to the degree of structural renal injury.21 However, no renal biopsy studies in humans have been conducted to ascertain the exact relationship between structural renal injury and the urinary biomarkers measured in this study. Many studies have, nevertheless, demonstrated a correlation between urinary biomarker concentration and functional severity,22, 23 and several have demonstrated that values above a given cutoff predict hard outcomes including dialysis and death.24, 25 Although it remains an assumption that increased urinary biomarker concentrations represent increased structural injury, the results presented here demonstrate that both transient (reversible) and prolonged (perhaps irreversible) increases in plasma creatinine were associated with increased urinary biomarker concentrations and that these increased with duration of AKI. The presence of retained tubular function demonstrated by a normal FENa (<1%) did not modify this outcome.
These data support the hypothesis that pre-renal AKI and what might be called ‘established' AKI represent a continuum of injury. The extent of increase in urinary biomarker concentration in TAKI and TAKI plus low FENa was significantly less than that seen with AKI lasting longer than 48 h. This suggests that reversibility attributed to ‘functional' rather than ‘structural' injury may simply reflect a lesser degree of structural injury, which is nevertheless present at both ends of the AKI continuum. This is consistent with the patchy (focal) nature of histological change observed in those rare cases of clinical AKI where renal biopsy is available.26 By analogy to non-ST segment elevation myocardial infarction, we speculate that injury to a limited number of nephrons will inevitably produce detectable increases in urinary biomarker concentration, but compensation by residual normally functioning nephrons will mask changes in function, although some distinction between glomerular and tubular function also needs to be drawn, as discussed below. Clearly, further studies are required to validate these suggestions.
The overall lack of correlation between FENa and biomarker concentration for all biomarkers except GGT (where there was a relatively poor correlation) suggests that these biomarkers are not surrogate measures of sodium handling. However, when sodium absorption was reduced (FENa >1) there was a modest correlation between FENa and CysC and NGAL. We suggest two possible explanations: (i) CysC and NGAL appear to have longer time courses than the other biomarkers,20 which may have allowed a linkage to FENa to be more obvious than with shorter time-course biomarkers; (ii) of the five biomarkers examined, only CysC and NGAL are filtered by the glomerulus and reabsorbed in the proximal tubules, suggesting, although different transport mechanisms are involved, that impaired proximal tubular transport functions may link increases in the urinary concentrations of these biomarkers to FENa, analogous to the effect of albuminuria and proteinuria on the reabsorption of these biomarkers.27
All patients were maximally hydrated on admission to ICU and received inotrope support as required. Fluid resuscitation is an important component in the assessment and treatment of critically ill patients in the ICU and often begins in the emergency department, where high fluid loading is a normal practice. The results suggest that demonstration of retained tubular function or waiting for a decrease in creatinine to diagnose pre-renal AKI is unnecessary and that the severity of renal injury will define the response to fluid loading. Thus, a separation of pre-renal ‘azotemia' from established AKI is unnecessary and is not useful in clinical practice, as it may delay additional investigations or intervention. As pre-renal AKI is associated with tubular injury, it is the degree of tubular injury reflected by the extent of increase in urinary biomarker concentration that is important. The extent of urinary biomarker increase is inversely related to the extent to which tubular function is retained, as reflected in the stepwise increase in early biomarker concentrations when progressing from normal to pre-renal to established AKI observed in this study.
Although other studies have not addressed this question directly, their results are consistent with these conclusions. In the emergency department study of Nickolas et al,8 30 of 635 patients were classified as having AKI according to the RIFLE criteria, and 88 others who met the RIFLE criteria were classified as pre-renal azotemia because FENa was <1% on presentation or they recovered normal plasma creatinine concentrations within 3 days. The urinary NGAL/urinary creatinine concentration was significantly higher in patients with AKI than those who recovered from AKI within 3 days (416±387 vs. 30.1±92.0), P<0.001; mean±s.d., μg/g creatinine). Similarly, urinary IL-18/creatinine (mean±s.e., pg/mg) was greater in patients with acute tubular necrosis (814±151, n=14) compared with patients classified as having pre-renal azotaemia (155±68, n=8), or healthy controls (23±9, n=11). In that study, patients with acute tubular necrosis or pre-renal azotemia were adjudicated by the renal consultant based on plasma creatinine changes in respond to fluid therapy, urine microscopy, and FENa<1%.28, 29
Recently, Singer et al.30 investigated whether urinary NGAL could be used to distinguish between pre-renal and intrinsic AKI in a cohort of 145 patients with AKI according to the RIFLE criteria on the day of enrolment. Patients were classified as pre-renal AKI (n=33) when plasma creatinine returned to baseline within 3 days and the cause of increase in creatinine was determined to be factors that compromised renal perfusion, and there was no exposure to nephrotoxins. Patients were classified as intrinsic AKI when potential tubular necrosis–inducing events could be identified and there was no response to fluid resuscitation and/or hemodynamic stabilization (n=75). A large number of patients (n=38) could not be classified. Urinary NGAL concentrations were greater in the intrinsic than the pre-renal AKI groups. NGAL was also greater in those who went on to increase their RIFLE severity class or to die. FENa did not discriminate between pre-renal and intrinsic AKI. Unfortunately, this study lacked a cohort of patients without AKI, which makes comparison with our study difficult, and the large number of unclassifiable patients calls the conclusions into some doubt as well. The approach of retrospectively determining the likely cause of increased creatinine and waiting to see which patients creatinine levels returned to normal may have resulted in choosing two cohorts with differing severity of parenchymal injury rather than a separate cohort with only a physiological response.
Thus, although recent studies could distinguish biomarker cutoffs that defined ‘established' from pre-renal AKI, they nevertheless documented evidence of structural injury in the pre-renal group. Similarly, evidence of structural injury was also observed in the earliest studies of reversible accumulation of nitrogenous wastes in healthy volunteers. In the study of Coller and Maddock,29 proteinuria and casts appeared in the urine after 2 days, and hematuria was also present after 3 days of water deprivation. These features resolved along with nitrogen retention when access to water was permitted, although proteinuria was the slowest to clear.
In this study, mortality in patients with TAKI was greater than in no-AKI patients (12% vs. 9%, P<0.01). This is consistent with the larger observational study of Uchino et al.10 of 20,126 hospital patients, where patients who recovered within 3 days had a greater than twofold (odds ratio: 2.26), and patients classified as acute tubular necrosis (no renal recovery within 3 days or received dialysis) a sixfold (odds ratio: 6.07), increase in hospital mortality compared with patients without AKI. Similarly, the study of Coca et al.31 of 35,302 diabetic veterans showed that long-term mortality increased with duration of AKI after noncardiac surgery.
It has previously been argued that the concept of pre-renal AKI is flawed in AKI associated with sepsis.2 Although renal underperfusion leading to necrosis does not appear to be the dominant injury paradigm in sepsis, our data additionally demonstrate that structural injury has occurred in these patients, as TAKI was associated with increased urinary biomarker concentrations in both patients with sepsis and without.
If pre-renal AKI simply represents the milder end of the spectrum of AKI with preservation of sodium handling, what use, then, are traditional markers of tubular function? We suggest that previous studies are consistent with the notion that tubular function is better preserved with briefer or less severe renal injury. For example, Pepin et al.9 showed that, in patients not receiving diuretics, FENa was better than FEurea in distinguishing persistent AKI from transient AKI (defined as recovery from a ⩾30% increase in plasma creatinine within 1 week; area under the curve, 0.83±0.07 vs. 0.56±0.11, respectively). In the same study, the FENa was three to four times higher in patients with persistent AKI compared with transient AKI, regardless of whether patients were receiving diuretics. Nevertheless, this combination of apparently preserved tubular function and an increased creatinine (indicating a reduction in GFR) coupled with an increase in injury biomarkers suggests that standard markers of tubular function are less sensitive to renal injury than markers of GFR, at least for the case distribution described. Consistent with the concept that injury is focal in human AKI, as already highlighted above, this further suggests that renal autoregulation of GFR is more sensitive to injury than markers of tubular transport function such as FENa. Tubular transport mechanisms are either more resilient or compensated for by normal nephrons. A consistent paradigm is that less severe ‘pre-renal mechanisms',32 which reduce nephron plasma flow, the glomerular hydraulic pressure gradient ultrafiltration coefficient, or the oncotic pressure gradient resulting in reduced single-nephron GFR, result in tubular injury to some but not all nephrons. This combination will result in apparent preservation of ‘overall' tests of tubular transport function, such as FENa, but with injury revealed by increasing urinary biomarker concentrations.
A recent retrospective analysis by Haase et al.33 of cohort studies of patients with cardio-renal disease demonstrated that an increase in plasma NGAL without an increase in plasma creatinine nevertheless predicted an increase in dialysis and mortality. Similarly, we observed in the no-AKI group that the addition of urinary NGAL increased the area under the curve (from 0.61 (95% confidence interval: 0.49–0.73) to 0.66 (0.54–0.78), P<0.05) for predicting death within 30 days in a logistic regression model of variables, which were associated with mortality under univariate analysis (age, weight, APACHE II score). This suggests that injury biomarkers have an important role in the classification and management of AKI in addition to stages defined by creatinine.
Only three patients matching our definition of pre-renal AKI appeared to have no increase in urinary biomarkers in this study. Although the formulaic classification (compared with a clinical adjudication) may have been responsible for classifying these as pre-renal AKI instead of as ‘no-AKI', we cannot rule out the possibility that they were true noninjury pre-renal AKI.
Our analysis, as with all biomarker analyses of urinary AKI biomarkers, treats the biomarkers as continuous variables. However, the pathophysiology suggests that some of these biomarkers are categorical. This may account for skewed concentration distributions and complicates the statistical analysis. For example, IL18, a pro-inflammatory cytokine, is upregulated in proximal epithelial cells and recruited macrophages during ischaemia/reperfusion injury. In our study, 55% of patients on entry to ICU had an IL18 concentration below the level of detection, leading to a highly skewed distribution. For this reason, we analyzed the results nonparametrically (Mann–Whitney U test comparison between each group), as well as parametrically following log transform (ANOVA with least significant difference post-hoc test).
Although patients were vigorously hydrated in accordance with ICU practice, our study was limited by a lack of fluid balance data to rule out volume depletion or objectify the response to deliberate fluid loading. Similarly, we lack data on diuretic use. By normalizing the biomarkers to urinary creatinine, we account, at least in part, for any potential differences in fluid loading.34 Although the largest study of its kind, the study remains limited by size; nearly 8% of patients were excluded because of insufficient follow-up plasma creatinine measurements or death before 48 h after admission. NGAL results were unavailable in another 4% of patients. Consequently, the modest number of patients in each group after stratification for duration and FENa means that the conclusions require validation in larger studies.
In summary, in critically ill patients, urinary biomarkers of injury increase in concentration with duration of AKI regardless of whether there is preservation of tubular function, as typified by a low FENa. This suggests that pre-renal AKI is simply a mild form of structural AKI rather than a unique functional form of AKI. Early return of renal function, as demonstrated by a decrease in creatinine, probably represents recovery, not reversibility, of a unique ‘physiological' increase in serum creatinine. Although it remains theoretically possible that GFR may decrease in the absence of renal tubular injury, until there are studies demonstrating that urinary biomarkers of renal injury are absent in such cases, we suggest that the term ‘pre-renal mechanisms' be used for classifying etiology but not type of AKI. Perhaps in the future AKI may be categorized according to extent of injury (biomarker concentration), extent and duration of glomerular functional change, and preservation or otherwise of sodium reabsorption or other tubular functions.
MATERIALS AND METHODS
The prospective observational study of FENa and FEurea and urinary biomarkers of AKI was a planned component of the combined observational and interventional study, EARLYARF, in two large general ICUs in two regional centers.19, 20 The study was approved by the Multiregional Ethics Committee of New Zealand (MEC/050020029) and registered under the Australian New Zealand Clinical Trials Registry (ACTRN012606000032550, http://www.actr.org.au). Inclusion/exclusion criteria, consent procedures, sample collection, and details of analysis for urinary GGT, CysC, IL-18, KIM-1, NGAL, and creatinine have been detailed elsewhere.19, 20 Briefly, eligible patients were all adults expected to remain in the ICU for >24 h, survive >72 h, and had not experienced a threefold or greater rise in creatinine on entry, were not anuric, on renal replacement therapy or assessed to need renal replacement therapy within 48 h, and did not have hematuria, rhabdomyolosis, myoglobinuria, or polycythemia, and were not receiving chemotherapy. Patients were randomized to a placebo-controlled trial of high-dose erythropoietin. As erythropoietin had no effect on outcome, the present analysis combines patients in both observation and intervention arms.19 Investigators blinded to patients' clinical characteristics performed all assays. Biomarker concentrations were indexed to urinary creatinine concentration in the same sample.
Urine and plasma sodium and urea were measured on entry to ICU. Other biomarkers were measured on entry, and at 12 and 24 h post entry. Plasma creatinine was measured daily for 7 days. Urine samples were stored at −80°C until batch analysis for CysC, KIM-1, IL-18, or NGAL. The maximum indexed urinary biomarker concentration within 24 h was used for this analysis. The FENa was calculated using the following formula: FENa=(plasma creatinine × urinary Na)/(plasma Na × urinary creatinine). Baseline creatinine was determined as previously described.19 Briefly, 51% of patients had a plasma creatinine before ICU admission; for the remaining patients, the lowest of the on-admission, final ICU, or follow-up creatinine was used. A similar approach in this cohort has been shown to be more accurate than using the Modification of Diet in Renal Disease equation with the same assumed GFR to back-calculate a baseline creatinine.35
AKI was defined using the Acute Kidney Injury Network criteria as an absolute increase in plasma creatinine above baseline of at least 0.3 mg/dl (26.4 μmol/l) or a percentage increase of at least 50%.36 AKI status was determined at admission to the ICU, and 24 and 48 h later. Patients were stratified according to duration of AKI: (i) recovery within 24 h (AKI-24), (ii) recovery between 24 and 48 h (AKI 24–48), (iii) no recovery within 48 h or renal replacement therapy (AKI-48). TAKI was defined as AKI-24 or AKI 24–48. Recovery was defined as a reduction of plasma creatinine to <0.3 mg/dl above baseline. Patients with no-AKI within 48 h of entry served as the control group (no-AKI). Pre-renal AKI was defined as TAKI combined with a low fractional sodium excretion, FENa, <1%. Covariates were variables with P<0.1 following univariate analysis for the difference between no-AKI and pre-renal AKI or AKI-48. Covariates were age, sex, APACHE II score, and estimated baseline GFR (eGFR: using the Modification of Diet in Renal Disease formula) and sepsis.
Results were expressed as frequencies, mean±s.d., and medians and interquartile range. Differences between the distributions were assessed by ANOVA with Least Squares Difference for post-hoc analysis of the difference between groups, χ2 and Mann–Whitney U test, and sensitivity analysis. Analysis was performed with SPSS version 16 (SPSS, Chicago, IL) and GraphPad Prism 5.0a (GraphPad Software, San Diego, CA).
Acknowledgments
The EARLYARF trial was supported by the Health Research Council of New Zealand, grant 05/131. MN was supported by a University of Otago Post-Graduate Scholarship. JWP was supported by an Australian and New Zealand Society of Nephrology infrastructure and enabling grant. Some of this work was presented as a poster at the World Council of Nephrology 2011 conference, Vancouver, Canada, 8–12, April 2011.
PD is a coinventor on patents involving NGAL as a biomarker of CKD and AKI; CLE is a coinventor on patents involving IL-18; and JVB is a coinventor on patents involving KIM-1.
Footnotes
SUPPLEMENTARY MATERIAL
Table S1a. Urinary Biomarker Concentrations* in pre-renal AKI and AKI 48 in patients without sepsis.
Table S1b. Urinary Biomarker Concentrations* in pre-renal AKI and AKI 48 in patients with sepsis.
Table S2. Urinary and plasma sodium, urea, FENa, FEurea, and eGFR in total cohort and non-sepsis patients.
Table S3. Urinary Biomarker Concentrations* in pre-renal AKI defined by FEUrea and AKI 48.
Figure S1. Effect of duration of AKI on urinary biomarker concentration in patients without sepsis.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
Supplementary Material
References
- Parikh CR, Coca SG. Acute kidney injury: defining prerenal azotemia in clinical practice and research. Nat Rev Nephrol. 2010;6:641–642. doi: 10.1038/nrneph.2010.128. [DOI] [PubMed] [Google Scholar]
- Bellomo R, Bagshaw SM, Langenberg C, et al. Pre-renal azotemia: a flawed paradigm in critically ill septic patients. Contrib Nephrol. 2007;156:1–9. doi: 10.1159/000102008. [DOI] [PubMed] [Google Scholar]
- Kellum JA, Ronco C, Vincent JL. Unveiling current controversies in acute kidney injury. Contrib Nephrol. 2011;174:1–3. doi: 10.1159/000329814. [DOI] [PubMed] [Google Scholar]
- Yang L, Humphreys BD, Bonventre JV. Pathophysiology of acute kidney injury to chronic kidney disease: maladaptive repair. Contrib Nephrol. 2011;174:149–155. doi: 10.1159/000329385. [DOI] [PubMed] [Google Scholar]
- Macedo E, Mehta RL. Prerenal failure: from old concepts to new paradigms. Curr Opin Crit Care. 2009;15:467–473. doi: 10.1097/MCC.0b013e328332f6e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahr S, Miller SB. Acute oliguria. N Engl J Med. 1998;338:671–675. doi: 10.1056/NEJM199803053381007. [DOI] [PubMed] [Google Scholar]
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–1460. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- Nickolas TL, O'Rourke MJ, Yang J, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepin M-N, Bouchard J, Legault L, et al. Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kid Dis. 2007;50:566–573. doi: 10.1053/j.ajkd.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Uchino S, Bellomo R, Bagshaw SM, et al. Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol Dial Transplant. 2010;25:1833–1839. doi: 10.1093/ndt/gfp624. [DOI] [PubMed] [Google Scholar]
- Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: why drugs haven't worked and what is on the horizon. Clin J Am Soc Nephro. 2007;2:356–365. doi: 10.2215/CJN.03280906. [DOI] [PubMed] [Google Scholar]
- Macedo E, Bouchard J, Mehta RL. Renal recovery following acute kidney injury. Curr Opin Crit Care. 2008;14:660–665. doi: 10.1097/MCC.0b013e328317ee6e. [DOI] [PubMed] [Google Scholar]
- Carvounis C, Nisar S, Guro-Razuman S. Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int. 2002;62:2223–2229. doi: 10.1046/j.1523-1755.2002.00683.x. [DOI] [PubMed] [Google Scholar]
- Diskin CJ, Stokes TJ, Dansby LM, et al. The comparative benefits of the fractional excretion of urea and sodium in various azotemic oliguric states. Nephron Clin Pract. 2010;114:C145–C150. doi: 10.1159/000254387. [DOI] [PubMed] [Google Scholar]
- Musch W, Verfaillie L, Decaux G. Age-related increase in plasma urea level and decrease in fractional urea excretion: clinical application in the syndrome of inappropriate secretion of antidiuretic hormone. Clin J Am Soc Nephro. 2006;1:909–914. doi: 10.2215/CJN.00320106. [DOI] [PubMed] [Google Scholar]
- Coca SG, Yalavarthy R, Concato J, et al. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int. 2008;73:1008–1016. doi: 10.1038/sj.ki.5002729. [DOI] [PubMed] [Google Scholar]
- Parikh CR, Lu JC, Coca SG, et al. Tubular proteinuria in acute kidney injury: a critical evaluation of current status and future promise. Ann Clin Biochem. 2010;47:301–312. doi: 10.1258/acb.2010.010076. [DOI] [PubMed] [Google Scholar]
- Endre ZH, Westhuyzen J. Early detection of acute kidney injury: emerging new biomarkers. Nephrology. 2008;13:91–98. doi: 10.1111/j.1440-1797.2007.00905.x. [DOI] [PubMed] [Google Scholar]
- Endre ZH, Walker RJ, Pickering JW, et al. Early intervention with erythropoietin does not affect the outcome of acute kidney injury (the EARLYARF trial) Kidney Int. 2010;77:1020–1030. doi: 10.1038/ki.2010.25. [DOI] [PubMed] [Google Scholar]
- Endre ZH, Pickering JW, Walker RJ, et al. Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int. 2011;79:1119–1130. doi: 10.1038/ki.2010.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer JS, Dieterle F, Troth S, et al. A panel of urinary biomarkers to monitor reversibility of renal injury and a serum marker with improved potential to assess renal function. Nat Biotechnol. 2010;28:486–494. doi: 10.1038/nbt.1627. [DOI] [PubMed] [Google Scholar]
- Haase-Fielitz A, Bellomo R, Devarajan P, et al. The predictive performance of plasma neutrophil gelatinase-associated lipocalin (NGAL) increases with grade of acute kidney injury. Nephrol Dial Transplant. 2009;24:3349–3354. doi: 10.1093/ndt/gfp234. [DOI] [PubMed] [Google Scholar]
- Haase M, Bellomo R, Devarajan P, et al. Novel biomarkers early predict the severity of acute kidney injury after cardiac surgery in adults. Ann Thorac Surg. 2009;88:124–130. doi: 10.1016/j.athoracsur.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Nejat M, Pickering JW, Walker RJ, et al. Urinary cystatin C is diagnostic of acute kidney injury and sepsis, and predicts mortality in the intensive care unit. Crit Care. 2010;14:R85. doi: 10.1186/cc9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh CR, Devarajan P, Zappitelli M, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22:1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenberg C, Bagshaw SM, May CN, et al. The histopathology of septic acute kidney injury: a systematic review. Crit Care. 2008;12:R38. doi: 10.1186/cc6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejat M, Hill JV, Pickering JW, et al. Albuminuria increases cystatin C excretion: implications for urinary biomarkers Nephrol Dial Transplant 2011. e-pub ahead of print 5 May 2011. [DOI] [PubMed]
- Parikh CR, Jani A, Melnikov V, et al. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kid Dis. 2004;43:405–414. doi: 10.1053/j.ajkd.2003.10.040. [DOI] [PubMed] [Google Scholar]
- Coller FA, Maddock WG. A study of dehydration in humans. Ann Surg. 1935;102:947–960. doi: 10.1097/00000658-193511000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer E, Elger A, Elitok S, et al. Urinary neutrophil gelatinase-associated lipocalin distinguishes pre-renal from intrinsic renal failure and predicts outcomes. Kidney Int. 2011;80:405–414. doi: 10.1038/ki.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coca SG, King JT, Rosenthal RA, et al. The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney Int. 2010;78:926–933. doi: 10.1038/ki.2010.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blantz RC, Singh P. Analysis of the prerenal contributions to acute kidney injury. Contrib Nephrol. 2011;174:4–11. doi: 10.1159/000329027. [DOI] [PubMed] [Google Scholar]
- Haase M, Devarajan P, Haase-Fielitz A, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752–1761. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralib AM, Pickering JW, Shaw GM, et al. Test characteristics of urinary biomarkers depend on quantitation method in acute kidney injury. J Am Soc Nephrol. 2012;23:322–333. doi: 10.1681/ASN.2011040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering JW, Endre ZH. Back-calculating baseline creatinine with MDRD misclassifies acute kidney injury in the intensive care unit. Clin J Am Soc Nephro. 2010;5:1165–1173. doi: 10.2215/CJN.08531109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.