Abstract
Generating a cytotoxic CD8+ T-cell response that can eradicate malignant cells is the primary objective of cancer vaccine strategies. In this study we have characterized the innate and adaptive immune response to the ISCOMATRIX adjuvant, and the ability of vaccine antigens formulated with this adjuvant to promote antitumor immunity. ISCOMATRIX adjuvant led to a rapid innate immune cell response at the injection site, followed by the activation of natural killer and dendritic cells (DC) in regional draining lymph nodes. Strikingly, major histocompatibility complex (MHC) class I cross-presentation by CD8α+ and CD8α− DCs was enhanced by up to 100-fold when antigen was formulated with ISCOMATRIX adjuvant. These coordinated features enabled efficient CD8+ T-cell cross-priming, which exhibited prophylactic and therapeutic tumoricidal activity. The therapeutic efficacy of an ISCOMATRIX vaccine was further improved when co-administered with an anti-CD40 agonist antibody, suggesting that ISCOMATRIX-based vaccines may combine favorably with other immune modifiers in clinical development to treat cancer. Finally, we identified a requirement for the myeloid differentiation primary response gene 88 (MyD88) adapter protein for both innate and adaptive immune responses to ISCOMATRIX vaccines in vivo. Taken together, our findings support the utility of the ISCOMATRIX adjuvant for use in the development of novel vaccines, particularly those requiring strong CD8+ T-cell immune responses, such as therapeutic cancer vaccines.
Keywords: ISCOMATRIX, cross-presentation, MyD88, CTL, vaccines, tumor
Tumor-associated antigens can be of oncogenic viral origin or derived from self-neoantigens that are mutated, overexpressed or ectopically expressed by tumor cells.1 The potential immunogenicity of tumor-associated antigen has stimulated decades of research to develop efficacious therapeutic cancer vaccines. The effectiveness of a cancer vaccine hinges on its ability to induce cytotoxic CD8+ T cells that can infiltrate primary tumors, eradicate disseminated malignant cells and protect patients from relapse.2, 3 Therapeutic cancer vaccines are typically comprised of a well-vetted TA-Ag and an immune modulator, such as an adjuvant, to condition the microenvironmental context and boost the immunogenic potential of the TA-Ag, and to facilitate its major histocompatibility complex (MHC) class I presentation by antigen-presenting cells to promote cytotoxic T-lymphocyte (CTL) cross-priming.4 Mechanisms underlying most vaccine adjuvants are incompletely understood; however, include prolonged half-life of the antigen, enhanced innate cell infiltration into the site of antigen deposition, improved antigen presentation by antigen-presenting cells and increased production of immunomodulatory cytokines and chemokines.5 However, most clinically used adjuvants (for example: alum, Montanide, MPL and MF59) are limited in their ability to elicit CD8+ CTL responses.6 The paucity of adjuvants that can promote tumor-specific CD8+ T-cell responses has led to the evaluation of many novel adjuvant technologies. ISCOMATRIX adjuvant (CSL Limited, Parkville, Australia) is a saponin-based particulate adjuvant (which forms cage-like structures approximately 40 nm in diameter).7 In pre-clinical models, ISCOMATRIX vaccines have been demonstrated to generate broad humoral and cellular immune responses; importantly, this includes CD8+ T-cell immunity.8 However, the mechanistic details of how ISCOMATRIX vaccines achieve CD8+ T-cell responses in vivo have not been fully elucidated.
Dendritic cells (DCs) present antigenic peptides to CD4+ T cells via MHC class II molecules and CD8+ T cells through MHC class I molecules. In most instances MHC class I presentation is restricted to endogenously derived proteins; however, certain DC subsets possess the ability to deliver exogenously derived proteins into the MHC class I presentation pathway, a process termed cross-presentation.4, 9 In mice, cross-presentation has been identified as a feature of the CD8α+ subset of lymphoid organ DCs (CD8 DCs hereafter),10, 11, 12, 13 although a second population of tissue-derived CD103+ DCs may support CD8+ T-cell cross-priming during certain pathogenic infections.14, 15
Pathogen-associated antigens captured by antigen-presenting cells are generally associated with pathogen-associated molecular patterns that are detected by pattern recognition receptors, such as those of the toll-like receptor (TLR) and inflammasome pathways.16, 17 Under these conditions, antigen cross-presentation and appropriate DC activation favor CD8+ T-cell cross-priming.4 In contrast, tumor-associated antigen can be cross-presented in the absence of appropriate immune activation, and in the context of tumor-mediated immune suppression.18, 19 As such, DCs cross-presenting tumor-associated antigen often fail to mount an effective antitumor CD8+ CTL immune response. Cancer vaccine strategies likely require an adjuvant to potentiate the immunogenicity of the vaccine antigen by concomitantly activating cross-presenting DCs.3 However, immune activation without efficient cross-presentation may result in a failed or suboptimal antitumor response. Therefore, a desirable feature of an immune adjuvant is to combine both immune modulation and efficient antigen delivery into the MHC class I cross-presentation pathway.
In this study we have characterized the innate and adaptive immune responses elicited by ISCOMATRIX vaccines in mice. We propose that the integrated capacity of ISCOMATRIX adjuvant to enhance antigen cross-priming, combined with immune activation, supports its clinical development as a cancer vaccine adjuvant.
Results
ISCOMATRIX adjuvant promotes an innate immune response in vivo
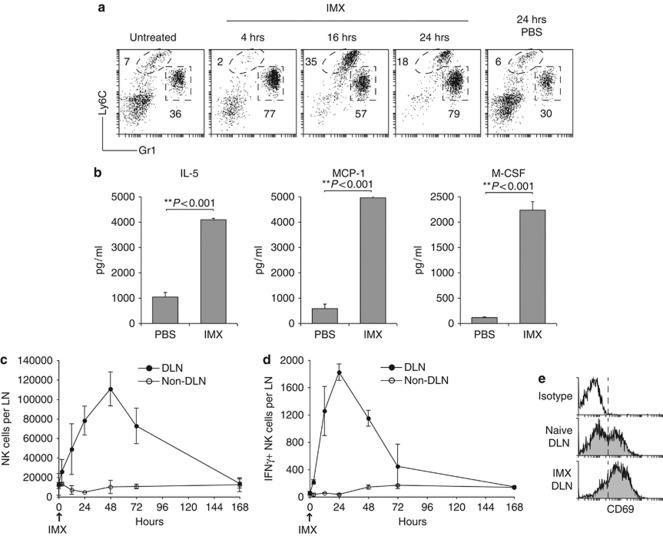
The innate immune response to injected ISCOMATRIX adjuvant was evaluated using the sterile subcutaneous air-pouch technique.20 Gr1high neutrophils rapidy accumulated in the air-pouch within 4 h of ISCOMATRIX adjuvant administration, Ly6Chigh monocyte infiltration peaked at 16 h, relative to phosphate-buffered saline (PBS)-injected control air-pouches (Figure 1a). The influx in neutrophils and monocytes correlated with a dramatic increase in the number of myeloid (CD11bhigh) cells recovered from the air-pouch exudate (data not shown). To assess the local cytokine and chemokine response to ISCOMATRIX adjuvant, immune cell infiltrates were isolated and cultured overnight. Interleukin (IL)-5, monocyte chemotactic protein-1 and macrophage-colony-stimulating factor (CSF) levels were significantly elevated in the supernatant collected from immune cells obtained from ISCOMATRIX adjuvant-treated mice (Figure 1b). No significant differences were observed in the other chemokines or cytokines analyzed (data not shown).
Figure 1.
ISCOMATRIX adjuvant activates innate immune cells in vivo. (a) Immune cell infiltrates were gated on the myeloid marker CD11b. Monocyte (CD11b+Ly6C+) and neutrophil (CD11b+Gr1+) recruitment into subcutaneous air-pouches was evaluated 4, 16 and 24 h following subcutaneous ISCOMATRIX adjuvant (IMX) administration. Profiles are representative for n=3–6 mice per time-point. (b) Levels of IL-5, monocyte chemotactic protein-1 α and macrophage CSF (macrophage-CSF) detected in cultured air-pouch immune exudates. Error bars show the s.e.m. (n=3 per group). Student's t-test was used to calculate statistical significance. (c) Time-course of NK cell accumulation in the DLN (brachial) and non-DLN (inguinal) following subcutaneous IMX injection. (d) Ex vivo NK cell IFN-γ production in the DLN and non-DLN after IMX administration. Error bars represent the s.e.m. (n=4–8 individual LN per time-point). (e) Representative profiles showing NK cell expression of CD69 in the DLN of naïve or 24 h after IMX injection. No increase in CD69 expression was observed on NK cells, or other cell populations, from the non-DLN (data not shown). All results are representative of at least two independent experiments.
We next characterized the innate immune cell response in the brachial (draining) and inguinal (non-draining) lymph nodes (LNs). Correlating with an overall increase in cellularity (Supplementary Figure 1A), the number of natural killer (NK) cells, NK T cells, monocytes and neutrophils were elevated in the draining LN (DLN), compared with the non-DLN (Figure 1c, and Supplementary Figure 1B). To evaluate innate immune cell activation, we examined NK cell interferon (IFN)-γ—production and CD69 upregulation following a single dose of ISCOMATRIX adjuvant. IFN-γ-producing NK cells were detected in the DLN within 3 h of subcutaneous adjuvant delivery, and peaked at 24 h (Figure 1d). NK cell IFN-γ production correlated with increased surface expression of the early activation marker, CD69 (Figure 1e). Increased CD69 expression was restricted to the DLN, and was also elevated on other immune cell populations including B cells and DCs (data not shown). Given the ability of ISCOMATRIX adjuvant to recruit a local inflammatory response, we sought to investigate adaptive immune responses to ISCOMATRIX adjuvant formulated with two model vaccine antigens, given as a prime and boost regimen.
ISCOMATRIX vaccines facilitate cellular and humoral immune responses
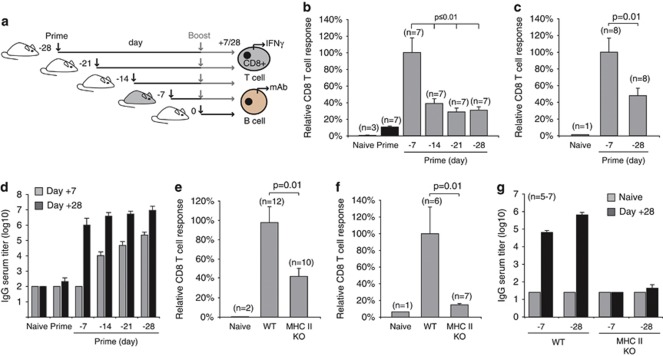
To characterize the adaptive immune response to ISCOMATRIX vaccines, we utilized antigens with well-defined H-2 Kb-restricted MHC class I epitopes in C57Bl6 mice: ovalbumin (OVA) and a fusion protein based on herpes simplex virus (HSV) coat proteins glycoprotein B (HSV-1) and D (HSV-2) (gB:gD). The endogenous antigen-specific CD8+ T-cell response was quantified in the spleen following a prime, or prime-boost vaccination regimen (Figure 2a, schematic). The percentage of vaccine antigen-specific IFN-γ-producing CD8+ T cells is shown relative to percentage obtained with the day −7, 0 vaccination protocol (Figures 2b and c). The percentage of antigen-specific IFN-γ-producing CD8+ T cells was routinely between 3 and 8% of the endogenous CD8+ T cells (Supplementary Figure 2A). This percentage correlated with the number of CD44high OVA-specific CD8+ T cells quantified using an H-2 Kb-SIINFEKL tetramer (Supplementary Figures 2B and 2C). The humoral response was evaluated with the same dosing regimens used to assess the CD8+ T-cell response (Figure 2a, schematic). OVA-specific antibody titers were compared in sera collected from naive mice or 7 or 28 days after the final vaccination. Initial titers were highest 7 days after the −28, 0 regimen; however, total levels were comparable after 28 days with all dosing combinations (Figure 2d).
Figure 2.
Cellular and humoral immune responses to ISCOMATRIX vaccines are dependent on CD4+ T-cell help. (a) Schematic showing the dosing regimen used to evaluate vaccine antigen-specific CD8+ T cell and antibody response. (b) IFN-γ production by endogenous OVA-specific CD8+ T cells was determined in the spleen 7 days after the boost vaccination. (c) IFN-γ production by endogenous gB (HSV-1)-specific CD8+ T cells was determined in the spleen 7 days after the boost vaccination. Results in (b, c) are expressed relative to the response obtained with a day −7, 0-dosing regimen. Mean values are expressed ±s.e.m.. Student's t-test was used to calculate statistical significance. (d) OVA-specific antibody titers (total IgG) in serum collected from mice vaccinated with the dosing protocols shown in (a). OVA-specific titers were quantified 7 or 28 days after the final vaccine dose. (e) OVA-specific CD8+ T-cell responses were compared in wild type (WT) and MHC class II-deficient mice (MHC II KO) vaccinated on days −7, 0. (f) The OVA-specific ‘recall' CD8+ T-cell response was assessed using a 5-day ex vivo re-stimulation protocol. (g) OVA-specific IgG titers in serum collected from naïve WT or MHC II KO vaccinated with two different vaccine dosing regimens. All results are representative of at least two or more independent experiments. ‘Student's' t-test was used to calculate statistical significance.
Depending on the challenge model, cytotoxic CD8+ T-cell priming can require assistance by CD4+ T helper (TH) cells.21, 22 To investigate whether CD4+ TH participated in ISCOMATRIX adjuvant-mediated cellular immunity, we compared effector and recall CD8+ T-cell responses in wild type and MHC class II-deficient mice, which lack CD4+ T cells.23 MHC class II-deficient mice generated 60% fewer antigen-specific IFN-γ-producing CD8+ T cells compared with wild-type control mice (Figure 2e). Consistent with this finding, the CD8+ T-cell recall response was severely compromised in MHC class II-deficient mice (Figure 2f). Vaccinated MHC class II-deficient mice also failed to develop vaccine-antigen-specific antibodies (Figure 2g).
Antitumor responses require components of innate and adaptive immunity
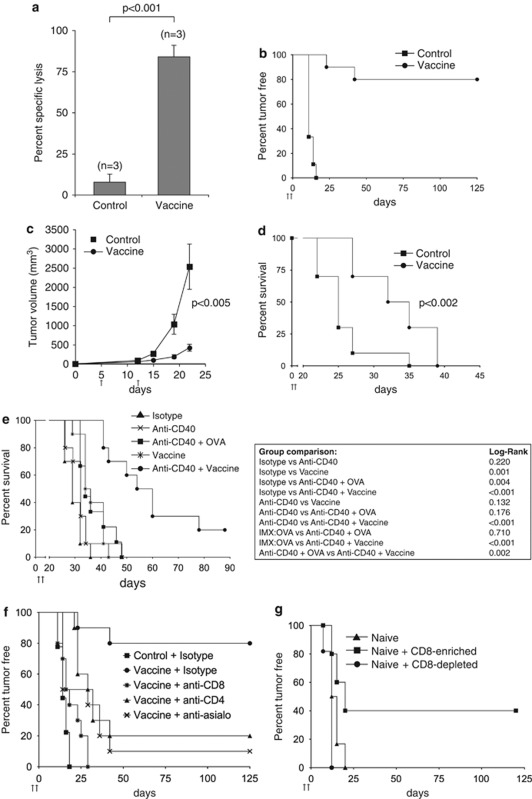
We next evaluated the cytotoxic activity of CD8+ T cells generated by an ISCOMATRIX vaccine by monitoring the lysis of target cells pulsed with SIINFEKL peptide in pre-vaccinated versus control (OVA)-treated animals (Figure 3a). To confirm the antitumor activity of the vaccine antigen-specific CTLs, we used the B16 melanoma tumor challenge model.24 In all, 80% of vaccinated mice remained tumor-free after 125 days, as compared with a 100% tumor incidence in mice that received ISCOMATRIX alone (control) (Figure 3b). All tumor-free mice rejected a subsequent challenge of OVA-expressing melanoma cells but succumbed to parental B16 tumor cell growth (data not shown). To evaluate the impact of an ISCOMATRIX vaccine in the therapeutic tumor model, mice were inoculated with OVA-expressing melanoma cells 5 days before prime and boost vaccination (Figure 3c). A significant delay in tumor growth was observed only in the vaccinated cohort, which correlated with improved survival, although all mice eventually succumbed to tumor burden (Figure 3d). ISCOMATRIX or OVA alone did not extend survival, as compared with untreated animals (Supplementary Figure 3A). We next assessed whether ISCOMATRIX vaccines could combine with an immune modifier to augment the therapeutic antitumor activity. Anti-CD40 agonists are recognized as potent immune modifiers, and as such, represent an attractive immunotherapy in treating neoplastic disease.25, 26 We found that the anti-CD40 antibody, FGK45, combined effectively with an ISCOMATRIX vaccine to significantly extend survival beyond what was achieved with vaccine or anti-CD40 plus antigen formulations alone (Figure 3e).
Figure 3.
The antitumor response mediated by ISCOMATRIX vaccines requires components of innate and adaptive immunity. (a) The CTL response was evaluated in animals that received the ISCOMATRIX (IMX) vaccine or antigen (OVA) alone on day −7, 0. SIINFEKL-pulsed CFSEhigh-labeled target cells were injected intravenously on day +7, and specific-lysis was evaluated ex vivo 4 h later by flow cytometry. Specific lysis was calculated relative to a control (non-pulsed) CFSElow-labeled cell population. The mean specific lysis is shown ±s.e.m. Student's t-test was used to calculate statistical significance. (b) Tumor incidence in mice that received IMX vaccine (n=9) or IMX alone (control, n=10) on day −7, 0. All animals were challenged 7 days after boost dose with 1.5 × 105 B16:OVA melanoma cells. (c) Tumor cells were inoculated in mice 5 days before receiving the IMX vaccine (vaccine) or IMX alone (control) on day 5 and 12. Tumor volumes were monitored until the first tumor reached the 3000 mm3, which was nominated as the survival end-point. ‘Student's' t-test was used to calculate statistical significance; n=10 per group. (d) Kaplan–Meier curves showing survival end-points for the cohorts in (c) (P<0.002 by the log-rank test) (n=10 per group). (e) Tumor cells were inoculated into mice 5 days before the indicated vaccination regimens (day 5 (prime) and 12 (boost)). Kaplan–Meier curves showing survival end-points for each cohort (n=10 per group; vaccine versus anti-CD40+vaccine=P<0.002 by the log-rank test). (f) Mice were administered anti-CD4, anti-CD8 or anti-asialo-GM1 (anti-asialo) on days −8, −5, −2, +1 and +4 during the day −7, 0 vaccine regimen. Mice were challenged on day +7 with 1.5 × 105 B16:OVA cells and tumor incidence was monitored. (g) CD8-enriched or CD8-depleted spleen cell fractions from pre-vaccinated mice were adoptively transferred into naïve recipients. After 1 day of adoptive transfer, mice were challenged with 5 × 105 B16:OVA melanoma cells. Tumor incidence was monitored out to day 125 (n=10–12 per group). All results are representative of two or more independent experiments.
To evaluate the contribution of innate and adaptive immune cell populations in ISCOMATRIX vaccine-mediated tumor protection, we depleted CD8+, CD4+ or NK cells during the prime and boost vaccinations. Vaccine-mediated tumor protection was severely attenuated when T cell or NK cell populations were depleted (Figure 3f). To test the hypothesis that cross-primed CD8+ T cells could confer tumor protection in our model, we adoptively transferred CD8-enriched and CD8-depleted splenocyte fractions from previously vaccinated mice into naive recipients. All mice were challenged 1 day later with B16-OVA melanoma cells (Figure 3g). Only the CD8+ splenocyte fraction was able to provide tumor protection in 40% of the naive recipients. Taken together, these results indicate that ISCOMATRIX vaccines require the coordinated function of the innate and adaptive response to generate an antitumor CTL response in vivo.
DCs are required for NK cell activation and CD8+ T-cell immunity in response to an ISCOMATRIX vaccine
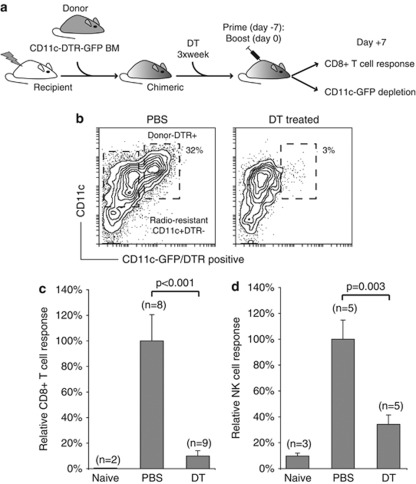
DCs are potent stimulators of naïve T cells and NK cells, and as such, provide a critical link between innate and adaptive immunity.4, 27, 28 To evaluate the importance of DCs for vaccine-mediated CD8+ T-cell cross-priming, we utilized transgenic mice expressing the diphtheria toxin receptor (DTR) fused with green fluorescent protein under control of the DC-associated CD11c promoter (CD11c-DTR).29 Recipient mice were reconstituted with bone marrow from CD11c-DTR transgenic (Figure 4a, schematic). This rendered DTR-expressing CD11c+ cells sensitive to diphtheria toxin (DT) (Figure 4b). Notably, radio-resistant Langerhans cells,30, 31 represented the green fluorescent protein-negative CD11c-expressing cells in DLN of the chimeric mice. CD11c+ DC ablation significantly impaired the generation of antigen-specific CD8+ T cells (Figure 4c and Supplementary Figure 4A). Depletion of CD11c-expressing cells had no effect on ISCOMATRIX adjuvant-mediated NK cell accumulation in the DLN but significantly reduced NK cell IFNγ production (Figure 4d and data not shown). Given the dependence on DCs for ISCOMATRIX adjuvant-mediated NK cell and CD8+ T-cell immune responses, we next sought to evaluate the effect of ISCOMATRIX adjuvant on DC activation in vitro and in vivo.
Figure 4.
CD11c-expressing cells are required for ISCOMATRIX adjuvant -mediated NK cell and CD8+ T-cell responses. (a) Schematic showing the generation of CD11c-DTR mice, and the CD11c+ depletion protocol used during the prime and boost IMX vaccine regimen. (b) Representative profile showing the depletion of radiosensitive CD11c/DTR-green fluorescent protein-expressing DCs at the time the antigen-specific CD8+ T-cell immune responses were quantified. (c) CD11c-DTR mice were either treated with PBS or DT 1 day before the prime vaccine dose, and then three times per week. The OVA-specific CD8+ T-cell response was assessed ex vivo in the spleen 7 days after the boost vaccination. The magnitude of the response is shown relative to the PBS-treated cohort ±s.e.m. Results are pooled from two separate experiments. (d) CD11c-DTR mice were treated with PBS or DT −3 and −1 days before a single dose of ISCOMATRIX adjuvant. Ex vivo NK cell IFN-γ production was measured in the DLN after 24 h. The magnitude of the response is shown relative to the PBS-treated cohort, ±s.e.m. The results are representative of at least two separate experiments. Student's t-test was used to calculate statistical significance.
ISCOMATRIX adjuvant administration results in potent DC activation in vivo
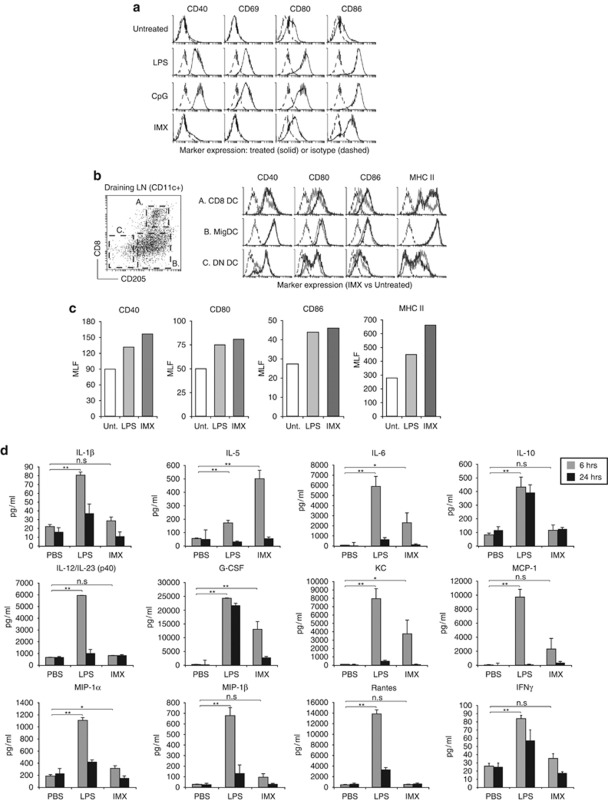
A characteristic feature of TLR-induced DC activation is increased cell surface expression of T-cell co-stimulatory molecules and MHC class II.13, 32 To establish whether ISCOMATRIX adjuvant directly activated DCs, we generated DCs in vitro using the FMS-like tyrosine kinase 3 (Flt3) ligand culture system.33, 34 Compared with TLR4 (lipopolysaccharide, LPS) or TLR9 (CpG) stimulation, ISCOMATRIX adjuvant failed to induce CD40, CD69, CD80 or MHC class II expression (Figure 5a and data not shown). A modest increase in CD86 was observed but to a lesser extent than TLR4 or 9 stimulation. Similar results were obtained with DCs generated with granulocyte-macrophage CSF and IL-4 (data not shown). Plasmacytoid DCs in the Flt3L-treated cultures did not respond to ISCOMATRIX adjuvant (data not shown). Consistent with the lack of phenotypic activation, ISCOMATRIX adjuvant failed to induce pro-inflammatory cytokine production by macrophages or DCs, as compared with lipopolysacaride (LPS) stimulation (Supplementary Figure 5A and data not shown).
Figure 5.
In vivo DC activation and cytokine responses to ISCOMATRIX adjuvant administration. (a) Flt3L-derived DCs were cultured overnight in the presence of IMX (5 μg ml−1), CpG (1 μM) or LPS (1 μg ml−1). CD40, CD69, CD80 and CD86 upregulation (black lines) was monitored on conventional (CD11c+CD45RA−) DCs by flow cytometry, as compared with an isotype control antibody (dashed lines). (b) CD8 and double-negative (DN) lymphoid DCs were distinguished from ‘tissue-derived' MigDCs based on the expression of CD8 and CD205 (left dot plot).53 Right histogram; expression of CD40, CD80, CD86 and MHC class II expression (black lines) on DCs following a single subcutaneous dose of ISCOMATRIX adjuvant, as compared with DCs isolated from untreated DLN (gray lines). Isotype control antibody staining is shown as dashed lines. Data are representative of 3–5 experiments. (c) Activation marker expression by CD8 DCs isolated from the DLN of untreated mice, or 24 h after a subcutaneous dose of IMX (5 μg) or LPS (3 μg). The mean linear fluorescence (MLF) is shown on the y-axis, with isotype controls MLF values subtracted for each sample. (d) Cytokine levels in the serum collected 6 or 24 h after subcutaneous IMX or LPS administration: shown are the levels of IL—1β,IL-5,IL-6, IL-10, IL-12/23(p40), granulocyte-CSF, keratinocyte chemoattractant (KC or CXCL1), monocyte chemotactic protein-1 (MCP-1 or CCL2), macrophage inflammatory protein-1α (MIP-1α or CCL3) and β (MIP-1β or CCL4), Rantes (or C) and IFN-γ. Error bars show the s.e.m. (n=6 mice per treatment group). Student's t-test was used to calculate statistical significance (*P<0.05, **P<0.001, n.s., not significant). All results are representative of two or more independent experiments.
DC activation is considered a key event in T-cell priming.35 Given our in vitro results, we questioned whether ISCOMATRIX adjuvant injection caused DC activation in vivo. DCs isolated from the DLN 24 h post-ISCOMATRIX adjuvant administration were evaluated for activation marker expression (Figure 5b). In contrast to our in vitro results, CD8+CD205+ DCs (CD8 DCs) showed consistent upregulation of CD40, CD80, CD86 and MHC class II. Activation markers on CD205−CD8− DCs (double negative, DN DCs) or tissue-derived migratory CD8−CD205+ DCs (MigDCs) did not significantly change with treatment. Plasmacytoid DCs in the DLN showed modest increases in activation marker expression (data not shown). DC activation was not evident in the spleen or in the non-DLN (data not shown). In comparison with LPS administration, ISCOMATRIX adjuvant induced similar or greater activation marker upregulation by the DLN CD8 DCs (Figure 5c). Consistent with immune cell activation, systemic cytokines were detected in the serum of ISCOMATRIX adjuvant-treated mice; although at much lower levels compared with LPS-treated mice, with the exception of IL-5 (Figure 5d). In the DLN, elevated levels of IL-6 and the IL-6-type cytokine family member leukemia inhibitory factor,36 along with monocyte chemotactic protein-1, macrophage-CSF and tumor necrosis factor-α (TNF-α) were detected (Supplementary Figure 6a).
ISCOMATRIX adjuvant facilitates antigen cross-presentation by CD8 and non-CD8 DCs
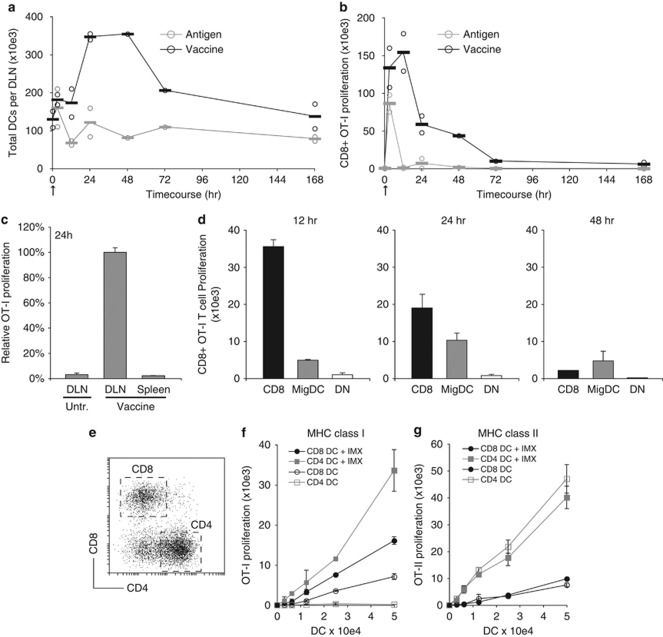
Given the ability of ISCOMATRIX adjuvant to promote DC activation and provide a pro-inflammatory milieu in vivo, we next assessed its effects on DC migration into DLN and MHC antigen presentation. The increase in DLN cellularity (Supplementary Figure 1A) correlated with a three–four-fold increase in the number of CD11cint-high DCs at 24 h, as compared with mice that had received antigen alone (Figure 6a). To evaluate the kinetics of MHC I and II presentation, DCs were isolated at the indicated time-points from the DLN and co-cultured with carboxyfluorescein succinimidyl ester (CFSE)-labeled OVA-specific CD8+ (OT-I) or CD4+ (OT-II) T cells (Figure 6b and Supplementary Figure 7A). DCs from mice that were vaccinated or received OVA antigen alone induced comparable OT-I proliferation at 3 h. By contrast, at 12 h there was a 100-fold difference in T-cell proliferation by DCs from vaccinated mice. No systemic MHC class I antigen cross-presentation was detected in the spleen of immunized mice (Figure 6c), and MHC class II antigen presentation was only modestly improved by formulating OVA with the ISCOMATRIX adjuvant (Supplementary Figure 7A).
Figure 6.
ISCOMATRIX adjuvant facilitates antigen entry into the MHC class I cross-presentation pathway in DCs. (a) Time-course showing the number of CD11c+ DCs isolated from the DLN of mice injected with vaccine or OVA (antigen) alone. (b) MHC class I cross-presentation was quantified in the DLN ex vivo after a single dose of ISCOMATRIX vaccine or OVA (antigen) alone. The CD11c+ DC fraction (>90%) was purified from the DLN at the indicated times. A total of 5 × 103 DCs were then co-cultured with 5 × 104 CFSE-labeled OT-1 CD8+ T cells. Proliferation was quantified 60 h later by flow cytometry. Circles in (a) and (b) represent data points from independent experiments (an average of n=4–8 DLN per sample), and bars show the pooled average. (c) MHC-I presentation by CD11c+ DCs purified from the spleen or DLN 24 h after a single ISCOMATRIX vaccine dose. OT-I proliferation was determined as in (b). (d) Highly purified (>95%) populations of CD8, DN and MigDC were purified from the DLN 12, 24 and 48 h after a single vaccine dose. Cross-presentation was assessed by co-culturing each DC population with 5 × 104 CFSE-labeled OT-1 cells and quantifying proliferation 60 h later. (e) CD11c-enriched spleen DCs were separated into CD8 and CD4 populations by flow cytometry (>95% purity). (f) CFSE-labeled OT-I T cells were co-cultured with CD8 or CD4 DCs pulsed for 30 min with OVA (antigen) alone (open symbols) or antigen+ISCOMATRIX (closed circles). Pulsed DCs were cultured with CFSE-labeled OT-I cells, and proliferation was determined after 60 h as described. (g) The same DCs used in (f) were co-cultured with CFSE-labeled CD4+ OT-II T cells. Proliferation was quantified as described. Error bars represent the s.d. of triplicate samples. Results are representative of two or more independent experiments.
To identify the DC subset(s) cross-presenting antigen in vivo, we isolated by flow cytometry CD8, MigDCand DN DC populations at 12, 24 and 48 h after a single vaccine dose (Figure 6d). By 12 h, the CD8 DC subset dominated MHC class I cross-presentation to OT I cells; however, by 24 h the MigDC population efficiently cross-presented antigen. This second wave of cross-presentation coincided with an influx of CD8−CD205int DCs into the DLN (Figure 6a and Supplementary Figure 7b).
Given the ability of ISCOMATRIX adjuvant to facilitate the translocation of antigen from endosomes into the cytosol of human DCs in vitro,37 we hypothesized that cytosolic translocation of antigens might enable cross-presentation by non-specialized DC subsets. To evaluate this, we purified CD8 and CD4 DCs from the spleen of naïve animals (Figure 6e). Consistent with our earlier study,11 only CD8 DCs efficiently cross-presented soluble antigen to naïve CD8+ T cells (Figure 6f). Surprisingly, CD4 DCs were even more efficient than CD8 DCs at cross-presentating soluble antigen formulated with ISCOMATRIX adjuvant. Finally, consistent with our in vivo observation, ISCOMATRIX adjuvant did not significantly improve soluble antigen delivery into the MHC class II antigen presentation (Figure 6g).
ISCOMATRIX vaccines are dependent on a myeloid differentiation primary response gene 88 (MyD88) signaling axis in vivo
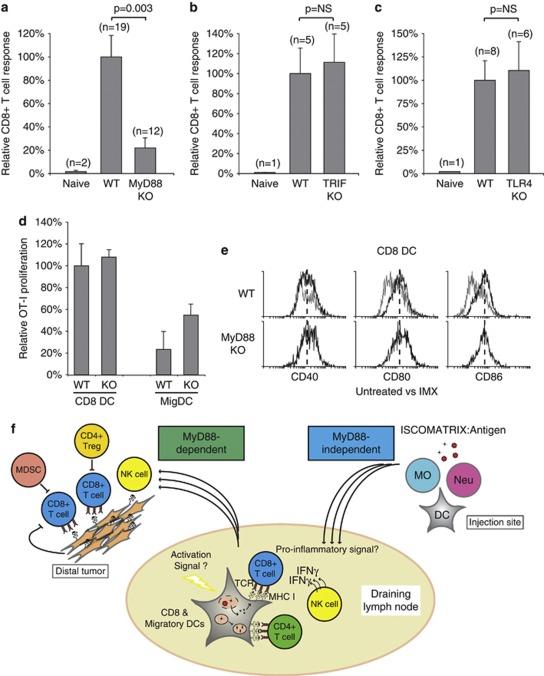
Finally, to further understand how ISCOMATRIX vaccines mediate cellular immunity in vivo, we investigated whether CD8+ T-cell cross-priming was dependent on adapters of the TLR and IL-1R pathway.16 Strikingly, the CD8+ T-cell response was significantly impaired in MyD88-deficient mice (Figure 7a). By contrast, mice deficient for TIR-domain-containing adapter-inducing IFN-β (TRIF) gave a comparable CD8+ T-cell response to wild-type controls (Figure 7b). Interestingly, the increased DLN cellularity following ISCOMATRIX adjuvant administration was indistinguishable between wild-type, TRIF or MyD88-deficient mice (data not shown). NK cell IFN-γ production and vaccine antigen-specific antibody responses were also strongly impaired in MyD88-deficient mice (Supplementary Figure 8A and data not shown). To evaluate if an endogenous TLR4 ligand, signaling through the MyD88 adapter, may reproduce this phenotype, we assessed the vaccine-mediated CD8+ T-cell response in TLR4-deficient mice (Figure 7c).7 Unlike MyD88, TLR4 was dispensable for ISCOMATRIX vaccine-mediated cellular responses in vivo. Interestingly, MyD88-deficiency had no effect on vaccine antigen cross-presentation by CD8 or MigDCs, or ISCOMATRIX-induced DC maturation in the DLN (Figures 7d and e).
Figure 7.
ISCOMATRIX vaccines are dependent on a MyD88-signaling axis in vivo (a) CD8+ T-cell responses were compared in wild type (WT) and MyD88-deficient mice (MyD88 KO) vaccinated with an ISCOMATRIX vaccine on day −7, 0, with the magnitude of the CD8+ T-cell response shown relative to WT mice. (b, c) Same as in (a) except the CD8+ T-cell response was evaluated in TRIF or TLR4-deficient mice. (d) Purified CD8 and MigDCs from wild type or MyD88-deficient (KO) mice were isolated from the DLN 24 h after vaccine administration. MHC class I cross-presentation was assessed by co-culturing each population with 5 × 104 CFSE-labeled OT-1 cells and quantifying proliferation 60 h later, as described. (e) CD40, CD80 and CD86 expression (black lines) was assessed for CD8 DCs isolated from the DLN of WT or MyD88 KO mice dosed with ISCOMARTIX adjuvant, compared with CD8 DCs from untreated WT mice (gray lines). Dashed lines illustrate the median fluorescence for each marker. (f) Schematic illustrating the interaction between DCs, T cells and NK cells in the DLN following ISCOMATRIX vaccine delivery. ISCOMATRIX vaccines initiate a localized inflammatory response at the subcutaneous injection site, and efficient DC activation and MHC class I cross-presentation in the DLN (MyD88-independent). Although the precise DC activation signal(s) is currently unknown, a distinct pro-inflammatory milieu was detected locally and systemically following ISCOMATIRX adjuvant administration. In the DLN, NK cell activation and CD8+ T-cell cross-priming was dependent on DCs, as well as an intact MyD88 signaling network. Cross-primed CD8+ T cells exhibit potent antitumor activity in prophylactic tumor challenge models. However, in the case of pre-established tumor burden, the effectiveness of the vaccine is likely to be blunted by immune suppressive networks, such as myeloid-derived suppressor cells (MDSC), regulatory T cells (Treg) and tumor-derived factors that prevent complete tumor eradication.
Discussion
Therapeutic cancer vaccines are typically comprised of three components: an antigen, to give specificity to the cellular response; a delivery modality, to promote antigen capture and transfer into the MHC class I pathway; and an immune-modulating agent, to enhance the immunogenicity of the vaccine antigen.38 Therapeutic vaccines are intended to generate CTLs that can infiltrate tumors and selectively eliminate malignant cells.3
ISCOMATRIX adjuvant-based vaccines have been shown to generate robust humoral and cellular immune responses in a range of pre-clinical models, and have a good safety profile in human subjects.8 How ISCOMATRIX adjuvant facilitates immunity to co-delivered vaccine antigens remains poorly understood. The particulate nature of ISCOMATRIX adjuvant likely promotes efficient endocytosis by DCs; both at the injection site and by DCs in the DLNs (Figure 7f).39 The earliest detectable response to ISCOMATRIX adjuvant administration was a rapid influx of innate immune cells into the injection site and a distinct localized cytokine and chemokine response. The initial pro-inflammatory response led to innate immune cell accumulation and activation in the LNs draining the injection site. Coordinated NK cell and DC functionalities culminated in TH-dependent CD8+ T-cell cross-priming. We observed that optimal vaccine antigen-specific CD8+ T-cell responses were achieved when the boost vaccination was delivered 7 days after the initial prime dose. This observation was consistent with our earlier observation that DC antigen cross-presentation is restored 7 days after exposure to potent maturation stimuli.11, 13 Indeed, a boost vaccination delivered earlier than 7 days was found to negatively impact the magnitude of the CD8+ T-cell response (data not shown).7 Our results suggest that an accelerated 7-day interval between the prime and boost regimen may enhance efficacy, and be beneficial in the instance of treating aggressively growing tumors.
ISCOMATRIX adjuvant contains the ISCOPREP saponin, a purified fraction of Quillaia saponin, which may alter the biophysical properties of the endosomal membrane in cells, thereby facilitating the cytosolic translocation of co-delivered vaccine antigens.37 Consistent with this hypothesis, ISCOMATRIX adjuvant enabled efficient MHC class I cross-presentation of a vaccine antigen in vitro by a population of DCs that typically do not cross-present soluble antigens. The ability of the ISCOMATRIX adjuvant to enable cross-presentation by non-specialized DC subsets may explain the efficient MHC class I cross-presentation observed by tissue-derived DCs trafficking into DLNs from the vaccination site. Conversely, cross-presentation by the CD205highCD11cintCD8neg DC population identified in this study may reflect an intrinsic feature of a dermal or an inflammatory-induced DC subset.14, 15, 40 Consistent with an earlier report,30 Langerhans were not sufficient for CD8+ T-cell cross-priming in vivo. The precise identification of the cross-presenting tissue-derived DC population following ISCOMATRIX vaccine administration requires further phenotypic and functional characterization.
Many therapeutic cancer vaccines have failed to achieve primary endpoints in clinical studies.41 A likely caveat is that pre-clinical models used to assess efficacy do not adequately reproduce the immunosuppressive mechanisms that pre-exist in cancer patients such as; regulatory T-cell networks, immature myeloid cells and tumor cell-derived immune suppressive factors (Figure 7f).3, 18 In addition, most current standard of care options for controlling cancer involves chemotherapies that may impair effective cellular immune responses.42, 43 Despite this, several emerging Phase III studies indicate that immune-modulatory strategies may have clinical activity in certain cancer indications.44, 45 Encouragingly, we have demonstrated that the therapeutic effect of ISCOMATRIX adjuvant-based vaccines can be enhanced through combination with clinically relevant immune modifiers: melanoma (anti-CD40 agonist antibody); and in pancreatic cancer (CpG oligodeoxynucleotide).46 A clear mechanistic understanding of the cooperativity between these agents and ISCOMATRIX vaccines remains to be elucidated. For example, CD40 signaling can modulate many facets of the immune response, including DC, macrophage and T-cell functions.25, 47, 48, 49 Therefore, it is feasible that ISCOMATRIX vaccines and CD40 agonists function independently to activate distinct immune-signaling nodes, which combine effectively to enhance therapeutic efficacy. Importantly, our findings suggest that the single agent activity of immunotherapeutics may benefit through combination with an ISCOMATRIX adjuvant-based cancer vaccine.
In comparison to TLR4 agonists, ISCOMATRIX adjuvant administration produced a unique systemic and localized cytokine and chemokine signature. This pro-inflammatory response was transient and coincided with NK cell and DC activation in LNs draining the injection site. Inconsistent with direct pattern recognition receptor stimulation, ISCOMATRIX adjuvant failed to activate macrophages or DCs in vitro, in contrast to TLR4 or 9 stimulation. These results support a mechanism of indirect immune cell activation in vivo, most likely via specific cytokine cascades. To address the possibility that an endogenous TLR ligand may indirectly facilitate immune activation, we vaccinated mice deficient for the TLR-signaling adapters MyD88 or TRIF. Strikingly, innate and adaptive immune responses to ISCOMATRIX vaccines were severely compromised in MyD88-deficient mice. We reasoned that an endogenous TLR ligand ‘danger signal' released upon ISCOMATRIX adjuvant administration might account for the impaired vaccine response in MyD88-deficient mice;43, 50 however, TLR4 was found to be dispensable for cellular immunity, as was IL-1R signaling (data not shown). Instead we propose that innate immune cells responding to ISCOMATRIX adjuvant at the injection site initiate a cascade-localized inflammatory events, which culminate in CTL cross-priming in DLNs. The exact mechanisms underlying these events remain to be elucidated, and are the focus of continuing research efforts. Interestingly, DC activation and cross-presentation were MyD88-independent, lending to the possibility that MyD88-dependence is downstream of apical inflammatory events but required for DC-dependent NK cell activation and adaptive immune responses (Figure 7f).
In conclusion, our study has characterized the immune responses to ISCOMATRIX adjuvant in vivo. We show that ISCOMATRIX vaccines promote significant cross talk between the innate and adaptive compartments, which culminate in tumorcidal CD8+ T-cell cross-priming. These findings have clear implications for therapeutic cancer vaccine design; however, they also implicate a novel pattern recognition receptor-independent pro-inflammatory pathway that may be linked to the biophysical properties of the ISCOMATRIX adjuvant. Taken together, our results support the clinical evaluation of combination approaches that incorporate ISCOMATRIX adjuvant-based vaccines with immune modifiers to treat cancer.
Methods
Mice
Unless otherwise stated, all C57BL/6 experimental mice were 6–8-weeks old. Wild-type, MHC class II-deficient,23 MyD88-deficient,51 TRIF-deficient,52 C57Bl6.TLR4(Lps-d)-deficient (C3H/HEJ backcrossed n=8 generations to C57Bl6 background) and CD11c-DTR transgenic mice29 were maintained under specific pathogen-free conditions. All experiments were subject to approval by the institutional Ethics Committee according at Genentech Inc. (South San Francisco, CA, USA), CSL Limited (Parkville, Victoria, Australia) or the Walter and Eliza Hall Institute (WEHI, Melbourne, Victoria, Australia). All animal work was undertaken in accordance with institutional and national guidelines and conformed to the provisions of the Declaration of Helsinki (as revised in Edinburgh 2000).
Air-pouch method
The air-pouch model was adapted from the method previously described.20 Briefly, anesthetized mice were injected subcutaneously with 5 ml of sterile air into the intravascular area. On day 3, 2–3 ml of sterile air was injected to maintain the integrity of the air-pouch. On day 7, 5 μg ISCOMATRIX adjuvant in 100 μl of PBS or PBS alone was injected into the air-pouch. At the indicated time, animals were euthanized by CO2 inhalation and 1 ml of PBS was injected into the air-pouch. Exudates, typically 500–800 μl, were collected and analyzed for infiltrating immune cells. For cytokine and chemokine determinations, infiltrating cells were isolated 4 h after the indicated treatment, and cultured overnight in serum-free media (Invitrogen, Carslbad, CA, USA). Supernatants were concentrated using Amicon Ultra centrifugal filters (3 kDa) (Millipore, Billerica, MA, USA) before chemokine and cytokine analysis.
Cytokine determinations
Cytokines and chemokines were measured with the Bio-Plex cytokine assay (Bio-Rad, Hercules, CA, USA) and analyzed with the Luminex 100 system (Luminex, Austin, TX, USA). The full list of chemokines and cytokines included in the 32-plex assay were: IL-1α, IL-1β, IL2, IL4, IL-5, IL-6, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17, IL-18, Eotaxin, granulocyte-CSF, macrophage-CSF, granulocyte-macrophage-CSF, IFNγ, KC, monocyte chemotactic protein-1, macrophage inflammatory protein-1α, macrophage inflammatory protein-1β, CCL5, tumor necrosis factorα, basic fibroblast growth factor, leukemia inhibitory factor, chemokine (C–X–C motif) ligand 9 (CXCL9), macrophage inflammatory protein-2, platelet-derived growth factor and vascular endothelial growth factor.
Dendrtic cell preparations
DCs were isolated from the spleen and LNs, as previously described.53 Enriched DC fractions (typically >95%) were stained with a combination of anti-CD11c (clone N418), anti-CD205 (clone NLDC145) and anti-CD8 (clone YTS16.4) to identify resident and migratory subsets. Bone marrow-derived DCs were generated as previously described using FMS-like tyrosine kinase 3 ligand.33 Anti-CD11c and anti-CD45RA (clone 14.8) were used in combination to identify conventional (CD11c+CD45RA−) and plasmacytoid (CD11c+CD45RA+) DC populations. Anti-CD40 (clone FGK-45), anti-CD69 (clone H1.2F3), anti-CD80 (clone 16–10A1), anti-CD86 (clone GL1) and anti-MHC class II (clone M5/114) expression was determined relative to isotype control antibodies. ISCOMATRIX adjuvant (5 μg ml−1), CpG1668 (1 μM) (GeneWorks, Hindmarsh, SA, Australia or Invivogen, San Diego, CA, USA) or LPS and Escherichia coli serotype 0111:B4 (LPS) (1 μg ml−1) (Invivogen) were used to stimulate DCs or macrophages in vitro. For in vivo DC activation marker evaluation or cytokine determinations, mice were injected subcutaneously with 3 μg of LPS or 5 μg of ISCOMATRIX adjuvant in 100 μl of PBS.
Immunization protocol OVA-specific CD8+ T-cell and antibody response
ISCOMATRIX adjuvant was prepared as described previously.54 The vaccine xantigens were soluble, endotoxin low preparations of chicken OVA (<0.1 EU mg−1) (Worthington Biochemical Corporation, Lakewood, NJ, USA) or HSV-1gB:HSV-2gD fusion protein (gB:gD <0.1 EU mg−1). The gB:gD fusion protein was generated by adding the HSV-1 gB CTL epitope SSIEFARL to the N-terminal of HSV-2 gD glycoprotein (CSL Limited). Alternatively, endotoxin-low OVA (0.007 EU mg−1) was generated at Genentech Inc. from chicken egg-white (specific pathogen-free eggs, Charles River, Willmington, MA, USA), as previously described.55 All prime or boost immunizations were administered subcutaneously into the scruff of the neck in 100 μl of PBS. Each vaccine dose contained 5 μg of ISCOMATRIX adjuvant (equivalent to 3.75 ISCOUNITS) and 30 μg of antigen. Mice were terminally bled via cardiac puncture at the time points indicated. Sera was assayed for reactivity to OVA, as previously described.56
B16 melanoma challenge and adoptive CD8 and non-CD8 cell transfer
Mice were vaccinated on days −7 and 0 with OVA+ISCOMATRIX adjuvant and then challenged 7 days after the boost dose with 1 × 105 B16.F10 melanoma cells expressing full-length chicken egg OVA (B16-OVA). Tumor-incidence was monitored out to day 125. Tumor-free animals were re-challenged on day 150 with 1 × 105 parental B16 melanoma (B16-F10) or B16-OVA cells (data not shown). For adoptive CD8 and non-CD8 cell transfer experiments into naïve recipients: the spleens were harvested from donor mice 21 days after the prime-boost vaccine regimen, and CD8 expressing cells were positively selected using magnetic beads (Miltenyi Biotec, Auburn, CA, USA). Purified CD8 (>85% purity) and CD8-depleted cell fractions were adoptively transferred intravenously (10 × 106) into naïve C57Bl6 recipients. After 1 day of the transfer, mice were challenged with 5 × 105 B16 cells and tumor incidence was monitored. For the therapeutic melanoma cancer model, all mice were inoculated with 1 × 105 B16-OVA tumor cells on day 0. After 5 days of tumor cell inoculation the mice were either untreated or dosed with ISCOMATRIX, OVA or the ISCOMATRIX vaccine (adjuvant+antigen), followed by a boost dose on day 12. Tumor volumes were monitored until the first tumor reached 3000 mm3, which was nominated as the survival end-point. 10 mg kg−1 of anti-CD40 (clone FKG45, BioXcell, West Lebanon, NH, USA, <0.14 EU mg−1) or control rat IgG2a isotype antibody (<0.07 EU mg−1) were co-administered by intraperitoneal injection on day 5 and 12. For T cell and NK cell depletion experiments; 10 mg kg−1 of anti-CD8 (clone 2.43), anti-CD4 (clone GK1.5), anti-asialo-GM1 or anti-NK1.1. (clone PK136, data not shown) were delivered by intraperitoneal injection on days −7, −4, −1, +2 and +5 during the day 0, 7 prime-boost vaccine regimen. After 7 days of the boost dose, all mice were challenged with B16:OVA melanoma cells, as described.
Innate immune cell recruitment and intracellular IFN-γ cytokine staining
To measure the vaccine antigen-specific CD8+ T-cell response, splenocytes were cultured ex vivo for 4 h in the presence of brefeldin A (5 μg ml−1) with SIINFEKL (OVA), SSIEFARL (gB) or an irrelevant peptide (1 μg ml−1). Briefly, splenocytes were stained with anti-CD3 (clone 17A2) and anti-CD8 (clone 2.43), washed, fixed and permeabilized (BD Biosciences, Franklin, NJ, USA as per manufacturer's instructions) followed by staining with anti-IFN-γ (clone XMG1.2). CD8+ T cells were analyzed by flow cytometry for the expression of IFN-γ.21 To evaluate the recall CD8+ T-cell response, splenocytes were co-cultured with EG7-OVA cells (a mouse thymoma EL4 cells stably transfected with OVA) for 5 days. Cells were cultured for a further 4 h with brefeldin A, then stained as described above. Similar results were obtained using the 4-h ex vivo re-stimulation protocol, although the magnitude of the response was reduced (data not shown). H2-Kb/Ova-specific tetramer staining was performed as previously described.57 OVA-tetramer+ CD8+ T cells were also co-stained for CD44 expression (clone IM7). NK cell IFN-γ production was evaluated in the draining and non-DLNs at the indicated time-points after a single dose of ISCOMATRIX adjuvant. Briefly, lymphocytes were cultured for 4 h in the presence of brefeldin A, before staining with anti-NK1.1 (clone PK136) and anti-CD49b (clone DX5), and then fixed and intracellular stained with anti-IFN-γ, as described above. CD69 expression was determined on freshly isolated NK cells, using 7-aminoactinomycin to exclude dead cells.
In vivo CTL assays
Mice were immunized with the ISCOMATRIX vaccine or antigen (OVA) alone on day −7 and 0. On day 7 mice were injected intravenously with 2 × 107 CFSEhigh-labeled SIINFEKL peptide-pulsed cells and CFSElow-labeled, control-unpulsed cells in equal ratios. After 4 h spleens were analyzed by flow cytometry and specific-lysis was calculated as previously described.13
CD11c-DTR bone marrow chimeras
Recipient C57BL/6 mice were irradiated with two doses of 550 cGy 3 h apart, and were reconstituted with 3–5 × 106 T cell-depleted bone marrow cells extracted from the femurs and tibias of CD11c-DTR transgenic mice, as described.29, 58 Briefly, mature T cells were depleted from the donor bone marrow with anti-CD4 (clone RL172), anti-CD8 (clone 3.168) and anti-Thy1 (clone J1) antibodies, followed by treatment with rabbit complement. After 1 day of reconstitution, mice were injected intraperitoneally with 100 μg anti-Thy1 (clone T24) to deplete radio-resistant T cells. All antibodies were kindly provided by Ken Shortman, Walter and Eliza Hall Institute (WEHI). Mice were rested for 5–7 weeks before use. For systemic DC depletion, chimeras were injected intraperitoneally with 4 ng g body weight diphtheria toxoid (DT) (in PBS) every 2 days for the duration of the experiment. MHC class II-depleted splenocytes from Ly5.1 congenic donors were adoptively transferred 24 h before the first vaccine dose. The CD8+ T-cell response was comparable when gating on either transferred Ly5.1 or host Ly5.2 cells (data not shown).
Preparation of CFSE-labeled T cells
OT-I T cells (H-2 Kb-restricted anti-OVA257–264) or OT-II T cells (I-Ab-restricted anti-OVA323–339) were purified from pooled LNs (inguinal, axillary, sacral, cervical and mesenteric) of transgenic mice by depletion of non-CD8 T cells (OT-I) or non-CD4 T cells (OT-II) and were labeled with CFSE as described.13 The T-cell preparations were routinely 85–95% pure, as determined by flow cytometry.
Co-culture assays—DCs and OVA-specific CD8 or CD4 T cells
DC co-culture experiments with transgenic OVA-specific OT-I (CD8) or OT-II (CD4) T cells were performed as previously described.13, 59 Briefly, enriched CD11c-expressing DCs were isolated from DLNs following ISCOMATRIX vaccine or antigen alone (OVA) administration. A total of 5 × 103 DCs were co-cultured in Roswell Park Memorial Institute+10% heat-inactivated fetal bovine serum with 5 × 104 CFSE-labeled naïve OT-I or OT-II T cells. Proliferation was quantified by flow cytometry after 60 h using blank calibration beads, as described.13 A total of 5 × 103 highly purified (by flow cytometry sorting) (>95%) CD8 (CD8+CD205+) MigDC (CD8−CD205+) and DN (CD8−CD205−) DCs were co-cultured directly ex vivo with CFSE-labeled OT-I or OT-II cells. Highly purified (by flow cytometry sorting) (>95%) splenic CD8 or CD4 expressing DCs were pulsed with soluble OVA (100 μg ml−1) or OVA+ISCOMATRIX (5 μg ml−1) for 30 min and washed in media. The indicated number of each DC population was then co-cultured with CFSE-labeled OT-I or OT-II T cells and proliferation quantified, as described above.
Thioglycollate-induced macrophages
Thioglycollate-induced macrophages were generated as previously described.60 Briefly, 1 ml of thioglycollate broth was injected into the peritoneal cavity. Peritoneal cells, (>80%) F4/80+ macrophages were isolated in 10 ml of macrophage-serum-free media (Invitrogen).
Acknowledgments
We thank Max Schnurr and Peter Duewell for helpful discussions. We thank A. Strasser, J. Villadangos, W Heath, K. Shortman and A Lew (The WEHI, Melbourne, Victoria, Australia), and O. Wijburg (Melbourne University, Victoria, Australia) for access to the various knockout mice used in this study. We thank flow cytometry core facilities at Genentech, CSL and the WEHI for their assistance. We thank C. Quinn and S.Edwards for generating the HSV-1gB:HSV-2gD fusion protein.
EM, JB, ABM, LP, HB, DA, SK, LP and DD are employees and shareholders of CSL Limited. NSW, BY, SL, SC, AY, PH and AA are employees of Genentech, a member of the Roche Group. The remaining authors declare no conflict of interest.
Footnotes
The Supplementary Information that accompanies this paper is available on the Immunology and Cell Biology website (http://www.nature.com/icb)
Supplementary Material
References
- Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake RA, Robinson BW. Immunotherapy and chemotherapy—a practical partnership. Nat Rev Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- Tritto E, Mosca F, De Gregorio E. Mechanism of action of licensed vaccine adjuvants. Vaccine. 2009;27:3331–3334. doi: 10.1016/j.vaccine.2009.01.084. [DOI] [PubMed] [Google Scholar]
- Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30:23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Drane D, Gittleson C, Boyle J, Maraskovsky E. ISCOMATRIX adjuvant for prophylactic and therapeutic vaccines. Expert Rev Vaccines. 2007;6:761–772. doi: 10.1586/14760584.6.5.761. [DOI] [PubMed] [Google Scholar]
- Maraskovsky E, Schnurr M, Wilson NS, Robson NC, Boyle J, Drane D. Development of prophylactic and therapeutic vaccines using the ISCOMATRIX adjuvant. Immunol Cell Biol. 2009;87:371–376. doi: 10.1038/icb.2009.21. [DOI] [PubMed] [Google Scholar]
- Wilson NS, Villadangos JA. Regulation of antigen presentation and cross-presentation in the dendritic cell network: facts, hypothesis, and immunological implications. Adv Immunol. 2005;86:241–305. doi: 10.1016/S0065-2776(04)86007-3. [DOI] [PubMed] [Google Scholar]
- Smith CM, Belz GT, Wilson NS, Villadangos JA, Shortman K, Carbone FR, et al. Cutting edge: conventional CD8 alpha+ dendritic cells are preferentially involved in CTL priming after footpad infection with herpes simplex virus-1. J Immunol. 2003;170:4437–4440. doi: 10.4049/jimmunol.170.9.4437. [DOI] [PubMed] [Google Scholar]
- Schnorrer P, Behrens GM, Wilson NS, Pooley JL, Smith CM, El-Sukkari D, et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc Natl Acad Sci U S A. 2006;103:10729–10734. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz GT, Smith CM, Eichner D, Shortman K, Karupiah G, Carbone FR, et al. Cutting edge: conventional CD8 alpha+ dendritic cells are generally involved in priming CTL immunity to viruses. J Immunol. 2004;172:1996–2000. doi: 10.4049/jimmunol.172.4.1996. [DOI] [PubMed] [Google Scholar]
- Wilson NS, Behrens GM, Lundie RJ, Smith CM, Waithman J, Young L, et al. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat Immunol. 2006;7:165–172. doi: 10.1038/ni1300. [DOI] [PubMed] [Google Scholar]
- Belz GT, Bedoui S, Kupresanin F, Carbone FR, Heath WR. Minimal activation of memory CD8+ T cell by tissue-derived dendritic cells favors the stimulation of naive CD8+ T cells. Nat Immunol. 2007;8:1060–1066. doi: 10.1038/ni1505. [DOI] [PubMed] [Google Scholar]
- Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Inflammasomes: guardians of cytosolic sanctity. Immunol Rev. 2009;227:95–105. doi: 10.1111/j.1600-065X.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X. Regulatory T cells and immune tolerance to tumors. Immunol Res. 2009;46:79–93. doi: 10.1007/s12026-009-8124-7. [DOI] [PubMed] [Google Scholar]
- Edwards JC, Sedgwick AD, Willoughby DA. The formation of a structure with the features of synovial lining by subcutaneous injection of air: an in vivo tissue culture system. J Pathol. 1981;134:147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- Smith CM, Wilson NS, Waithman J, Villadangos JA, Carbone FR, Heath WR, et al. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol. 2004;5:1143–1148. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, et al. Mice lacking MHC class II molecules. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- Ramirez-Montagut T, Chow A, Hirschhorn-Cymerman D, Terwey TH, Kochman AA, Lu S, et al. Glucocorticoid-induced TNF receptor family related gene activation overcomes tolerance/ignorance to melanoma differentiation antigens and enhances antitumor immunity. J Immunol. 2006;176:6434–6442. doi: 10.4049/jimmunol.176.11.6434. [DOI] [PubMed] [Google Scholar]
- French RR, Chan HT, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat Med. 1999;5:548–553. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- van Mierlo GJ, den Boer AT, Medema JP, van der Voort EI, Fransen MF, Offringa R, et al. CD40 stimulation leads to effective therapy of CD40(−) tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proc Natl Acad Sci U S A. 2002;99:5561–5566. doi: 10.1073/pnas.082107699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: ‘l'union fait la force'. Blood. 2005;106:2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, et al. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- Merad M, Sugie T, Engleman EG, Fong L. In vivo manipulation of dendritic cells to induce therapeutic immunity. Blood. 2002;99:1676–1682. doi: 10.1182/blood.v99.5.1676. [DOI] [PubMed] [Google Scholar]
- De Smedt T, Pajak B, Muraille E, Lespagnard L, Heinen E, De Baetselier P, et al. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med. 1996;184:1413–1424. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M, et al. Cutting edge: generation of splenic CD8+ and CD8- dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol. 2005;174:6592–6597. doi: 10.4049/jimmunol.174.11.6592. [DOI] [PubMed] [Google Scholar]
- Brasel K, De Smedt T, Smith JL, Maliszewski CR. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood. 2000;96:3029–3039. [PubMed] [Google Scholar]
- Reis e Sousa C. Activation of dendritic cells: translating innate into adaptive immunity. Curr Opin Immunol. 2004;16:21–25. doi: 10.1016/j.coi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Metcalfe SM. LIF in the regulation of T-cell fate and as a potential therapeutic. Genes Immun. 2011;12:157–168. doi: 10.1038/gene.2011.9. [DOI] [PubMed] [Google Scholar]
- Schnurr M, Orban M, Robson NC, Shin A, Braley H, Airey D, et al. ISCOMATRIX adjuvant induces efficient cross-presentation of tumor antigen by dendritic cells via rapid cytosolic antigen delivery and processing via tripeptidyl peptidase II. J Immunol. 2009;182:1253–1259. doi: 10.4049/jimmunol.182.3.1253. [DOI] [PubMed] [Google Scholar]
- Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat Med. 2005;11 (Suppl 4:S63–S68. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, et al. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J Immunol. 2004;173:3148–3154. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethinking therapeutic cancer vaccines. Nat Rev. 2009;8:685–686. doi: 10.1038/nrd2994. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–170. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse MA, Whelan M. A year of successful cancer vaccines points to a path forward. Curr Opin Mol Ther. 2010;12:11–13. [PubMed] [Google Scholar]
- Jacobs C, Duewell P, Heckelsmiller K, Wei J, Bauernfeind F, Ellermeier J, et al. An ISCOM vaccine combined with a TLR9 agonist breaks immune evasion mediated by regulatory T cells in an orthotopic model of pancreatic carcinoma. Int J Cancer. 2011;128:897–907. doi: 10.1002/ijc.25399. [DOI] [PubMed] [Google Scholar]
- Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signaling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. Role of adapter TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- Wilson NS, El-Sukkari D, Belz GT, Smith CM, Steptoe RJ, Heath WR, et al. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood. 2003;102:2187–2194. doi: 10.1182/blood-2003-02-0513. [DOI] [PubMed] [Google Scholar]
- Maraskovsky E, Sjolander S, Drane DP, Schnurr M, Le TT, Mateo L, et al. NY-ESO-1 protein formulated in ISCOMATRIX adjuvant is a potent anticancer vaccine inducing both humoral and CD8+ t-cell-mediated immunity and protection against NY-ESO-1+ tumors. Clin Cancer Res. 2004;10:2879–2890. doi: 10.1158/1078-0432.ccr-03-0245. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Miyazaki Y, Zimmerman GA, Albertine KH, McIntyre TM. Endotoxin contamination of ovalbumin suppresses murine immunologic responses and development of airway hyper-reactivity. J Biol Chem. 2003;278:42361–42368. doi: 10.1074/jbc.M307752200. [DOI] [PubMed] [Google Scholar]
- Lenarczyk A, Le TT, Drane D, Malliaros J, Pearse M, Hamilton R, et al. ISCOM based vaccines for cancer immunotherapy. Vaccine. 2004;22:963–974. doi: 10.1016/j.vaccine.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapoznikov A, Jung S. Probing in vivo dendritic cell functions by conditional cell ablation. Immunol Cell Biol. 2008;86:409–415. doi: 10.1038/icb.2008.23. [DOI] [PubMed] [Google Scholar]
- Wilson NS, El-Sukkari D, Villadangos JA. Dendritic cells constitutively present self antigens in their immature state in vivo and regulate antigen presentation by controlling the rates of MHC class II synthesis and endocytosis. Blood. 2004;103:2187–2195. doi: 10.1182/blood-2003-08-2729. [DOI] [PubMed] [Google Scholar]
- Hopper KE. Kinetics of macrophage recruitment and turnover in peritoneal inflammatory exudates induced by Salmonella or thioglycollate broth. J Leuk Biol. 1986;39:435–446. doi: 10.1002/jlb.39.4.435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.