Abstract
Fifty strains of bacteria were isolated from six isolates of the nematode Bursaphelenchus mucronatus (Bm) from China and Russia and identified using the BioMerieux Vitek 32 system. In bioassay, 3 bacterial strains showed the high levels of phytotoxin production while 19, 16, and 12 strains showed moderately, low and no phytotoxin production, respectively. Inoculation of 2-month-old Pinus thunbergii seedling with each of the six Bm isolates showed that the mean number of days from inoculation to death of 80% of the seedlings was significantly related to the ratio of the total number of bacterial strains for a nematode isolate to the number of pathogenic bacterial strains of the nematode isolate. The results of inoculation of 3-year-old P. thunbergii seedlings showed that inoculation with either axenic Bm (ABm) or axenic B. xylophilus (ABx) and the pathogenic bacterial strain together were essential for inducing pine wilt. These findings demonstrate that wilt symptoms caused by Bm conform to our earlier hypothesis (Zhao et al., 2003) that pine wilt disease, induced by certain Bx or Bm isolates, is caused by a complex of both the nematodes and their associated pathogenic bacteria. The results also account for the variation in pathogenicity of Bm populations from different parts of the world.
Keywords: axenic nematode, Aeromonas, Acinetobacter, bioassay, pathogenicity, Pinus thunbergii, Pseudomonas
Introduction
The nematode Bursaphelenchus mucronatus (Bm), a close relative of the pine wood nematode, Bursaphelenchus xylophilus (Bx), is a wood-inhabiting nematode native to northern Eurasia. Nematodes in Bursaphelenchus are mycophagic and phytophagic (mostly on conifers) and the specialized entomophilic nematode juvenile (dauerlarva) are vectored to conifers by wood-inhabiting beetles. Inoculations with Bm isolates from different regions of the world have resulted in varying pathogenicities to pine species. Mamiya (1998) stated that the occurrence of B. mucronatus has never been related to epidemic disease anywhere within its distribution area. Kanzaki and Futai (2006) reported that when they inoculated shaded and unshaded Pinus densiflora seedlings with B. mucronatus, the nematode was only pathogenic on shaded seedlings. However, other authors have reported the potential pathogenicity of B. mucronatus (Kulinich et al., 1994; Kishi, 1995). Damage caused by B. mucronatus may be related to special environmental conditions of susceptible pine species (Braasch et al., 1998). Li et al. (2007) reported that more than 20,000 trees of Pinus yunanensis died of pine wilt caused by B. mucronatus in 2005 in five forests in Huaping county, Yunnan Province, China. Zhang (2003) indicated that more than 65 million pine trees died of pine wilt disease caused by B. mucronatus in the local pine forests in Guiyang City, Guizhou Province, China. Caroppo et al. (2000) used three Bursaphelenchus species to inoculate conifer seedlings in a growth chamber and outdoors. They found that inoculation with Bm isolates resulted in 30-100% mortality to P. sylvestris, P. pinaster, and Larix decidua. The highest mortality was recorded for P. sylvestris seedlings. A similar result was reported in Turkey for P. sylvestris seedlings (Akbulut et al., 2007). At Nanjing, China, Zhang and Lin (2004) inoculated 2-year-old Pinus thunbergii seedlings with 20 Bm isolates from China, Japan, Canada, Korea, France and Norway. The results showed that the most pathogenic Bm isolates, with seedling mortality rates above 85%, were BmFr (from France), BmCGHH (from China), BmCFJ2 (from China) and BmCFJ3 (from China). The second most pathogenic group of Bm isolates, with a significant higher seedling mortality relative to the control, included 11 populations from China and Canada. A third group of Bm isolates, which caused no significant seedling mortality, included BmJan (from Japan), BmK1 (from Korea), BmK2 (from Korea) and BmH (from Norway).
These differences in results raise the question as to why Bm isolates vary in their pathogenicity. There is currently no generally accepted answer to the question. To investigate whether the bacteria carried by Bm play a role in pine wilt disease, we isolated and identified the bacterial strains associated with the following six Bm isolates: Ru1 (from Russia), Ru2 (from Russia), Ru3 (from Russia), SC (from Sichuang Provice, China), JX1 (from China) and JX2 (from China), and then determined the ability of the bacterial strains to produce phytotoxins using a bioassay comprised of bacterial cell free cultures and 2-month-old P. thunbergii seedlings. In addition, the pathogenicity of various combinations of Bm isolates, Bx isolates and their associated bacterial strains were determined in 3-year-old P. thunbergii seedlings.
Materials and Methods
Bacteria carried by Bm: Six Bm isolates were used in the studies. Three originated in Russia (all isolated in 2002) including Ru1 from Abies sp. tree logs in the Ural Mountains, Sverdlovski region, Ru2 from Larix sibirica in the Irkusky Region, and Ru3 from Picea sp. in the Krasnoyarsky Territory and three (all isolated from P. massoniana in 2004) from China: JX1, JX2 from Jiangxi Province, China and SC from Sichuan Province. JX2 was collected from a log of a healthy P. massoniana, which was cut down in 2003 in a pine forest in Jingde Zhen, Jiangxi Province, China, where pine wilt disease has never been reported. The other Bm isolates from China were from naturally-affected pine forests, where surveys for Bx before collecting the wood samples of Bm showed that the nematode was not present.
Isolation and identification of bacteria: The wood from which the nematodes were isolated was surface sterilized with 70% ethanol and the sapwood was removed with a sterilized knife. Four blocks (1 cm × 0.5 cm × 0.5 cm each) were removed from the centre of the wood and plated onto nutrient broth (NB) medium (10 g of peptone, 5 g of NaCl, 12 g of agar per liter, pH 7.2-7.4) in petri dishes and incubated at 28°C. Three replicate plates were made for each sample. Two to three days later bacterial colonies were selected from the trails made by nematodes on the medium. To avoid losing bacteria species, colonies with different characters from those already selected were intentionally chosen and kept for further purification. The selected and purified colonies were stored at −28°C.
To ensure all the colonies of the bacteria came from Bm, the nematodes were collected from the dishes in which bacterial colonies had been isolated, and identified morphologically using a microscope. Only those colonies from dishes in which all the nematodes were identified as Bm were selected for further identification.
Characteristics such as color, transparency, prominence, edge, and viscosity of the isolated and purified colonies were observed while Gram and oxidase reactions were performed (Noel & Bergey, 1984). These cultures were then identified using a bioMérieux Vitek 32 Auto-BacteriaI Analyzer (bioMérieux Inc., Marcy-Etoile, France). The VITEK GNI+ or VITEK GPI cards were adopted according to the Gram, catalase, and oxidase reactions of the bacterial strains. The results were then subjected to analysis using the related software (bioMerieux Inc., Marcy-Etoile, France).
To further identify the Bm and Bx used in this study, genomic DNA was extracted from the six Bm isolates and one Bx isolate. Duplex PCR was then applied according to the method outlined by Jiang (2005).
Aseptic culture of P. thunbergii seedlings: Seeds of P. thunbergii, were washed in running water for 4 hr, dipped in 0.1% potassium permanganate for 2 hr and then washed three times in sterile water. The seeds were afterwards treated with 70% ethanol for 30 sec, then with 0.1% mercuric chloride for 1 min and washed five times in sterile water. Under aseptic conditions the seed coat was removed, the seed contents were washed three times in sterile water, treated with 5% hydrogen peroxide solution for 3 min, then washed five times in sterile water. The treated seeds were soaked for 24 hr, treated with 3% hydrogen peroxide solution for 3 min, and washed five times in sterile water. They were then put on a potato dextrose agar (PDA) plate for germination at 26°C. If a plate was contaminated, the seeds or seedlings were discarded. When new seedlings sprouted, they were transferred to 20 mm diameter test tubes containing MS media (Sigma Chemicals), incubated at 25°C and grown using a 14 hr, 10,000 lux light followed by 10 hr dark regime.
Bioassay of toxins produced by the bacteria: All the identified strains of bacteria were separately inoculated into nutrient broth liquid medium (3 g of nutrient broth, 10 g of peptone, 5 g of NaCl, pH 7.2-7.4). The cultures were then grown as shake cultures (110 rotations/min at 28°C) for 5 days. The original liquid medium was then adjusted to a final concentration of 1×106 bacteria/ml and was centrifuged at 800 g for 15 min. Then, the supernatant liquid was filtered through a 0.45 μm filter (ME25STL, Whatman Inc.) and the liquid without bacterial cells was used in the bioassay tests.
All glassware and materials were sterilized and the bioassay was conducted in a 300 ml glass bottle with a 4.5 cm diameter mouth that contained three 1.5 ml ampoules. Under aseptic conditions, a 2-month-old aseptic seedling with the root cut off was put into an ampoule containing the bacteria-free supernatant from an identified bacterium. Enough water was added to the bottle to maintain a high humidity level. There were six replicates for each strain of the identified bacteria. The blank nutrient broth was medium treated identically to the inoculated media used in the control. Each bottle with three seedlings was sealed and kept at 27°C under 14 hr light (10000 lux) and 10 hr dark. For 10 days the appearance of the seedlings was recorded, along with the number of days from beginning of the bioassay to the wilting of a seedling. The mean time for the six replicates was used as an indicator of the phytotoxicity of each bacterium. If all six inoculated seedlings wilted within 2, 2-4, or 4-6 days, the strain was considered to produce the strong (+++), moderate (++) or weak (+) phytotoxic reactions, respectively. For those assays with no wilted seedlings within 6 days, the strain was considered as nontoxic (−).
Inoculation of 2-month-old P. thunbergii seedlings with Bm isolates: After culturing the wild Bm isolates on a Botrytis cinerea culture, the nematodes were extracted using Baermann funnels, then washed three times with a sterile, physiological salt solution (0.9% w/v NaCl) with the suspension adjusted to 2000 nematodes/ml. The upper stem of a 2-month-old P. thunbergii seedling was lightly punctured with a needle. Then, a small piece of sterilized cotton was introduced onto the wound and 500 nematodes pipetted onto it. Each treatment consisted of 20 replicates. Inoculation with a sterile, physiological salt solution was used as the control.
The seedlings were observed daily for 30 days, while the symptoms which developed and the days at which 80% of the seedlings had wilted were recorded for each treatment.
Inoculation of 3-year-old P. thunbergii seedling with Bm isolates: The JX2 isolate of Bm was cultured on the fungus Botrytis cinerea growing on PDA medium in a petri dish kept at 28°C for 1 week. Bx (Nanjing isolate, NJ) was isolated from naturally diseased and wilted Pinus thunbergii in Nanjing, Jiangsu, China. The infested wood was cut into 2 mm × 10 mm × 10 mm chips and the nematodes were extracted from 20 g (fresh weight) samples using a Baermann funnel. The nematodes were cultured on B. cinerea, growing on PDA.
Bm and Bx nematodes were washed off the culture plates with sterilized water and individuals were isolated and identified using a dissecting microscope. An adult female and a male adult were placed on a B. cinerea culture grown on PDA and incubated at 28°C. Adult nematodes of the two species were extracted from the resulting culture using a Baermann funnel and placed into a petri dish containing a thin layer of 2% agar and 1% ethanol (Shuto and Watanabe, 1988) and kept at 28°C for 24 hr. The nematodes and eggs were then washed with a physiological salt solution and centrifuged in a test tube at 800 g for 6 min. The eggs were then removed from the precipitate with a 10 μl syringe and used as the non-sterilized egg control.
The remaining precipitate, containing nematodes and eggs, was sterilized with 15% hydrogen peroxide for 10 min at 20°C. After the precipitate was washed five times and centrifuged at 800 g, the nematodes were removed. The remaining axenic eggs were cultured on bacteria-free B. cinerea. To confirm that the nematodes were free of bacteria, a few eggs were placed on NB medium and incubated at 28°C for 2 days and examined for bacterial colonies. If any bacteria were present, the nematodes from the same batch were discarded. The bacteria free nematodes were propagated for use as inoculum on B. cinerea growing on PDA in petri dishes kept at 28°C.
The experiments were conducted between July and October, 2006. The bacteria-free Bm JX2 isolate (ABm) and the bacteria free Bm NJ isolate (ABx) were used for inoculation. The bacterial strains used in the inoculation were SC7 (Pseudomonas putida) and GcM6-1A (P. putida) (Zhao et al., 2003), which were the strong phytotoxin producers and ZM2C (Pantoea sp.) (Wang, 2003), Ru25 (Acinetobacter lwoffi/junii) and JX23 (Pantoea agglomerans), which were not phytotoxin producers. The following isolate assays were used in the inoculation experiments: wild Bm (JX2), wild Bx (NJ), ABm (axeptic Bm JX2), ABx (axeptic Bx NJ), Ru25, JX23, SC7, GcM6-1A, ZM2C (Zhao et al., 2003; Wang, 2003), ABm and Ru25, ABm and JX23, ABm and SC7, ABm and ZM2C, ABm and GcM6-1A, ABx and ZM2C, ABx and GcM6-1A, ABx and Ru25, ABx and JX23, ABx and SC7, and Control (physiological salt solution, 0.9% w/v NaCl) (see Table 1).
Table 1.
Bacterial strains identified from the six Bursaphelenchus mucronatus isolates and their phytotoxicity to 2-month-old Pinus thunbergii seedlings.
A bacterial colony was removed from the slant nutrient broth agar media after incubation for 48 hr, transferred into nutrient broth liquid medium, and grown as a shake culture for 48 hr at 28°C. The bacterial culture was then centrifuged at 15000 g for 30 min and the supernatant discarded. For inoculations with bacteria and nematodes, the suspension was left for 1 hr to allow the bacteria to combine with the nematodes. Untreated nematodes were obtained using Baermann funnels after culturing wild BX on a mat of B. cinerea and washing them three times with sterile physiological salt solution. The nematode suspension was adjusted to 10,000 nematodes/ml. To ensure that the nematodes were axenic, they were checked again before use as described previously. Tests using 3-year-old P. Thunbergii seedlings were conducted in a P. thunbergii forest on the campus of Nanjing Forestry University, China. Inoculations were made with 5000 wild or axenic Bm or Bx in 0.5 ml and/or 2 × 106 bacteria in 0.5 ml of sterile physiological salt solution and a control treatment with 0.5 ml of sterile physiological salt solution. Twelve trees were used for each treatment. All tools and materials used in the experiments were sterilized. A cut, about 5 mm deep, was made on the tree trunk after surface sterilizing the bark with 70% alcohol, after which, the inoculum was introduced onto a piece of sterilized cotton and inserted into the cut. Then, the cotton was covered with a piece of five-layer-thick gauze and a second piece, surface–treated with streptomycin paste, was added and both were bound tightly onto the trunk with a bandage. Symptoms which developed on the trees were observed and recorded 3 months after inoculation. Two branch samples were cut from an inoculated seedling and were used to recover bacteria and nematodes by the protocol outlined in the “Isolation and identification of bacteria” paragraph.
Results
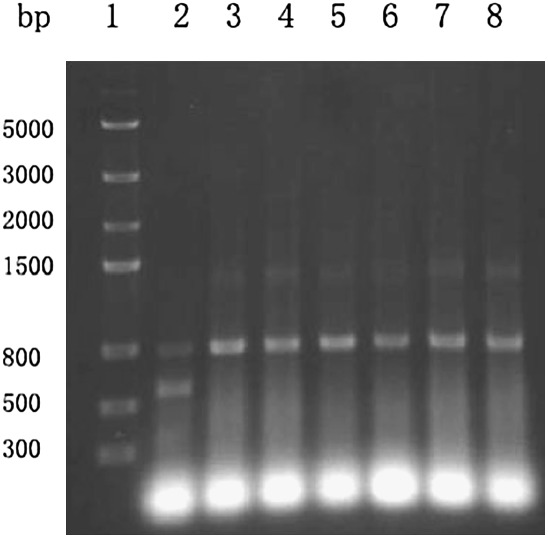
As expected, PCR of the six Bm isolates yielded a fragment of approximately 800 bp, while the duplex PCR of Bx isolate yielded two fragments with sizes of approximately 800 bp and 570 bp (Fig. 1). This result was consistent with previously reported findings (Jiang, 2005), which indicated that the Bm isolates used in this study were quite different from the Bx isolates with respect to the size of the amplified DNA.
Fig. 1.
Duplex PCR with Bm and Bx isolates. 1, DNA marker; 2, Bx; 3, Ru1; 4, Ru2; 5,Ru3; 6, JX1; 7, JX2; 8, SC.
Table 1 shows the bacterial strains isolated from the six isolates of Bm and their phytotoxicity to pine seedlings. Three strains produced the strong phytotoxic reaction (+++), while strains 19, 16 and 12 produced a moderate (++), weak (+) and no (−) phytotoxin effects, respectively (Tables 1 and 2).
Table 2.
The number of bacterial strains carried by the nematode isolates and their pathogenicity.
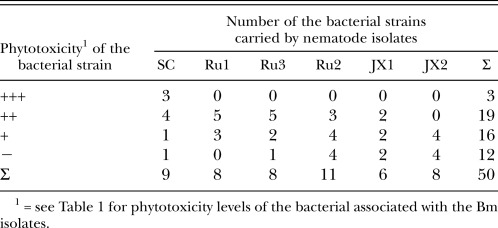
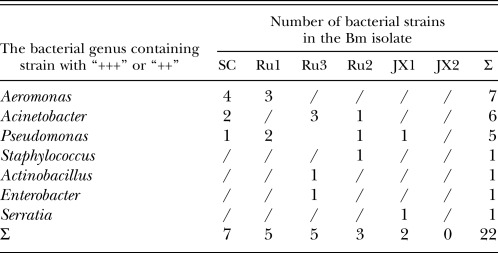
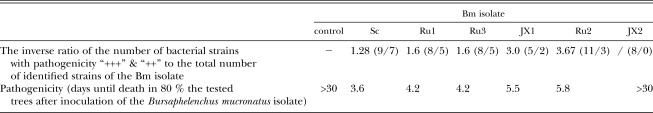
Table 2 gives the number of bacterial strains from the six Bm isolates, grouped by the level of their phytotoxin production. Table 3 shows the number of bacterial strains with “+++” and “++” phytotoxicity levels and their distribution among the Bm isolates. The dominant bacterial genera isolates which exhibited the strong pathogenicity levels were Aeromonas, Acinetobacter and Pseudomonas (Table 3). Table 4 shows the inverse ratio of the number of bacterial strains with pathogenicity “+++” and “++” to the total number of identified strains for each of the six Bm isolates and the pathogenicity of each of six Bm isolates based on the mean number of days from nematode inoculation until the death of 80% of the 2-month-old P. thunbergii seedlings. In descending order, the ratio values for the Bm isolates were SC, Ru1=Ru3, JX1, Ru2, and JX2. Their relative pathogenicities followed the same sequence. The JX2 isolates not exhibiting strong or moderate phytotoxic bacteria in our bioassay system did not show pathogenicity to the 2-month-old P. thunbergii seedlings (Table 3 and Table 4). This indicates that the pathogenicity of each Bm isolate on P. thunbergii seedlings is related to the inverse ratio of the pathogenic bacterial strains in the isolate.
Table 3.
The number of bacterial strains with “+++” and “++” phytotoxicity levels and their distribution among the Bursaphelenchus mucronatus isolates.
Table 4.
The relationship between the inverse ratio of the number of bacterial strains and pathogenicity (“+++” and “++”) to the total number of the identified strains in the Bursaphelenchus mucronatus isolate and its pathogenicity to the tested two-month-old Pinus thunbergii.
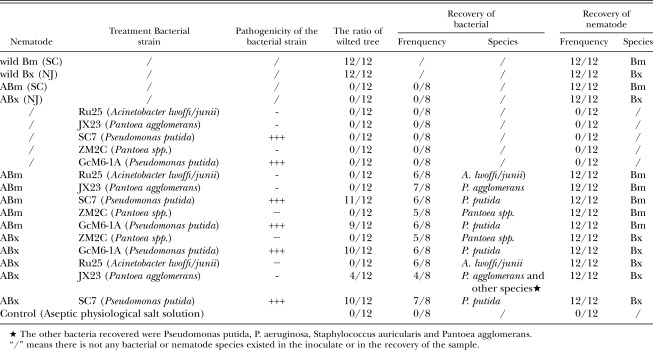
In the inoculation experiments with 3-year-old seedlings (Table 5), the strong phytotoxin producing bacteria used were SC7 (Pseudomonas putida) (Table 1), and GcM6-1A (P. putida) (Zhao et al. 2003), followed by the non-phytotoxin producing Ru25 (Acinetobacter lwoffi/junii) (Table 1), JX23 (Pantoea agglomerans) (Table 1), and ZM2C (Pantoea spp.) (Wang, 2003). The bacterial strains in the experiment were combined with Bm SC and BX NJ nematode isolates, respectively. The results of the inoculation experiment with 3-year-old P. thunbergii seedlings showed that the wild Bm SC and wild Bx NJ isolates were the most pathogenic, resulting in death of all 12 test trees (Table 5). However, the axenic Bm SC isolate (ABm) and the BX NJ isolate (ABx) did not cause wilting in any of the 12 inoculated trees. The trees inoculated with all five bacterial strains showed no symptoms. In the ABm or ABx plus the bacterial strains of non-phytotoxin producing treatments, the test trees did not show any wilting symptoms except for trees inoculated with ABx plus JX23 (Pantoea agglomerans) showed wilting symptom in 4 trees of the 12 treated ones. However, 9 to 11 seedlings wilted in the groups of 12 trees treated with ABm or ABx plus strong phyottoxin producing bacterial strains. At the end of the experiment, all the components of inoculum (bacteria, nematodes, or both) were recovered from the affected tree seedlings, except in the trees inoculated with only a bacterial strain, which showed no trace of the inoculum.
Table 5.
Percentage of wilted, 3-year-old P. thunbergii and recovery of the inoculated bacterium and nematode.
Discussion
The dominative pathogenic bacterial strains isolated from the six Bm isolates belong to the genera Aeromonas, Acinetobacter and Pseudomonas. Thus, Bm carries much more pathogenic bacterial diversity compared to Bx, which mainly carries the Pseudomonas bacteria (Zhao et al., 2003). The observation that some Bm isolates carry only non-pathogenic bacteria implies that the relationship between this nematode species and its associated pathogenic bacteria is not as close as that of Bx, which carries primarily pathogenic bacteria.
As described in the introduction of this paper, there are different theories regarding the varying pathogenicity of Bm. However, the cause of these differences has not been clarified. Earlier, Zhao et al. (2003) proposed that pine wilt disease is induced by the complex of both the nematode Bx and the pathogenic bacteria it carries. The results of the present study show that pine wilt symptoms caused by certain Bm isolates were induced by the nematode and associated pathogenic bacteria. The Bm isolate JX2 which did not carry moderate pathogenic bacteria, did not induce pine wilt symptoms. Pine wilt caused by the Bm isolates has the same etiology as that caused by Bx. Thus, we conclude that pine wilt disease is induced by the complex of both Bm and the pathogenic bacteria it carries. The differences between Bm and Bx in their pathogenicity levels may reflect the relationship between the nematode and the associated pathogenic bacteria. The relationship between Bm and its associated pathogenic bacteria is more diverse than that of Bx. Some of the Bm associated pathogenic bacteria may have forged a commensal relationship with their nematodes or even act as parasites. Our findings might explain why some wild isolates of Bm can induce pine wilt symptoms, while other populations of this nematode species cannot.
In the inoculation experiment with 3-year-old P. thunbergii seedlings, the only exceptions were the four trees from a group of 12, inoculated with the nematode ABx and bacterial strain JX23 (Pantoea agglomerans), that wilted. This may have occurred because the ABx was re-contaminated with pathogenic bacteria in the field during or after inoculation (Zhao et al., 2003; Kawazu & Kaneko, 1997). In the recovery, the other pathogenic bacteria appeared in the 4 wilted seedlings, but not in the healthy seedlings (Table 5), which supports this explanation.
In this study, bacteria were not recovered from trees inoculated with only a bacterial strain. It indicates that the bacterial strain could not survive in the tested trees if bacteria were inoculated without nematodes. However, ABm or ABx inoculated without bacteria survived in the pine seedlings. Therefore, it was suggested that both the nematodes and their associated strong phytotoxin producing bacteria were required to induce pine wilt. The phytotoxin producing bacteria play an essential role in the pine wilt disease caused by Bm or Bx.
To understand the inner processes of the wilt disease caused by Bm, further research is needed on the relationship between Bm and associated pathogenic bacteria, what factors cause the bacteria carried by Bm to increase their combined pathogenicity and on the molecular etiology of wilt disease caused by Bm.
Footnotes
The work was supported by a key project of the Natural Science Foundation of China (No: 30430580) and the State Forestry Administration, People's Republic of China (No: 20070430); Russian Foundation for Basic Research (grant No 03-04-39022). We thank Dr. Jack Sutherland, Victoria, British Columbia, Canada, for helping correct the English.
This paper was edited by Edwin Lewis.
Literature Cited
- Akbulut S, Yüksel B, Serin M, Baysal I, Erdem M. Pathogenicity of Bursaphelenchus mucronatus in pine seedlings under greenhouse conditions Turk. J. Agric. For. 2007;31:169–173. [Google Scholar]
- Caroppo S, Cavalli M, Coniglio D, Ambrogioni L. Pathogenicity studies with various Bursaphelenchus populations on conifer seedlings under controlled and open air conditions. Redia LXXXIII. 2000:61–75. [Google Scholar]
- Braasch H, Caroppo S, Ambrogioni L, Michalopoulos H, Skarmoutsos G, Tomiczek CH. Pathogenicity of various Bursaphelenchus species and implications to European forests. In: Futai K, Togashi K, Ikeda T, editors. Sustainability of pine forests in relation to pine wilt and decline. Tokyo, Japan: 1998. pp. 14–22. [Google Scholar]
- Jiang L, Zheng J, Waeyenberge L, Subbotin SA, Moens M. Duplex based PCR identification Bursaphelenchus xylophilus (Steiner & Buhrer, 1934) Nickel, 1970. Russian Journal of Nematology. 2005;13(2):115–121. [Google Scholar]
- Kawazu K, Kaneko N. Asepsis of the pine wood nematode isolate OKD-3 causes it to lose its pathogenicity. Japanese Journal of Nematology. 1997;27:76–80. [Google Scholar]
- Kanzaki N, Futai K. Is Bursaphelenchus mucronatus a weak pathogen to the Japanese red pine? Nematology. 2006;8:485–489. [Google Scholar]
- Kishi Y. Tokyo: Thomas Company Limited; 1995. The Pine Wood Nematode and the Japanese Pine Sawyer; p. 302. [Google Scholar]
- Kulinich OA, Kruglic IA, Eroshenko AS, Kolossova NV. Occurrence and distribution of the nematode Bursaphelenchus mucronatus in the Russian Far East. Russian Journal of Nematology. 1994;2:113–119. [Google Scholar]
- Li GX, Li DL, Yu SF. Preliminary investigation on the primary reason of pine wilt disease in Huaping. Journal of Yunnan Agricultural University. 2007;22:225–228. [Google Scholar]
- Mamiya Y. Review on the pathogenicity of Bursaphelenchus mucronatus. In: Futai K, Togashi K, Ikeda T, editors. Sustainability of pine forests in relation to pine wilt and decline; Proceedings of International Symposium; 26-30 October; Tokyo. Tokyo: 1998. pp. 57–64. [Google Scholar]
- Noel RK, Bergey S. Baltimore: Williams & Wilkins; 1984. Bergey's manual of systematic bacteriology. [Google Scholar]
- Shuto Y, Watanabe H. Stimulating effect of ethanol on oviposition of the pine wood nematode, Bursaphelenchus xylophilus. Agric. Biol. Chem. 1988;52:2927–2928. [Google Scholar]
- Wang HL. Nanjing, China: Najing Forestry University; 2003. Study on the species and distribution of bacteria carried by pine wood nematode. M.S. thesis, [Google Scholar]
- Zhang ZY, Lin MS. Pathogenicity of Bursaphelenchus mucronatus on the seedlings of black pine. Journal of Nanjing Agricultural University. 2004;27:46–50. [Google Scholar]
- Zhang JH. Damages by and preventing pine forests from pine wilt disease caused by Bursaphelenchus mucronatus. Practical Forestry Technology. 2003;10:32. [Google Scholar]
- Zhao BG, Wang HL, Han SF. Distribution and pathogenicity of bacteria species carried by Bursaphelenchus xylophilus in China. Nematology. 2003;5:899–906. [Google Scholar]
- Zhao BG, Lin F. Mutualistic symbiosis between Bursaphelenchus xylophilus and bacteria of the genus Pseudomonas. Forest Pathology. 2005;35:39–345. [Google Scholar]