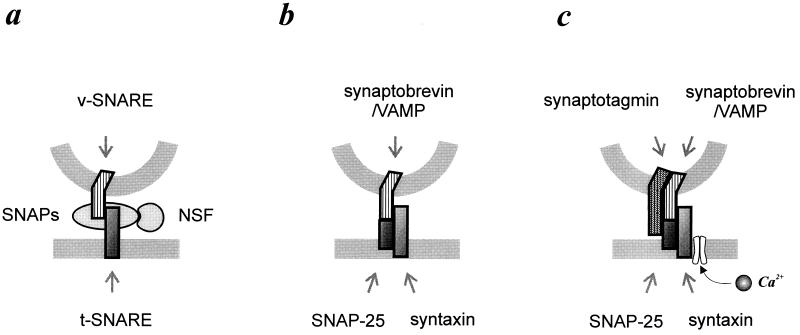
Synaptic vesicle exocytosis—the basis for neurotransmitter release at nerve terminals—is at the heart of nervous system functioning. The molecular analysis of this special form of exocytosis was recently greatly stimulated by the finding that the key molecules in various intracellular vesicular transport steps, including neurotransmitter release, are conserved from yeast to man (1–3). Prompted by this generality of vesicular transport machinery Rothman and colleagues proposed a universal “docking and fusion particle” to explain vesicle docking and fusion at all locations, including synapses (4). The Rothman proposal, also called the SNARE hypothesis, identifies four key components: (i) a vesicle membrane protein named v-SNARE, (ii) a target membrane protein dubbed t-SNARE, (iii) a cytosolic protein required for membrane fusion N-ethylmaleimide-sensitive fusion protein (NSF), and (iv) adaptors for NSF termed SNAPs (soluble NSF attachment proteins) (Fig. 1a). Vesicle docking is accounted for by the complementarity between the v- and t-SNAREs. The assembled v- and t-SNARE then acts as a receptor for the SNAPs, which in turn incorporates the fusion protein, NSF. The docking and fusion particle containing all four basic parts, thus formed, is called the SNARE complex. In this scheme, vesicle fusion is achieved by the energy liberated from the hydrolysis of ATP by NSF, which is an ATPase. By virtue of its simplicity the SNARE hypothesis has gained popularity and become a familiar term for those drawn to the intricacies of intracellular membrane traffic. The popularity, however, has also attracted intense scientific scrutiny to the SNARE hypothesis from every imaginable angle. This commentary provides a brief perspective on the docking and fusion particle as it applies to synapse function and places recent findings (5, 6) in context.
Figure 1.
(a) Schematic diagram indicating four essential components of the docking and fusion particle, also called the SNARE complex. Complementarity between the v- and t-SNAREs directs vesicle docking, and the SNAREs act as a receptor for the SNAP–NSF fusion protein complex. (b) The original v-SNARE, synaptobrevin/VAMP and t-SNAREs, SNAP-25, and syntaxin are shown. (c) Synaptotagmin is the new v-SNARE at the synaptic terminal. N-type Ca2+ channel, which mediates synchronous fusion of docked synaptic vesicle, associates with syntaxin.
Elements of the SNARE Complex
Before delving into the nerve terminal a short introduction to the four key components of the docking and fusion particle follows. The story of the SNAREs starts with the ATPase NSF. NSF was originally identified as the soluble factor required for the fusion of Golgi-derived transport vesicles; in its absence, docked vesicles accumulate on the surface of the Golgi membrane (7). It is now known that NSF is required for many intracellular membrane fusion events in the exocytic and endocytic pathways and is the universal ATPase, which supplies the driving force for membrane fusion in the docking and fusion particle mentioned above (1). To fulfill its requirement at membrane junctions, NSF, which is a cytosolic protein, must be brought to membranes by binding to the adaptor molecules, SNAPs. There are three isoforms of SNAP: α-SNAP and γ-SNAP are ubiquitously present in all cell types, whereas β-SNAP is brain specific (1).
Characterization of the universal fusion protein NSF, and its equally ubiquitous adaptors SNAPs, was soon followed by identification of the other two members of the SNARE complex. A search for a membrane receptor(s) for NSF–SNAP revealed a complex consisting of three proteins already well known at the presynaptic terminal: a synaptic vesicle protein synaptobrevin (initially called VAMP) and two plasma membrane proteins syntaxin and SNAP-25. [Not to be confused with NSF adaptor SNAPs. SNAP-25 stands for synaptosome associated protein of 25K. It is an amusing coincidence that two molecules carrying an identical acronym, characterized independently in different experimental systems, are later found to be functionally associated (4).] In nerve terminals, synaptobrevin, therefore, is the v-SNARE, and syntaxin and SNAP-25 are the t-SNAREs (Fig. 1b). It is not coincidental that the original SNAREs thus identified are synaptic proteins, since a preparation highly enriched in nerve terminals—bovine brain particulate extract—was used as the source for potential NSF–SNAP receptor. Subsequent identification of v-SNARE and t-SNARE homologues on many different membranes of the secretory and endocytic pathways underscores the generality of the SNARE complex (1–3).
In addition to its universality, there are two important observations that justify the popularity of SNARE complex as the general machinery underlying vesicle docking and fusion. First, in vitro complex of NSF–SNAP–SNARE proteins—the docking and fusion particle—disassembles upon ATP hydrolysis by NSF (4, 8). This finding, although from a test tube experiment, reinforces the view that membrane fusion catalyzed by NSF is the result of a conformational change in the fusion particle triggered (or perhaps enabled) by ATP hydrolysis. Second, each one of the three SNAREs is a specific target of lethal Clostridial toxins: tetanus toxins that causes spastic paralysis by blocking inhibitory neurons in the spinal cord and botulinum toxins that induce muscle fatigue by inhibiting synaptic transmission at the neuromuscular junction (9). These very specific toxins block transmitter release by proteolytically cleaving the SNAREs, so nature has provided dramatic testimony for the physiological significance for the v- and t-SNAREs in vesicle fusion, at least for the synaptic terminal.
Constitutive vs. Regulated Vesicle Fusion
The SNARE complex is aptly called the docking and fusion particle, because the roles for v- and t-SNAREs in the docking and subsequent fusion events are inseparable. That is, the SNAREs not only provide the specificity for docking but they also control fusion by acting as a scaffold for recruiting the NSF–SNAP complex. In constitutive vesicular transport pathways fusion follows vesicle docking without delay, and a bona fide fusion competent docked intermediate has never been isolated. Presumably, such a docked intermediate is in an energetically unfavorable state, and the SNARE complex assembly and its dissolution by NSF apparently proceed as a single-step reaction. In synaptic vesicle exocytosis, however, docked vesicles do not fuse until triggered by the influx of Ca2+ ions. Such Ca2+ regulation of transmitter release requires the modification of the general docking and fusion particle to accommodate the following hallmark features of synaptic vesicle fusion: (i) docked vesicles must be prevented from undergoing immediate fusion and (ii) the fusion machinery must very rapidly respond to the influx of Ca2+ ions.
How could the universal docking and fusion complex be altered to incorporate regulated vesicle exocytosis? Two limiting cases come immediately to mind. One extreme possibility would be that synaptic vesicles first predock by a different mechanism (i.e., not using the complementarity between the original SNAREs). Then, Ca2+ influx would rapidly permit formation of the universal docking and fusion particle, thereby effecting transmitter release. In this case, the original SNARE complex would operate as the specialized “fusion particle,” and have no role in docking. In an alternative scenario, the docking and fusion particle would form in the usual way as the SNARE complex; however, the final fusion step(s) would be prevented by stabilizing some postdocking–prefusion intermediate, which would be only very briefly present in constitutive vesicular transport pathways. A nerve-terminal-specific protein may act as a gate to trap the docking and fusion particle in such a prefusion intermediate, and the gate would only open in response to Ca2+ influx.
Recent Modifications of the SNARE Hypothesis
Two recent findings elaborate on some possible adaptations of the synaptic SNARE complex to serve requirements imposed by the nerve terminal. In the first study, Rothman and colleagues propose an additional v-SNARE which, together with the old v-SNARE synaptobrevin, directs synaptic vesicle docking (5, 10). This new v-SNARE is synaptotagmin, which is widely believed to act as a Ca2+ sensor for transmitter release (11, 12). In addition to a role in docking, synaptotagmin may thus act as a Ca2+-sensitive gate that traps the SNARE complex in a prefusion intermediate. In the second study, Catterall and colleagues demonstrate physiological interaction between a t-SNARE syntaxin and a Ca2+ channel that mediates transmitter release (6). Their study indicates how Ca2+ influx may be tightly coupled to transmitter release. Altogether, these two reports give us a glimpse into the highly complex protein–protein interactions regulating the basic working parts of the docking and fusion particle in synaptic vesicle exocytosis.
First, we examine the proposal for the new v-SNARE, synaptotagmin (Fig. 1c). Recently synaptotagmin I, an abundant synaptic vesicle protein, has been proposed as a specialized v-SNARE based on the following observations: synaptotagmin I binds brain-specific NSF adaptor, β-SNAP, and recruits NSF (10), and synaptotagmin I interacts stoichiometrically—in a Ca2+-independent manner—with a t-SNARE, SNAP-25 (5). Synaptotagmin, therefore, satisfies all the features expected of a v-SNARE, and it may clarify the issue of whether the nerve terminal SNAREs mediate docking and fusion or only fusion. A frequently cited instance of evidence against a role for original SNAREs in docking is the fact that synaptic vesicles remain morphologically docked after proteolytic cleavage of SNAREs with Clostridial toxins (ref. 13; also see below). As synaptotagmin is not a substrate for any of the Clostridial toxins and binds to SNAP-25 (remember that SNAP-25 is one of the t-SNAREs) missing the C terminus 9 or 26 amino acids after cleavage with botulinum toxins A or E, the interaction between synaptotagmin and SNAP-25 would provide a stable link for vesicle docking even in the presence of the toxins. It has therefore been suggested that synaptotagmin is the basis by which docked vesicles are still apparent in toxin treated cells (5). This is a readily testable hypothesis as follows: Although deficient in fast Ca2+-coupled synaptic transmission, synaptotagmin I mutant mice display terminals with a normal complement of morphologically docked vesicles (12). Since these mutant mice completely lack the domain of synaptotagmin I required for specific binding to SNAP-25, it would be of interest to test the effect of toxin treatment on vesicle docking at mutant synapses.
The proposal of synaptotagmin I as an additional v-SNARE brings the Ca2+-triggering mechanism closer to the core of the SNARE hypothesis. Although not directly proven, the bulk of biochemical and genetic evidence to date indicate synaptotagmin I as the Ca2+ sensor for triggering synaptic vesicle fusion (11, 12). The observed Ca2+-independent association of synaptotagmin with SNAP-25 would position the Ca2+ sensor in direct contact with the fusion machinery. Then ATP hydrolysis by NSF may rearrange the SNARE complex—including the Ca2+ sensor—into a metastable intermediate; the destabilization of such a prefusion intermediate caused by Ca2+ binding to synaptotagmin would then rapidly force membrane fusion. A recent report shows that vesicle fusion lags behind Ca2+ influx by only 60 μs at physiological temperatures (14). The exceedingly rapid induction of fusion, therefore, presents a formidable mechanical constraint to the fusion machinery, leaving no time for sequential enzymatic reactions.
Another more recent finding involving the SNARE complex and Ca2+ sensitivity of synaptic vesicle fusion is the interaction between the t-SNARE syntaxin and α1B subunit of a Ca2+ channel that mediates fast transmitter release (known as N-type Ca2+ channel). Because influx of extracellular Ca2+ ions rapidly triggers transmitter release, it has been thought that Ca2+ channels must be located very close to docked, release-ready synaptic vesicles. Such notion is supported by the observed in vitro interaction between syntaxin and N-type Ca2+ channels (15). Catterall and colleagues now demonstrate that injection of peptides into presynaptic neurons, which cause dissociation of Ca2+ channel-SNARE complex in vitro, reversibly attenuates synchronous transmitter release (6). The inhibitory effect of the peptide on Ca2+-triggered release is consistent with a physiological role for SNARE complex in positioning synaptic vesicles and the Ca2+ sensor at the site of Ca2+ influx, thereby ensuring efficient coupling of Ca2+ signal to vesicle fusion. Interestingly, coexpression of SNAREs and Ca2+ channel subunits in oocytes demonstrates a syntaxin-dependent inactivation of N-type Ca2+ channels (16, 17). The SNARE complex, therefore, may also negatively regulate the probability of vesicle fusion by controlling the gating properties of Ca2+ channels. Such negative modulation of the release machinery by SNAREs could play a significant role in the alterations of transmitter release properties observed during synaptic plasticity. For instance, paired-pulse facilitation, the best studied example of short-term synaptic plasticity, is an enhancement of transmitter release to the second of a pair of pulses delivered at short intervals. The facilitation of second response relative to the first is due to the prior exposure of presynaptic terminal to a build-up of Ca2+ ions during the first pulse. Interestingly, the peptides that disassemble SNARE–Ca2+ channel protein complex significantly enhance paired-pulse facilitation, while attenuating transmitter release to the first pulse. The stimulatory effect of the peptides on paired-pulse facilitation is suggested to be caused by the increased Ca2+ influx during the second pulse from lack of channel inactivation following the first pulse (6). The SNARE complex, therefore, may regulate many facets of transmitter release, in addition to directing vesicle docking and fusion.
In short, recently identified interactions involving the members of the SNARE complex and proteins that uniquely expedite Ca2+-triggered transmitter release exemplify the adaptation of the SNARE complex at the synaptic terminal to satisfy the requirements of regulated synaptic vesicle exocytosis. Synaptotagmin as a v-SNARE defines a unique docking mechanism, and also recruits Ca2+ sensor directly into the SNARE complex. Syntaxin, while acting as a t-SNARE, also interacts with Ca2+ channels to couple incoming Ca2+ signal to rapid vesicle fusion. A challenge now is to incorporate into the “core SNARE hypothesis ”the molecular basis to account for all of the special features of synaptic vesicle exocytosis.
Open Questions
At present there are two major issues regarding the SNARE complex at the nerve terminal. The first question is whether the SNAREs specify vesicle docking or whether they are dispensable for docking and solely provide for vesicle fusion. The following observations have questioned the role of SNAREs in docking of synaptic vesicles: (i) as mentioned above, docked vesicles remain after proteolytic cleavage of the v- and t-SNAREs with Clostridial toxins (13) and (ii) although enriched in active zones, t-SNAREs are not strictly localized to sites of exocytosis (18). As to the first point, the toxins do not efficiently cleave the SNARE complex formed in vitro (19). It is thus possible that vesicles that are already docked when the toxin gains access to the presynaptic terminal may be resistant to proteolysis and may explain the presence of docked vesicles in the electron micrographs of toxin-treated nerve terminals. In that case, the docked vesicles would be fusion competent, and one would thus expect to see a single round of transmitter release upon Ca2+ influx from toxin-treated synapses. Interestingly, in adrenal chromaffin cells, limited ATP-independent exocytosis—thought to represent a readily releasable pool of vesicles that have been primed by NSF (20) and that may be the metastable intermediate discussed above—can be observed in the presence of tetanus or botulinum A toxins (ref. 21 but also see ref. 22). In addition, synaptotagmin I as a v-SNARE may provide a toxin-insensitive link between docked vesicle and the active zone membrane as discussed above. With respect to the second point, the presence of t-SNAREs in regions other than the sites of exocytosis may simply be due to inefficient intracellular protein sorting machinery. If v-SNAREs could recognize the concentration gradient of t-SNAREs then a strict localization of t-SNAREs is not necessary as long as there is considerable enrichment. It thus remains to be seen whether SNAREs provide specificity for docking and act as a docking and fusion particle or whether they play a more exclusive role as a specialized fusion particle at the synapse.
A major obstacle for addressing whether particular components are required for docking and not in fusion is the difficulty in assessing various states of docked vesicles. That is, some docked vesicles may be functionally docked and be fusion competent, whereas others may be fusion incompetent. For example, at central synapses, most docked vesicles do not fuse upon Ca2+ entry, even under conditions that are optimal for transmitter release (23). Although such functional heterogeneity among docked vesicles may represent adaptation of the fusion machinery at the synapse, it does provide a starting point for dissecting the molecular basis for functional docking. The nerve terminal may therefore provide some of the fundamental answers for the vesicle docking mechanisms that are central to all transport steps.
The second important issue regarding the current state of SNAREs is the following: what is the minimal set of proteins required for fusion and how is its assembly regulated? Biochemical approaches have been the most popular in addressing the relevant interactions between the SNAREs and the associated factors. This is reflected in the number of documented interactions that always seems to be on a rise. Take synaptotagmin for example: the same domain of synaptotagmin that is required for binding to SNAP-25 discussed above, also binds to itself and to five other presynaptic proteins and polyinositol phosphates (11). Such findings are facilitated by binding reactions, which can be readily performed using purified components and tissue extracts. Nevertheless, the orientation and the oligomeric state of the components involved are left ambiguous in such in vitro binding reactions. A typical interaction between v- and t-SNAREs must involve their cytosolic ends in the opposite orientation (unless the cytosolic portion can be folded into floppy domains); nevertheless, the orientation of membrane proteins cannot be controlled in binding assays performed in detergent extracts. As for the oligomeric state of the SNAREs and its interacting molecules, synaptotagmin, for instance, which forms homooligomers and binds to many other factors, may allow simultaneous association with other partners, thereby increasing the complexity of regulation. It thus remains to be established how many of these associations characterized in vitro are physiologically relevant.
In summary, we have gained much information about who’s who in the presynaptic terminal in the past several years. The next advance will come from determining the precise mechanism of action of each of the SNARE components and their partners in a functional assay for synaptic vesicle docking and fusion.
References
- 1.Rothman J E. Nature (London) 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 2.Südhof T C. Nature (London) 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 3.Scheller R H. Neuron. 1995;14:893–897. doi: 10.1016/0896-6273(95)90328-3. [DOI] [PubMed] [Google Scholar]
- 4.Söllner T, Whiteheart S W, Brunner M, Erdjument-Bromage H, Germanos S, Tempst P, Rothman J E. Nature (London) 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 5.Schiavo G, Stenbeck G, Rothman J E, Söllner T H. Proc Natl Acad Sci USA. 1997;94:997–1001. doi: 10.1073/pnas.94.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mochida S, Sheng Z-H, Baker C, Kobayashi H, Catterall W A. Neuron. 1996;17:781–788. doi: 10.1016/s0896-6273(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 7.Malhotra V, Orci L, Glick B S, Block M R, Rothman J E. Cell. 1988;54:221–227. doi: 10.1016/0092-8674(88)90554-5. [DOI] [PubMed] [Google Scholar]
- 8.Söllner T, Bennett M K, Whiteheart S W, Scheller R H, Rothman J E. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 9.Montecucco C, Schiavo G. Q Rev Biophys. 1995;28:423–472. doi: 10.1017/s0033583500003292. [DOI] [PubMed] [Google Scholar]
- 10.Schiavo G, Gmachl M J S, Stenbeck G, Söllner T H, Rothman J E. Nature (London) 1995;378:733–736. doi: 10.1038/378733a0. [DOI] [PubMed] [Google Scholar]
- 11.Südhof T C, Rizo J. Neuron. 1996;17:379–388. doi: 10.1016/s0896-6273(00)80171-3. [DOI] [PubMed] [Google Scholar]
- 12.Geppert M, Goda Y, Hammer E R, Li C, Rosahl T W, Stevens C F, Südhof T C. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 13.Hunt J M, Bommert K, Charlton M P, Kistner A, Habermann E, Augustine G, Betz H. Neuron. 1994;12:1269–1279. doi: 10.1016/0896-6273(94)90443-x. [DOI] [PubMed] [Google Scholar]
- 14.Sabatini B L, Regehr W G. Nature (London) 1996;384:170–172. doi: 10.1038/384170a0. [DOI] [PubMed] [Google Scholar]
- 15.Bennett M K, Calakos N, Scheller R H. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- 16.Bezprozvanny I, Scheller R H, Tsien R W. Nature (London) 1995;378:623–626. doi: 10.1038/378623a0. [DOI] [PubMed] [Google Scholar]
- 17.Wiser O, Bennett M K, Atlas D. EMBO J. 1996;15:4100–4110. [PMC free article] [PubMed] [Google Scholar]
- 18.Walch-Solimena C, Blasi J, Edelmann L, Chapman E R, Fischer von Mollard G, Jahn R. J Cell Biol. 1995;128:637–645. doi: 10.1083/jcb.128.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi T, McMahon H, Yamasaki S, Binz T, Hata Y, Südhof T C, Niemann H. EMBO J. 1994;13:5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banerjee A, Barry V A, DasGupta B R, Martin T F J. J Biol Chem. 1996;271:20223–20226. doi: 10.1074/jbc.271.34.20223. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence G W, Weller U, Dolly J O. Eur J Biochem. 1994;222:325–333. doi: 10.1111/j.1432-1033.1994.tb18871.x. [DOI] [PubMed] [Google Scholar]
- 22.Banerjee A, Kowalchyk J A, DasGupta B R, Martin T F J. J Biol Chem. 1996;271:20227–20230. doi: 10.1074/jbc.271.34.20227. [DOI] [PubMed] [Google Scholar]
- 23.Stevens C F, Wang Y. Neuron. 1995;14:795–802. doi: 10.1016/0896-6273(95)90223-6. [DOI] [PubMed] [Google Scholar]