Abstract
Alveolar macrophages are known to express a variety of growth factors and neurotrophins. Fibroblast growth factor-1 (FGF-1) is abundantly present in the lung and has mitogenic and neurotrophic activities similarly to neurotrophins. In order to determine whether FGF-1 associates with neurotrophins in alveolar macrophages, we investigated the immunocytochemical colocalization of FGF-1 with neurotrophins, nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin-3 (NT-3), in mouse alveolar macrophages. The results showed that 34% of macrophages were immunoreactive for FGF-1, 10% for NGF, 9% for BDNF, and 17% for NT-3. Of FGF-1-immunoreactive (IR) macrophages, 16% were immunoreactive for NT-3, but only small percentages were immunoreactive for NGF (0.8%) and for BDNF (0.3%). FGF-1 and neurotrophins were all localized in the intracellular vesicles. In the vesicles, FGF-1 and NT-3 were frequently colocalized. All macrophages expressed lysosome-associated protein-2 (LAMP-2), a late endosomal and lysosomal marker, and early endosomes antigen 1 (EEA1), an early endosomal marker. FGF-1 and NT-3 were predominantly colocalized with LAMP-2 rather than with EEA1, whereas NGF and BDNF were colocalized with EEA1 rather than with LAMP-2. These results indicate that FGF-1 and NT-3 are substantially expressed in mouse alveolar macrophages and colocalized in vesicles, predominantly in late endosomes and lysosomes.
Keywords: FGF-1, NGF, BDNF, NT-3, mouse alveolar macrophages
I. Introduction
Macrophages are a major cellular component of the lung. They provide local defense against a variety of pathogenic particulates passing through the airway and play a role in airway inflammation. Macrophages ingest foreign substances in a phagocytic manner and release chemical factors, such as interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α), in response to inflammatory stimuli [10, 14]. Besides these classical inflammatory chemicals, alveolar macrophages have been recently known to express growth factors and neurotrophins [6, 14, 20].
Fibroblast growth factor-1 (FGF-1) is highly expressed in the normal human lung [8] including alveolar macrophages as well as bronchial epithelium [12]. This growth factor is likely to have a cell proliferation effect on various types of cells in the lung. Treatment with FGF-1 has been shown to promote proliferation of human airway smooth muscle cells [12], rat alveolar type II cells [15], rodent lung epithelial cells [3, 5], mouse fibroblast cell lines [11, 22], and rabbit ligament fibroblasts [21]. Besides these mitogenic effects, FGF-1 has been found to exhibit neurotrophic activity, such as the promotion of neurite outgrowth in cultured rat sensory neurons [16], the survival and regeneration of neurons in the rat vagal preganglion nerve after injury [9], and the recovery of sensory function after transsection of cervical roots in rats [7]. Thus, FGF-1 is likely a regulator of the structure modeling in the lung via cell proliferation of various lung cells and maintenance of neurons.
Alveolar macrophages also express the neurotrophin family, which comprises nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4/5 (NT-4/5). Ricci et al. [20] showed immunocytochemical expression of NGF and neurotrophin receptors TrkA, TrkB, and TrkC in alveolar macrophages obtained from bronchoalveolar lavage (BAL) fluid of normal human subjects. They therefore suggest that macrophage-derived NGF acts on macrophages in an autocrine or paracrine manner and regulates their functions including phagocytosis, IL-1 production, and macrophage differentiation. We have also previously shown that mRNAs for NT-3 and NT-4/5 are expressed in alveolar macrophages of intact mice and their receptors TrkB and TrkC are located in interstitial macrophages, suggesting the presence of the signal communication via neurotrophins in the peripheral lung [6]. Physiologically, the neurotrophins in the lung, whose sources are sensory neurons (non-adrenergic and non-cholinergic neurons), epithelial cells, fibroblasts, and immune cells including macrophages, are possibly required for differentiation and maintenance of peripheral sensory neurons [17]. Recently, more information on the physiological action of neurotrophins in the lung has been described. The neurotrophins play roles in the normal lung development and lung health at least in part through the cell proliferation of airway smooth muscle cells, pulmonary endothelial cells, and epithelial cells [18].
Thus, FGF-1 and neurotrophins are expressed in alveolar macrophages and have overlapping actions, such as mitogenic and neurotrophic actions. Therefore, FGF-1 in alveolar macrophages might associate with neurotrophins in various processes such as storage, release, and action upon the target. Knowledge of their intracellular storage will help us to understand the capacity and manner of their release. Accordingly, we investigated the intracellular colocalization of FGF-1 with neurotrophins, NGF, BDNF, and NT-3, in mouse alveolar macrophages. We further investigated the intracellular colocalization of these factors with lysosome-associated protein-2 (LAMP-2), a late endosomal and lysosomal marker, and with early endosome antigen 1 (EEA1), an early endosomal marker, to determine their precise intracellular localization.
II. Materials and Methods
Animals
Animal care was in accordance with the guidelines of the Animal Care Committee of Kitasato University School of Medicine. C57BL/6 male mice, 12–20 weeks of age, were deeply anesthetized and euthanized with diethyl ether, and BAL fluid was obtained as described below. A total of 30 mice were used in this study.
Preparation of alveolar macrophages
The trachea was cannulated with a nylon tube, and the lung was lavaged five times with 1 ml Earle’s balanced salt solution (Life Technologies, Carlsbad, CA, USA) for collecting alveolar macrophages. The BAL fluid specimens were centrifuged at 300 g for 5 min. The pelleted cells were suspended in Roswell Park Memorial Institute (RPMI) 1640 medium (Life Technologies). Cells were plated on coverslips in dishes and incubated at 37°C for 90 min in an atmosphere of 5% CO2-95% air, in an attempt to purify the adherent cells as macrophages. After incubation, non-adherent cells were removed by swirling the dish to resuspend these cells and aspirating them off with a Pasteur pipette. The dish was then sprayed with medium by using a pipette to resuspend loosely adherent cells. The suspension was aspirated off again, and the adherent macrophages were used for preparation of immunocytochemistry. The purity of macrophages was 99.6±0.3% (Mean±SEM, N=23 microscopic fields, total 638 cells) as determined by morphological analysis combined with toluidine blue (0.1% in PBS) staining. The remaining 0.4% were fibroblasts. There were no mast cells whose cytoplasmic granules are stained red-violet by toluidine blue.
Immunocytochemical procedure
Alveolar macrophages were fixed with 4% paraformaldehyde for 5 min at room temperature. After fixation, they were washed with 0.025 M phosphate-buffered saline (PBS) containing 0.3% Triton X-100 (PBST) for 10 min, and treated for 10 min with protein blocking agent (Immunon, Pittsburgh, PA, USA) at room temperature to block nonspecific protein sites. Cells were incubated for 1 hr at room temperature with the primary antibody, goat anti-human FGF-1 antibody (4 µg/ml, reacts with mouse, rat, and human FGF-1, Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-mouse NGF antibody (1:500, Serotec, Oxford, UK), chicken anti-mouse NGF antibody (2 µg/ml, Chemicon International, Temecula, CA, USA), rabbit anti-mouse BDNF antibody (1:1000, Chemicon International), chicken anti-human BDNF antibody (2 µg/ml, reacts with human and mouse BDNF, General Laboratories, Madison, WI, USA), sheep anti-mouse NT-3 antibody (10 µg/ml, Chemicon International), or chicken anti-human NT-3 antibody (1:100, reacts with human and mouse NT-3, Abcam, Tokyo). For each of NGF, BDNF, and NT-3, we used two antibodies derived from different species indicated above, and there was virtually no difference in the results between them. Rat monoclonal anti-mouse LAMP-2 antibody (1:100, Santa Cruz Biotechnology) and rabbit anti-human EEA1 antibody (1:100, reacts with mouse, rat, and human EEA1, Sigma-Aldrich, St. Louis, MO, USA) were also used. Double labeling was performed using two primary antibodies derived from different species. After washing with PBS, the macrophages were incubated for 1 hr at room temperature with the secondary antibodies, Alexa Fluor 568 IgG (1:100, Life Technologies) and Alexa Fluor 488 IgG (1:100, Life Technologies), corresponding to the primary antibodies. No staining was observed with isotype-matched negative controls (goat IgG, rabbit IgG, sheep IgG, chicken IgY, and rat IgG2a). The secondary antibodies without the primary antibody did not stain alveolar macrophages. The immunostained cells on coverslips were usually examined with an inverted microscope (Axiovert 135 TV Carl Zeiss, Oberkochen, Germany) equipped with excitation filters (546 nm for Alexa Fluor 568, 450–490 nm for Alexa Fluor 488) and emission filters (590 nm for Alexa Fluor 568, 515–565 nm for Alexa Fluor 488). The photomicrographs were taken by digital camera (AxioCam MRm, Carl Zeiss) driven by AxioVision 4.7 software (Carl Zeiss).
Confocal microscopic observation
Intracellular localization of FGF-1, NGF, BDNF, NT-3, LAMP-2, and EEA1 was investigated under a Zeiss LSM510 confocal microscope equipped with argon (488 nm) and helium-neon (543 nm) lasers to excite green and red fluorescence, respectively. A 100×/1.4 oil immersion objective was used.
Analysis
The number of macrophages immunoreactive for each of FGF-1 and neurotrophins was counted in the microscopic field, and the percentage of immunoreactive cells to total cells in the field was calculated. One to 3 fields were examined for each coverslip. The mean±SEM percentage was then calculated from all microscopic fields examined. The degree of colocalization of FGF-1 with neurotrophins at the cellular level was expressed as the mean (±SEM) percentage of double-labeled cells to total FGF-1-IR cells in the microscopic field. Colocalization in intracellular vesicles (colocalization of FGF-1 with NT-3 and colocalization of FGF-1, NGF, BDNF, or NT-3 with LAMP-2 or EEA1) was also analyzed. The mean (±SEM) percentage of double-labeled vesicles to vesicles immunoreactive for each of FGF-1 and neurotrophins in the cell were calculated. Significance of difference was determined by repeated measures analysis of variance (ANOVA) followed by the Bonferroni-Dunn post hoc test. Statistical significance was determined as P<0.05.
III. Results
Immunocytochemical expression of FGF-1 and neurotrophins in mouse alveolar macrophages
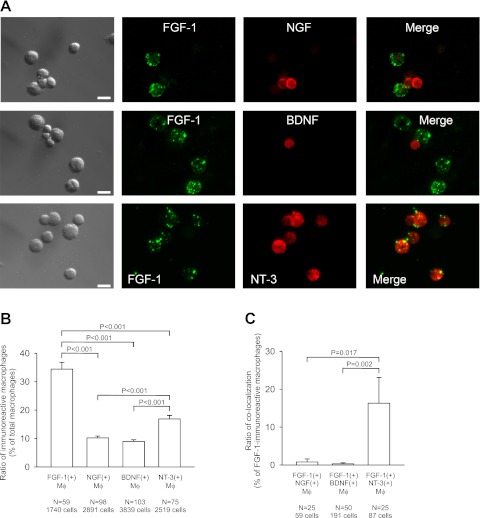
Immunocytochemistry revealed that some alveolar macrophages were stained with antibodies against FGF-1, NGF, BDNF, or NT-3 (Fig. 1A). Mean percentages of the immunoreactive alveolar macrophages to total macrophages were 34% for FGF-1, 10% for NGF, 9% for BDNF, and 17% for NT-3 (Fig. 1B). The percentage of FGF-1-immunoreactive (IR) macrophages and that of NT-3-IR macrophages were significantly higher than that of NGF-IR macrophages or of BDNF-IR macrophages (Fig. 1B). Immunoreactivities for FGF-1, NGF, BDNF, and NT-3 were all located in intracellular vesicles (Figs. 2A, 3A, and 4A).
Fig. 1.
Expression and colocalization of FGF-1 with neurotrophins in mouse alveolar macrophages. (A) Double immunocytochemical staining of macrophages with FGF-1 and NGF (upper panels), with FGF-1 and BDNF (middle panel), and with FGF-1 and NT-3 (lower panels). Panels in the first column show differential interference contrast (DIC) images. Panels in the fourth column represent merged images of the second and third columns. Bars=10 µm. (B) Expression ratio of FGF-1, NGF, BDNF, and NT-3 in alveolar macrophages. Mean percentages of the number of macrophages immunoreactive for FGF-1, NGF, BDNF, and NT-3 to the number of total macrophages are shown. (C) Ratio of colocalization of FGF-1 with neurotrophins in alveolar macrophages. The percentages of double-immunoreactive macrophages to FGF-1-immunoreactive macrophages are shown. (B, C) Error bars represent standard error of the mean. (+), the presence of immunoreactivity; Mϕ, macrophages; N, number of microscopic fields examined (total cell numbers are indicated below); P, probability of significance.
Fig. 2.
Colocalization of FGF-1 and NT-3 in vesicles of mouse alveolar macrophages. (A) Confocal images showing colocalization of FGF-1 and NT-3 in the vesicles. The first panel shows DIC image and the fourth panel shows a merged image of the second and third panels. Bars=5 µm. (B) Ratio of colocalization of FGF-1 and NT-3 in vesicles of alveolar macrophages. The 36 cells immunoreactive for both FGF-1 and NT-3 were examined. (+), the presence of immunoreactivity; (–), the absence of immunoreactivity.
Fig. 3.
Colocalization of FGF-1, NGF, BDNF, and NT-3 with LAMP-2 in vesicles of mouse alveolar macrophages. (A) Confocal images showing colocalization of FGF-1, NGF, BDNF, and NT-3 with LAMP-2, a late endosomal and lysosomal marker. Panels in the first column show DIC images. Panels in the fourth column represent merged images of the second and third columns. Bars=5 µm. (B) Ratio of colocalization of FGF-1, NGF, BDNF, and NT-3 with LAMP-2 in the vesicles of alveolar macrophages. The percentages of double-immunoreactive vesicles to the vesicles immunoreactive for FGF-1, NGF, BDNF, or NT-3 are shown. (+), the presence of immunoreactivity; P, probability of significance.
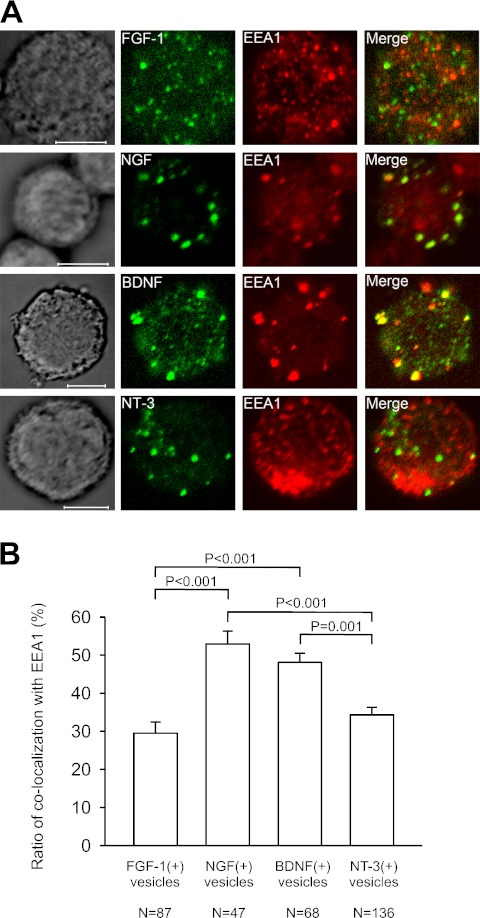
Fig. 4.
Colocalization of FGF-1, NGF, BDNF, and NT-3 with EEA1 in vesicles of mouse alveolar macrophages. (A) Confocal images showing colocalization of FGF-1, NGF, BDNF, and NT-3 with EEA1, a early endosomal marker. Panels in the first column show DIC images. Panels in the fourth column represent merged images of the second and third columns. Bars=5 µm. (B) Ratio of colocalization of FGF-1, NGF, BDNF, and NT-3 with EEA1 in the vesicles of alveolar macrophages. The percentages of double-immunoreactive macrophages to the vesicles immunoreactive for FGF-1 NGF, BDNF, or NT-3 are shown. (+), the presence of immunoreactivity; P, probability of significance.
Cellular colocalization of FGF-1 with neurotrophins
Double labeling with FGF-1 and one of the neurotrophin family members showed that some FGF-1-IR macrophages were immunoreactive for NT-3 (Fig. 1A, upper panels) and almost no FGF-1-IR macrophages were immunoreactive for NGF or BDNF (Fig. 1A, middle and lower panels). The quantitative analysis indicated that 16% of FGF-1-IR macrophages were immunoreactive for NT-3, whereas only small percentages of FGF-1-IR macrophages were immunoreactive for NGF (0.8%) and for BDNF (0.3%) (Fig. 1C). The ratio (percentage) of colocalization of FGF-1 with NT-3 was significantly higher than that with NGF and with BDNF (Fig. 1C).
Vesicular colocalization of FGF-1 with NT-3
In macrophages double-labeled with FGF-1 and NT-3, some intracellular vesicles were observed to be immunoreactive for both (Fig. 2A). Results of quantitative analysis for colocalization at the vesicular level are shown in Figure 2B. The majority (89%) of FGF-1-IR vesicles were immunoreactive for NT-3, and reversely, 64% of NT-3-IR vesicles were immunoreactive for FGF-1. Since FGF-1 was rarely colocalized with NGF or BDNF at cellular level as described above, quantitative analysis of vesicular colocalization of FGF-1 with these two neurotrophins could not be determined.
Vesicular colocalization with LAMP-2 and EEA1
We further investigated the precise intracellular localization of FGF-1 and neurotrophins using antibodies against LAMP-2, a late endosomal and lysosomal marker and against EEA1, an early endosomal marker. All alveolar macrophages had immunoreactivity for LAMP-2 and for EEA1, and the immunoreactivities for LAMP-2 and EEA1 were located in intracellular vesicles.
As shown in Figure 3A, some of the FGF-1-IR, NGF-IR, BDNF-IR, and NT-3-IR vesicles were immunoreactive for LAMP-2. Quantitative analysis showed that two-thirds of FGF-1-IR (65%) and of NT-3-IR (66%) vesicles were immunoreactive for LAMP-2, and one-third of NGF-IR (33%) and of BDNF-IR (37%) vesicles had immunoreactivity for LAMP-2 (Fig. 3B). The ratio of colocalization of FGF-1 with LAMP-2 and that of NT-3 with LAMP-2 were significantly higher than that of NGF with LAMP-2 or of BDNF with LAMP-2 (Fig. 3B).
Vesicular colocalization with EEA1 was also observed (Fig. 4A), but its degree was different from that with LAMP-2 (Fig. 4B). One-third of FGF-1-IR vesicles (30%) and of NT-3-IR vesicles (34%) were immunoreactive for EEA1, and a half of NGF-IR vesicles (53%) and of BDNF-IR vesicles (48%) were immunoreactive for EEA1 (Fig. 4B). The percentage of colocalization of FGF-1 with EEA1 and that of NT-3 with EEA1 were significantly lower than that of NGF with EEA1 or that of BDNF with EEA1 (Fig. 4B).
IV. Discussion
The present immunocytochemical study indicated that mouse alveolar macrophages express FGF-1 and neurotrophins, NGF, BDNF, and NT-3, in their vesicles.
The previous immunohistochemical study on the normal human lung tissue demonstrated that FGF-1 is constitutively expressed in alveolar macrophages [12], though its quantitative analysis has not been performed. In the present study, we analyzed the number of immunoreactive alveolar macrophages and obtained the result that 34% of macrophages expressed FGF-1. This indicates that mouse alveolar macrophages store a substantial amount of FGF-1.
Mouse alveolar macrophages also appear to contain high amounts of NT-3. The present result showed that 17% of macrophages were immunoreactive for NT-3 and this was higher than the ratio of NGF-expressing macrophages (10%) or of BDNF-expressing macrophages (9%). These results are somewhat different from those obtained from normal human alveolar macrophages. Ricci et al. [20] have shown that 2.5% of normal human alveolar macrophages were immunoreactive for NGF and that no macrophages were immunoreactive for BDNF or NT-3. The difference between their results and ours might depend on the species observed. Indeed, the species differences in the localization of neurotrophins have been found. For example, NGF is immunohistochemically detected in salivary glands in mice but not in humans [4]. Enzyme-linked immunoassay detects BDNF in human and rat but not mouse serum [19]. If the localization differences are attributed to species specificity, the data from mice may not be applicable to humans. However, it is true that both humans and mice constitutively express neurotrophins in alveolar macrophages.
Our previous RT-PCR study has shown that mouse alveolar macrophages express mRNA for NT-3 but not for NGF or BDNF [6]. This is not inconsistent with the present immunocytochemical results showing that NGF, BDNF, and NT-3 were all expressed in alveolar macrophages but the ratio of expression was different. Together with our present and previous results, NT-3-synthesizing macrophages are more in number than NGF- or BDNF-synthesizing macrophages. In addition, as discussed below, the expression of NGF and BDNF in alveolar macrophages may be attributed to the uptake from the extracellular milieu rather than synthesis. The amount of mRNAs for NGF and BDNF was thus demonstrated to be under detectable levels in our previous study.
The present colocalization analysis showed that 16% of FGF-1-expressing macrophages co-expressed NT-3, whereas only a few FGF-1-expressing macrophages co-expressed NGF (0.8%) or BDNF (0.3%). Also at the intracellular level, the frequent colocalization of FGF-1 and NT-3 in vesicles was observed (89% of FGF-1-IR vesicles were immunoreactive for NT-3 and 64% of NT-3-IR vesicles were immunoreactive for FGF-1). Thus, FGF-1 can be stored together with NT-3 in the same vesicles in macrophages. The colocalization of FGF-1 with neurotrophins in the same cell has previously been found in other samples. Bizon et al. [1] have shown that FGF-1 and NGF are colocalized in cells of the rat striatum, although it is unclear whether or not the colocalization is found in intracellular vesicles.
The present study on the vesicular colocalization with LAMP-2 and EEA1 indicated that FGF-1 and NT-3 are colocalized with LAMP-2 rather than EEA1, whereas NGF and BDNF are colocalized with EEA1 rather than LAMP-2. Thus, it is likely that FGF-1 and NT-3 are preferentially located in late endosomes or lysosomes, whereas NGF and BDNF are preferentially located in early endosomes. Macrophages as well as other immune cells have lysosomes that function as secretory compartments, called secretory lysosomes [2]. Different from secretory granules in general secretory cells, secretory lysosomes contain not only secretory proteins but also lysosomal proteins such as LAMP-1, LAMP-2, CD63, and lysosomal hydrolases [2]. Thus, FGF-1 and NT-3 are possibly located not only in ordinary lysosomes but also in the secretory lysosomes. The presence of NGF and BDNF in macrophages, both of which tend to be located in early endosomes, may predominantly result from the uptake (endocytosis) from the extracellular milieu. In nervous system cells (neurons and astrocytes), neurotrophins released from their own or neighboring cells can be endocytosed and then recycled for exocytosis [13]. Thus, if such a neurotrophin transport system is also present in macrophages, the source of NGF and BDNF is most likely to be the alveolar macrophages themselves in addition to epithelial cells and fibroblasts. However, direct evidence for uptake, recycling, and release of FGF-1 and neurotrophins is needed to confirm this speculation.
In summary, the present study demonstrates that FGF-1 as well as NT-3 are both substantially expressed in alveolar macrophages, and that they are colocalized in the vesicles, predominantly in late endosomes and lysosomes. The colocalization of FGF-1 with NT-3 would suggest their possible coordinated storage, release, and action in alveolar macrophages.
V. Disclosure of Conflict of Interest
None of the authors has any conflict of interest to disclose.
VI. Acknowledgments
This work was partially supported by a Grant-in-Aid for Scientific Research (C) (Kakenhi 20591418) from the Japan Society for the Promotion of Science (JSPS) to H.H.
VII. References
- 1.Bizon J. L., Lauterborn J. C., Gall C. M. Subpopulations of striatal interneurons can be distinguished on the basis of neurotrophic factor expression. J. Comp. Neurol. 1999;408:283–298. [PubMed] [Google Scholar]
- 2.Blott E. J., Griffiths G. M. Secretory lysosomes. Nat. Rev. Mol. Cell Biol. 2002;3:122–131. doi: 10.1038/nrm732. [DOI] [PubMed] [Google Scholar]
- 3.Cardoso W. V., Itoh A., Nogawa H., Mason I., Brody J. S. FGF-1 and FGF-7 induce distinct patterns of growth and differentiation in embryonic lung epithelium. Dev. Dyn. 1997;208:398–405. doi: 10.1002/(SICI)1097-0177(199703)208:3<398::AID-AJA10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 4.De Vicente J. C., Garcia-Suárez O., Esteban I., Santamaria J., Vega J. A. Immunohistochemical localization of neurotrophins and neurotrophin receptors in human and mouse salivary glands. Ann. Anat. 1998;180:157–163. doi: 10.1016/S0940-9602(98)80016-2. [DOI] [PubMed] [Google Scholar]
- 5.Deterding R. R., Jacoby C. R., Shannon J. M. Acidic fibroblast growth factor and keratinocyte growth factor stimulate fetal rat pulmonary epithelial growth. Am. J. Physiol. 1996;271:L495–L505. doi: 10.1152/ajplung.1996.271.4.L495. [DOI] [PubMed] [Google Scholar]
- 6.Hikawa S., Kobayashi H., Hikawa N., Kusakabe T., Hiruma H., Takenaka T., Tomita T., Kawakami T. Expression of neurotrophins and their receptors in peripheral lung cells of mice. Histochem. Cell Biol. 2002;118:51–58. doi: 10.1007/s00418-002-0426-y. [DOI] [PubMed] [Google Scholar]
- 7.Huang M.-C., Chang P.-T., Tsai M.-J., Kuo H.-S., Kuo W.-C., Lee M.-J., Lo M.-J., Lee I.-H., Huang W.-C., Lee L.-M., Shih Y.-H., Lee L.-S., Cheng H. Sensory and motor recovery after repairing transected cervical roots. Surg. Neurol. 2007;68:S1:17–S1:24. doi: 10.1016/j.surneu.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 8.Hughes S. E., Hall P. A. Immunolocalization of fibroblast growth factor receptor 1 and its ligands in human tissues. Lab. Invest. 1993;69:173–182. [PubMed] [Google Scholar]
- 9.Jacques T. S., Skepper J. N., Navaratnam V. Fibroblast growth factor-1 improves the survival and regeneration of rat vagal preganglionic neurones following axon injury. Neurosci. Lett. 1999;276:197–200. doi: 10.1016/s0304-3940(99)00832-0. [DOI] [PubMed] [Google Scholar]
- 10.Kelley J. Cytokines of the lung. Am. Rev. Respir. Dis. 1990;141:765–788. doi: 10.1164/ajrccm/141.3.765. [DOI] [PubMed] [Google Scholar]
- 11.Komi A., Suzuki M., Imamura T. Permeable FGF-1 nuclear localization signal peptide stimulates DNA synthesis in various cell types but is cell-density sensitive and unable to support cell proliferation. Exp. Cell Res. 1998;243:408–414. doi: 10.1006/excr.1998.4176. [DOI] [PubMed] [Google Scholar]
- 12.Kranenburg A. R., Willems-Widyastuti A., Mooi W. J., Saxena P. R., Sterk P. J., de Boer W. I., Sharma H. S. Chronic obstructive pulmonary disease is associated with enhanced bronchial expression of FGF-1, FGF-2, and FGFR-1. J. Pathol. 2005;206:28–38. doi: 10.1002/path.1748. [DOI] [PubMed] [Google Scholar]
- 13.Lessmann V., Gottmann K., Malcangio M. Neurotrophin secretion: current facts and future prospects. Prog. Neurobiol. 2003;69:341–374. doi: 10.1016/s0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 14.Lohmann-Matthes M.-L., Steinmüller C., Franke-Ullmann G. Pulmonary macrophages. Eur. Respir. J. 1994;7:1678–1689. [PubMed] [Google Scholar]
- 15.Mason R. J., Leslie C. C., McCormick-Shannon K., Deterding R. R., Nakamura T., Rubin J. S., Shannon J. M. Hepatocyte growth factor is a growth factor for rat alveolar type II cells. Am. J. Respir. Cell Mol. Biol. 1994;11:561–567. doi: 10.1165/ajrcmb.11.5.7524567. [DOI] [PubMed] [Google Scholar]
- 16.Mohiuddin L., Fernyhough P., Tomlinson D. R. Acidic fibroblast growth factor enhances neurite outgrowth and stimulates expression of GAP-43 and Tα1 α-tubulin in cultured neurones from adult rat dorsal root ganglia. Neurosci. Lett. 1996;215:111–114. [PubMed] [Google Scholar]
- 17.Nockher W. A., Renz H. Neurotrophins in inflammatory lung diseases: modulators of cell differentiation and neuroimmune interactions. Cytokine Growth Factor Rev. 2003;14:559–578. doi: 10.1016/s1359-6101(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 18.Prakash Y., Thompson M. A., Meuchel L., Pabelick C. M., Mantilla C. B., Zaidi S., Martin R. J. Neurotrophins in lung health and disease. Expert Rev. Respir. Med. 2010;4:395–411. doi: 10.1586/ers.10.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radka S. F., Holst P. A., Fritsche M., Altar C. A. Presence of brain-derived neurotrophic factor in brain and human and rat but not mouse serum detected by a sensitive and specific immunoassay. Brain Res. 1996;709:122–130. doi: 10.1016/0006-8993(95)01321-0. [DOI] [PubMed] [Google Scholar]
- 20.Ricci A., Greco S., Mariotta S., Felici L., Amenta F., Bronzetti E. Neurotrophin and neurotrophin receptor expression in alveolar macrophages: an immunocytochemical study. Growth Factors. 2000;18:193–202. doi: 10.3109/08977190009003244. [DOI] [PubMed] [Google Scholar]
- 21.Scherping S. C. Jr., Schmidt C. C., Georgescu H. I., Kwoh C. K., Evans C. H., Woo S. L.-Y. Effect of growth factors on the proliferation of ligament fibroblasts from skeletally mature rabbits. Connect. Tissue Res. 1997;36:1–8. doi: 10.3109/03008209709160209. [DOI] [PubMed] [Google Scholar]
- 22.Tassi E., Al-Attar A., Aigner A., Swift M. R., McDonnell K., Karavanov A., Wellstein A. Enhancement of fibroblast growth factor (FGF) activity by an FGF-binding protein. J. Biol. Chem. 2001;276:40247–40253. doi: 10.1074/jbc.M104933200. [DOI] [PubMed] [Google Scholar]