Abstract
Meloidogyne incognita causes more damage to cotton in the US than any other pathogen. The objective of this study was to document the cumulative effect of moderate resistance on M. incognita population density, root galling, and yield suppression in the southern United States on a moderately resistant cotton genotype grown continuously for three years. Cotton genotypes were Phytogen PH98-3196 (77% suppression of M. incognita), Acala NemX (85% suppression of M. incognita), and Delta and Pine Land DP458 B/R (susceptible standard, 0% suppression). Cotton was grown in fumigated and non-fumigated plots to measure yield loss. Each genotype and nematicide combination was planted in the same place for three years at two sites to document cumulative effects. In 2006, following three years of the different genotypes, all plots at one site were planted with susceptible cotton to document residual effects of planting resistant genotypes. Root galling and nematode population densities in the soil were significantly lower, and percentage yield suppression was numerically lower, when moderately resistant cotton was grown compared to the susceptible standard in both fields in all three years. Differences between susceptible and moderately resistant genotypes are established quickly (after only one season) and then either maintained at similar levels or slightly increased in subsequent years depending on initial nematode levels. However, when susceptible cotton was grown following three years of the moderately resistant genotypes, the nematode suppression provided by moderate resistance was undetectable by the end of the first season. Moderately resistant cotton genotypes are more beneficial than previously reported and should be pursued for nematode management. Rotation of moderately resistant and susceptible cotton could be used along with nematicides to manage root-knot nematodes in a continuous cotton cropping system and reduce selection pressure on the nematodes.
Keywords: Cotton, Gossypium hirsutum, host-plant resistance, Meloidogyne incognita, nematode management, southern root-knot nematode
Host-plant resistance, the ability of a plant to suppress nematode reproduction, is a consistent and effective means of reducing damage from plant-parasitic nematodes (Meyer et al., 2006). Resistance can be an integral method of nematode suppression in crops for which highly-resistant cultivars are available. For example, resistance is a primary management tool for the soybean cyst (Heterodera glycines), reniform (Rotylenchulus reniformis), and root-knot (Meloidogyne spp.) nematodes in soybean (Glycine max), and for some root-knot nematodes in tobacco (Nicotiana tobacum) (Koenning et al. 1999; Young, 1998).
In practice, host plant resistance to a nematode species is identified by comparing genotypes to a susceptible standard. Genotypes supporting less than 10% of the nematode reproduction on the susceptible standard are typically considered to be highly resistant, and genotypes supporting more than 10% but less than the susceptible standard are moderately resistant (Hussey and Janssen, 2002). Although cotton (Gossypium hirsutum) germplasm with a high level of resistance to Meloidogyne incognita has been available to breeders for years, no cultivars adapted to the southern United States with a high level of resistance have been released (Robinson et al., 2001; Shen et al., 2006). Acala NemX has resistance to M. incognita, but it is an Acala-type cotton developed for California and it is poorly adapted to the southern United States (Koenning et al., 2001; Zhou and Starr, 2003). A few cultivars with moderate levels of M. incognita resistance have been available in the southern United States in the past. However, since nematicide use can often increase yield of cotton with moderate resistance to M. incognita (Colyer et al., 1997; Davis and May, 2003; Koenning et al., 2001), there was concern that moderate resistance was inadequate.
Highly resistant genotypes provide the greatest benefit, but a moderate level of resistance also can reduce yield loss in the current crop (Adee et al., 2008; Davis and May, 2003; Koenning et al., 2001; Zhou and Starr, 2003). One study with resistant Acala NemX cotton in California documented increased yield in a subsequent susceptible Acala-type cotton crop (Ogallo et al., 1999), but NemX was reported to be highly resistant and it is not known how applicable those findings are to the southeastern United States where Acala-type cotton is not grown. The objective of this study was to document the cumulative effect of a moderately resistant cotton genotype grown continuously for three years on M. incognita population density and cotton yield suppression in the southern United States.
Materials and Methods
Evaluation of resistance: The level of resistance for the genotypes in this study was documented in two greenhouse trials. Each trial had 6 replications in a randomized complete block design. The cultivar ‘Delta and Pine Land DP458 B/R’ was used as a susceptible standard, and M-120 RNR germplasm was used as a resistant standard. Cotton seeds (the two standards plus ‘Acala NemX’ and ‘Phytogen PH98-3196’) were planted into 15-cm-diam. pots, and seedlings were thinned to one plant per pot prior to inoculation. The soil used was a Tifton Sandy Loam (82% sand, 7% silt, 11% clay, >1% organic matter).
Inoculum was collected from tomato roots (Solanum lycopersicum ‘Rutgers’) by agitating roots in 0.6% sodium hypochlorite solution for two minutes (Hussey and Barker, 1973) approximately 1 hour before inoculation. Inoculum of 8,000 M. incognita eggs/pot (approximately 600 eggs/150cm3 soil) was distributed into two holes (approximately 2.5 cm deep) and covered with soil. Pots were watered immediately following inoculation.
Nematode eggs were extracted from the entire root system 28 days after inoculation. Roots were washed free of soil, weighed, cut into 5-cm pieces, and agitated in a 1.2% sodium hypochlorite solution in a 1-liter flask for four minutes. Eggs were collected and rinsed with tap water on nested 150- over 25-μm-pore sieves. Egg counts were subjected to a square-root transformation to equalize the error variances prior to statistical analysis. Reproductive factors were calculated as RF = Pf/Pi = final egg count/inoculum level. Data from the two trials were pooled for a combined analysis of variance and means separation by Fisher's protected least significant difference (LSD0.05).
Field tests: Two field sites (Field A and Field B) were established at the University of Georgia Gibbs Farm in Tifton, GA. At both sites, treatments were replicated six times in a split-plot design. Treatments consisted of three genotypes (whole plots) with differing levels of nematode resistance: Acala NemX, Phytogen PH98-3196, and DP458 B/R. Each genotype was grown in both fumigated (1,3-dichloropropene at 56 l/ha) and non-fumigated plots (subplots) so that yield suppression due to nematodes could be measured. Plots were fumigated two to three weeks prior to planting. For thrip control, all plots received aldicarb at 2.2 kg/ha (Jost et al., 2003). Each genotype and nematicide combination was planted in the same place for three consecutive seasons so that the cumulative effects could be documented. The first year at Field A was 2003, and the first year at Field B was 2004. During a fourth year (2006) at Field A, all plots were planted with the susceptible cultivar DP458 B/R.
Cotton was planted on 15 May 2003, 6 May 2004, 12 May 2005, and 4 May 2006 at Field A and 6 May 2004, 13 May 2005, and 4 May 2006 at Field B. All plots were managed identically except for cotton genotype, which varied by treatment as described above. Soil samples for nematode analysis were collected in early, mid, and late season (28 May, 8 July, and 5 November 2003; 13 May 24 June, 5 August, and 15 October 2004; 24 may, 8 July, 31 August, and 31 October 2005; and 18 May, 7 July, 16 August, and 18 October 2006). Plots were harvested on 24 October 2003, 6 October 2004, 20 October 2005, and 12 October 2006 at Field A, and 7 October 2004, 20 October 2005, and 13 October 2006 at Field B. Following harvest, 10 plants per plot were carefully excavated, and a root gall rating was assigned to each plant based on a linear 0 to 10 scale (0 = no galling, 1 = 10% of the root system galled, 2 = 20%, etc., and 10 = 100% galled). Gall ratings were performed on 6 November 2003, 13 October 2004, 1 November 2005, and 18 October 2006.
Percentage yield suppression was calculated for each plot as (yield in fumigated plots – yield in non-fumigated plots) / (yield in fumigated plots). Plots treated with 1,3-dichloropropene were used to provide an estimate of yield when nematode damage was minimized, so those plots were excluded from the analysis of variance. A split-plot analysis of variance was calculated for each field site in each year for nematode population densities, and appropriate LSD values were calculated to compare genotypes within a fumigation treatment.
Results
Evaluation of resistance: The two greenhouse trials showed no interaction between trials for the square root of egg counts or for root galling. The mean number of eggs on the susceptible standard (DP458 B/R) was 61,550 eggs/plant and the resistant standard (M-120 RNR) was 4,000 eggs/plant. The mean number of eggs/plant on PH98-3196 was 14,150, and the number on NemX was 9,491. Reproductive factors (RF = Pf/Pi = final egg count/inoculum level) were 7.7 for DP458 B/R, 1.8 for PH98-3196, and 1.2 for NemX. All genotypes had significantly fewer eggs/plant than DP458 B/R, but M-120 RNR, NemX, and PH98-3196 were statistically similar to each other.
Gall ratings generally were reflective of egg counts. The mean gall ratings were 6.9 for DP458 B/R, 2.3 for PH98-3196, 2.1 for NemX, and 1.4 for M-120 RNR. All genotypes had significantly less root galling than DP458 B/R, but M-120 RNR, NemX, and PH98-3196 were statistically similar to each other.
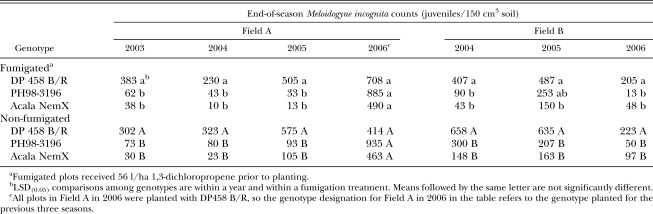
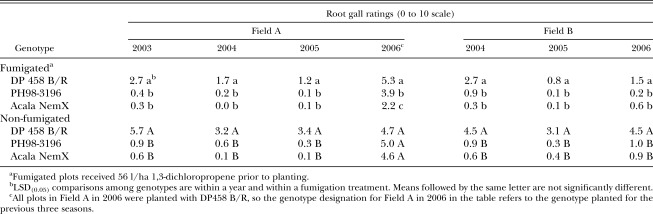
Field tests: End-of-season nematode population densities in both fields were lower following PH98-3196 or NemX than following DP458 B/R after the first year at each field site, and levels following PH98-3196 and NemX did not differ from each other (Table 1). In Field A, nematode levels in non-fumigated plots were reduced below the University of Georgia Extension Service recommended action threshold of 150 juveniles/150 cm3 soil (Jost et al. 2003) by one year of PH98-3196 or NemX, but not by DP458 B/R. After the first year in Field B, nematode population densities were significantly higher following DP458 B/R than following PH98-3196 or NemX. The effect of genotype on end-of-season population densities was not affected by fumigation (there was no genotype×fumigation interaction) in either field, though fumigation significantly reduced end-of-season population densities in Field B. Root galling (Table 2) in the first year in both fields was reflective of end-of-season soil population densities: resistant genotypes and fumigation reduced nematode population densities and galling, but the effect of genotype was not influenced by fumigation (no interaction).
Table 1.
Number of Meloidogyne incognita in soil at harvest after growing three cotton genotypes in two fields.
Table 2.
Root gall ratings for three cotton genotypes following harvest in two fields.
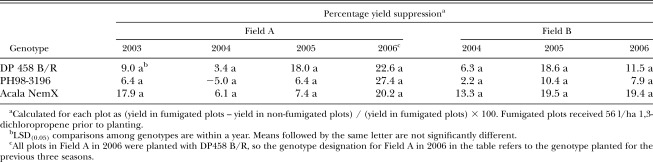
At the end of both the second and third year at each location, the results for nematode population densities and root galling were similar to results following the first year (Tables 1 and 2). In both fields, the two resistant genotypes reduced end-of-season root-knot nematode population densities compared to DP458 B/R, and both genotype and fumigation reduced galling compared to the susceptible standard. In both fields, root galling of PH98-3196 and NemX was statistically similar. End-of-season nematode population densities were similar for PH98-3196 and NemX in Field B in both the second and third years, but population densities were lower for NemX in Field A in the second year. In Field A, end-of-season nematode population densities following PH98-3196 and NemX were below action threshold levels in both the second and third years, but levels were below the threshold in Field B only after the third year. As in the first year, fumigation did not influence the effect of genotype on either nematode population densities or root galling in the second year, but root galling was reduced by fumigation more on DP458 B/R than on either resistant genotype in both fields in the third year (a statistical genotype×fumigation interaction). The percentage yield suppression did not differ (P ≤ 0.10) among genotypes in either field in any year (Table 3).
Table 3.
Percentage yield suppression caused by M. incognita for three cotton genotypes in two fields.
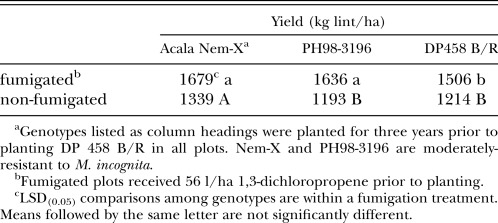
In the fourth year in Field A (all plots planted with the susceptible DP458 B/R), the end-of-season nematode population densities were similar among plots regardless of which genotype had been planted in that plot during the previous three seasons (Table 1). Root galling was not affected by the previous genotype in non-fumigated plots, but root galling was affected by the previous genotype in fumigated plots (Table 2) with the highest rating following DP458 B/Rand the lowest following NemX. In the fourth year, the greatest yield was measured in plots which had been planted to NemX for the previous three years (Table 4). Yield following PH98-3196 was greater than yield following DP458 B/R only in fumigated plots. Percentage yield suppression in the fourth year was not affected by previous genotype (Table 3).
Table 4.
Yield of Meloidogyne incognita-susceptible DP458 B/R cotton following three years of cotton genotypes with differing levels of resistance to M. incognita.
Discussion
A commonly-used system for classifying the level of resistance in a genotype requires a 90% decrease in nematode reproduction compared to a susceptible standard for a genotype to be considered highly resistant, and genotypes with significantly less reproduction than the standard but less than a 90% reduction are considered to be only moderately resistant (Hussey and Janssen, 2002). Though cotton germplasm with a high level of resistance to M. incognita has been available for decades, a high level of resistance has not yet been successfully incorporated into a commercially available cultivar because the genetics of nematode resistance in cotton is complex (Robinson et al., 2001; Shen et al., 2006). However, a few cultivars with moderate resistance were available in recent years in the southern United States, including Stoneville LA887, Paymaster H1560, Stoneville 5599 B/R (Davis and May, 2005; McPherson et al., 2004; Nui et al., 2007; Robinson et al., 2001).
NemX has been reported to be either moderately resistant (Koenning et al., 2001; McPherson et al., 2004; Nui et al., 2007) or highly resistant (Ogallo et al., 1997; Ogallo et al., 1999). Greenhouse evaluation of M. incognita reproduction documented differences in levels of resistance among the genotypes used in this study. PH98-3196 was moderately resistant with a reduction of 77% in the number of eggs produced compared to DP458 B/R. We had intended for NemX to be a highly-resistant standard in our study with PH98-3196 as the moderately-resistant genotype, but NemX also was only moderately resistant with nematode reduction of 85%. Both PH98-3196 and NemX allowed M. incognita levels to increase with reproductive factors (Pf/Pi) in greenhouse testing of 1.8 and 1.2, respectively.
A poor host can be as effective as a non-host or fallow in reducing nematode populations (Caswell et al., 1991), and moderate resistance makes a plant a poor host. A moderately-resistant soybean cultivar reduced population levels of Heterodera glycines and also reduced damage to the next susceptible crop (Adee et al., 2008). Research on the utility of moderate resistance in cotton has been limited and has focused primarily on nematode suppression and yield losses in a single season (Colyer et al., 1997; Davis and May, 2003; Koenning et al., 2001). One study in California showed that suppressed levels of M. incognita following Acala NemX resulted in increased yield in a subsequent susceptible cotton crop, and one year of NemX was as effective as two successive years of NemX (Ogallo et al., 1999). The end-of-season nematode population densities in our study also showed that a single year of a moderately resistant genotype (PH98-3196) significantly reduced nematode levels in a field after a single year. There was, however, some evidence of a cumulative benefit in Field B which had higher initial nematode population densities. In that field, nematode levels were suppressed below the action threshold (150 juveniles/150 cm3 soil) when the moderately resistant genotype was grown for two additional years.
In nematicide-treated plots, we documented increased yield in susceptible cotton following three years of either NemX or PH98-3196: this is the least level of resistance in cotton that has been shown to result in reduced nematode damage on a following susceptible crop. However, in plots which did not receive a nematicide, yields were increased only following NemX. Our results are consistent with previous work that reported yield increases with nematicide use even following two years of an effective rotation crop (Rodríguez-Kábana and Touchton, 1984). Although moderately resistant genotypes reduced nematode population densities to near or below the action threshold level recommended in Georgia, yield in non-fumigated plots was significantly reduced compared to fumigated plots. Additionally, genotypes with moderate resistance apparently support enough reproduction to maintain nematode populations for at least three years at levels that can rebound quickly: the end-of-season nematode population densities in the fourth year at Field A were similar to continuous susceptible cotton in both fumigated and non-fumigated plots despite the significant and persistent suppression of M. incognita levels.
Because nematode population densities increased to the level of the continuous susceptible plots during a single season, the use of cotton with moderate resistance to M. incognita as a rotation crop with susceptible cotton appears to provide no more than a single year of benefit in the southern United States. Although we only planted susceptible cotton after the resistant genotypes in Field A, nematode population densities in Field B were generally greater than in Field A, so we speculate that results in Field B would have been similar. It is possible that beneficial effects may last longer in places with different soil environments, winter attrition rates, and other factors that can influence nematode population levels and the damage they cause.
Moderate resistance in cotton suppresses nematode reproduction, but the yield of moderately resistant genotypes typically is still reduced when plants are parasitized by M. incognita, and the percentage yield suppression has been shown by regression analysis to be inversely related to the level of resistance (Davis and May 2003). In this study, analysis of variance showed that the percentage yield suppression did not differ statistically among genotypes in either field in any year, but yield suppression for the susceptible DP458 B/R was numerically greater than for the moderately resistant PH98-3196 in all years in both fields. Such consistency suggests that the means may have been different but a large degree of variation in the data may have prevented the difference from being statistically significant. Percentage yield suppression for NemX was numerically greatest in all years because it is not adapted to Southeastern growing conditions (Koenning et al., 2001; Zhou and Starr, 2003).
Although crop rotation can be an effective means of nematode management, the primary crops rotated with cotton in Georgia have limitations: peanut (Arachis hypogaea) effectively suppresses M. incognita, but peanut acreage is limited, and corn (Zea mays) is an excellent host for M. incognita (Davis and Timper, 2000). Cotton is grown in some fields without rotation for years. Moderate levels of resistance, such as that in PH98-3196, is sufficient to provide significant suppression of M. incognita after one season thereby providing the short-term benefit of reducing damage in the current season's crop and the longer-term benefit of reducing nematode population levels for the next year's crop. Differences in nematode population levels between susceptible and moderately-resistant genotypes are established quickly (after only one season) and then maintained at similar levels or increased slightly in subsequent years. Unfortunately, growing resistant cotton continuously can cause the nematode population to become more virulent on the resistant cotton (Ogallo et al., 1997). Our results suggest that rotation of moderately resistant cotton and susceptible cotton could be used along with nematicides to manage root-knot nematodes in a continuous cotton cropping system and to reduce selection pressure on the nematodes.
Footnotes
The authors thank Thomas Hilton, A. Kyle Montfort, David Clements, and William Wilson for their technical assistance. Mention of trade names or commercial products is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the United States Department of Agriculture.
This paper was edited by Ekaterini Riga.
Literature Cited
- Adee EA, Johnson ML, Niblack TL. Effect of soybean cultivars moderately resistant to soybean cyst nematode on SCN populations and yield. Online. Plant Health Progress. 2008 doi: 10.1094/PHP-2008-0618-03-RS. [DOI] [Google Scholar]
- Caswell EP, DeFrank J, Apt WJ, Tang CS. Influence of non-host plants on population decline of Rotylenchulus reniformis. Journal of Nematology. 1991;23:91–98. [PMC free article] [PubMed] [Google Scholar]
- Colyer PD, Kirkpatrick TL, Caldwell WD, Vernon PR. Influence of nematicide application on the severity of the root-knot nematode—Fusarium wilt disease complex in cotton. Plant Disease. 1997;81:66–70. doi: 10.1094/PDIS.1997.81.1.66. [DOI] [PubMed] [Google Scholar]
- Davis RF, May OL. Relationships between tolerance and resistance to Meloidogyne incognita in cotton. Journal of Nematology. 2003;35:411–416. [PMC free article] [PubMed] [Google Scholar]
- Davis RF, May OL. Relationship between yield potential and percentage yield suppression caused by the southern root-knot nematode in cotton. Crop Science. 2005;45:2312–2317. [Google Scholar]
- Davis RF, Timper P. Resistance in selected corn hybrids to Meloidogyne arenaria and M. incognita. Supplement to the Journal of Nematology. 2000;32:633–640. [PMC free article] [PubMed] [Google Scholar]
- Hussey RS, Janssen GJW. Root-knot nematodes: Meloidogyne species. In: Starr JL, Cook R, Bridge J, editors. Plant resistance to parasitic nematodes. Wallingford, UK: CABI Publishing; 2002. pp. 43–70. [Google Scholar]
- Hussey RS, Barker KR. A comparison of methods of collecting inocula for Meloidogyne spp., including a new technique. Plant Disease Reporter. 1973;57:1025–1028. [Google Scholar]
- Jenkins WR. A rapid centrifugal flotation technique for separating nematodes from soil. Plant Disease Reporter. 1964;48:692. [Google Scholar]
- Jost PJ, Brown SM, Culpepper S, Harris G, Kemerait B, Roberts P, Shurley D, Williams J. Tifton, GA: University of Georgia Cooperative Extension Service; 2003. 2003 Georgia Cotton Production Guide. Publication No. CSS-03-01; p. 98. [Google Scholar]
- Koenning SR, Barker KR, Bowman DT. Resistance as a tactic for management of Meloidogyne incognita on cotton in North Carolina. Journal of Nematology. 2001;33:126–131. [PMC free article] [PubMed] [Google Scholar]
- Koenning SR, Overstreet C, Noling JW, Donald PA, Becker JO, Fortnum BA. Survey of crop losses in response to phytoparasitic nematodes in the United States for 1994. Supplement to the Journal of Nematology. 1999;31:587–618. [PMC free article] [PubMed] [Google Scholar]
- McPherson MG, Jenkins JN, Watson CE, McCarty JC. Inheritance of root-knot nematode resistance in M-315 RNR and M78-RNR cotton. Journal of Cotton Science. 2004;8:154–161. [Google Scholar]
- Meyer SLF, Kokalis-Burelle N, Davis RF, Thies JA, Zasada IA. USDA-ARS Research on practices compatible with organic agriculture for management of plant-parasitic nematodes on vegetable crops. Journal of Vegetable Science. 2006;12:47–81. [Google Scholar]
- Nui C, Hinchliffe DJ, Cantrell RG, Wang C, Roberts PA, Zhang J. Identification of molecular markers associated with root-knot nematode resistance in upland cotton. Crop Science. 2007;47:951–960. [Google Scholar]
- Ogallo JL, Goodell PB, Eckert J, Roberts PA. Evaluation of NemX, a new cultivar of cotton with high resistance to Meloidogyne incognita. Journal of Nematology. 1997;29:531–537. [PMC free article] [PubMed] [Google Scholar]
- Ogallo JL, Goodell PB, Eckert JW, Roberts PA. Management of root-knot nematodes with resistant cotton cv. NemX. Crop Science. 1999;39:418–421. [Google Scholar]
- Robinson AF, Bowman DT, Cook CG, Jenkins JN, Jones JE, May OL, Oakley SR, Oliver MJ, Roberts PA, Robinson M, Smith CW, Starr JL, Stewart JM. Nematode Resistance. In: Kirkpatrick TL, Rothrock CS, editors. Compendium of cotton diseases. second edition. St. Paul, MN: APS Press; 2001. pp. 68–72. [Google Scholar]
- Rodríguez-Kábana R, Touchton JT. Corn and Sorghum as rotational crops for management of Meloidogyne arenaria in peanut. Nematropica. 1984;14:26–36. [Google Scholar]
- Shen X, Van Becelaere G, Kumar P, Davis RF, May OL, Chee P. QTL mapping for resistance to root-knot nematodes in the M-120 RNR upland cotton line (Gossypium hirsutum L.) of the Auburn 623 RNR source. Theoretical and Applied Genetics. 2006;113:1539–1549. doi: 10.1007/s00122-006-0401-4. [DOI] [PubMed] [Google Scholar]
- Young LD. Breeding for nematode resistance and tolerance. In: Barker KR, Pederson GA, Windham GL, editors. Plant nematode interactions. Madison, WI: American Society of Agronomy; 1998. pp. 187–207. [Google Scholar]
- Zhou E, Starr JL. A comparison of the damage functions, root galling, and reproduction of Meloidogyne incognita on resistant and susceptible cotton cultivars. Journal of Cotton Science. 2003;7:224–230. [Google Scholar]