Abstract
Oscillating microbubbles of radius 20–100 μm driven by ultrasound initiate a steady streaming flow around the bubbles. In such flows, microparticles of even smaller sizes (radius 1–5 μm) exhibit size-dependent behaviors: particles of different sizes follow different characteristic trajectories despite density-matching. Adjusting the relative strengths of the streaming flow and a superimposed Poiseuille flow allows for a simple tuning of particle behavior, separating the trajectories of particles with a size resolution on the order of 1 μm. Selective trapping, accumulation, and release of particles can be achieved. We show here how to design bubble microfluidic devices that use these concepts to filter, enrich, and preconcentrate particles of selected sizes, either by concentrating them in discrete clusters (localized both stream- and spanwise) or by forcing them into narrow, continuous trajectory bundles of strong spanwise localization.
INTRODUCTION
Trapping, sorting, and focusing of micron-sized objects such as biological cells, droplets and particles in microfluidic environments is an important preprocessing step in micro total analysis systems (μTAS)1, 2 for single cell detection and diagnostic analysis. Many applications require control over position and concentration of the objects in order to induce mixing,3 chemical reactions,4 biomolecular tagging,5 or a host of other processes. The necessary manipulations of the objects often rely on actively applying various external force fields, including hydrodynamic (inertial),6, 7, 8 electrokinetic,9, 10, 11, 12 dielectrophoretic,13, 14, 15, 16 magnetic,17, 18 optical19, 20 or acoustic21, 22, 23 (ultrasound standing waves, surface acoustic waves) forces. Such forces will usually act differently on particles depending on their size, geometry, and mechanical or electromagnetic properties; as a result, particle separation and sorting become possible. A passive alternative to active forces is the integration of geometric features such as obstacles and side channels into the microfluidic network.24, 25, 26 Particle sensitivity is less obvious here, but size-dependent, directional and selective transports of particles and cells in controlled fashion can be achieved in such setups as well.27, 28 Usually, this requires the introduction of very small structural elements on the order of the particle size.24, 25, 26, 27, 28
Microbubbles, with a size of tens to hundred microns, have been used as powerful actuating elements for microfluidic applications in various contexts.29, 30, 31, 32, 33, 34 Cyclic generation, growth and collapse of vapor bubbles from thermal heating can pump liquid.29 Bubbles from chemical electrolysis are used as active valves.30Ultrasound driven microbubbles rectify rapid oscillatory motion of the air/liquid interface into steady streaming flow in the bulk liquid around the bubbles. Such steady streaming flows are useful in deforming and lysing vesicles,31 directional transport of liquid,32 and enhancing mixing in microfluidics.33, 34 The latter type of bubble streaming takes a unique position in that the bubble interface is doubtless an active element, but its amplitude is usually very small compared with the flow dimensions, so that for the purposes of flow description it is merely a passive boundary, albeit one that adds an important component to the microfluidic flow and thus shapes the flow domain inside the micro devices. Microparticles exhibit size-dependent behaviors in bubble streaming flows and the size-dependent characteristics allow us to manipulate the particles, including trapping and focusing, without either external forces or small-scale structural elements.35 This technique is novel and has a number of advantages over the existing methods, including very simple manufacturing, interactive control, general applicability, and negligible heat generation. Bubble-induced microstreaming had been previously used to trap particles in an H-shaped device with a single bubble for switching and sorting of micro-particles to different outlets.36 In the present work, we strategically place several bubbles along the sides of a rectangular micro-channel to shape the flow and achieve focusing. Specifically, we show that this multi-bubble system can be tuned for optimum focusing. In addition, we discuss how these devices can be used to trap particles continuously for applications such as pre-concentration of particles, filtering, and particle enrichment.
EXPERIMENTAL SETUP
We fabricate the microfluidic devices in Polydimethylsiloxane (PDMS) following standard soft lithography procedures.36 The master molds are made of 100 μm thick SU-8 on silicon wafers using photolithography. A liquid mixture of PDMS (Sylgard 184, Dow Corning) is poured onto the SU-8 molds and is allowed to crosslink for 24 h at room temperature. Fully cured PDMS replicas are peeled off from the molds and bonded to a layer of flat PDMS after oxygen plasma treatment to form a closed microfluidic network. Inlets and outlets are interfaced through 1/32 in. ID tubing (SmallParts Inc) for liquid access. As shown schematically in Fig. 1, a typical device consists of a main channel with a depth of D = 100 μm from the predefined SU-8 thickness and height H = 250 μm in the image plane (top view). One or more blind side channels are positioned perpendicular to the main channel with a typical opening w = 80 μm. When aqueous solution is introduced into the main channel, air is trapped in these blind side channels to form semicylindrical air bubbles protruding into the main channel.35, 37 The device is bonded to a glass slide. A piezoelectric transducer (Physik Instrumente, Germany) is glued on the glass slide to provide ultrasonic excitation using sinusoidal signals from a function generator (model 7075, Hioki) and an amplifier (model 7500, Krohn-Hite). Polystyrene microparticles (Magsphere Inc) are suspended in a density-matched water-glycerol solution (23% gly w/w). Different sizes are used, with the most common being particle radii of ap = 1, 2.5, and 5 μm. The polydispersity of each kind of particle is < 5% (coefficient of variation of the radii). The surfactant Tween 20 (Fisher Scientific) is added at 1% w/w to the liquid solution to prevent sticking of particles to the microchannel walls, as well as to avoid particle aggregation (the concentration of surfactant is well above the critical micelle concentration, so that surface concentrations should remain stable). A syringe pump (PHD Ultra, Harvard Apparatus) imposes a Poiseuille flow at constant flow rate in the main channel. We use a high-speed camera (Phantom v310, Vision Research) to capture images and videos through an inverted microscope (IX71, Olympus). The frame rate of the camera allows both visualization of the fast motion of the oscillating bubble and the resulting streaming flow. In the experiment, light from a halogen source is used to illuminate from the top of the microscope for transmitted light bright-field observation. The issues related to the thermal effects of the light source are negligible. However, the bubble size, which is sensitive to temperature change, is maintained to within± 2% by controlling the external room temperature. We use imagej (Ref. 38) software for particle detection. First, the recorded images are converted to binary images by thresholding. Then, the built-in function “analyze particles” is used to detect the outline and to calculate the center of mass of the particles. The program works well to track isolated particles. When particles touch each other or the boundaries, the program fails to identify the correct size and therefore the particles. In our experiments, the particle concentrations are low enough and frame rates are high enough to ensure that trajectory tracking works well.
Figure 1.
(a) Perspective schematic of experiment setup. Different soft lithography devices can be mounted on the same base (glass slide). (b) Top-view schematic of an elementary setup with one bubble, one inlet (I) and one outlet (O). (c) Placing bubbles on alternate sides of the main channel improves efficiency of the setup.
RESULTS AND DISCUSSION
Microbubble streaming and particles
The set-up is mounted on a microscope glass slide base onto which a piezoelectric transducer is glued, inducing a periodic pressure variation in the liquid at a driving frequency of f = 1 – 100 kHz. Due to its compressibility, the bubble (typically of radius a ≈ w/2 = 40 μm) oscillates at the driving frequency f and with oscillation amplitude , typically in a combination of a dominant volume mode and surface modes.39, 40 The fast oscillatory motion of the bubble surface (liquid/air interface) produces a second order streaming flow with a steady component. When sampling the resulting flows at rates much slower than f, the effective time averaging leaves only this steady flow observable (the amplitude of the fast, oscillatory motion is also very small). The steady streaming around the bubble has two symmetric closed-loop vortices above the bubble, in Fig. 2a, similar to the flows generated from hemispherical bubbles.31, 32 The streaming flow velocity reaches its maximum us near the bubble surface and increases quadratically with the increasing oscillation amplitude (i.e., driving voltage), as shown in Fig. 2c. This conforms to the theoretical expectation that, as a second-order effect in ε, the scale of streaming should be , where ω = 2πf. The best-fit prefactor of the quadratic law shows that dimensional analysis also predicts the prefactor of us to about 15% accuracy (Fig. 2c).
Figure 2.
Streaming flow from a cylindrical microbubble: (a) streak image (f = 16.8 kHz) without superimposed Poiseuille flow; (b) streamlines from RNW singularity theory; (c) streaming velocity scale us at different driving voltages. The prefactor of 0.83 is close to the dimensional-analysis expectation of 1.
Dimensionless oscillation amplitudes of can be easily obtained, resulting in a streaming velocity on the order of mm/s to cm/s. Compared with electrokinetic and electrophoretic methods,11, 12 microbubble streaming is a powerful and efficient way to transport liquid in microfluidic environment. Importantly, the streaming is characterized by the Reynolds number Res ≡ usa/v, where v is the kinematic viscosity (v ≈ 1.8 × 10−6 Pa s for our water/glycerol mixture).41 If , the flow is described as Rayleigh-Nyborg-Westervelt (RNW) streaming,42 i.e., as a Stokes flow far from the bubble. It can then be modeled using the method of images and singularity theory.31, 43 The condition is only met for the smallest of our experimental frequencies and/or small driving voltages. For the most typical frequencies f used in experiment, between 15 kHz and 50 kHz, Res ∼ 1 for , and a quantitative description of the flow needs a more sophisticated theory, essentially involving the full Navier-Stokes equations.44 Nevertheless, RNW theory quite accurately describes qualitative aspects such as the streamline picture, see Fig. 2b.
Finite size particles show size-dependent behavior around the bubble, when they are present in bubble streaming flows. In Fig. 3, an ap = 2.5 μm particle and an ap = 5 μm particle are at almost the same initial position when the bubble is excited. As the particles are transported in the streaming flow, they start to settle to different stable characteristic closed trajectories (loops) around the bubble. The large particle orbits on a smaller trajectory; while the small particle orbits on a larger loop. Though small in size and density-matched, the microparticles are not completely passive tracers. When the particles are far from the bubble, they are able to follow the streamlines like passive tracers. However, near the bubble surface the streamlines are denser. The presence of the bubble restricts the particle’s ability to stay on the same streamline, because the particles cannot penetrate the bubble surface.35 Consequently, particles are forced to move to another streamline, schematically shown in Fig. 4. A further distinction between particles of different sizes is the induction of secondary streaming around a particle exposed to an oscillatory flow field,32 which can contribute to the crossing of trajectories.45 Ideally, particles of the same size have a unique characteristic trajectory in bubble streaming flows.
Figure 3.
Particle trajectories of large and small particles. (a) Initial positions of two different-sized particles; (b) and (c) Resulting closed trajectories, showing that large particles stay closer to the bubble.
Figure 4.
Schematic illustration of characteristic loops of large and small particles.
Shaping flow domain and selective trapping of microparticles
For practical applications, microfluidics often requires throughput through a device. In this subsection, we show how to use microbubble streaming together with a pressure gradient flow driven by a syringe pump (i.e., a Poiseuille flow in rectangular channels) to achieve such throughput and at the same time manipulate particles in these combined flows. The parameter used to regulate the flow is the relative streaming strength, , defined as the ratio of the mean Poiseuille velocity to the streaming velocity us. Fig. 5 shows the evolution of the flow field as s varies. In all images, the Poiseuille flow is directed from right to left (− x direction). When the bubble is not excited (s → ∞), the flow assumes its Poiseuille characteristic with a parabolic velocity profile at the imaging plane. As the streaming velocity increases, the streamlines bend towards the bubble upstream of the bubble and a closed loop region forms downstream of the bubble. With further increase of streaming velocity (smaller s), the streaming flow dominates near the bubble with two vortex loop structures. At the upper edge of the upstream loop, the flow has a hyperbolic point P (Fig. 5d) with an associated critical streamline separating closed (vortex) and open (transport) streamlines. Far upstream, this critical streamline must be horizontal at a height z = h(s). The bubble streaming flow thus shapes the Poiseuille flow domain into two partitions divided by the critical streamline. In the flow region above this critical streamline, the Poiseuille flow is affected little with only slightly bent streamlines. In the flow region below this critical streamline, the open streamlines of the Poiseuille flow have to pass through the gap between the upstream closed loop and the bubble surface. Without introducing moving parts, the flow is thus divided into two domains, one of which experiences an extreme constriction, which is not imposed by a material boundary. By increasing the streaming velocity (smaller s), this constricted flow domain passing through the gap increases.
Figure 5.
Flow field of combined bubble streaming and main-channel Poiseuille flow (Poiseuille flow from right to left): (a) bubble is not excited; (b) s = 0.17; (c) s = 0.043; (d) s = 0.021; (e) s = 0.014; (f) schematic detail of the boxed region of (d).
When particles are introduced to the combined flow, we observe selective trapping of microparticles. In Fig. 6, at larger s, both the large and small particles pass by the bubble without being trapped; at smaller s, the large particle is trapped around the bubble. The trapping mechanism can be explained from a geometry argument:35 Continuity requires that the streamlines below the critical streamline with flow rate must pass through a narrow gap of width dgap between the bubble surface and the critical streamline. The Poiseuille flow is here given by using plane Poiseuille flow approximation. The factor of 6 for plane Poiseuille flow is slightly modified by the finite cross-sectional area of the channel, but the plane Poiseuille flow formula is a very good approximation. The smaller the parameter s, the larger is h, and the greater a fraction of the entire channel flow is funneled through the gap. Since the particles cannot penetrate the bubble, particles with radius ap > dgap will be pushed away from the bubble and into the vortex—these particles are then trapped. The velocity within the narrow gap is close to uniform, ugap ≈ us, so that Q = usdgap. Equating this to the Poiseuille flow rate to height h yields a theoretical prediction for the gap width, dgap = sh[3(h/H) − 2(h/H)2], which equals the predicted critical particle radius for trapping. We have shown in previous work35 that this prediction holds quantitatively true for typical particle sizes in our experiments.
Figure 6.
Trajectories of large and small particles at different s: (a) s = 0.031; (b) s = 0.022 (f = 23.1 kHz).
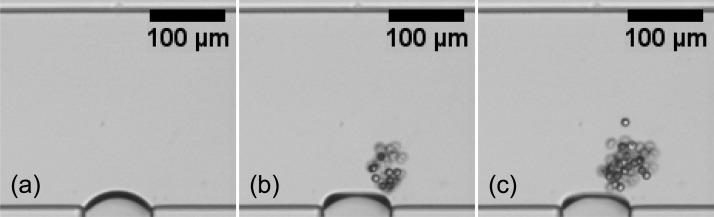
As the Poiseuille flow transports more particles towards the bubble, the bubble can continuously trap particles of size ap > dgap into a cluster, see Fig. 7. However, if too many particles are trapped in this fashion, particle-particle interactions eventually lead to the expulsion of particles from this cluster. The release of these particles does not occur at random positions of the loop trajectory, but only from a very narrow region.35 Therefore, the released particles follow a narrowly focused bundle of trajectories downstream. Previously, we have showed size-based switching and sorting of microparticles using this phenomenon by integrating a single bubble into a H-shaped microfluidic device.35 Here, we demonstrate other applications: preconcentrating and focusing of microparticles in Sec. 4.
Figure 7.
Accumulation of microparticles around an oscillating bubble. Snapshots (b) and (c) are taken 2 s and 4 s after (a), respectively.
APPLICATIONS
Preconcentrating/filtering/enrichment of microparticles
One direct application based on the trapping of selected-size particles is enrichment or preconcentrating of these microparticles. Our goal here is to avoid release of trapped particles and instead use the enrichment of particle concentration present in the clusters for purposes of preconcentration of the trapped species (and thus filtering with respect to undesired smaller species of particles). For this purpose, we use a programmable function generator to excite the bubbles periodically, i.e., with a certain duty cycle. A multi-bubble setup with a total of five microbubbles placed alternately on the sides of the main channel is used for this application. During the times when the bubbles are excited, the bubbles trap clusters of particles. Upon deactivation of the bubbles, these trapped particles are released all at once and carried downstream by the Poiseuille flow. In this way, the particles are enriched and dispatched downstream periodically. Fig. 8 shows the number of particles per time going through a view window downstream of the bubbles under different excitation cycles. The pattern is chosen as (τon;τoff), where the ultrasound is turned on for the duration τon, then remains off for τoff, and then the pattern repeats. Fig. 8 combines results of experiments with (10 s;10 s), (20 s;10 s), and (30 s;10 s). When the bubbles are not excited, the average number of particles per second is about 5. The release of the accumulated particles results in peaks of 10-40 times of this background average. The peak value increases with increasing time of trapping as more particles are trapped during the excitation cycle. By integrating more bubbles and increasing the trapping time, the enrichment can be further increased. Directly after the peak, for a duration of ≈ τoff, a background signal can be observed. Enrichment of cells or other micron-sized objects can be accomplished in this fashion, yielding discrete clusters or bunches of particles localized both in x (the downstream direction) and z (the spanwise direction). After such preconcentration, the application of reactants or particle probes can be done much more efficiently.46 As particles smaller than the critical size are passed through the device, a simultaneous filtering effect is also in evidence.
Figure 8.
Preconcentration of microparticles: (a) snapshot of the 5-bubble setup. The traces show the number of particles per time detected downstream of the last of five bubbles. The duty cycles are given by (τon;τoff) values of (b) (10 s;10 s); (c) (20 s;10 s); (d) (30 s;10 s).
Focusing of microparticles
In contrast to the previous section, we make explicit use of the release of trapped particles here. Because of the narrow width of the released trajectory bundles, the trajectories that far from a bubble span the interval (0, h) in the z-direction are now confined to a much narrower interval after having been trapped. A simple modification to the setup can likewise focus those particles (at initial positions in the interval (h, H)) that have not been trapped in this first step: Positioning side channels (and thus bubbles) at equally-spaced distances alternatingly on the two walls of the main channel ensures that the entire width H of the channel is subject to the trapping and focused release. The overall result is that the bubbles effectively force the particles to flow in a fraction of the channel, enforcing continuous concentration of particles in the z-direction. Fig. 9 shows streak images at different streaming strength as well as the normalized histogram of particle position in z across the channel width. In fact, for a given particle size, the focusing of particles happens in two stages: In the first stage (weaker streaming velocity, i.e., larger s), the particle size is smaller than the critical (gap) size, and particles are not trapped. Nevertheless, see Fig. 9b, the bubbles compress the streamlines to a narrower region than the whole channel width to effect a limited focusing of the particles. In the second stage, s becomes small enough for the particle size to be larger than dgap: the particles are trapped and released into a narrow bundle of trajectories, see Figs. 9f, 9g. The transition from the first stage to the second happens between Figs. 9e, 9f (i.e., s between 0.052 and 0.041).
Figure 9.
Focusing of microparticles (ap = 5 μm) in microchannel: (a) bubbles are not excited, s = ∞; (b) s = 0.23; (c) s = 0.092; (d) s = 0.066; (e) s = 0.051; (f) s = 0.043; (g) s = 0.029.
As is evident from the histograms, upon decreasing s the focusing ability improves dramatically just before the trapping of particles sets in, with the eventual trajectory bundles reaching z-widths as small as 2Δz ≈ 5.4ap, where Δz is the standard deviation of z-coordinates in the trajectory bundle exiting the field of view. As preconcentration or prepositioning of particles at this level is, again, very helpful for applications where cells are supposed to be transfected or tagged by contact with an agent that is only available or affordable in very small quantities. As s decreases towards zero, the width Δz is limited by the increased violence of particle-particle interactions (whose scale is set by the bubble streaming flow strength), which enables the release of particles from a wider region, thus widening the bundle of allowed escape trajectories. We observe an optimal s of ≈ 0.05 (in Table TABLE I.), which can be achieved through driving the piezo transducer at 70 Vrms with a Poiseuille flow of , well within the range of parameters accessible in our setup, as well as in a practical device.
TABLE I.
Experimental mean position and standard deviation Δz of the microparticle trajectory bundles.
| s | ∞ | 0.24 | 0.094 | 0.068 | 0.052 | 0.041 | 0.034 |
| Mean position (μm) | 116.6 | 119.6 | 122.2 | 125.4 | 136.7 | 164.2 | 184.3 |
| Standard deviation Δz (μm) | 50.6 | 50.6 | 29.2 | 18 | 13.5 | 18.1 | 18 |
Focusing simulations
In order to investigate the continuous focusing mechanism further, we have employed numerical simulations to study the process. The bubble streaming flow was modeled through Stokes flow singularities in the RNW formalism, and the Poiseuille flow superimposed through addition, consistent with the low- Res approximation of this theory. The finite channel height, H, and multiple bubbles on either sides of the channel were simulated by the method of images.31, 43 While the experimental situation depicted in Figure 9 is characterized by Res = 0.6 at optimal focusing, the RNW theory (which is strictly valid for ) will show some quantitative shortcomings. However, the mechanism of focusing that is observed in the experiments can be understood using these simulations.
To simulate random perturbations to the particles, we add velocity perturbations at every time step, with randomly chosen direction and a magnitude proportional to the local velocity. Hence, the velocity of a particle (u) at a given time is given by , where, uf is the unperturbed velocity of the particle, α is the strength of the perturbation, and is a unit vector pointed at a random angle. In our simulations, we have used two bubbles on opposite sides of the channel of height H = 6.25 a and separated by a distance 12 a. Approximately 50-60 particles all having radius ap = 0.125 a (corresponding to ap = 5 μm particles in the experiments) and evenly distributed across the height of the channel were released one at a time from a distance of 5 bubble radii upstream of the first bubble. The only interactions considered between particles themselves are the random velocity perturbations described above. The numerical algorithm also ensures that the impenetrability condition of the bubble surface is enforced by repositioning any particle colliding with the bubble.
Once the particles reach a distance of 15 radii downstream of the second bubble, the z coordinate values of the particles are used to compute the standard deviation Δz. The variation of Δz with s is compared with that from the experiments in Fig. 10. The simulations capture the essential character of the Δz versus s curve, which has a minimum for a non-zero s, and is observed for a very similar value of s as in the experiments. We have chosen α = 2 in our simulations to best illustrate the focusing mechanism. Varying α around 2 only changes the magnitude of Δz but retains the value of optimum s. This indicates that the effect of focusing is due to the intrinsic nature of the bubble streaming and does not significantly depend on the magnitude of the perturbations.
Figure 10.
Comparison of the particle focusing as a function of s between experiments and simulations for ap = 5 μm, H = 250 μm, and a = 40 μm. Both experiments and simulations show a finite value of s ≈ 0.05 for optimum focusing.
From our simulations, we note that the criterion for optimum focusing is when all the particles hitting the first bubble also hit the second bubble and escape into the Poiseuille flow. This can occur if the critical streamline that passes close to the first bubble to within a distance of ap also has the closest approach of ap to the second bubble. From our computations, we find that this condition is satisfied for s = 0.049, which is in very good agreement with the values of optimum s seen in the experiments and simulations (Fig. 10). Similar to the experimental observations, even in the simulations we see that below the optimum value of s, the perturbations kick the particles into streamlines that diverge more and more with decreasing s, and hence result in a decrease in the quality of focusing.
CONCLUDING REMARKS
We have shown how to use the ability of bubble microstreaming to manipulate microparticles in two qualitatively different ways to effect spatial concentration of the particles, for purposes of enrichment and processing in microfluidic devices. Using the trapping abilities of the individual bubbles, we were able to accumulate the particles in discrete clusters around the bubbles, which could then be “read out” of the device as enriched bunches of enhanced particle concentration. Using the well-controlled release from trapping, we find a continuous enrichment in the spanwise direction from the property of narrow bundling of particle escape trajectories. The latter process lacks focusing in the streamwise direction (or, equivalently, in time) but reaches much greater alignment in the spanwise direction, down to bundle widths on the order of the particle diameter. In this fashion, the second method of continuous focusing is also potentially applicable for particle positioning, as the position of the bundle changes with the flow parameters.
The applications demonstrated in the present work highlight the importance of characteristic flow geometry for particle manipulation, a general principle behind bubble microstreaming flows: not only does the location of the critical streamline (Fig. 5) determine the critical particle size for trapping, but the setup with alternating bubbles on both sides of the main channel relies on the location of the respective critical streamlines for the desired focusing effect. As the streamline portrait can be easily and quickly changed through changes in ultrasound driving amplitude and/or frequency, interactive modification of the setup is an additional potential feature of such devices, adding an active component to control on top of the passive driving of the bubble-induced flow. We have demonstrated one instance of such interactive control through particle enrichment (where the control is a selection of “on” and “off” duty cycles), but a variety of more sophisticated control patterns can be envisioned. Bubble microstreaming thus offers a versatile toolbox, whether focusing on a simple passive device with no need for feedback, or on an active device with feedback for immediate selection of a desired flow.
References
- Andersson H. and van den Berg A., Sens. Actuators, B 92, 315 (2003). 10.1016/S0925-4005(03)00266-1 [DOI] [Google Scholar]
- Dittrich P. S., Tachikawa K., and Manz A., Anal. Chem. 78, 3887 (2006). 10.1021/ac0605602 [DOI] [PubMed] [Google Scholar]
- El-Ali J., Gaudet S., Günther A., Sorger P. K., and Jensen K. F., Anal. Chem. 77, 3629 (2005). 10.1021/ac050008x [DOI] [PubMed] [Google Scholar]
- DeMello A. J., Nature (London) 442, 394 (2006). 10.1038/nature05062 [DOI] [PubMed] [Google Scholar]
- Buranda T., Huang J., Perez-Luna V. H., Schreyer B., Sklar L. A., and Lopez G. P., Anal. Chem. 74, 1149 (2002). 10.1021/ac0109624 [DOI] [PubMed] [Google Scholar]
- Di Carlo D., Edd J., Irimia D., Tompkins R., and Toner M., Anal. Chem. 80, 2204 (2008). 10.1021/ac702283m [DOI] [PubMed] [Google Scholar]
- Wu Z., Willing B., Bjerketorp J., Jansson J., and Hjort K., Lab Chip 9, 1193 (2009). 10.1039/b817611f [DOI] [PubMed] [Google Scholar]
- Tanyeri M., Johnson-Chavarria E. M., and Schroeder C. M., Appl. Phys. Lett. 96, 224101 (2010). 10.1063/1.3431664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrum D., Culbertson C., Jacobson S., and Ramsey J., Anal. Chem. 71, 4173 (1999). 10.1021/ac990372u [DOI] [PubMed] [Google Scholar]
- Fu L.-M., Yang R.-J., Lin C.-H., Pan Y.-J., and Lee G.-B., Anal. Chim. Acta 507, 163 (2004). 10.1016/j.aca.2003.10.028 [DOI] [Google Scholar]
- Bazant M. Z. and Squires T. M., Phys. Rev. Lett. 92, 066101 (2004). 10.1103/PhysRevLett.92.066101 [DOI] [PubMed] [Google Scholar]
- Kang Y. and Li D., Microfluid. Nanofluid. 6, 431 (2009). 10.1007/s10404-009-0408-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K., Kerbage C., Hunt T., Westervelt R., Link D., and Weitz D., Appl. Phys. Lett. 88, 024104 (2006). 10.1063/1.2164911 [DOI] [Google Scholar]
- Lewpiriyawong N., Yang C., and Lam Y. C., Biomicrofluidics 2, 034105 (2008). 10.1063/1.2973661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommer M., Zhang Y., Keerthi N., Chen D., Thomson J., Meinhart C., and Soh H., Electrophoresis 29, 1213 (2008). 10.1002/elps.v29:6 [DOI] [PubMed] [Google Scholar]
- Valero A., Braschler T., Demierre N., and Renaud P., Biomicrofluidics 4, 7 (2010). 10.1063/1.3430542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijs M., Microfluid. Nanofluid. 1, 22 (2004). [Google Scholar]
- Pamme N. and Wilhelm C., Lab Chip 6, 974 (2006). 10.1039/b604542a [DOI] [PubMed] [Google Scholar]
- MacDonald M., Spalding G., and Dholakia K., Nature (London) 426, 421 (2003). 10.1038/nature02144 [DOI] [PubMed] [Google Scholar]
- Glückstad J., Nature Mater. 3, 9 (2004). 10.1038/nmat1041 [DOI] [PubMed] [Google Scholar]
- Nilsson A., Petersson F., Jönsson H., and Laurell T., Lab Chip 4, 131 (2004). 10.1039/b313493h [DOI] [PubMed] [Google Scholar]
- Tan M. K., Friend J. R., and Yeo L. Y., Lab Chip 7, 618 (2007). 10.1039/b618044b [DOI] [PubMed] [Google Scholar]
- Franke T., Abate A. R., Weitz D. A., and Wixforth A., Lab Chip 9, 2625 (2009). 10.1039/b906819h [DOI] [PubMed] [Google Scholar]
- Yang X., Yang J., Tai Y.-C., and Ho C.-M., Sens. Actuators, A 73, 184 (1999). 10.1016/S0924-4247(98)00269-6 [DOI] [Google Scholar]
- Huang L., Cox E., Austin R., and Sturm J., Science 304, 987 (2004). 10.1126/science.1094567 [DOI] [PubMed] [Google Scholar]
- Yamada M. and Seki M., Lab Chip 5, 1233 (2005). 10.1039/b509386d [DOI] [PubMed] [Google Scholar]
- Davis J., Inglis D., Morton K., Lawrence D., Huang L., Chou S., Sturm J., and Austin R., Proc. Nat. Acad. Sci. U.S.A. 103, 14779 (2006). 10.1073/pnas.0605967103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H., Samper V., Chen Y., Heng C., Lim T., and Yobas L., Biomed. Microdevices 10, 251 (2008). 10.1007/s10544-007-9131-x [DOI] [PubMed] [Google Scholar]
- Yin Z. and Prosperetti A., J. Micromech. Microeng. 15, 643 (2005). 10.1088/0960-1317/15/3/028 [DOI] [Google Scholar]
- Hua S. Z., Sachs F., Yang D. X., and Chopra H. D., Anal. Chem. 74, 6392 (2002). 10.1021/ac0259818 [DOI] [PubMed] [Google Scholar]
- Marmottant P. and Hilgenfeldt S., Nature (London) 423, 153 (2003). 10.1038/nature01613 [DOI] [PubMed] [Google Scholar]
- Marmottant P. and Hilgenfeldt S., Proc. Natl. Acad. Sci. U. S. A. 101, 9523 (2004); 10.1073/pnas.0307007101 [DOI] [PMC free article] [PubMed] [Google Scholar]; Marmottant P., Raven J. P., Gardeniers H., Bomer J. G., and Hilgenfeldt S., J. Fluid Mech. 568, 109 (2006). [Google Scholar]
- Liu R. H., Yang J., Pindera M. Z., Athavale M., and Grodzinski P., Lab Chip 2, 151 (2002). 10.1039/b201952c [DOI] [PubMed] [Google Scholar]
- Wang S., Jiao Z., Huang X., Yang C., and Nguyen N. T., Microfluid. Nanofluid. 6, 847 (2009). 10.1007/s10404-008-0357-6 [DOI] [Google Scholar]
- Wang C., Jalikop S., and Hilgenfeldt S., Appl. Phys. Lett. 99, 034101 (2011). 10.1063/1.3610940.1 [DOI] [Google Scholar]
- McDonald J. C., Duffy D. C., Anderson J. R., Chiu D. T., Wu H., Schueller O. J. A., and Whitesides G. M., Electrophoresis 21, 27 (2000). [DOI] [PubMed] [Google Scholar]
- Ahmed D., Mao X., Juluri B. K., and Huang T. J., Microfluid. Nanofluid. 7, 727 (2009). 10.1007/s10404-009-0444-3 [DOI] [Google Scholar]
- Abramoff M. D., Magelhaes P. J., and Ram S. J., Biophotonics Int. 11, 36 (2004). [Google Scholar]
- Brennen C. E., Cavitation and Bubble Dynamics (Oxford University Press, Oxford, 1995). [Google Scholar]
- Desai S. (unpublished).
- See http://www.dow.com/glycerine/resources/table18.htm for viscosity of aqueous glycerine solutions of different concentrations.
- Lighthill J., J. Sound Vib. 61, 391 (1978). 10.1016/0022-460X(78)90388-7 [DOI] [Google Scholar]
- Pozrikidis C., Boundary Integral and Singularity Methods for Linearized Viscous Flow (Cambridge University Press, Cambridge, 1992). [Google Scholar]
- Riley N., Mathematika 12, 161 (1965). 10.1112/S0025579300005283 [DOI] [Google Scholar]
- Jalikop S., Wang C., and Hilgenfeldt S., Preprint (2011).
- Lin C.-C., Hsu J.-L., and Lee G.-B., Microfluid. Nanofluid. 10, 481 (2010). 10.1007/s10404-010-0661-9 [DOI] [Google Scholar]