Abstract
This report studies the surface modification of cyclic olefin copolymer (COC) by 2-methacryloyloxyethyl phosphorylcholine (MPC) monomer using photografting technique for the purpose of biointerface applications, which demonstrate resistance to both protein adsorption and cell adhesion in COC-based microfluidic devices. This is essential because the hydrophobic nature of COC can lead to adsorption of specific compounds from biological fluids in the microchannel, which can affect the results during fluidic analysis and cause clogging inside the microchannel. A correlation was found between the irradiation time and hydrophobicity of the modified substrate. Static water contact angle results show that the hydrophilicity property of the MPC-grafted substrate improves with increasing irradiation time. The contact angle of the modified surface decreased to 20 ± 5° from 88 ± 3° for the untreated substrate. The surface characterization of the modified surface was evaluated using x-ray photoelectron spectroscopy (XPS) and Fourier transform infrared spectroscopy (FTIR spectroscopy). Attenuated total reflection-FTIR and XPS results show the presence of the phosphate group (P-O) on modified COC substrates, indicating that the hydrophilic MPC monomer has successfully grafted on COC. Finally, it was demonstrated that cell adhesion and protein adsorption on the MPC modified COC specimen has reduced significantly.
INTRODUCTION
Polymer based microfluidic devices have been widely used in lab-on-chip application due to their low cost, disposability, availability of a wide range of materials with different physical properties and flexibility in the choice of micro-manufacturing method. There are a large number of polymeric materials, e.g., poly(methyl methacrylate) (PMMA),1 polycarbonate (PC),2 polydimethylsiloxane (PDMS),3 cyclic olefin polymers (COP),4 and cyclic olefin copolymers (COCs),5 which are available for fabrication of microfluidic devices. The COCs are of special interest because they possess some unique properties when compared to PDMS, PMMA, and PC.6, 7, 8 COC is a thermoplastic copolymer consisting of ethylene and norbornene using a metallocene catalyst. Through its characteristic molecular structure and superior catalyst technology, COC offers a wide range of grade variations, in terms of flow properties and heat resistance, such as TOPAS-8007 (Tg = 78 °C), TOPAS-5013 (Tg = 130 °C), TOPAS-6015 (Tg = 160 °C), and TOPAS 6017 (Tg = 130 °C). As a result, depending on the specific application, an appropriate substrate material can be selected for the microdevice. Thus, TOPAS 6015 and TOPAS 6017 would be more suitable for polymerease chain reaction (PCR) devices requiring higher heat resistant material. Moreover, due to the olefinic character, COC has good resistance to hydrolysis, acids, and alkalis and most organic polar solvents such as acetone, methanol, and isopropyl alcohol. COC is easier to emboss, which is especially important when using soft PDMS tools because the density of COC is lower than that of PMMA. The water absorption of COC is <0.01%, which is 10 times less than that of PMMA. Thus, the relative humidity changes in the environment will not significantly affect devices replicated in COC. Moreover, COC material exhibit high transparency to light with wavelengths above 300 nm and has low autofluorescence. Access to phase and fluorescent imaging is one of the big advantages of using microfluidic devices for cell culture. The transparency of COC to UV-light makes it an interesting material for lab-on-a-chip systems designed for bio-detection using integrated circuits.
Although COC based microfluidic devices have been widely used, some limitations exist. Devices made up of COC are not optimal for studies involving pharmaceuticals because of its hydrophobic surface, which makes it prone to spontaneous nonspecific protein adsorption and cell adhesion when exposed to biological tissues or fluids. The concentration of a specific molecule cannot be determined exactly if the device material absorbs small molecules, and protein adsorption on devices can result in inaccurate signaling or cell differentiation. In some instances, nonspecific protein/cell adsorption can be detrimental to device performance, such as in the case of invivo biosensor and cardiovascular stent surfaces.9, 10
In order to minimize adsorption of analytes such as proteins and to reduce the adhesion of cells, it is necessary to chemically modify the COC surfaces. The wide variety of polymer surface modification techniques that have been developed are covered in several excellent reviews.11, 12, 13, 14, 15 While a few other methods have been used,16, 17, 18, 19 modification of COC surfaces has primarily been accomplished using photografting.20, 21, 22, 23, 24 Photografting is typically initiated by a photoinitiator, such as benzophenone (BP); UV excitation promotes abstraction of hydrogen atoms from the polymer surface, leading to the formation of surface-bound radicals that may then initiate a surface graft polymerization process.25 During photografting, suitable graft monomers can be used to modify the polymer surface to suit the required end application without changing the properties of the polymer substrates.
Ishihara et al. have investigated the preparation of novel surfaces on polyethylene membrane, which demonstrated resistance to both protein adsorption and cell adhesion.26, 27, 28 The fundamental concept was inspired by biomembrane surfaces, which are mainly constructed from neutral phospholipids which contain phosphorylcholines. The surface of phospholipids assemblies, such as liposomes, is quite inert to biological systems and proteins, and cells only interact with them mildly.29 In order to make biomembrane-like surfaces on the substrate, they prepared many polymers consisting of 2-methacryloyloxyethyl phosphorylcholine (MPC) units, which have a phosphorylcholine group in the side chain.30, 31, 32 The MPC polymers coated on to the medical devices could suppress serious biological reactions even when they were in contact with living organisms.28
Using this concept, in this paper, we attempted to modify the surface of COC based microfluidic devices using UV-photografting technique by incorporating MPC monomers containing neutral phospholipids (i.e., phosphorylcholines), which can reduce protein adsorption as well as cell adhesion and increase the surface hydrophilicity. The COC surfaces were modified using the sequential photografting technique with covalent attachment of the initiator. The photografted surfaces were characterized and evaluated for their ability to resist protein adsorption and cell adhesion.
EXPERIMENTAL SECTION
Materials and chemicals
In this study, an amorphous COC, namely, commercially available TOPAS-8007; Tg = 78 °C from TICONA was used. Rectangular TOPAS-8007 samples of 1 mm thickness, 25 mm width, and a length of 75 mm were molded using an injection molding machine (Battenfeld HM 25/60). The water-soluble monomer MPC (molar mass: 295.27 g/mol) with 96% purity was used without purification. The chemically pure BP photoinitiator was used as received. FITC-BSA (fluorescein isothiocyanate labelled bovine serum albumin) was purchased from Sigma–Aldrich, Singapore. Acetone (reagent grade) was used as solvent. All chemicals were obtained from (Sigma–Aldrich, Singapore).
Molding parameters
For the preparation of 1 mm thick COC substrate, the molding parameters such as nozzle temperature injection speed and injection pressure were optimized for part quality and cycle time. The temperatures for the back, middle, and front section of the screw cylinder were set at 230 °C, 240 °C, and 250 °C, respectively, and the nozzle temperature was 250 °C. An injection pressure of 120 MPa and an injection speed of 100 mm/s were used.
Microfabrication
Capillary electrophoresis microchip containing microchannels that were 100 μm wide and 37 μm high (aspect ratio around 3) were embossed in the injection molded COC substrate using a silicon die. The silicon die was prepared using the DRIE technique that is commonly used in micro electro-mechanical systems (MEMS) processing.33 Prior to embossing, the polymer substrates were annealed at 90 °C for 1 h so as to reduce the residual stresses. Just before embossing, the substrates were cleaned using acetone and then dried at ambient temperature to remove any surface contamination. The embossing was carried out at 95 °C at an embossing pressure of 2.94 MPa with a holding time of 4 min using a Specac hydraulic press. Details of the optimum conditions for microembossing on TOPAS are outside the scope of the present work, and the details are presented elsewhere.34
MPC graft polymerization
The smaller 1 cm × 1 cm substrates were used for experiments to optimize the photografting processing parameters such as MPC monomer concentration, initiator concentration, and the irradiation time. This was because it was easier to handle smaller substrates in the optimization experiments. Moreover, the smaller substrates could readily be used in characterization studies using x-ray photoelectron spectroscope (XPS) and cell adhesion study. Once the parameters were optimized, the larger size embossed microfluidic substrate with microchannels was utilized in the ensuing studies. TOPAS-8007 specimens (measuring 1 cm by 1 cm) were immersed in an acetone solution containing 0.2 mol/L benzophenone for 20 min after which they were dried in the dark at room temperature to remove acetone. The MPC was dissolved in degassed pure water to attain concentrations ranging from 0.1 to 0.4 mol/L. Subsequently, the COC specimens that had first been coated with benzophenone were immersed in the aqueous MPC solutions. Photo-induced graft polymerization on the COC surface was performed using UV lamp (Techno Digm, Singapore, λ = 365 nm) with an irradiation intensity of 130 mW/cm2 for 10–30 min. After polymerization, the MPC-grafted COC substrates were removed, washed with pure water and ethanol, and dried at room temperature.
The extent of grafting, in μg/cm2, was expressed as the weight increase per surface area of the sample and was calculated from the following equation:
Extent of grafting = (Wg − W0)/S, where Wg and W0 are the weights of the Topas sample after and before photografting, and S is the surface area of sample.
Surface characterizations
A Perkin-Elmer GX Fourier transform infrared spectroscope (FTIR) with an ATR (attenuated total reflection) unit was utilized to investigate the chemical changes between the untreated and surface grafted substrate. It was used to confirm the attachment of functional group such as carboxylic ester and phosphate groups on the MPC grafted Topas surface. The FTIR-ATR resolution was 4 cm−1, and 32 scans were carried out for each treated sample.
The wettability of the surfaces were characterized by static water contact angles that were determined using a video-based FTA 200 Series contact angle apparatus (with FTA32 video 2.0) with 1 μL of DI water deposited programmatically onto the polymer surface. For each sample, at least six different spots were measured, and the data were averaged.
The atomic composition of the unmodified and grafted TOPAS surfaces was analyzed using an XPS. A Kratos Ultra XPS system, equipped with a monochromatic Al Kα x-ray source (1486.6 eV) operated at 150 W (15 kV, 10 mA) and a concentric hemispherical electron energy analyzer, was used. The samples were mounted on the standard sample holder using double-sided adhesive tape. The pressure in the analysis chamber was maintained at or below 3.0 × 10−9 Torr during the measurements. The core-level spectra were obtained at a photoelectron take-off angle of 90° measured with respect to the plane of the sample surface. All binding energies (BEs) were referenced to the neutral C 1 s peak at 285.0 eV to compensate for surface charging effects. The atomic concentrations of these elements were determined by their corresponding peak areas. Curve fitting of the raw XPS data was performed using kratos vision software to determine the relative atomic concentrations of surface carbon (C), oxygen (O), phosphorus (P), and nitrogen (N).
The AFM setup of the Nanoscope III Scanning Probe Microscope, California, was used in tapping mode to study the roughness of the unmodified and modified COC substrate under ambient atmospheric condition with a scanning rate of 0.7 Hz and a scan area of 10 μm × 10 μm.
Cell adhesion
Mouse 3T3 fibroblasts (ATCC, USA) were used for the cell adhesion on the modified and unmodified COC substrate surfaces. Light microscopy was employed to observe cell adhesion of unmodified and MPC grafted surfaces. After dissociation, a cell suspension with cell concentration of 5 × 104 cells/mL was initially plated on unmodified and modified substrates and incubated at 37 °C overnight to determine their propensity for mediating cell adhesion. After 24 h, unattached cells were removed, and plates were washed with a phosphate buffered saline (PBS), fixed in 10% formaldehyde, and imaged by phase-contrast microscopy.
Protein adsorption
To measure the nonspecific adsorption of protein, invitro protein adsorption experiments were conducted in a PBS solution. Prior to adsorption, the unmodified and modified samples were hydrated in PBS for 6 h. The surfaces were then exposed to 1 mg/ml of FITC-BSA for 1 h at 37 °C. The samples exposed to FITC-BSA were then rinsed by fresh PBS for 3–5 times and dried at room temperature for 4 h. After that the surfaces were observed using a fluorescent microscope and analyzed the fluorescence intensity using imagej software; the fluorescence intensities were quantified as pixel intensities from the 8-bit images. The relative fluorescent intensity of the adsorbed protein on the grafted surface was compared with the unmodified COC sample.
RESULTS AND DISCUSSION
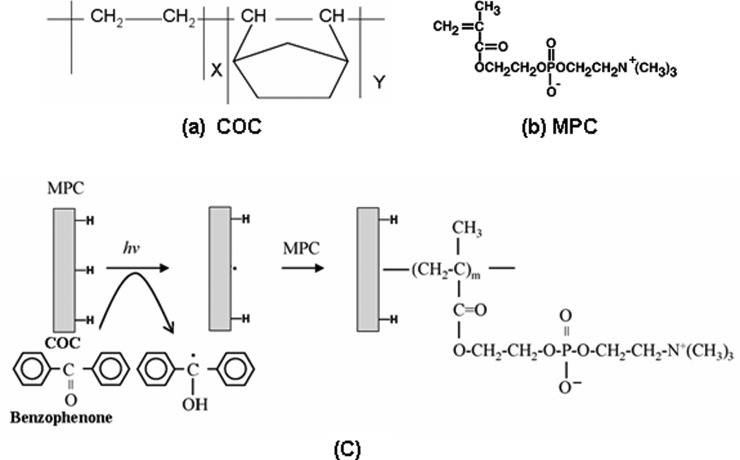
The reaction mechanism of MPC graft polymerization on COC is illustrated in Fig. 1. At first, the physically adsorbed BP on the COC surface was excited by UV irradiation to the triplet state. Then, it extracts a hydrogen atom from the COC surface to generate a radical that is capable of initiating the graft polymerization of MPC. Thus, sequential graft polymerization was achieved. As the extent of BP that is activated depends on the duration of irradiation, the grafting efficiency mainly depends on the UV irradiation time. Grafting efficiency also depends on other factor such as solvent, the type of monomer, photoinitiator used, and the distance between the sample holder and UV light source.
Figure 1.
Chemical structure of (a) COC, (b) MPC, and (c) schematic representation of the MPC grafting on COC, i.e., MPC solution containing benzophenone coated COC when exposed to UV light generate a free radical on the COC substrate by abstraction of H-atom from the substrate surface by the benzophenone followed by free radical polymerization of MPC.
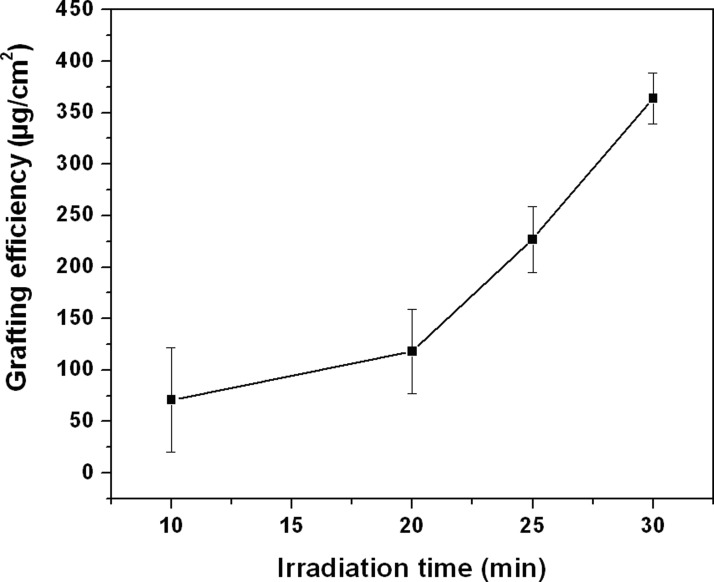
Fig. 2 shows the effect of irradiation time on the grafting efficiency of MPC onto the Topas substrate for irradiation times from 10 to 30 min at a fixed concentration of monomer (0.2 mol/L) and initiator (0.2 mol/L). It is apparent from Fig. 2 that with increase in irradiation time, the grafting efficiency increases. This may be due to the increase in number of excited triplet state electron of benzophenone, which initiates more free radical reactive sides on the COC surface. As a result, more MPC monomer can easily be attached on the COC surface.
Figure 2.
Effect of irradiation time on the extent of grafting on COC substrate [monomer concentration = 0.2 mol/L and initiator (BP) = concentration 0.2 mol/L].
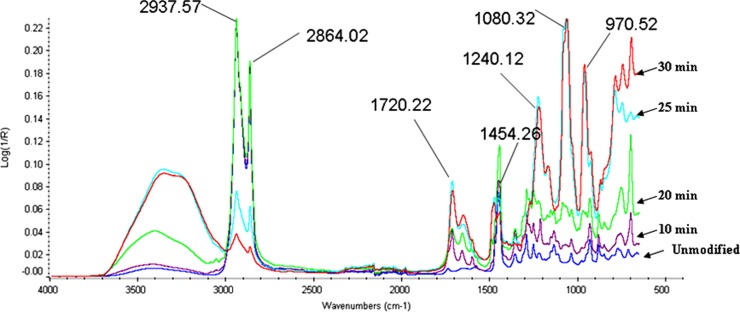
In order to confirm the attachment of the functional groups on the COC surface, ATR FTIR was performed. Fig. 3 shows the FTIR spectra of MPC grafted COC substrate for varying irradiation times compared to the unmodified COC. For the unmodified COC, the observed absorption peaks in the range 2937–2864 cm−1 for CH stretching and the peaks around 1454 cm−1 for bending mode are in good agreement with previous reports.35 For modified substrate, it was observed that no change in absorption peaks was obtained within the irradiation time up to 9 min. However, after irradiation times of 10, 20, 25, and 30 min, absorption peaks could be observed for MPC modified COC surfaces and strong absorption peak was found above 25 min of irradiation.
Figure 3.
IR spectra of the original TOPAS substrate (unmodified) and MPC-grafted TOPAS substrates photografted using varying irradiation times [monomer concentration = 0.2 mol/L and BP concentration = 0.2 mol/L].
The absorption peaks at 1240, 1080, and 970 cm−1 were observed in MPC modified COC substrate (see Fig. 3). These peaks correspond to the phosphate group in the MPC. The absorption peak at 1720 cm−1 observed in the MPC modified COC substrate corresponds to the carbonyl group of the carboxylic ester group. These additional absorption peaks, which correspond to the carboxylic ester group and phosphate group in the MPC modified COC substrate confirmed the presence of MPC unit on the modified COC substrate. It is apparent that the MPC monomer was successfully grafted onto the TOPAS surface.
XPS analysis was carried out to further investigate the attachment of MPC monomer on to the COC surface. XPS provides both quantitative and qualitative information regarding the elements that exist at depths within ≤10 nm on a polymeric surface.36 Table TABLE I. shows the elemental compositions on the MPC-grafted COC substrate at takeoff angles of 90° determined by peak areas of C1s, O1s, N1s, and P2p. The existence of N and P elements were confirmed on the grafted surface only. The higher compositions of N and P were due to an increase in the amount of graft polymer.
TABLE I.
XPS analysis of elemental composition for untreated Topas and MPC grafted Topas substrates with varying irradiation time [monomer concentration 0.2 mol/L and initiator (BP) concentration 0.2 mol/L].
| Irradiation time (min) | C 1s (at. %) | O 1s (at. %) | P 2p (at. %) | N 1s (at. %) |
|---|---|---|---|---|
| 0 (unmodified) | 98.88 | 1.22 | 0.00 | 0.00 |
| 10 | 76.79 | 21.15 | 0.72 | 1.35 |
| 20 | 74.65 | 21.63 | 1.12 | 2.49 |
| 25 | 74.74 | 21.97 | 1.26 | 2.53 |
| 30 | 73.31 | 22.42 | 1.58 | 2.69 |
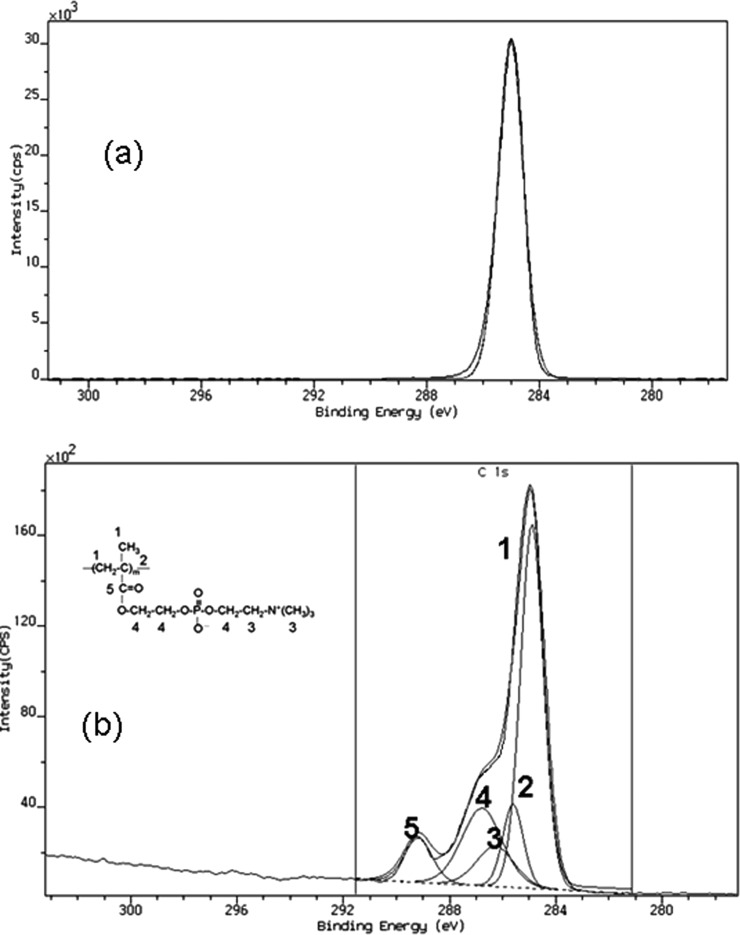
Core level chemical shifts provide a reasonable degree of information regarding its organic structure. In order to understand the effect of the photografting on the chemical structure of the modified surface more precisely the shape of the C 1s peak was evaluated. In the unmodified TOPAS substrate, only one peak corresponding to C–C, C–H (binding energy at 285 eV) was observed (see Fig. 4a), whereas the C1s spectrum for the grafted surface was smoothly fitted into five peaks. Fig. 4b displays the high resolution C1s XPS spectra of the MPC-grafted COC at a photoelectron takeoff angle of 90° for 25 min irradiation time. The carbon peaks at 285.0, 285.7, 286.3, 286.7, and 288.8 eV were associated with each functional moiety in PMPC (see Fig. 4b). The C1s peaks of the PMPC were associated with each carbon as shown in Fig. 4b), and the ratios of the peak areas were almost proportional to the number of carbon atoms in the MPC. This again confirms that MPC monomer has been successfully attached on the modified substrate in comparison to the unmodified substrate where no such component was observed. Treated chips rinsed in DI water for 4 h were also measured to evaluate whether the charged surface groups may be lost by dissolution. However, the XPS data for the rinsed specimens indicated no loss of oxygen-containing species.
Figure 4.
Peak fitting of the C1s XPS spectra for (a) unmodified and (b) a typical MPC-grafted TOPAS substrate surface at a photoelectron takeoff angle of 90° [monomer concentration = 0.2 mol/L; initiator concentration = 0.2 mol/L; and irradiation time = 25 min].
The surface roughness of unmodified COC was 20 ± 5 nm and that for the modified COC with an irradiation time up to 30 min remained unchanged. However, the specimen with an irradiation time above 30 min exhibited a very high surface roughness of 400 ± 50 nm. In general, a lower surface roughness is believed to help flow through the channel without affecting the velocity of the fluid.
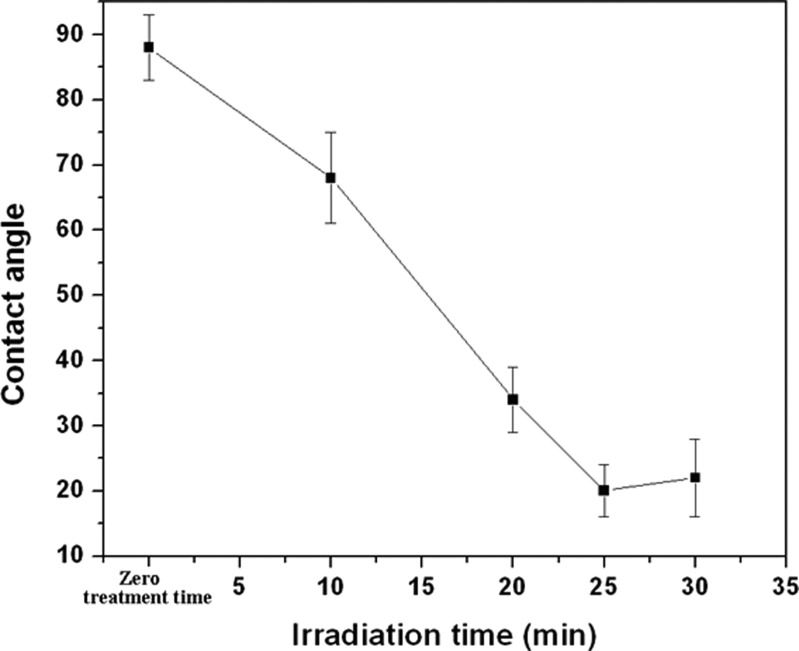
The water contact angles of the untreated and the MPC-grafted COC surfaces are as shown in Fig. 5. The untreated COC substrates were hydrophobic with an average contact angle of 88° ± 3°, which is comparable to that reported previously.37 The contact angle decreased with increasing irradiation time, and minimum contact angles of 20° ± 3° and 22° ± 5° were achieved for MPC modified COC substrates with UV exposure times of 25 and 30 min, respectively. There was no change in contact angles in three modified COC samples (with an irradiation time of 25 min) that were stored in ambient atmosphere for 15 days. Moreover, there was also no change in contact angle in several modified COC chips that were heated to 50 °C for 1 h. These observations indicated that the properties of the surfaced obtained using UV-grafting utilizing MPC monomer were permanent and did not change with ageing to 15 days and heating to 50 °C for 1 h.
Figure 5.
Effect of irradiation time on the surface contact angle of MPC modified TOPAS substrates [monomer concentration = 0.2 mol/L; initiator (BP) concentration = 0.2 mol/L].
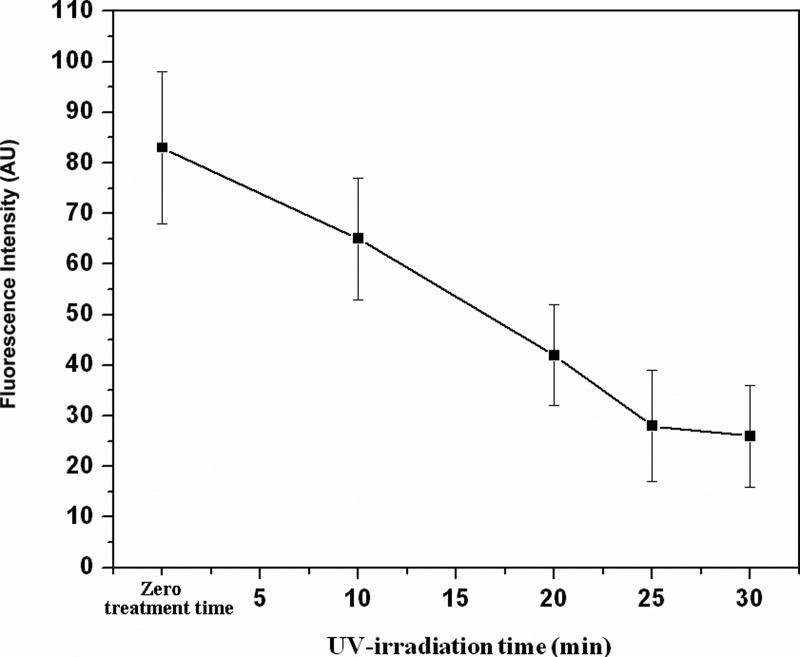
Fig. 6 shows the amount of protein adsorption through the measurement of fluorescent intensity. The lower intensity of fluorescent light from the surface of grafted specimens indicated that the surfaces that had been modified with MPC are less sensitive to protein adsorption. On the other hand, the high fluorescent intensity from unmodified COC surface indicated substantial protein adsorption on that surface. Thus, such reduction in protein revealed that COC surface grafted with MPC will be good for the inhibition of thrombus formation; hence, it would be an important surface modification technique in the field of capillary electrophoretic separation of proteins and peptides.
Figure 6.
Fluorescence intensity of adsorbed fluorescein labeled BSA on unmodified and MPC grafted COC substrate. Average fluorescent intensity (n = 5) is plotted as a function of MPC grafting time [monomer concentration = 0.2 mol/L; initiator (BP) concentration = 0.2 mol/L].
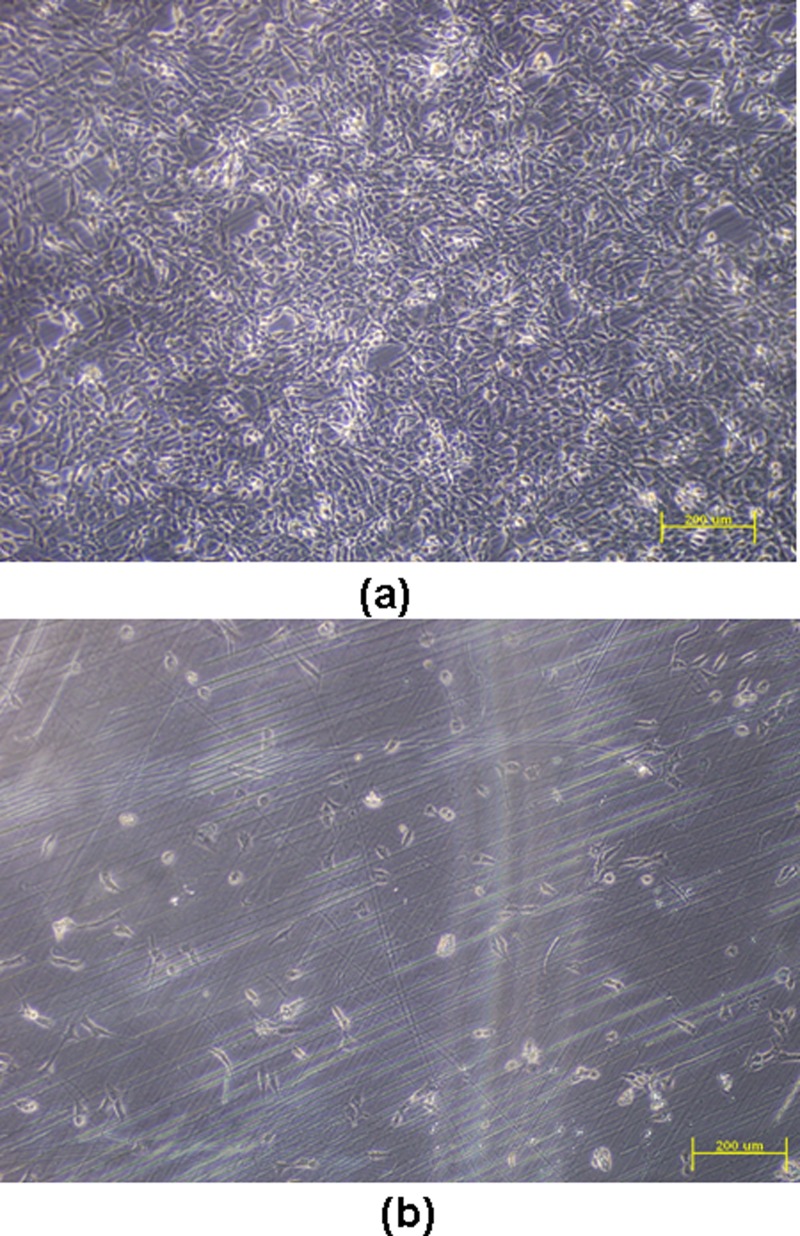
Cell adhesion analysis was carried out for both the unmodified COC and the MPC-grafted COC. Fig. 7 shows the microscopic images of cell adhesion on modified and unmodified COC specimen surfaces on which mouse 3T3 cells were cultured. MPC modified COC substrate shows minimal cell adhesion, whereas unmodified Topas COC exhibited strong cell adhesion. The cell adhesion test confirms that the MPC surface grafting technique is effective in reducing cell adhesion in COC based microfludic chips that are used for studies on cell separation.
Figure 7.
Cells attached on (a) unmodified COC substrate and (b) MPC grafted COC surface [monomer concentration = 0.2 mol/L; initiator (BP) concentration = 0.2 mol/L; and irradiation time = 25 min].
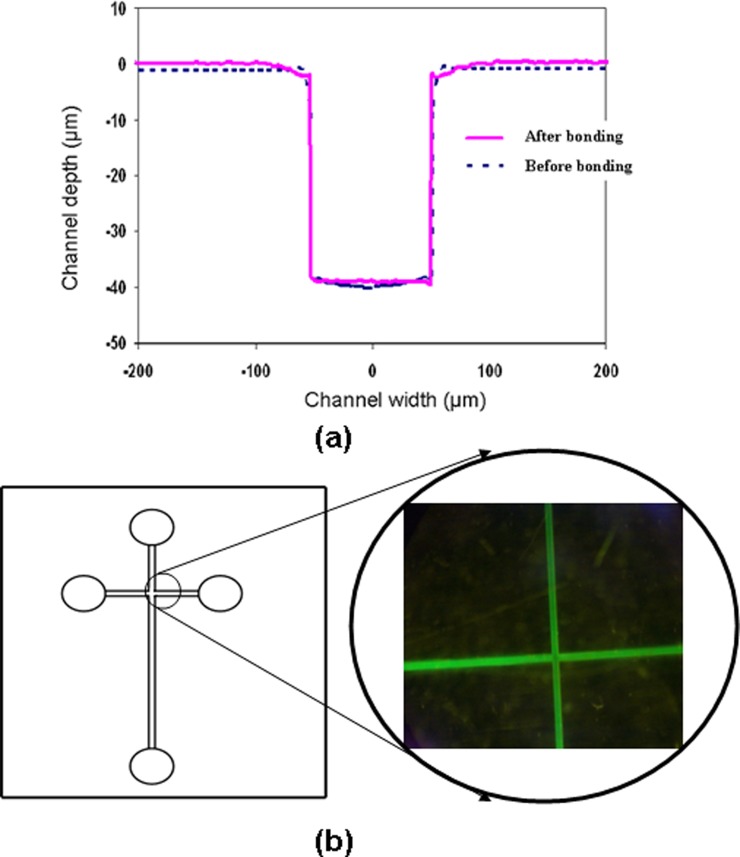
Finally, we conducted experiments to demonstrate that the MPC grafted COC could still be readily sealed by thermal bonding to produce a microfluidic device. For this purpose, embossed COC substrates were photo grafted with the optimized grafting conditions (monomer concentration = 0.2 mol/L, initiator concentration = 0.2 mol/L, and irradiation time = 25 min) were thermally bonded at a temperature of 70 °C with a bonding pressure of 2 MPa for 5 min with another grafted flat COC substrate. It can be seen from Fig. 8a that no changes in microchannel dimensions were observed in the COC capillary electrophoresis microchip by comparing the cross-section profile of the embossed microchannels before bonding and after grafting followed by thermal bonding. Furthermore, no clogging of microchannels was observed when the COC chip was used for tests using sample solutions which contained proteins. Fig. 8b shows a micrograph of a microchannel that has been injected with a fluorescent protein solution of FITC-BSA that was prepared using PBS (pH 7.4). The purpose of using the fluorescent proteins was twofold: to determine the protein adsorption and to facilitate assessment of any leakage during passage of fluid. It was apparent from Fig. 8 that good sealing was obtained without causing any leak or shape distortion of the microchannel during the encapsulation process by thermal bonding.
Figure 8.
(a) Comparison of cross-sectional channel profile of the grafted capillary electrophoresis microchip before and after thermal bonding [bonding temperature = 70 °C; bonding pressure = 2 MPa; and bonding time = 5 min]; (b) microchip that were filled with FITC-BSA solution indicating good sealing with no leakage as well as for protein adsorption measurement.
CONCLUSIONS
We have demonstrated the UV-photografting of MPC on to COC substrates in order to improve the surface hydrophilicity as well as resistance to both protein adsorption and cell adhesion in COC based microfluidic devices. The surface wettability is an important aspect such that it improves biocompatibility for the microchannel in microfluidic devices. The results show that MPC can attach on to COC surfaces, resulting in a reduction in water contact angle to ∼20° from 88° and hence enhance in the surface hydrophilicity. It has been observed that attachment of MPC monomer on to the surface of COC has significantly reduced the protein adsorption and cell adhesion, which improved the surface biocompatibility. Since the protein adsorption and cell adhesion study are essential for biosensors, immunoassays, and cell biology studies, we believe that the present method of surface modification on COC substrate using MPC monomer will have important applications for the development of microfluidics in bio-analysis, cell sorting, and cell culture.
References
- Lee G. B., Chen S. H., Huang G. R., Sung W. C., and Lin Y. H., Sens. Actuators B 75, 142 (2001). 10.1016/S0925-4005(00)00745-0 [DOI] [Google Scholar]
- Liu Y., Ganser D., Schneider A., Liu R., Grodzinski P., and Kroutchinina N., Anal. Chem. 73, 4196 (2001). 10.1021/ac010343v [DOI] [PubMed] [Google Scholar]
- Fujii T., Microelectron. Eng. 61–62, 907 (2002). 10.1016/S0167-9317(02)00494-X [DOI] [Google Scholar]
- Yi L., Xiaodong W., and Fan Y., J. Mater. Process. Technol. 208, 63 (2008). 10.1016/j.jmatprotec.2007.12.146 [DOI] [Google Scholar]
- Mair D. A., Geiger E., Pisano A. P., Fréchet J. M. J., and Svec F., Lab Chip 6, 1346 (2006). 10.1039/b605911b [DOI] [PubMed] [Google Scholar]
- See http://www.polyplastics.com/en/product/lines/topas/TOPAS.pdf for information about the physical and chemical properties of TOPAS materials.
- Piruska A., Nikcevic I., Lee S. H., Ahn C., Heineman W. R., Limbacha P. A., and Seliskar C. J., Lab Chip 5, 1348 (2005). 10.1039/b508288a [DOI] [PubMed] [Google Scholar]
- Chang T. Y., Yadav V. G., Leo S. D., Mohedas A., Rajalingam B., Chen C. L., Selvarasah S., Dokmeci M. R., and Khademhosseini A., Langmuir 23, 11718 (2007) 10.1021/la7017049 [DOI] [PubMed] [Google Scholar]
- Wisniewski N. and Reichert M., Colloids Surf., B 18, 197 (2000). 10.1016/S0927-7765(99)00148-4 [DOI] [PubMed] [Google Scholar]
- McPherson T. B., Shim H. S., and Park K., J. Biomed. Mater. Res. 38, 289 (1997). [DOI] [PubMed] [Google Scholar]
- Liu J. K. and Lee M. L., Electrophoresis 27, 3533 (2006). 10.1002/elps.v27:18 [DOI] [PubMed] [Google Scholar]
- Dolnik V., Electrophoresis 25, 3589 (2004). 10.1002/elps.v25:21/22 [DOI] [PubMed] [Google Scholar]
- Belder D. and Ludwig M., Electrophoresis 24, 3595 (2003). 10.1002/elps.v24:21 [DOI] [PubMed] [Google Scholar]
- Makamba H., Kim J. H., Lim K., Park N., and Hahn J. H., Electrophoresis 24, 3607 (2003). 10.1002/elps.v24:21 [DOI] [PubMed] [Google Scholar]
- Kato K., Uchida E., Kang E. T., Uyama Y., and Ikada Y., Prog. Polym. Sci. 28, 209 (2003). 10.1016/S0079-6700(02)00032-1 [DOI] [Google Scholar]
- Gaudioso J. and Craighead H. G., J. Chromatogr. A 971, 249 (2002). 10.1016/S0021-9673(02)00843-9 [DOI] [PubMed] [Google Scholar]
- Sohn Y. S., Kai J., and Ahn C. H., Sens. Lett. 2, 171 (2004). 10.1166/sl.2004.054 [DOI] [Google Scholar]
- Lim Y. T., Kim S. J., Yang H., and Kim K., J. Micromech. Microeng. 16, N9 (2006). 10.1088/0960-1317/16/7/N01 [DOI] [Google Scholar]
- Marie R., Beech J. P., Voros J., Tegenfeldt J. O., and Hook F., Langmuir 22, 10103 (2006). 10.1021/la060198m [DOI] [PubMed] [Google Scholar]
- Rohr T., Ogletree D. F., Svec F., and Frechet J. M. J., Adv. Funct. Mater. 13, 264 (2003). 10.1002/adfm.200304229 [DOI] [Google Scholar]
- Li C., Yang Y. N., Craighead H. G., and Lee K. H., Electrophoresis 26, 1800 (2005). 10.1002/elps.v26:9 [DOI] [PubMed] [Google Scholar]
- Yang Y. N., Li C., Lee K. H., and Craighead H. G., Electrophoresis 26, 3622 (2005). 10.1002/elps.v26:19 [DOI] [PubMed] [Google Scholar]
- Lin R. and Burns M. A., J. Micromech. Microeng. 15, 2156 (2005). 10.1088/0960-1317/15/11/023 [DOI] [Google Scholar]
- Bhattacharyya A. and Klapperich C. M., Anal. Chem. 78, 788 (2006). 10.1021/ac051449j [DOI] [PubMed] [Google Scholar]
- Ranby B., Yang W. T., and Tretinnikov O., Nucl. Instrum. Methods Phys. Res. B 151, 301 (1999). 10.1016/S0168-583X(99)00158-5 [DOI] [Google Scholar]
- Ishihara K., Iwasaki Y., Ebihara S., Shindo Y., and Nakabayashi N., Colloids Surf., B 18, 325 (2000). 10.1016/S0927-7765(99)00158-7 [DOI] [PubMed] [Google Scholar]
- Nakabayashi N. and Ishihara K., Macromol. Symp. 101, 405 (1996). 10.1002/masy.19961010146 [DOI] [Google Scholar]
- Ishihara K., Trends Polym. Sci. 5(12 ), 401 (1997). [Google Scholar]
- Liposomes and Their Uses in Biology and Medicine, edited by Papahadjopoulos D. (New York Academy of Sciences, 1978), Vol. 308, pp. 1–462. [PubMed] [Google Scholar]
- Ishihara K., Ueda T., and Nakabayashi N., Polym. J. 22, 355 (1990). 10.1295/polymj.22.355 [DOI] [Google Scholar]
- Ueda T., Oshida H., Kurita K., Ishihara K., and Nakabayashi N., Polym. J. 24, 1259 (1992). 10.1295/polymj.24.1259 [DOI] [Google Scholar]
- Ishihara K., Hanyuda H., and Nakabayashi N., Biomaterials 16, 873 (1995). 10.1016/0142-9612(95)94150-J [DOI] [PubMed] [Google Scholar]
- Jena R. K., Yue C. Y., Lam Y. C., and Wang Z. Y., Sens. Actuators B 150, 692 (2010). 10.1016/j.snb.2010.08.018 [DOI] [Google Scholar]
- Jena R. K., Chester S. A., Srivastava V., Yue C. Y., Anand L., and Lam Y. C., Sens. Actuators B 157, 518 (2010). 10.1016/j.snb.2011.05.012 [DOI] [Google Scholar]
- Forsyth J., Perena J. M., Benavente R., Perez E., Tritto I., Boggioni L., and Britzinger H. H., Macromol. Chem. Phys. 202, 614 (2001). [DOI] [Google Scholar]
- Ruiz L., Hilborn J. G., Leonard D., and Mathieu H. J., Biomaterials 19, 987 (1998). 10.1016/S0142-9612(97)00197-X [DOI] [PubMed] [Google Scholar]
- Stachowiak T. B., Mair D. A., Holden T. G., Lee L. J., Svec F., and Frechet J. M. J., J. Sep. Sci. 30, 1088 (2007). 10.1002/jssc.v30:7 [DOI] [PubMed] [Google Scholar]