Abstract
Background
The Renin-Angiotensin-Aldosterone-System plays a pivotal role in hypertension. Angiotensin II (Ang II) is a major regulator of aldosterone synthesis and secretion, and it is known to facilitate reactive oxygen species (ROS) generation in many cell types. Aims: Here, we assessed the role of ROS signaling in Ang II-induced aldosterone synthesis by focusing on the regulation of aldosterone synthase (CYP11B2), a cytochrome P450 oxidase that catalyzes the final step in aldosterone biosynthetic pathway. Results: Ang II increased CYP11B2 activity, mRNA and protein with a concomitant elevation of 6-Carboxy- 2′,7′-dichlorodihydrofluorescein diacetate fluorescence, malondialdehyde and protein carbonyl levels (indices of ROS), NADPH oxidase (Nox) activity, and H2O2 levels in human and rat adrenal cortical cells. The expression of nuclear receptor related 1 protein, a transcription factor known to regulate CYP11B2 expression, was also augmented by Ang II. These Ang II-evoked effects were either abolished or attenuated by pretreatment of cells with either Ang II type I receptor (AT1R) antagonist, or antioxidants or Nox inhibitor or siRNA silencing of Nox1, 2 and 4, or inhibitors of phospholipase C and protein kinase C. Exogenous H2O2 mimicked the facilitatory effects of Ang II on CYP11B2 activity, mRNA, and protein expression, and these changes were significantly reduced by PEG-catalase. Innovation: ROS, particularly H2O2, is identified as a key regulator of aldosterone production. Conclusion: Our results suggest that Ang II facilitates CYP11B2 activity and the ensuing aldosterone production via activation of AT1R-Nox-H2O2 signaling pathway. Antioxid. Redox Signal. 17, 445–459.
Introduction
Aldosterone, a mineralocorticoid primarily produced and secreted by zona glomerulosa of the adrenal cortex, plays important roles in the control of blood pressure via regulation of sodium and water homeostasis. It is synthesized from cholesterol via a series of hydroxylation and oxidation reactions involving members of the cytochrome P450 super family that include cholesterol desmolase, 3β-hydroxysteroid dehydrogenase, CYP21 hydroxylase, 11β-hydroxylase, and aldosterone synthase (CYP11B2) [Fig. 1A; Ref. (24)]. CYP11B2, a cytochrome P450 oxidase localized to the inner mitochondrial membrane, catalyzes the final step of aldosterone synthesis wherein deoxycorticosterone (via the intermediate, corticosterone) is converted to aldosterone in an O2-dependent reaction (31). In adults, excessive production and secretion of aldosterone, due to either primary or secondary disorders, result in sodium retention and systemic arterial hypertension (39, 44). On the other hand, disorders of aldosterone synthesis in infants are associated with severe dehydration, electrolyte disturbances, and growth retardation (47). Given the importance of aldosterone in the control of renal and cardiovascular function, several studies have examined the cellular mechanisms regulating aldosterone production.
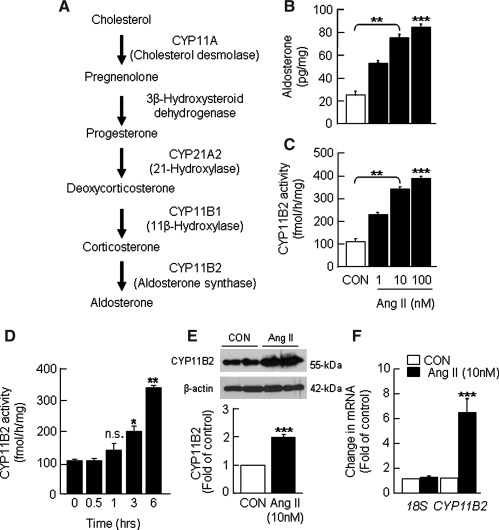
FIG. 1.
Ang II-evoked changes in aldosterone production and CYP11B2 activity, mRNA, and protein expression in human H295R cells. (A) Aldosterone biosynthetic pathway. Cells grown to 90% confluence were treated with indicated concentrations of Ang II for 6 h. Cells not treated with Ang II served as control (CON). Changes in aldosterone levels in the culture medium (B) and CYP11B2 activity in cell homogenates (C) as a function of concentration of Ang II were determined using procedures as described in Materials and Methods section. Ang II (10 nM)-induced changes in CYP11B2 activity as a function of incubation time (D), CYP11B2 protein as determined by western blot (E), and CYP11B2 mRNA expression as assessed by real-time PCR (F) are shown. The protein and mRNA data were obtained with cells treated with either vehicle or Ang II for 6 h and normalized using β-actin and 18S, respectively. Results derived from six independent experiments are presented as mean±SEM. *p<0.05, **p<0.01, ***p<0.001 and n.s., not significant. Ang II, angiotensin II; CYP11B2, aldosterone synthase; PCR, polymerase chain reaction.
Innovation.
The Renin-Angiotensin-Aldosterone-System (RAAS) plays a pivotal role in hypertension. Although it is well known that angiotensin II (Ang II), the major effector of the RAAS, regulates aldosterone synthesis and can increase reactive oxygen species (ROS) in several cell types, the contribution of ROS to Ang II-induced aldosterone synthesis has not been investigated. The analysis of aldosterone synthase, the rate-limiting enzyme in aldosterone biosynthesis, revealed a functional link between Ang II, Nox-, and mitochondria-derived O2.− and the ensuing H2O2 and aldosterone production. The identification of O2.−/H2O2 as a key regulator of aldosterone synthesis provides a new platform for the development of antioxidant-based combination therapy in RAAS-dependent arterial hypertension.
Studies on rat and bovine zona glomerulosa (5, 6, 48) as well as human adrenocarcinoma H295R cell cultures (4, 34, 42) have identified angiotensin II (Ang II), the effector of the renin-angiotensin system, and K+ as the major regulators of aldosterone synthesis. Ang II derived from circulation stimulates the synthesis and release of aldosterone in the adrenal cortex by inducing CYP11B2 transcription in an angiotensin II type I receptor (AT1R)-dependent manner (27, 29). Multiple AT1R activated signaling pathways that contribute to enhanced aldosterone production and release have been described (1, 2). For instance, stimulation of AT1R by Ang II activates phospholipase C-β (PLC-β) and the inositol trisphosphate/Ca2+ pathway, leading to transcriptional up regulation of CYP11B2 mRNA and enhanced aldosterone synthesis (2). On the other hand, studies in vascular smooth muscle cells showed that AT1R-dependent activation of NADPH oxidase (Nox) and the ensuing increase in superoxide anion production (O2.−) mediate Ang II-induced vascular damage and smooth muscle cell proliferation via activation of the reactive oxygen species (ROS) signaling pathway (13, 52). Furthermore, Ang II-mediated increase in ROS levels in cardiomyocytes and renal mesangial cells contributes to cardiac hypertrophy (3, 14) and progression of renal dysfunction (19), respectively.
Currently, the role of ROS signaling in Ang II-dependent stimulation of aldosterone synthesis is not known. In the present study, we examined whether Nox-derived ROS mediates the enhanced aldosterone production by Ang II. The effects of Ang II on ROS production, CYP11B2, and Nox activities as well as the effects of antioxidants and Nox inhibition on Ang II-mediated stimulation of aldosterone synthesis were studied in vitro in H295R cells and rat adrenal cortical slices containing zona glomerulosa. Our results showed that Ang II treatment increases CYP11B2 activity, aldosterone levels, and Nox-derived H2O2. Moreover, administration of Nox inhibitor or antioxidants or H2O2 scavenger markedly attenuates Ang II-induced increases in CYP11B2 transcription, protein levels and activity, as well as aldosterone levels in adrenal cortical cells.
Results
Ang II increases CYP11B2 activity
CYP11B2 catalyzes the final step in aldosterone biosynthesis (Fig. 1A). To assess the effects of Ang II on aldosterone production and CYP11B2 activity, H295R cells were treated with Ang II (1, 10 and 100 nM) for 6 h at 37°C, and aldosterone levels in the culture medium as well as CYP11B2 activity in the cell-free extracts were determined. Preliminary analysis showed that cell pellets contain a negligible amount of aldosterone, whereas >98% of the aldosterone occurs in the culture medium. Therefore, the changes in aldosterone level in the culture medium were used as an index of aldosterone synthesis. Ang II increased aldosterone levels (Fig. 1B) and CYP11B2 activity (Fig. 1C) in a dose-dependent manner; a maximum increase of ∼3.5-fold was seen for both at a 10 nM concentration. The increase in CYP11B2 activity evoked by Ang II seems to be a time-dependent phenomenon. Up to 1 h, Ang II (10 nM) had no effect on CYP11B2 activity, whereas it induced ∼2.0 and 3.5-fold increases in CYP11B2 activity after 3 and 6 h of treatment, respectively (Fig. 1D). The increases in CYP11B2 activity and the ensuing aldosterone production by Ang II (10 nM for 6 h) were due in part to elevations in CYP11B2 protein (∼2-fold; Fig. 1E; Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/ars) and mRNA expression (∼6.8-fold; Fig. 1F). The findings just mentioned are consistent with the previously reported Ang II-evoked increases in aldosterone levels and CYP11B2 mRNA expression in H295R cells (26, 27, 50).
Ang II stimulates ROS generation in H295R cells
Studies in cardiomyocytes (49), vascular smooth muscle cells (52), and renal mesangial cells (19) showed that Ang II-induced cellular responses are mediated via ROS signaling. To assess whether Ang II elevates ROS levels in adrenal cortical cells, H295R cells were loaded with 6-Carboxy-2′,7′-dichlorodihydrofluorescein diacetate (DCFDA) for 30 min before Ang II treatment. The change in DCFDA fluorescence due to ROS-mediated oxidation was monitored by fluorescence microscopy (45, 46). Ang II increased DCFDA fluorescence in a concentration- (Fig. 2A; Supplementary Fig. S2A) and time-dependent manner (Supplementary Fig. S2B). A maximum increase in DCFDA fluorescence was seen at a 10 nM concentration (Fig. 2A), and exposure to Ang II for as short as 10 min was sufficient to induce DCFDA fluorescence (∼20-fold; Supplementary Fig. 2B) with a maximum increase seen at 40 min. At a higher incubation time, there was a progressive decrease in fluorescence intensity, which may be, in part, due to deterioration of fluorescence signals. These results suggest that within several minutes of treatment, Ang II facilitates ROS generation in H295R cells.
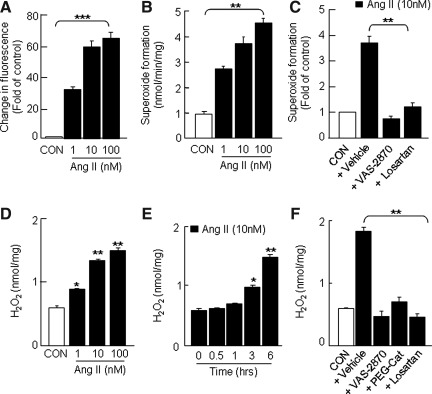
FIG. 2.
Induction of ROS generation in H295R cells by Ang II. Cells, grown in an eight-well glass slide chamber, were loaded with DCFDA (5 μM for 30 min) and then treated with either Ang II (1, 10, and 100 nM) or vehicle (CON) for various periods. Ang II-induced changes in DCFDA fluorescence was monitored by fluorescence microscopy. Concentration-dependent Ang II-induced changes in DCFDA fluorescence (A) and superoxide formation (B) are shown. Effects of VAS-2870 (20 μM; a Nox-specific inhibitor) and losartan (10 μM, an AT1R-specific antagonist) on Ang II (10 nM for 6 h)-dependent superoxide formation (C), concentration- (D), and time (E)-dependent Ang II (10 nM)-induced changes in H2O2 levels. Effects of VAS-2870 (20 μM), PEG-catalase (PEG-Cat, 350 U/ml), and losartan (10 μM) on Ang II (10 nM for 6 h)-dependent H2O2 formation (F) are shown. The incubation time for DCFDA fluorescence and superoxide/H2O2 measurements was 1 and 6 h, respectively. The DCFDA fluorescence intensity was calculated from four different fields, and the data are expressed as fold increase of control (CON=1). Superoxide and H2O2 generation was measured spectrophotometrically by monitoring SOD (2000 U/ml)-inhibitable, NADPH-dependent oxidation of cytochrome C (80 μM) and catalase-inhibitable oxidation of Amplex Red (100 μM) to resorufin, respectively, as described in Materials and Methods section. Results derived from six independent experiments are presented as mean±SEM. *p<0.05, **p<0.01, and ***p<0.001. AT1R, angiotensin II type I receptor; DCFDA, 6-Carboxy- 2′,7′-dichlorodihydrofluorescein diacetate; Nox, NADPH oxidase; ROS, reactive oxygen species; SOD, superoxide dismutase.
Since exposure to 10 nM of Ang II induced a maximum increase in aldosterone levels (Fig. 1B), CYP11B2 activity (Fig. 1C), and ROS generation (Fig. 2A), H295R cells treated with 10 nM of Ang II were used for the following mechanistic studies.
Source and chemical identity of ROS generated by Ang II in H295R cells
Several reports suggested that Ang II-induced ROS can be either superoxide anion or its dismutated product, H2O2 (8, 13, 17). They can either be generated via activation of oxidases including Nox (37, 45) or formed in the mitochondria (9, 21). To delineate these possibilities, we examined the effects of Ang II on (i) superoxide formation in the cell homogenates as an index of Nox activity, (ii) H2O2 levels in the cell homogenates, and (iii) mitochondrially derived ROS levels.
Superoxide formation by Ang II via Nox
Analysis of Nox activity (measured as cellular superoxide generation) showed that Ang II (10 nM) stimulates superoxide formation by ∼3.5-fold compared with untreated controls (Fig. 2B). Both VAS-2870 (20 μM), a recently reported specific inhibitor of Nox (43), and losartan (10 μM), an AT1R-specific competitive antagonist, blocked the increase in Nox activity (Fig. 2C). These findings suggest that Ang II induces superoxide formation via AT1R-dependent activation of Nox.
Nox-dependent H2O2 generation by Ang II
H2O2 levels in cell homogenates were determined spectrophotometrically by monitoring catalase-inhibitable oxidation of Amplex Red. In un-stimulated cells, low levels of H2O2 were detected (0.59±0.01 nmol/mg). Ang II increased H2O2 levels in a dose-dependent manner with a maximum increase (∼2.8-fold) seen at a 10 nM concentration (Fig. 2D). Ang II (10 nM)-induced increase in H2O2 levels is time-dependent. Significant increases in H2O2 levels were seen only after 3 h of treatment (∼2-fold; Fig. 2E) and increased further (∼3-fold) with 6 h incubation. Pretreatment with either Nox inhibitor (VAS-2870) or H2O2 scavenger (PEG-catalase, 350 U/ml) or AT1R antagonist (losartan) effectively prevented Ang II-induced elevation of H2O2 levels (Fig. 2F).
Nox-dependent mitochondrial ROS generation by Ang II
To assess whether mitochondria contributes to Ang II-induced ROS generation in H295R cells, we employed two approaches; in one of them, we monitored Ang II-induced changes in MitoSOX Red fluorescence in live cells and in the other, we measured aconitase activity in the mitochondria-enriched fraction. The activity of aconitase has been reported to be sensitive and inversely related to ROS levels [Supplementary Ref. (1)].
In the first set of experiments, cells were loaded with MitoSOX Red (2.5 μM) before treatment with either vehicle or Ang II (10 nM) or antimycin A (100 μM) for 40 min. Ang II had no significant effect on MitoSOX Red fluorescence, whereas antimycin A, which induces ROS in the mitochondria via inhibition of complex III, produced a robust increase in fluorescence (positive control; Supplementary Fig. S3A) compared with untreated control.
In the second set of experiments, aconitase activity in the mitochondria-enriched fraction was determined. In control cells, the aconitase activity was found to be 1.7±0.2 μmoles/min/mg. Treatment with Ang II (10 nM) for 1 h was without any effect, whereas a significant decrease in aconitase activity was seen after 3 and 6 h of Ang II treatment (∼25% and ∼50% inhibition, respectively; Supplementary Fig. S3B). Moreover, Ang II-induced reduction in aconitase activity was prevented by VAS-2870, a potent inhibitor of Nox, manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin pentachloride (MnTMPyP) (which traps superoxide), and losartan (AT1R antagonist) (Supplementary Fig. S3C).
AT1R-dependent Nox-derived H2O2 mediates upregulation of CYP11B2 by Ang II
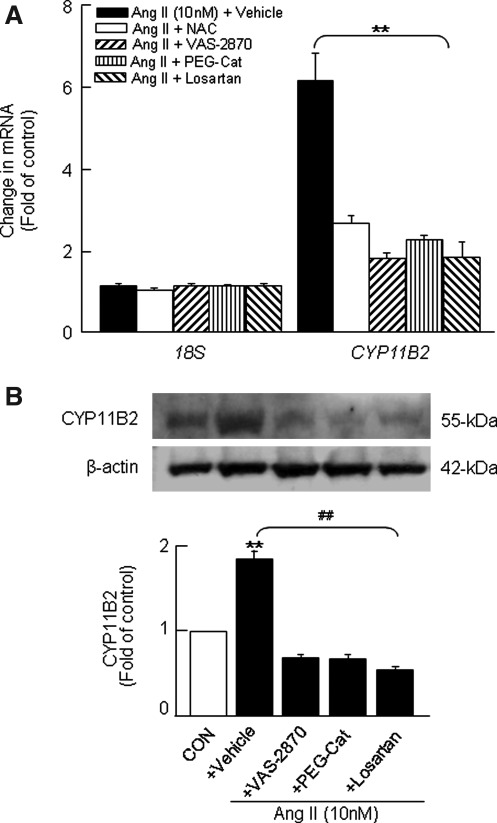
The results just mentioned suggest that Ang II facilitates an initial AT1R-Nox-dependent formation of H2O2 (via dismutation of superoxide), which significantly accumulates after 3 h treatment paralleling with Ang II-induced time-dependent changes in CYP11B2 activity (Fig. 1D) and mitochondrially derived ROS (as evidenced by decrease in aconitase activity; Supplementary Fig. S3B). We, therefore, tested the effects of inhibitors of the pathway just mentioned on Ang II-induced increase in aldosterone synthesis. Pretreatment of cells with antioxidants (N-acetyl cysteine [NAC], an antioxidant/precursor of glutathione; MnTMPyP, which traps superoxide anion) or H2O2 scavenger (PEG-catalase) or Nox inhibitor (VAS-2870) or AT1R antagonist (losartan) drastically reduced not only an Ang II-induced increase in DCFDA fluorescence (Fig. 3A; Supplementary Fig. S4) but also the increase in CYP11B2 activity (Fig. 3B), aldosterone levels (Fig. 3C), CYP11B2 mRNA (Fig. 4A), and protein expression (Fig. 4B; Supplementary Fig. S1) evoked by Ang II. Neither Nox inhibitors nor antioxidants affected CYP11B2 activity and aldosterone levels in the control cells (data not shown).
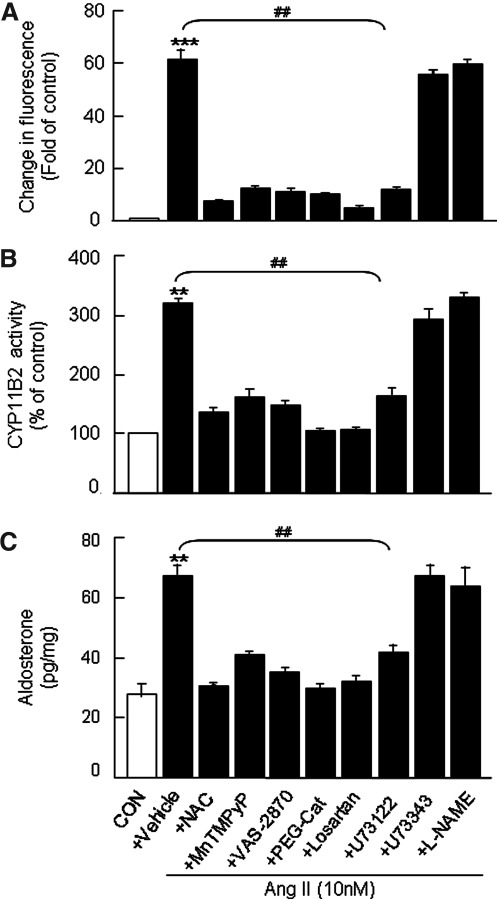
FIG. 3.
Effects of antioxidants, inhibitors of Nox, NOS, and PLC and AT1R antagonist on Ang II-induced elevation of ROS, CYP11B2 activity, and aldosterone levels in human H295R cells. Cells were treated with Ang II (10 nM for 6 h) in the absence (vehicle) and presence of either antioxidants (NAC, 200 μM; MnTMPyP, 100 μM), or H2O2 scavenger (PEG-Cat, 350 U/ml) or Nox inhibitor (VAS-2870, 20 μM) or NOS inhibitor (L-NAME, 10 μM) or PLC inhibitor and its inactive analog (U73122 and U73343, respectively, 10 μM each) or AT1R antagonist (losartan, 10 μM). Cells not treated with Ang II served as CON. Changes in DCFDA fluorescence (A), CYP11B2 activity in the homogenates (B), and aldosterone levels in the culture medium (C) are shown. Results derived from six independent experiments are presented as mean±SEM. ** and ##p<0.01 and ***p<0.001. Either ** or *** denotes a significant difference between the control and Ang II treated samples. ## denotes a significant difference between Ang II and Ang II+drug-treated samples. L-NAME, L-NG-Nitroarginine methyl ester; MnTMPyP, manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin pentachloride; NAC, N-acetyl cysteine; PLC, phospholipase C; NOS, nitric oxide synthase.
FIG. 4.
Effects of antioxidants, Nox inhibitor, H2O2 scavenger, and AT1R antagonist on Ang II-induced changes in CYP11B2 mRNA and protein expression in H295R cells. Cells were treated with Ang II (10 nM for 6 h) in the absence and presence of either antioxidant (NAC, 200 μM) or H2O2 scavenger (PEG-Cat, 350 U/ml) or Nox inhibitor (VAS-2870, 20 μM) or losartan (10 μM). Cells not treated with Ang II were used as CON. Cell lysates were analyzed for CYP11B2 mRNA expression by real-time PCR (A), and CYP11B2 protein expression was analyzed by western blot analysis (B). The protein and mRNA expression were normalized using β-actin and 18S, respectively. Results derived from six independent experiments are presented as mean±SEM. ** and ##p<0.01. ** denotes a significant difference between the control and Ang II treated samples. ## denotes a significant difference between Ang II and Ang II+drug-treated samples.
On the other hand, allopurinol, an inhibitor of xanthine oxidase (another source of cytosolic ROS), had no significant effect on DCFDA fluorescence in Ang II-treated cells (data not shown). Likewise, L-NG-Nitroarginine methyl ester (L-NAME), an inhibitor of nitric oxide synthase (NOS; another putative source of ROS), was without any significant effect on the increase in ROS, CYP11B2 activity, and aldosterone levels facilitated by Ang II (Fig. 3).
Next, we examined the possible role of phopholipase C (PLC), a well-known upstream effector of AT1R-mediated activation of protein kinase C (PKC, via diacylglycerol [DAG])-Nox pathway in Ang II-induced facilitation of aldosterone synthesis. PLC inhibitor (U73122, 10 μM) but not its inactive analog (U73343; 10 μM) markedly attenuated the increase in DCFDA fluorescence (Fig. 3A; Supplementary Fig. S4), CYP11B2 activity (Fig. 3B), and aldosterone levels (Fig. 3C) by Ang II. Likewise, PKC inhibitor (bisindolylmaleimide [BIS], 10 μM) also blocked an Ang II–induced increase in ROS (Supplementary Fig. S4).
Role of Nox2-derived H2O2 in Ang II-induced nuclear receptor related 1 protein expression
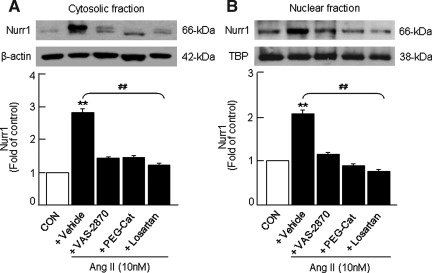
In vitro studies have shown that regulation of hCYP11B2 by Ang II is mediated in part by up-regulation of orphan nuclear receptors nuclear receptor related 1 protein (Nurr1) and NGF1-B, members of the neuronal growth factor-induced clone B (NGFI-B) family of transcription factors that bind to NBRE-1 and Ad5 cis-elements (28, 29, 38). Therefore, we examined whether Nox-derived H2O2 contributes to Ang II-induced changes in Nurr1 expression in H295R cells. Nurr1 expression was examined in the cytosolic and nuclear-enriched fractions isolated from control and Ang II-treated cells by western blot analysis. As shown in Figure 5, Ang II (10 nM) induced a nearly threefold increase in Nurr1 expression in the cytosolic fraction (Fig. 5A), whereas a twofold increase was seen in the nuclear-enriched fraction (Fig. 5B). Pretreatment with either AT1R antagonist (losartan) or Nox inhibitor (VAS-2870) or H2O2 scavenger (PEG-catalase) prevented this facilitatory effect of Ang II on Nurr1 expression seen in the cytosolic (Fig. 5A) and nuclear-enriched fractions (Fig. 5B).
FIG. 5.
Analysis of Ang II-induced changes in Nurr1 expression in H295R cells. Cells were treated with Ang II (10 nM for 6 h) in either the absence or presence of H2O2 scavenger (PEG-Cat, 350 U/ml), Nox inhibitor (VAS-2870, 20 μM) and AT1R antagonist (losartan, 10 μM). Cells not treated with Ang II were used as CON. Nuclear and cytosolic fractions were isolated by differential centrifugation of cell homogenates as described in Materials and Methods section. Changes in Nurr1 protein expression in the cytosolic (A) and nuclear (B) fractions of H295R cells as assessed by western blot are shown. Nurr1 expression in the cytosol and nucleus was normalized based on the expression level of β-actin and TATA binding protein (TBP), respectively. Results derived from six independent experiments are presented as mean±SEM. ** and ##p<0.01. ** denotes a significant difference between the control and Ang II treated samples. ## denotes a significant difference between Ang II and Ang II+drug-treated samples. Nurr1, nuclear receptor related 1 protein.
Evidence for the involvement of multiple Nox isoforms in Ang II-induced aldosterone synthesis
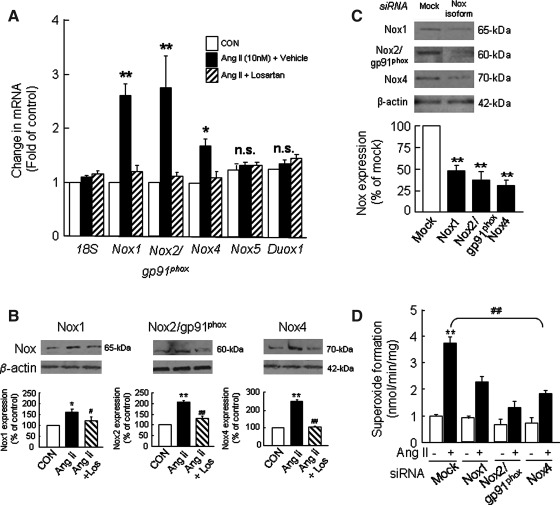
The increase in superoxide formation via Nox activity as observed in Ang II-treated H295R cells may arise from one or more Nox isoforms that include Nox1, Nox2/gp91phox, Nox4, and Nox5 as well as DUOX1 and 2 (12). To begin to delineate the relative contribution of various Nox isoforms to the regulation of aldosterone synthesis by Ang II in H295R cells, we first determined the expression of Nox isoforms in this cell line and then assessed the effect of Ang II on Nox expression. Real-time-polymerase chain reaction (PCR) analysis showed that Nox1, Nox2/gp91phox, Nox4, and Nox5 isoforms as well as Duox1 are expressed in H295R cells; Nox4 occurred at a higher abundance than Nox2 and Nox1. Stimulation of cells with Ang II (10 nM for 6 h) increased Nox1 and Nox2 mRNA by ∼2.7-fold each and Nox4 mRNA by ∼1.7-fold compared with control cells and pretreatment with losartan ameliorated these effects; however, Ang II had no effect on Nox5 and Duox1 mRNA expression (Fig. 6A). Western blot analysis showed that Nox1, Nox2, and Nox4 protein levels are also elevated by Ang II treatment compared with controls; a greater increase was seen with Nox4 and Nox2 than with Nox1 (Fig. 6B).
FIG. 6.
Ang II-induced alterations of Nox isoform expression in H295R cells. Cells treated with Ang II (10 nM for 6 h) in the absence and presence of AT1R antagonist (losartan, 10 μM) were analyzed for Nox isoform mRNA expression by real-time PCR (A), and protein expression was analyzed by western blot analysis (B). Cells not treated with Ang II were used as CON. Effects of transfection of siRNA of mock and specific Nox isoforms on corresponding Nox protein expression (C) and superoxide formation in H295R cells (D) are presented. The protein and mRNA data were normalized using β-actin and 18S, respectively. Superoxide was measured in cell homogenates spectrophotometrically by monitoring SOD (2000 U/ml)-inhibitable, NADPH-dependent oxidation of cytochrome C (80 μM). Results derived from six independent experiments are presented as mean±SEM. * and #p<0.05; ** and ##p<0.01. * and ** denote a significant difference between the control and Ang II treated samples. # and ## denote a significant difference between Ang II and Ang II+drug-treated samples. Los, losartan.
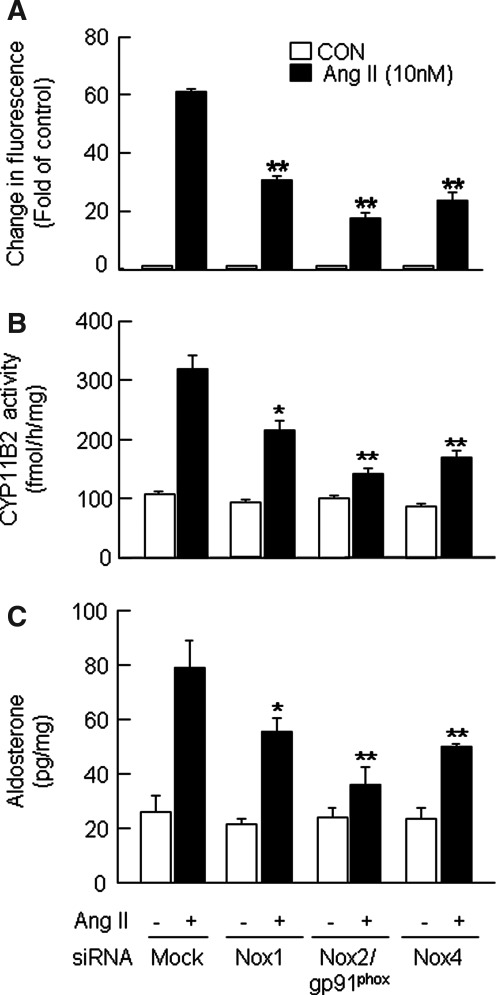
To delineate the relative contribution of Nox isoforms to Ang II-induced aldosterone synthesis, we used the siRNA approach to silence individual Nox isoforms. Cells transfected with siRNA specific to Nox1, 2, and 4 showed significantly reduced expression of corresponding proteins (Fig. 6C). Furthermore, deficiency in Nox isoforms resulted in the attenuation of Ang II-induced increases in superoxide formation (Fig. 6D), DCFDA fluorescence (Fig. 7A), CYP11B2 activity (Fig. 7B), and aldosterone production (Fig. 7C). However, the magnitude of the decrease differs among various Nox isoforms examined. Thus, a greater attenuation of Ang II-induced responses was observed with Nox2 deficiency than that of either Nox4 or Nox1 (Fig. 7).
FIG. 7.
Differential effects of siRNA silencing of Nox isoforms on Ang II-induced changes in ROS, CYP11B2 activity, and aldosterone production in H295R cells. Cells transfected with either mock or Nox1 or Nox2/gp91phox or Nox4 siRNA were treated with Ang II (10 nM for 6 h) or vehicle (CON). Live cells, cell lysates, and culture medium were analyzed for DCFDA fluorescence (A), CYP11B2 activity (B), and aldosterone levels (C). Results derived from six independent experiments are presented as mean±SEM. *p<0.05 and **p<0.01.
Exogenous H2O2 increases CYP11B2 activity and aldosterone production
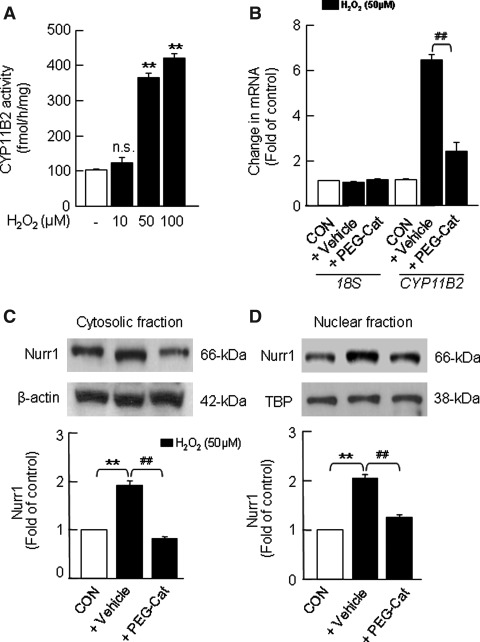
Thus far, the results just presented suggest that H2O2 derived from Ang II-mediated activation of Nox contributes to aldosterone synthesis. To assess the direct effect of H2O2 on aldosterone synthesis, H295R cells were treated with increasing concentrations of H2O2 (10, 50 and 100 μM) for 6 h at 37°C. H2O2, in a concentration-dependent manner, increased DCFDA fluorescence (Supplementary Fig. S5A, B), and CYP11B2 activity with a maximum response elicited at a 50 μM concentration (Fig. 8A). H2O2 (50 μM)-induced increase in CYP11B2 activity was in part due to increases in CYP11B2 mRNA (∼6.2-fold; Fig. 8B) and protein expression (Supplementary Fig. S6). Moreover, H2O2-treated cells showed a robust up regulation of Nurr1-like immunoreactivity in the cytosolic and nuclear-enriched fractions (Fig. 8C, D). In cells pretreated with PEG-catalase (350 U/ml), the H2O2-induced effects were either absent or markedly attenuated (Fig. 8B–D and Supplementary Figs. S5B, right panel and S6).
FIG. 8.
Effects of exogenous H2O2 on CYP11B2 activity and mRNA expression and Nurr1 expression in H295R cells. Cells were treated with H2O2 in the absence and presence of H2O2 scavenger (PEG-Cat, 350 U/ml) for 6 h at 37°C. Cells not treated with H2O2 served as CON. Cell lysates were analyzed for CYP11B2 activity (A) and CYP11B2 mRNA expression (B). Nurr1 protein expression in the cytosolic (C) and nuclear (D) fractions of H295R cells is shown. Nurr1 expression in the cytosol and nucleus was normalized based on the expression level of β-actin and TBP, respectively. Results derived from six independent experiments are presented as mean±SEM. ** and ##p<0.01; n.s., not significant. ** denotes a significant difference between the control and H2O2 treated samples. ## denotes a significant difference between H2O2 and H2O2+drug-treated samples.
Studies in rat adrenal cortex
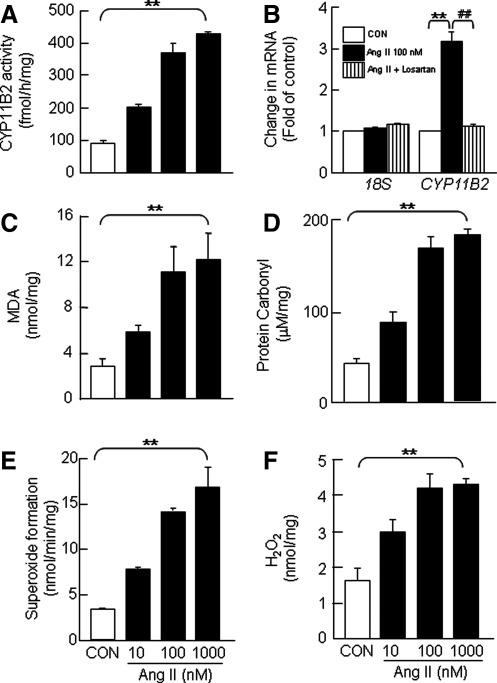
The described studies just mentioned showed that Ang II increases aldosterone production in H295R cells via mechanisms involving activation of Nox-derived H2O2 signaling pathways. In order to test the validity of these cell line-derived findings in rodent tissues, we examined the effects of Ang II on aldosterone synthesis in vitro in rat adrenal cortical slices containing zona glomerulosa, a major site of aldosterone synthesis. Exposure of adrenal cortical sections to Ang II increased DCFDA fluorescence (Supplementary Fig. S7A), CYP11B2 activity (Fig. 9A; Supplementary Fig. S8A), CYP11B2 mRNA (Fig. 9B), CYP11B2 protein (Supplementary Fig. S7B), indices of ROS generation, including malondialdehyde (MDA; Fig. 9C) and protein carbonyl (Fig. 9D) levels, superoxide formation via Nox activity (Fig. 9E), and H2O2 levels (Fig. 9F; Supplementary Fig. S8B) in a concentration- and time-dependent manner. Unlike in H295R cells, a 10-fold higher concentration of Ang II was required to elicit a maximum increase in these variables.
FIG. 9.
Ang II-induced changes in CYP11B2 activity and mRNA expression and ROS levels in rat adrenal cortex. Rat adrenal cortical slices treated with increasing concentrations of Ang II (10, 100, and 1000 nM for 6 h) were analyzed for CYP11B2 activity (A), CYP11B2 mRNA (B), and MDA levels as assessed by T-BARS assay (C), protein carbonyl levels (D), superoxide formation (E), and H2O2 levels (F). Slices not treated with Ang II served as CON. Superoxide and H2O2 generation was measured spectrophotometrically by monitoring SOD (2000 U/ml)-inhibitable, NADPH-dependent oxidation of cytochrome C (80 μM) and catalase-inhibitable, oxidation of Amplex Red (100 μM) to resorufin, respectively, as described in Materials and Methods section. Results derived from six independent experiments are presented as mean±SEM. ** and ##p<0.01. ** denotes a significant difference between the control and Ang II treated adrenal cortical slices. ## denotes a significant difference between Ang II and Ang II+drug-treated adrenal cortical slices. MDA, malondialdehyde.
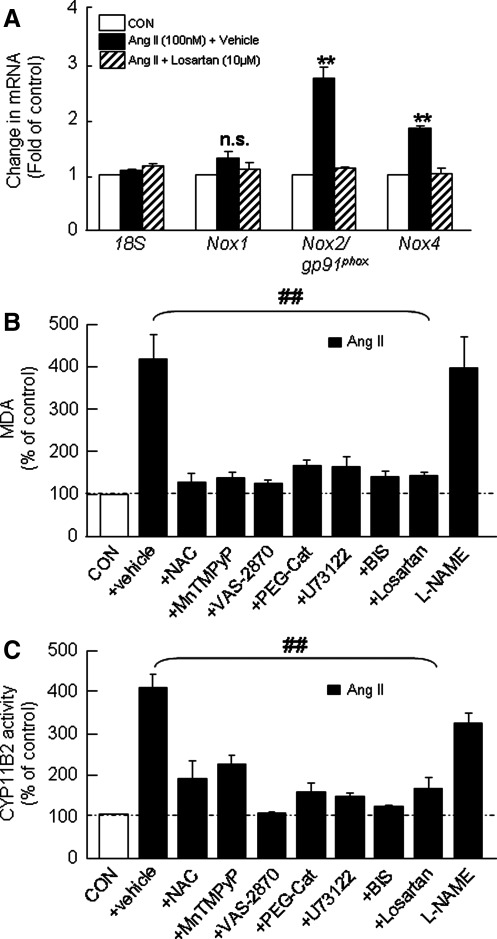
Rat adrenal cortex expresses Nox1, Nox2, and Nox4 mRNAs, whereas Nox5, DUOX1, and 2 mRNAs are not detected. Similar to H295R cells, Ang II upregulated Nox2 and Nox4 but not Nox1 mRNA expression in the rat adrenal cortex, and losartan abolished this effect (Fig. 10A). Pretreatment of adrenal cortex with either antioxidants (NAC and MnTMPyP) or H2O2 scavenger (PEG-catalase) or Nox inhibitor (VAS-2870) or PLC inhibitor (U73122) or PKC inhibitor (BIS) either prevented or significantly attenuated Ang II-evoked elevation of MDA levels and CYP11B2 activity, whereas NOS inhibitor (L-NAME) was without any significant effect (Fig. 10B, C).
FIG. 10.
Differential induction of Nox isoforms by Ang II and effects of inhibitors of AT1R-PLC-PKC-Nox pathway on Ang II-induced changes in ROS and CYP11B2 activity in rat adrenal cortical slices. The changes in Nox1, Nox2, and Nox4 mRNA expression (A) are shown. Comparison of the effects of antioxidants (NAC, 200 μM and MnTMPyP, 100 μM), H2O2 scavenger (PEG-Cat, 350 U/ml), inhibitor of Nox (VAS-2870, 20 μM), NOS (L-NAME, 10 μM), PLC (U73122, 10 μM) and PKC (BIS, 10 μM), and AT1R antagonist (losartan, 10 μM) on Ang II (100 nM for 6 h)-induced changes in MDA levels (B) and CYP11B2 activity (C) are shown. Results derived from six independent experiments are presented as mean±SEM. ** and ##p<0.01, and n.s., not significant. ** denotes a significant difference between the control and Ang II treated adrenal cortical slices. ## denotes a significant difference between Ang II and Ang II+drug-treated adrenal cortical slices. PKC, protein kinase C; BIS, bisindolylmaleimide.
Discussion
The overall goal of the present study was to determine whether the regulation of aldosterone synthesis by Ang II involves ROS signaling and to identify the underlying mechanisms. Our results demonstrate that Ang II-induced aldosterone production in adrenal cortical cells is mediated by Nox-derived H2O2 and the ensuing transcriptional and translational activation of CYP11B2.
Ang II increases ROS generation in adrenal cortical cells
Our data showed that Ang II increases DCFDA fluorescence, MDA, and protein carbonyl levels in human H295R cells and rat adrenal cortex in a concentration- and time-dependent manner involving AT1R activation. These results along with our findings of higher levels of superoxide formation via Nox activation and increased H2O2 levels in Ang II treated cells compared with control cells suggest that Ang II induces a rise in ROS comprising both superoxide and its dismutated product, H2O2 in adrenal cortical cells.
The agonist-evoked ROS elevation could be due to either an increase in ROS synthesis via upregulation of ROS-generating enzymes or a reduction in ROS degradation via down regulation of antioxidant enzymes. Nox family (15), xanthine oxidase, and uncoupled eNOS contribute to ROS production (10), whereas ROS degradation is mediated by antioxidant enzymes that include superoxide dismutase 1 and 2 (SOD-1 and SOD-2), catalase, and glutathione peroxidase. Ang II is shown to facilitate ROS generation in cardiac fibroblasts (23), vascular smooth muscle cells (45), adventitial cells (40), and renal mesangial (19) cells. On the other hand, other studies showed that Ang II selectively reduces the activity and expression of catalase in cardiac fibroblasts (51) and that of Mn-SOD in aortic fibroblasts without impairing the activities of other antioxidant enzymes (23). The studies just mentioned suggest that depending on the cell type, Ang II can either facilitate ROS production or attenuate ROS degradation contributing to elevated cellular ROS levels. Our results showed that Ang II increases superoxide generating Nox activity, and Nox inhibitors prevented Ang II-induced increase in ROS levels, suggesting that Ang II facilitates ROS generation. Interestingly, pretreatment with PEG-catalase, which degrades ROS, prevented Ang II-mediated increase in ROS levels. These findings raise the possibility that additional mechanisms involving a possible reduction in the antioxidant capacity of adrenal cortical cells may contribute to Ang II-induced raise in ROS levels.
Involvement of ROS signaling in Ang II-mediated aldosterone synthesis
A novel finding of the present study is that Ang II-induced aldosterone synthesis in adrenal cortical cells involves activation of ROS signaling pathways. Although it is well established that Ang II elevates ROS levels in several cell lines (12, 15) and that it stimulates aldosterone synthesis via activation of AT1R-mediated signaling pathways (1, 2), the possible involvement of ROS in Ang II-mediated aldosterone synthesis has not been directly examined. Several lines of evidence from this study suggest that induction of ROS signaling via AT1R activation contributes to Ang II-evoked aldosterone synthesis. These include (i) Ang II increased the activity and expression of CYP11B2, the rate-limiting enzyme in aldosterone biosynthetic pathway with a concomitant elevation in superoxide generation via Nox activity and H2O2 levels via dismutation of superoxide; (ii) AT1R antagonist prevented not only Ang II-mediated activation of CYP11B2 but also the rise in superoxide and H2O2 levels, and (iii) Antioxidants, Nox inhibitor, and H2O2 scavenger, which prevented the rise in superoxide and H2O2 levels also markedly attenuated Ang II-induced increases in the activity and expression of CYP11B2 and aldosterone levels. Collectively, these correlative observations suggest that superoxide/H2O2 signaling contributes to Ang II-mediated up regulation of CYP11B2 transcription, translation, and activity as well as the ensuing aldosterone production.
Evidence for the role of Nox-derived H2O2 in Ang II-induced aldosterone synthesis
ROS including superoxide and hydrogen peroxide have been identified as important signaling molecules in biological systems. The activation of oxidases, especially that of Noxs, which are a family of seven widely distributed enzymes, generate superoxide (12). We found that human H295R cells and rat adrenal cortex express both inducible (Nox1 and Nox2) and constitutively active (Nox4) Nox isoforms. Additionally, H295R cells also express Nox5 and DUOX1. Ang II treatment induced a ∼3-fold increase in Nox activity in H295R cells and adrenal cortical cells, which is consistent with an earlier report in rat aorta wherein Ang II is shown to increase Nox activity (35). The increase in Nox activity by Ang II in H295R cells seems to occur primarily via up regulation of Nox1, Nox2, and Nox4 mRNA and protein expression whereas it occurs in rat adrenal cortical cells via elevation of Nox2 and Nox4 mRNAs. Since losartan prevented this response, it is likely that signaling pathways activated by AT1R are required for increased Nox expression and activation. AT1R is known to activate PLC-DAG-PKC signaling in several cell types. The finding that inhibitors of both PLC and PKC prevented Ang II-induced elevation in ROS (as evidenced by DCFDA fluorescence) suggests that AT1R-PLC-PKC signaling contributes to activation and upregulation of Nox isoforms and the ensuing ROS generation in adrenal cortical cells.
It is conceivable that Nox-derived H2O2 may mediate the increase in CYP11B2 activity by Ang II. Such a possibility is supported by the followings findings: (i) Ang II-induced increase in DCFDA fluorescence in H295R cells could be detected as early as 10 min after Ang II treatment and was prevented by Nox inhibitor (VAS-2870). (ii) The increase in the steady-state level of H2O2 was discernible only after 3 h of Ang II exposure. Nox inhibitor and PEG-catalase, which metabolize H2O2, attenuated Ang II-induced rise in H2O2. (iii) Ang II-mediated increases in CYP11B2 activity, mRNA and protein expression, and aldosterone production were markedly attenuated by both Nox inhibitor and PEG-catalase. (iv) Exogenous H2O2 dose-dependently augmented CYP11B2 activity, protein, and mRNA in H295R cells as well as rat adrenal cortical cells, and these effects were markedly attenuated by PEG-catalase. Interestingly, a significant increase in H2O2 levels was seen only after 3 h of exposure to Ang II, coinciding with Ang II-induced rise in aldosterone levels and CYP11B2 activity (which are not evident at earlier time points). It is, therefore, conceivable that Nox1, 2, and 4-dependent increase in superoxide either via enzyme activation or increased expression facilitates a sustained increase in the steady-state levels of H2O2, which after reaching a threshold level increases aldosterone synthesis.
We found that mitochondrial aconitase activity, which is a sensitive measure of mitochondrial ROS levels, is significantly reduced only after 3 h treatment with Ang II, suggesting that ROS levels in the mitochondria are also elevated by Ang II. Given that significant increases in H2O2 levels, CYP11B2 activity, and aldosterone levels are also seen after 3 h, it is likely that mitochondria-derived ROS also contributes to Ang II-induced aldosterone synthesis. Notably, allopurinol, an inhibitor of xanthine oxidase, and L-NAME, an NOS inhibitor, showed no significant effect on either ROS levels or aldosterone production in Ang II-treated cells. Therefore, it is likely that the contribution of ROS derived from either xanthine oxidase or NOS to aldosterone synthesis by Ang II is rather negligible. Collectively, our results lend support to the involvement of H2O2 derived from Nox1, 2, and 4 and also from mitochondria in the regulation of aldosterone synthesis by Ang II. However, the mechanism(s) by which Ang II activates various Nox isoforms and induces mitochondrial ROS in adrenal cortical cells remains to be established. It is also possible that a positive feed forward mechanism involving interactions between either Nox2 or Nox4 with CYP11B2, which is a cytochrome P450 enzyme, may contribute to ROS generation by Ang II in adrenocortical cells, similar to that recently reported (11).
The signaling pathways associated with the transcriptional regulation of CYP11B2 by Ang II via AT1R have been well documented (27). These pathways include PLC-Gq/11 (2), src family of tyrosine kinase (41), and the 12-lipoxygenase pathway (16) and converge on the synthesis of Nurr1 and NGF1-B family of transcription factors to facilitate CYP11B2 expression (28, 29, 38). Among them, the PLC pathway is also coupled to Nox activation via activation of PKC (12). Losartan and inhibitors of PLC and PKC blocked Ang II-induced generation of superoxide, suggesting that AT1R-PLC-PKC signaling contributes to Nox-derived H2O2 formation in adrenal cortical cells. Since H2O2 stimulated Nurr1 protein expression in H295R cells and PEG-catalase inhibited these effects, it is conceivable that by directly stimulating Nurr1 expression, Nox-derived H2O2 facilitates the transcription and translation of CYP11B2 and the ensuing aldosterone synthesis, a possibility that remains to be investigated.
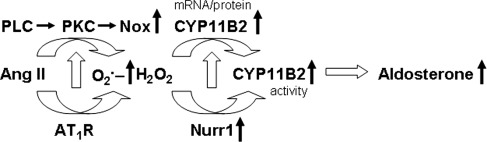
In summary, we present evidence for the involvement of AT1R-dependent, Nox-derived H2O2 signaling in Ang II-mediated augmentation of CYP11B2 activity and aldosterone production in the human and rat adrenal cortical cells (Fig. 11). Available information suggests that 20%–30% of adult populations are affected by hypertension (20, 30). Ang II is an important component of the renin-angiotensin-aldosterone-system (RAAS), which is a major regulator of blood pressure. Our finding that Nox-derived H2O2 mediates Ang II-induced aldosterone synthesis provides new insights into the regulatory mechanisms of the RAAS. This newly identified functional link between Ang II, AT1R, Nox-derived H2O2, CYP11B2 activity, and aldosterone production will aid in the development of antioxidant-based combination therapy for the treatment of excessive aldosterone-dependent systemic arterial hypertension.
FIG. 11.
Proposed model of Nox-derived H2O2 in Ang II-induced aldosterone synthesis in adrenal cortical cells. Upward arrows denote an increase. ETC, electron transport chain; H2O2, hydrogen peroxide; O2.−, superoxide anion.
Materials and Methods
Cell cultures
H295R human adrenocortical tumor cells were obtained from the American Type Culture Collection. Cells were cultured in DME/Ham's F-12 medium supplemented with 2.5% Nu-Serum I, 1% ITS/Premix, 1% penicillin/streptomycin, and 0.01% gentamycin. Cells were maintained and grown on 75-cm2 flasks in a humidified atmosphere of 5% CO2 at 37°C. Experiments were performed on cells grown to ∼80%–90% confluence between passages 7 and 11. Before treatment with various reagents (for 30 min), the cells were grown in serum- and antibiotic-free medium for 16 h.
Animals
The Institutional Animal Care and Use Committee of the University of Chicago approved animal handling and experimental protocols. The studies were performed on adult, male Sprague-Dawley rats weighing 200–250 g. Animal were housed two per cage in a temperature-controlled room on a 12-h light/dark cycle (6AM/6PM) and were given food and water ad libitum. Adrenal glands from anesthetized rats were harvested, and adrenal cortical layers containing zona glomerulosa were dissected and stored at −80°C until further analysis.
Extraction and enzyme immunoassay of aldosterone
Aldosterone, released into the culture medium or formed during in vitro enzymatic reactions, was determined using an aldosterone specific EIA kit (Cayman Chemical Company) as per the manufacturer's instructions. The detection limit of the enzyme immunoassay (EIA) for aldosterone was 20 pg/ml.
CYP11B2 assay
CYP11B2 activity was assayed using the procedure as previously described (36). Briefly, either H295R cells or adrenal cortical slices were homogenized in 100 μl of 50 mM Tris-HCl, pH 7.4 (assay buffer) containing protease inhibitor cocktail (Calbiochem). The reaction mixture containing 20 μg protein equivalent of tissue or cell-free extract, 10 μl assay buffer, and 20 μl substrate mix (2.5×NADPH Regeneration Solution A, 2.5×NADPH Regeneration Solution B and 1 μM 11-deoxycorticosterone) was incubated at 25°C for 4 h. The aldosterone formed during the reaction was determined by EIA as just described. CYP11B2 activity was expressed as the amount of aldosterone formed per hour per milligram protein. Protein concentration was determined using a Bio-Rad Protein Assay Kit.
Nox assay
To assess Nox activity, cellular superoxide formation was measured spectrophotometrically using the procedure as previously described (7). NADPH (200 μM)-dependent oxidation of cytochrome C (80 μM) in either the absence or presence of SOD (2000 U/ml) was monitored at 550 nm for 5 min. Nox activity was expressed as nmoles of SOD-inhibitable reduced cytochrome C (ɛ550nm=21 mM−1.cm−1) formed per minute per milligram protein. The specificity of the assay was assessed using VAS-2870 (a potent inhibitor of Nox activity) as well as apocynin, which was recently identified as an antioxidant rather than a Nox inhibitor (18).
Real-time PCR
Real-time PCR was performed using a MiniOpticon system (Bio-Rad) with SYBR GreenER two-step quantitative RT-PCR kit (Invitrogen) as previously described (32). The primer sequences used for real-time PCR analysis of 18S, CYP11B2, Nox1, Nox2, Nox4, Nox5, and Duox1 in human and rat adrenal cortical cells are shown in Supplementary Table S1. The changes in mRNA expression of various genes were calculated using the comparative threshold (CT) method. Values were compared with an internal standard gene 18S.
Knockdown of gp91phox in H295R cells
In order to knock down Nox1, Nox2/gp91phox, and Nox4, siRNAs against human Nox1, 2, and 4 were commercially purchased (Santa Cruz Biotechnology), and scramble siRNA was used as control. H295R cells (5×104) were plated in 24-well plates and transfected with Nox isoform siRNAs using siRNA transfection reagent according to the manufacturer's protocol. After 24 h, the culture medium was replaced with fresh supplemented medium, and the cells were maintained for an additional 24 h.
Preparation of nuclear and cytosolic fractions
Nuclear and cytosolic fractions of H295R cells were prepared as previously described (25). Briefly, cells (10×106) were extracted with 10 mM HEPES buffer, pH 7.9 containing complete protease inhibitor (Roche Diagnostics). Cell lysates were centrifuged at ∼450 g for 3 min at 4°C. The resulting supernatant and the pellet containing the cytosolic and nuclear-enriched fractions, respectively, were stored at −80°C till further analysis. Before analysis, the pellet containing the nuclear fraction was solubilized in the extraction buffer.
Western blot analysis
Immunoblot analysis was performed as previously described (33). The following antibodies from Santa Cruz Biotechnology were used: goat anti-Nox1 (1:250), goat anti-Nox-2/gp91phox (1:500), rabbit anti-Nox4 (1:500), goat anti-CYP11B2 (1:250), and rabbit anti-Nurr1 (1:1000). In addition, for data normalization, mouse anti-β-actin (1:10,000; Sigma) and mouse anti-TATA binding protein (TBP; 1:3000; Abcam) antibodies were also used. Either donkey anti-goat (1:4000) or goat anti-rabbit (1:4000) or goat anti-mouse (1:10,000) horseradish peroxidase-conjugated secondary antibody from Santa Cruz Biotechnology is used. The immunoreactive proteins were identified using the ECL detection kit from Amersham Biosciences. As loading controls, the expression levels of either β-actin (42-kDa; cytosol) or TBP (38-kDa; nuclear) were monitored.
Measurement of ROS using DCFDA fluorescence
Ang II-induced ROS generation in H295R cells was monitored by fluorescence microscopy as described in the Supplementary Data.
Measurement of MDA
Adrenal cortical slices were homogenized, and MDA levels in the homogenates were determined as a measure of lipid oxidation as previously described (32) and expressed as nanomoles per milligram of protein.
Protein carbonyl content
Carbonyl content in proteins was determined using the procedures as previously described (22). Protein homogenates (10–20 μg protein equivalent) were incubated with 2,4-dinitrophenylhydrazine (DNPH, 10 mM) at room temperature for 1 h with frequent mixing. Excess DNPH in the reaction medium was extracted with ethanol/ethylacetate mixture, and the remaining pellet was extracted with guanidine hydrochloride at 37°C. The absorbance of the extract at 380 nm was measured, and the carbonyl content was calculated using the molar extinction coefficient of DNP (ɛ380nm=22,000 M−1.cm−1).
Measurement of H2O2
H2O2 was measured using a commercially available Amplex Red assay kit (Molecular Probes) following the manufacturer's instructions (53). Briefly, either the cell lysate prepared in phosphate buffer or a known amount of H2O2 was incubated in the dark with Amplex Red (100 μM) and horseradish peroxidase (1 U/ml) in the presence and absence of catalase (2000 U/ml) for 30 min at room temperature, and the absorbance of the solution was measured at 560 nm. The concentration of H2O2 was determined based on the standard curve related to the concentration of H2O2 and absorbance at 560 nm and expressed as the amount of Amplex Red oxidized (peroxidase-dependent and catalase inhibitable) per milligram of protein.
Experimental protocols
Series 1
The effects of Ang II (1, 10, and 100 nM) on ROS levels in human H295R cells and rat adrenal cortical slices were determined by monitoring the changes in DCFDA fluorescence and MDA levels, respectively (n=6 experiments in each group). In parallel experiments, the effects of antioxidants (NAC, 200 μM; MnTMPyP, 100 μM), H2O2 scavenger (PEG-catalase, 350 U/ml), Nox inhibitor (VAS-2870, 20 μM), NOS inhibitor (L-NAME, 10 μM), PLC inhibitor and its inactive analog (U73122 and U73343, respectively, 10 μM each), PKC (BIS, 10 μM), xanthine oxidase inhibitor (allopurinol, 100 μM), and AT1R antagonist (losartan, 10 μM) on Ang II-induced changes in DCFDA fluorescence and MDA levels were determined (n=6 experiments in each group). In addition, changes in DCFDA fluorescence in response to Ang II were examined in mock, Nox1, Nox2/gp91phox, and Nox4 siRNA transfected H295R cells (n=6 experiments in each group).
Series 2
The changes in H2O2 levels were measured in H295R cells (∼5×105) treated with either vehicle, Ang II alone or in the presence of AT1R antagonist (losartan, 10 μM) or Nox inhibitor (VAS-2870, 20 μM) or H2O2 scavenger (PEG-catalase, 350 U/ml) (n=6 experiments in each group).
Series 3
CYP11B2 activity was determined in whole cell extracts prepared from either mock (control) or Nox1 or Nox2/gp91phox or Nox4 siRNA transfected H295R cells (∼5×105) or rat adrenal cortical slices (n=6 rats) treated with either Ang II (1–1000 nM) or vehicle for 6 h at 37°C (n=6 experiments in each group). In parallel experiments, the effects of AT1R antagonist, inhibitors of PLC, PKC, Nox, and NOS and antioxidants on Ang II-induced changes in CYP11B2 activity and protein expression were assessed (n=6 experiments in each group).
Series 4
Aldosterone levels were determined in cell culture medium of H295R cells (∼5×105) treated with either Ang II (1, 10, and 100 nM) or vehicle for 6 h at 37°C (n=6 experiments in each group). In parallel experiments, the effects of AT1R antagonist, inhibitors of PLC, PKC, Nox, and NOS and antioxidants on Ang II-induced changes in aldosterone levels were assessed (n=6 experiments in each group).
Series 5
Nox activity and mRNA and protein levels of Nox isoforms were determined in H295R cells, adrenal cortical slices, and mock, Nox1, Nox2/gp91phox, and Nox4 siRNA transfected H295R cells treated with vehicle or Ang II (10 nM for 6 h) (n=6 experiments in each group). The effects of losartan and Nox inhibitor (VAS-2870, 20 μM) on Nox enzyme activity were also investigated (n=6 experiments in each group).
Series 6
The effects of exogenous H2O2 (10, 50 and 100 μM for 6 h) on CYP11B2 activity, mRNA, and protein expression as well as on DCFDA fluorescence in untreated and H2O2 scavenger (PEG-catalase, 350 U/ml for 30 min)-pretreated H295R cells (∼5×105) were determined (n=6 experiments in each group).
Data analysis
In all experiments, the samples were analyzed in triplicate. All data are expressed as mean±SEM. The statistical significance was evaluated by an unpaired t-test. A difference with a p-value<0.05 was considered significant.
Supplementary Material
Abbreviations used
- Ang II
angiotensin II
- Apo
apocynin
- AT1R
angiotensin II type I receptor
- BIS
bisindolylmaleimide
- CYP11B2
aldosterone synthase
- DAG
diacylglycerol
- DAPI
4′, 6-diamidino-2-phenylindole
- DCFDA
6-Carboxy-2′,7′-dichlorodihydrofluorescein diacetate
- DNPH
2,4-dinitrophenylhydrazine
- EIA
enzyme immunoassay
- ETC
electron transport chain
- H2O2
hydrogen peroxide
- L-NAME
L-NG-Nitroarginine methyl ester
- MDA
malondialdehyde
- MnTMPyP
manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin pentachloride
- NAC
N-acetyl cysteine
- NGFI-B
neuronal growth factor-induced clone B
- NOS
nitric oxide synthase
- Nox
NADPH oxidase
- Nurr1
nuclear receptor related 1 protein
- O2.-
superoxide anion
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PEG-Cat
PEG-catalase
- PKC
protein kinase C
- PLC
phospholipase C
- RAAS
rennin-angiotensin-aldosterone-system
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TBP
TATA binding protein
- VAS-2870
3-Benzyl-7-(2-benzoxazolyl)thio-1,2,3-triazolo(4,5-d)pyrimidine
Acknowledgments
This study was supported by the National Heart, Lung, and Blood Institute grant HL-090554 (N.R.P.) and HL-089616 (G.K.K.).
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Bassett MH. Suzuki T. Sasano H. White PC. Rainey WE. The orphan nuclear receptors NURR1 and NGFIB regulate adrenal aldosterone production. Mol Endocrinol. 2004a;18:279–290. doi: 10.1210/me.2003-0005. [DOI] [PubMed] [Google Scholar]
- 2.Bassett MH. White PC. Rainey WE. The regulation of aldosterone synthase expression. Mol Cell Endocrinol. 2004b;217:67–74. doi: 10.1016/j.mce.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Bendall JK. Cave AC. Heymes C. Gall N. Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 4.Bird IM. Hanley NA. Word RA. Mathis JM. McCarthy JL. Mason JI. Rainey WE. Human NCI-H295 adrenocortical carcinoma cells: a model for angiotensin-II-responsive aldosterone secretion. Endocrinology. 1993;133:1555–1561. doi: 10.1210/endo.133.4.8404594. [DOI] [PubMed] [Google Scholar]
- 5.Capponi AM. Lew PD. Jornot L. Vallotton MB. Correlation between cytosolic free Ca2+ and aldosterone production in bovine adrenal glomerulosa cells. Evidence for a difference in the mode of action of angiotensin II and potassium. J Biol Chem. 1984;259:8863–8869. [PubMed] [Google Scholar]
- 6.Catt KJ. Balla T. Baukal AJ. Hausdorff WP. Aguilera G. Control of glomerulosa cell function by angiotensin II: transduction by G-proteins and inositol polyphosphates. Clin Exp Pharmacol Physiol. 1988;15:501–515. doi: 10.1111/j.1440-1681.1988.tb01108.x. [DOI] [PubMed] [Google Scholar]
- 7.Chamulitrat W. Stremmel W. Kawahara T. Rokutan K. Fujii H. Wingler K. Schmidt HH. Schmidt RA. Constitutive NADPH oxidase-like system containing gp91phox homologs in human keratinocytes. J Invest Dermatol. 2004;122:1000–1009. doi: 10.1111/j.0022-202X.2004.22410.x. [DOI] [PubMed] [Google Scholar]
- 8.Dikalov SI. Dikalova AE. Bikineyeva AT. Schmidt HH. Harrison DG. Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doughan AK. Harrison DG. Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 10.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 11.Eid AA. Gorin Y. Fagg BM. Maalouf R. Barnes JL. Block K. Abboud HE. Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes. 2009;58:1201–1211. doi: 10.2337/db08-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrido AM. Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol. 2009;302:148–158. doi: 10.1016/j.mce.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griendling KK. Minieri CA. Ollerenshaw JD. Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 14.Griendling KK. Sorescu D. Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 15.Groemping Y. Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu J. Wen Y. Mison A. Nadler JL. 12-lipoxygenase pathway increases aldosterone production, 3,5-cyclic adenosine monophosphate response element-binding protein phosphorylation, and p38 mitogen-activated protein kinase activation in H295R human adrenocortical cells. Endocrinology. 2003;144:534–543. doi: 10.1210/en.2002-220580. [DOI] [PubMed] [Google Scholar]
- 17.Hanna IR. Taniyama Y. Szöcs K. Rocic P. Griendling KK. NAD(P)H oxidase-derived reactive oxygen species as mediators of angiotensin II signaling. Antioxid Redox Signal. 2002;4:899–914. doi: 10.1089/152308602762197443. [DOI] [PubMed] [Google Scholar]
- 18.Heumüller S. Wind S. Barbosa-Sicard E. Schmidt HH. Busse R. Schröder K. Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 19.Jaimes EA. Galceran JM. Raij L. Angiotensin II induces superoxide anion production by mesangial cells. Kidney Int. 1998;54:775–784. doi: 10.1046/j.1523-1755.1998.00068.x. [DOI] [PubMed] [Google Scholar]
- 20.Kearney PM. Whelton M. Reynolds K. Muntner P. Whelton PK. He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 21.Kimura S. Zhang GX. Nishiyama A. Shokoji T. Yao L. Fan YY. Rahman M. Suzuki T. Maeta H. Abe Y. Role of NAD(P)H oxidase- and mitochondria-derived reactive oxygen species in cardioprotection of ischemic reperfusion injury by angiotensin II. Hypertension. 2005;45:860–866. doi: 10.1161/01.HYP.0000163462.98381.7f. [DOI] [PubMed] [Google Scholar]
- 22.Levine RL. Garland D. Oliver CN. Amici A. Climent J. Lenz AG. Ahn BW. Shaltiel S. Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 23.Lijnen PJ. van Pelt JF. Fagard RH. Downregulation of manganese superoxide dismutase by angiotensin II in cardiac fibroblasts of rats: association with oxidative stress in myocardium. Am J Hypertens. 2010;23:1128–1135. doi: 10.1038/ajh.2010.128. [DOI] [PubMed] [Google Scholar]
- 24.Lisurek M. Bernhardt R. Modulation of aldosterone and cortisol synthesis on the molecular level. Mol Cell Endocrinol. 2004;215:149–159. doi: 10.1016/j.mce.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Liu D. Jia H. Holmes DIR. Stannard A. Zachary I. Vascular endothelial growth factor–regulated gene expression in endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:2002–2007. doi: 10.1161/01.ATV.0000098644.03153.6F. [DOI] [PubMed] [Google Scholar]
- 26.Nogueira EF. Rainey WE. Regulation of aldosterone synthase by activator transcription factor/cAMP response element-binding protein family members. Endocrinology. 2010;151:1060–1070. doi: 10.1210/en.2009-0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nogueira EF. Bollag WB. Rainey WE. Angiotensin II regulation of adrenocortical gene transcription. Mol Cell Endocrinol. 2009a;302:230–236. doi: 10.1016/j.mce.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nogueira EF. Vargas CA. Otis M. Gallo-Payet N. Bollag WB. Rainey WE. Angiotensin-II acute regulation of rapid response genes in human, bovine, and rat adrenocortical cells. J Mol Endocrinol. 2007;39:365–374. doi: 10.1677/JME-07-0094. [DOI] [PubMed] [Google Scholar]
- 29.Nogueira EF. Xing Y. Morris CA. Rainey WE. Role of angiotensin II-induced rapid response genes in the regulation of enzymes needed for aldosterone synthesis. J Mol Endocrinol. 2009b;42:319–330. doi: 10.1677/JME-08-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paulis L. Unger T. Novel therapeutic targets for hypertension. Nat Rev Cardiol. 2010;7:431–441. doi: 10.1038/nrcardio.2010.85. [DOI] [PubMed] [Google Scholar]
- 31.Raff H. Ball DL. Goodfriend TL. Low oxygen selectively inhibits aldosterone secretion from bovine adrenocortical cells in vitro. Am J Physiol. 1989;256:E640–E644. doi: 10.1152/ajpendo.1989.256.5.E640. [DOI] [PubMed] [Google Scholar]
- 32.Raghuraman G. Kalari A. Dhingra R. Prabhakar NR. Kumar GK. Enhanced neuropeptide Y synthesis during intermittent hypoxia in the rat adrenal medulla: role of reactive oxygen species-dependent alterations in precursor peptide processing. Antioxid Redox Signal. 2011;14:1179–1190. doi: 10.1089/ars.2010.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raghuraman G. Prabhakar NR. Kumar GK. Post-translational modification of glutamic acid decarboxylase 67 by intermittent hypoxia: evidence for the involvement of dopamine D1 receptor signaling. J Neurochem. 2010;115:1568–1578. doi: 10.1111/j.1471-4159.2010.07063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rainey WE. Bird IM. Mason JI. The NCI-H295 cell line: a pluripotent model for human adrenocortical studies. Mol Cell Endocrinol. 1994;100:45–50. doi: 10.1016/0303-7207(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 35.Rajagopalan S. Kurz S. Münzel T. Tarpey M. Freeman BA. Griendling KK. Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rigel DF. Fu F. Beil M. Hu C-W. Liang G. Jeng AY. Pharmacodynamic and pharmacokinetic characterization of the aldosterone synthase inhibitor FAD286 in two rodent models of hyperaldosteronism: comparison with the 11β-hydroxylase inhibitor metyrapone. J Pharmacol Exp Ther. 2010;334:232–243. doi: 10.1124/jpet.110.167148. [DOI] [PubMed] [Google Scholar]
- 37.Rodríguez-Puyol M. Griera-Merino M. Pérez-Rivero G. Díez-Marqués ML. Ruiz-Torres MP. Rodríguez-Puyol D. Ang II induces a rapid and transient increase of reactive oxygen species. Antioxid Redox Signal. 2002;4:869–875. doi: 10.1089/152308602762197407. [DOI] [PubMed] [Google Scholar]
- 38.Romero DG. Rilli S. Plonczynski MW. Yanes LL. Zhou MY. Gomez-Sanchez EP. Gomez-Sanchez CE. Adrenal transcription regulatory genes modulated by angiotensin II and their role in steroidogenesis. Physiol Genomics. 2007;30:26–34. doi: 10.1152/physiolgenomics.00187.2006. [DOI] [PubMed] [Google Scholar]
- 39.Schalekamp MA. Wenting GJ. Man in't Veld AJ. Pathogenesis of mineralocorticoid hypertension. Clin Endocrinol Metab. 1981;10:397–418. doi: 10.1016/s0300-595x(81)80005-9. [DOI] [PubMed] [Google Scholar]
- 40.Shen WL. Gao PJ. Che ZQ. Ji KD. Yin M. Yan C. Berk BC. Zhu DL. NADPH oxidase-derived reactive oxygen species regulate angiotensin II-induced adventitial fibroblast phenotypic differentiation. Biochem Biophys Res Commun. 2006;339:337–343. doi: 10.1016/j.bbrc.2005.10.207. [DOI] [PubMed] [Google Scholar]
- 41.Sirianni R. Carr BR. Pezzi V. Rainey WE. A role for src tyrosine kinase in regulating adrenal aldosterone production. J Mol Endocrinol. 2001;26:207–215. doi: 10.1677/jme.0.0260207. [DOI] [PubMed] [Google Scholar]
- 42.Szekeres M. Turu G. Orient A. Szalai B. Supeki K. Cserzo M. Varnai P. Hunyady L. Mechanisms of angiotensin II- mediated regulation of aldosterone synthase expression in H295R human adrenocortical and rat adrenal glomerulosa cells. Mol Cell Endocrinol. 2009;302:244–253. doi: 10.1016/j.mce.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 43.ten Freyhaus H. Huntgeburth M. Wingler K. Schnitker J. Bäumer AT. Vantler M. Bekhite MM. Wartenberg M. Sauer H. Rosenkranz S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res. 2006;71:331–341. doi: 10.1016/j.cardiores.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 44.Tomaschitz A. Pilz S. Ritz E. Obermayer-Pietsch B. Pieberet TR. Aldosterone and arterial hypertension. Nat Rev Endocrinol. 2010;6:83–93. doi: 10.1038/nrendo.2009.263. [DOI] [PubMed] [Google Scholar]
- 45.Touyz RM. Schiffrin EL. Ang II-stimulated superoxide production is mediated via phospholipase D in human vascular smooth muscle cells. Hypertension. 1999;34:976–982. doi: 10.1161/01.hyp.34.4.976. [DOI] [PubMed] [Google Scholar]
- 46.Touyz RM. Yao G. Viel E. Amiri F. Schiffrin EL. Angiotensin II and endothelin-1 regulate MAP kinases through different redox-dependent mechanisms in human vascular smooth muscle cells. J Hypertens. 2004;22:1141–1149. doi: 10.1097/00004872-200406000-00015. [DOI] [PubMed] [Google Scholar]
- 47.Ulick S. Selective defects in the biosynthesis of aldosterone. In: New MI, editor; Levine LS, editor; Laron Z, editor. Adrenal Diseases in Childhood. Basel, Switzerland: Karger; 1984. pp. 145–155. [Google Scholar]
- 48.Vallotton MB. Rossier MF. Capponi AM. Potassium-angiotensin interplay in the regulation of aldosterone biosynthesis. Clin Endocrinol. 1995;42:111–119. doi: 10.1111/j.1365-2265.1995.tb01850.x. [DOI] [PubMed] [Google Scholar]
- 49.Wu S. Gao J. Ohlemeyer C. Roos D. Niessen H. Köttgen E. Gessner R. Activation of AP-1 through reactive oxygen species by angiotensin II in rat cardiomyocytes. Free Radic Biol Med. 2005;39:1601–1610. doi: 10.1016/j.freeradbiomed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 50.Yamashiro T. Kuge H. Zhang J. Honke K. Calcineurin mediates the angiotensin II-induced aldosterone synthesis in the adrenal glands by up- regulation of transcription of the CYP11B2 gene. J Biochem. 2010;48:115–123. doi: 10.1093/jb/mvq037. [DOI] [PubMed] [Google Scholar]
- 51.Yang W. Zhang J. Wang H. Gao P. Singh M. Shen K. Fang N. Angiotensin II downregulates catalase expression and activity in vascular adventitial fibroblasts through an AT1R/ERK1/2-dependent pathway. Mol Cell Biochem. 2011;358:21–29. doi: 10.1007/s11010-011-0915-1. [DOI] [PubMed] [Google Scholar]
- 52.Zafari AM. Ushio-Fukai M. Akers M. Yin Q. Shah A. Harrison DG. Taylor WR. Griendling KK. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- 53.Zhou M. Diwu Z. Panchuk-Voloshina N. Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem. 1997;253:162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.