Abstract
Vasoactive intestinal peptide (VIP) is a pleiotropic, highly conserved, peptide found in many different biological systems throughout invertebrate phyla. VIP is produced by cells of the immune system but also inhibits many different inflammatory products produced by these immune cells, including cytokines and chemokines. VIP inhibits these immune mediators by affecting transcriptional regulators such as NFκB and activator protein 1 which transcribes genes responsible for the production of inflammatory mediators in response to pathogens or cytokines. In this review, the therapeutic potential of VIP will be discussed in the context of transcriptional regulation of immune cells in in vitro and in vivo animal models.
Introduction
The biochemical structure and function of VIP
Vasoactive intestinal peptide (VIP) is a 28-amino acid peptide belonging to the Secretin family. It is co-synthesised from a VIP pro-peptide which also contains a sequence for peptide histidine isoleucine (Nishizawa et al. 1995), although in humans isolecine is replaced by methionine to form peptide histidine methionine (Itoh et al. 1983). The amino acid structure of VIP has been very highly conserved during the evolutionary radiation of different vertebrate phyla and the amino acid structure of VIP is identical in all mammals analysed to date, apart from guinea pigs which have four amino acid substitutions (Du et al. 1985).The amino acid sequence of VIP is also identical in frogs (Chartrel et al. 1995), alligators (Wang and Conlon 1993), and chickens (Nilsson 1975) and differs from the common mammalian sequence by only four amino acids (Table 1). VIP is a pleitoropic peptide which has many different functions in different bodily systems. It is a neurotransmitter which is highly expressed in both the central and peripheral nervous systems (Said and Rosenberg 1976) and is found in tissues such as lung, heart and urinary tract (Henning and Sawmiller 2001) and in the gastro-intestinal tract where it is the dominant inhibitory neurotransmitter (D’Amato et al. 1988; Grider and Rivier 1990).
Table 1.
Amino acid homology in different species of vertebrates
| Species | VIP amino acid recidues |
|---|---|
| Human, pig, cow, horse | HSDAV FTDNY TRLRK QMAVK KYLNS ILN |
| Dog, cat, rat mouse | |
| Guinea pig | HSDAL FTDTY TRLRK QMAMK KYLNS VLN |
| Chicken | HSDAV FTDNY SRFRK QMAVK KYLNS VLT |
| Alligator | HSDAV FTDNY SRFRK QMAVK KYLNS VLT |
| Frog | HSDAV FTDNY SRFRK QMAVK KYLNS VLT |
| Cod | HSDAV FTDNY SRFRK QMAAK KYLNS VLT |
Letters in bold denote variation in amino acid residue compared with the common mammalian amino acid sequence
Over the past 30 years, many studies have reported that VIP is not only produced by cells of the immune system but also that VIP has a significant biological effect on these cells (reviewed by Ganea and Delgado 2002; Smalley et al. 2009). In most cases, the effect of VIP is to inhibit the production of inflammatory mediators by cells of the innate immune system. However, VIP is also known to skew the differentiation of naieve T helper lymphocyte populations towards Th2 and stimulate the production of regulatory T cells. The broad effect of VIP results from the effect that VIP has on transcriptional regulation within these immune cells and this has generated a great deal of scientific interest and many studies have now reported the significant therapeutic potential of VIP in many different inflammatory diseases (see Table 2).
Table 2.
Human diseases in which VIP has been shown to have therapeutic potential
| Disease | Reference |
|---|---|
| Alzheimer’s disease | Gozes et al. (1996) |
| Asthma | Morice and Sever (1986) |
| Diabetes | Herrera et al. (2006) |
| Inflammatory bowel disease | Abad et al. (2003, 2005) |
| Graft versus host disease | Chorny et al. (2006) |
| Pancreatitis | Kojima et al. (2005) |
| Parkinson’s disease | Korkmaz et al. (2010) |
| Rheumatoid arthritis | Delgado et al. (2001) |
| Sepsis | Chorny and Delgado (2008); Delgado et al. (1999c) |
| Uveoretinitis | Keino et al. (2004) |
Studies using rodent models and, in some cases, human clinical trials are shown with relevant references
The aim of this review is to highlight the epigenetic effect of VIP on immune cells and discuss how this may translate into the development of novel therapeutics.
VIP receptors in the immune system
In 1992, a VIP-specific receptor was first identified in rat lung tissue known at the time as VIP1 (Ishihara et al. 1992) this was then followed by the identification of a homologous (VIP2) receptor from a rat olfactory bulb cDNA library (Lutz et al. 1993). The nomenclature of these receptors was then changed to VPAC1 (VIP1) and VPAC2 (VIP2; Harmar et al. 1998). VPAC1 and VPAC2 belong to the class II family of G-coupled protein receptors and both receptors have now been identified in a wide range of tissues in different animal phyla (Laburthe and Couvineau 2002). VIP shares 68% homology with pituitary adenylate cyclise-activating polypeptide (PACAP) which is another member of the Secretin family (Campbell and Scanes 1992; Segre and Goldring 1993) and both VPAC1 and VPAC2 bind VIP and PACAP with equivalent affinities (Rawlings and Hezareh 1996). Many of the same effects of VIP in the immune system have also been observed to occur due to PACAP and, as such, PACAP also has great therapeutic potential; however, in this review, only VIP will be considered.
Both VPAC1 and VPAC2 are expressed by some innate immune cell types, while others express one receptor or the other and the relative levels of VPAC1 or VPAC2 expression may alter as a result of stimulation. For example, human mast cells express only VPAC2 (Kulka et al. 2008), whereas human neutrophils (Harfi et al. 2004) and resting human peripheral blood monocytes (PBMs; Lara-Marquez et al. 2000) express only VPAC1 which is not upregulated when PBMs are cultured with lipopolysaccharide (LPS; El Zein et al. 2008). However, murine monocytes express both VPAC1 and VPAC2 (Kojima et al. 2005), while VPAC1 is also constitutively expressed by murine macrophages and VPAC2 is expressed following stimulation by LPS (Delgado et al. 1999a). In the case of human and murine dendritic cells (DCs), temporal expression of VPAC1 and VPAC2 occurs during the differentiation pathway. The first studies to report VPAC1 and VPAC2 expression in DCs were performed in human monocyte-derived DCs (Delneste et al. 1999) and this was followed by studies which showed that VPAC1 and VPAC2 were expressed in murine bone marrow-derived DC (Delgado et al. 2004). In both studies, VPAC1 was found to be expressed early in the differentiation pathway and this was followed by VPAC2 expression after about 6 days. VPAC1 and VPAC2 are also differentially expressed by human T lymphocytes with both CD4+ and CD8+ constitutively expressing relatively high levels of VPAC1, with CD4+ cells expressing significantly higher levels than CD8+ cells, but expressing much lower levels of VPAC2 (Lara-Marquez et al. 2001).
Which cells express VPAC1 and/or VPAC2, and in what context, of course has a huge bearing on the likely therapeutic use of VIP. For example, using a murine model of pancreatitis, Kojima et al. (2005) have shown that administration of VPAC1 agonist reduced production of TNFα, IL-6 and serum amylase with a subsequent reduction in histopathological damage associated with disease but that TNF-α, IL-6 and serum amylase levels were increased by VPAC2 agonists. In the murine LPS-induced model of sepsis, the inhibitory effect of VIP on inflammatory mediators (and subsequent reduction in mortality) also occurs via VPAC1 (reviewed by Ganea and Delgado 2002) and so the future development of VPAC1-specific agonists may have even greater therapeutic potential than using VIP. The effect VPAC1 ligation by VIP has a potent inhibitory effect on different cellular biochemical pathways which ultimately reduces the production of inflammatory mediators by immune effector cells (discussed below)
The effect of VIP on cyclic AMP accumulation and regulation of inflammatory mediators
The initial effect of VPAC1 or VPAC2 ligation by VIP is to significantly increase cyclic AMP (cAMP; Racusen and Binder 1977; Laburthe et al. 1978; Christophe et al. 1984; Robberecht et al. 1984), adenylate cylase (Salomon et al. 1993) and phospholipase C (MacKenzie et al. 1996). This can cause variable downstream effects on a variety of transcription factors, which may influence either the development or reduction of inflammatory pathology.
For example, VIP acting via VPAC1 stimulates cAMP accumulation in preosteoclast (MC373-E1) cell line with a subsequent release of IL-6 (which is involved in bone resorbtion) and inhibition of osteoblast development (Nagata et al. 2009). While VIP acting via both VPAC1 and VPAC2 increases the survival rate of Th2 lymphocytes due to cAMP-induced activation of exchange protein activated by cAMP (EPAC) and to a lesser degree via protein kinase A (PKA; Sharma et al. 2006). A proinflammatory effect of increased VIP/VPAC1-induced cAMP accumulation has also been reported in human monocytes. In this latter study, the cAMP-activated (PKA)/P38 pathway was shown to regulate exocytosis of matrix metalloproteinase 9 and complement receptor (CD35) while the cAMP-induced EPAC/PI3K/ERK pathway regulated expression of the β2 integrin,CD11b (El Zein et al. 2008). In murine macrophages, cAMP-induced PKA and Epac signalling pathways result in cell proliferation (Misra and Pizzo 2005; Misra et al. 2008) which, presumably, would also have a proinflammatory effect.
Mechanism and effect on the inhibitory effect of VIP on NFκB and activator protein-1 activity
VIP also inhibits the production of inflammatory mediators by monocytes and macrophages and this could be utilised in the treatment of a number of important human diseases. When immune cell receptors ligate pathogen molecules or cytokines, a cascade occurs which results in the activation of cytosolic transcription factors that cross the nuclear membrane and bind to DNA promoter sequences prior to production and release of inflammatory product. Transcription of TNF-α by nuclear factor κB (NFκB) in innate immune cells stimulated with LPS is given as an example (Fig. 1).
Fig. 1.
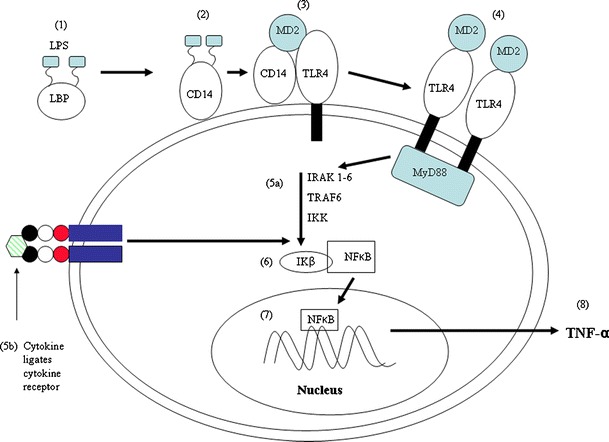
Transcription of TNF-α genes by NFκB following stimulation of innate immune cells by lipopolysaccharide (LPS) or cytokines. 1 LPS in fluids is bound by lipopolsaccharide binding protein (LBP); 2 LPS/LBP complexes bind to CD14 receptor; 3 CD14 receptor stimulates TLR4 via an accessory protein (MD2); 4 activation of MyD88 induces a biochemical cascade which (via phosphorylation) activates cytosolic enzymes; 5a including interleukin-1 receptor associated kinase (IRAK), tumour necrosis factor receptor associated factor (TRAF) and Inhibitory κB kinase (IKK); 5b shows that ligation of cytokine with cytokine receptor may have the same effect and activation of cytosolic enzymes can act as a convergence point whereby the effect of LPS on transcriptional regulation is potentiated by cytokine. 6 Phosphorylation and activation of IKK stimulates ubiquitination of NFκB which allows nuclear translocation of NFκB; 7 NFκB binds to NFκB promoter sequences on TNF-α gene; 8 newly synthesised TNF-α is released from the cell to mediate immunity
Delgado et al. (1999b) was the first to report that VIP inhibited LPS-induced inflammatory pathways in monocytes and macrophages via cAMP-dependent or independent mechanisms. The cAMP-dependent pathway and the subsequent activity of PKA has two different downstream effects. The first effect is to phosphorylate the cAMP response element binding protein (CREB) which then binds to the co-factor, CREB binding protein and prevents its interaction with NFκB (Delgado and Ganea 2001a) and thus reduces the activity of NFκB (Yang et al. 1996). This is likely to have a dramatic effect on the production of many immune mediators and a subsequent effect on inflammatory pathologies, since NFκB is known to transcribe genes for cytokines, chemokines and inducible nitric oxide synthetase which is needed for nitric oxide production in innate immune cells (reviewed Nam et al. 2009). Secondly, the cAMP-dependent pathway inhibits phosphorylation of mitogen-activated protein kinase/extracellular signal-regulated kinase (MAP/ERK; MEK kinase 1 or MEKK1) which in turn inhibits the MEKK3/6/p38 pathway and ultimately the phosphorylation of another NFκB co-factor, the TATA-box binding protein (Delgado and Ganea 2001a) which then has reduced affinity for both NFκB and DNA. The cAMP-independent pathway inhibits the activity of inhibitory κB kinase which prevents phosphorylation of the IκB and increases the stabilisation of IκB/NFκB complexes which prevents nuclear translocation of NFκB subunits (Delgado and Ganea 2001b).
In a murine model of Gram-negative sepsis (induced by LPS administration), VIP administration significantly reduced mortality (up to 20%) and this was associated with downregulation of inflammatory mediators such as TNF-α and IL-6 in serum (Delgado et al. 1999c). Our studies have also shown that in human THP1 monocytes, and peripheral blood monocytes, VIP inhibits LPS-induced nuclear translocation of NFκB (Fig. 2a) which significantly inhibits production of inflammatory cytokines such as TNF-α (Foster et al. 2005a). Thus, the inhibitory effect of VIP on inflammatory effectors via inhibition of gene transcription may have great potential in the treatment of human and animal sepsis. Similarly, modulation of murine IL-12 by VIP can also be cAMP-dependent or independent depending on the transcriptional regulators involved (Delgado et al. 1999b, 1999c). These studies were the first to show that VIP inhibited transcriptional regulation of cytokine and iNOS genes but that VIP also affected other transcription factors such as activator protein 1 (AP-1). AP-1 is another highly active transcriptional regulator which transcribes cytokine and chemokine genes. This can occur via nuclear translocation of heterodimers of Fos and Jun proteins (which constitute the AP-1 complex) or via nuclear translocation of monomeric c-Jun (Abate and Curran 1990). In studies in which VIP inhibited the inflammatory response of LPS-stimulated murine microglial cells (resident macrophages within the CNS), not only was AP-1 binding to DNA inhibited but also the heterodimeric composition of AP-1 was altered, changing from a c-JUN/c-FOS to a JUN-B/c-FOS which was mediated via MEK pathways (Delgado and Ganea 2000a). Our studies have also shown that VIP inhibits LPS-induced nuclear translocation of c-JUN in human monocytic THP1 cells (Foster et al. 2005a; Fig. 2b). Activation of murine microglial cells by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (a model for Parkinson’s disease) has been shown to be decreased in vivo by VIP administration and results in decreased nigrostriatal nerve fibre loss (Delgado and Ganea 2003). In murine models of Alzheimer’s disease (AD), VIP also inhibits b-amyloid induced microglia activation and subsequent neuronal death via inhibition of p38 MAPK and p42/p44 ERK (Delgado et al. 2008). However, an added consequence is to induce the neuroprotective glial protein activity-dependent neurotrophic factor (Gozes and Brenneman 2000) and in vivo studies have shown that nasal administration of a VIP analogue (stearyl-norleucine17, (st-Nle17)VIP) significantly protects mice against experimental AD (Gozes et al. 1996).
Fig. 2.
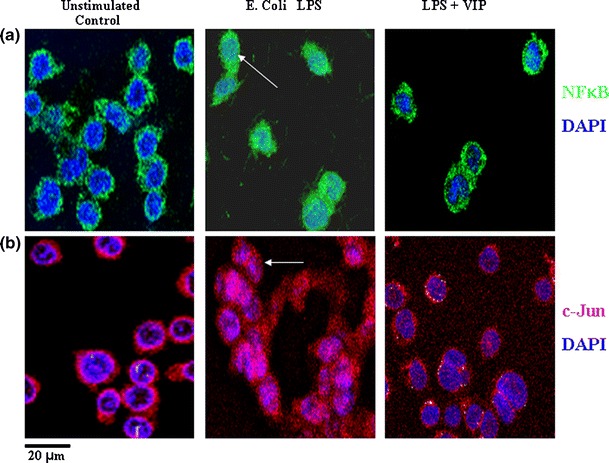
VIP inhibits LPS-induced nuclear translocation of NFκB and c-Jun in human THP1 monocytes. a THP1 monocytes stimulated with LPS from E. coli 0111:B4 (100 ng/ml) with or without VIP (10−8 M) 90 min after culture cells were permeabilised and fixed prior to staining with nuclear dye DAPI (blue) and either anti-human NFκB (green) or anti-human c-Jun (red). Colocalisation of transcription factors and THP1 cell nucleus is observed as turquoise (closed arrows) or pink (open arrows) only in cell exposed to LPS alone. Scale bar 20 μm. Data is representative of results obtained from five replicate experiments
Inhibition of the JAK/STAT pathway
The activation of the janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway initiates the transcription of a number of different inflammatory cytokine genes. Probably the best studied of these is IFN-γ which is produced by γδ T cells, CD8+ T cells, Th1 cells and NK cells and activates immune killing pathways in innate immune cells such as macrophages. The initial step occurs when IFN-γ ligates IFN-γ receptor alpha (IFN-γ Rα) which induces phosphorylation of associated JAK1. This induces IFN-γ receptor beta (IFN-γRβ) with associated JAK 2 to form a complex with IFN-γRα/JAK1 and this, in turn, induces phosphorylation of JAK2 (See Fig. 3). The next step in this pathway involves the interaction of STAT 1α and STAT 1β with the complex, these then become phosphorylated and disengage from the complex to form activated hetero or homodimers which translocate to the cell nucleus prior to gene transcription.
Fig. 3.
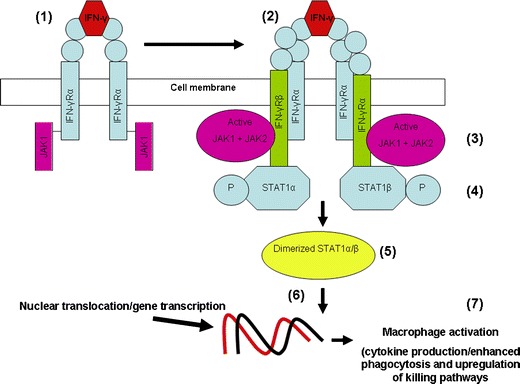
1 IFN-γ pathway leading to activation of macrophages. IFN-γ binds to IFN-γRα resulting in phosphorylation of associated JAK 1; 2 IFN-γRα interacts with IFN-γRβ; 3 IFN-γRα/IFN-γRβ interaction induces phosphorylation of IFN-γRβ-associated JAK2; 4 JAK2 phosphorylation provides a docking site for cytosolic STAT proteins which are then phosphorylated; 5 activated STAT proteins disengage the complex and dimerize; 6 active hetero or homodimers of STAT proteins enter the cell nucleus and transcribe many different genes; 7 IFN-γ-induced activation results in enhanced killing of microbes and antigen presentation
VIP inhibits IFN-γ on two different levels. Firstly, by preventing phosphorylation of JAK/STAT proteins, VIP prevents transcription of genes required for the production of inflammatory products, such as iNOS (Delgado and Ganea 2000b). Secondly, VIP inhibits production of IL-12 by antigen presenting cells, thus preventing differentiation of IFN-γ-producing Th1 lymphocytes and favouring differentiation of IL-4-producing Th2 lymphocytes (Delgado et al. 1999a). Although inhibition of IFN-γ could have a therapeutic effect in many different inflammatory diseases, it may also promote survival of bacteria.
The ability of Salmonella typhimurium to survive inside of macrophages determines virulence (Fields et al. 1986) and mutation of the PhoP regulon induce S. typhimurium attenuation because the bacteria cannot survive in macrophages (Miller and Mekalanos 1990; Groisman and Saier 1990). Reactive oxygen species (ROS) and subsequent oxidative burst is increased in Salmonella-infected macrophages cultured with IFN-γ and this significantly reduces the number of surviving bacteria when compared to cultures in which IFN-γ is not included (Foster et al. 2003). However, when Salmonella-infected macrophages are cultured with VIP, IFN-γ-induced upregulation of ROS is inhibited and this leads to an increase in the number of virulent and avirulent (PhoP) mutants which are recovered from cells (Foster et al. 2005b, 2006).
This does not necessarily negate the use of VIP in bacteria-induced diseases, such as Gram-negative sepsis, since VIP could be administered to patients as an adjunctive therapy to antibiotics, in the case of acute sepsis and in cases of severe sepsis (in which patients are suffering from the effect of dysregulated inflammatory cytokines after bacteria have been cleared form the system) the broad ranging inhibitory effect of VIP may have a potent therapeutic effect without antibiotic.
VIP inhibits expression of Toll-like receptors by preventing PU.1-stimulated TLR gene transcription
Since the activation of different TLRs is a pre-requisite for the production of inflammatory mediators in response to many conserved pathogen-associated molecular patterns, TLRs are a rational target for the control of a variety of diseases in which dysregulated cytokine production occurs as a result of TLR ligation.
Inflammatory bowel disease (IBD) which may occur in the form of Crohn’s colitis or ulcerative colitis affects about 3.6 million people in Europe and the USA alone (Loftus 2004). The intestine is known to express inhibitory factors which prevent intestinal TLRs being inappropriately activated by gut commensals (Abreu et al. 2005) and a possible reason for the development of IBD is due to dysregulated control of TLR expression in response to such commensal (reviewed by Kawai and Akira 2010). Suppression of TLR expression and/or activation is, therefore, a possible therapeutic avenue in IBD.
Gomariz et al. (2005) reported that daily intra-peritoneal administration of VIP (1 nM) downregulated TLR2 and TLR4 expression in colonic extracts obtained from a murine trinitrobenzene sulphonic acid (TNBS) model of human Crohn’s disease and that TLR4 expression was decreased on the surface of macrophages, DCs and lymphocytes within the mesenteric lymph nodes of these mice. This group then went on to show that VIP administration inhibited expression of Th1 cytokines in the colon and restored regulatory T cell populations to control levels (Arranz et al. 2008a). Upregulation of TLR4 by LPS in human rheumatoid synovial fibroblast has also been shown to be inhibited by VIP although the high constitutive expression of TLR 2 and TLR4 by these cells was unaffected by VIP (Gutiérrez-Cañas et al. 2006). Initially, it was speculated that the mechanism behind TLR modulation may be via inhibition of NFκB (Gomariz et al. 2005). Murine TLR2 gene expression is modulated via NFκB (Musikacharoen et al. 2001) and VIP does inhibit LPS-induced DNA binding of NFκB in murine RAW 264.7 macrophages (Delgado et al. 1998). VIP also inhibits LPS-induced NFκB/DNA interaction in human monocytic THP1 cells (Haehnel et al. 2002; Foster et al. 2005a) but NFκB promoter sequences have not been detected in either murine TLR4 gene or human TLR2 or TLR4 genes (Rehli 2002) and so inhibition of NFкB could not explain the inhibitory effect of VIP on upregulation of expression of human TLR2 and TLR4, or expression of murine TLR4.
We investigated the effect of VIP on translocation of the ets family transcription factor PU.1. PU.1 is required for expression of both human TLR2 (Haehnel et al. 2002) and TLR4 (Rehli et al. 2000) and is also required for differentiation of monocytes to macrophages (Shivdasani and Orkin 1996). When human THP1 cells or peripheral blood monocytes were stimulated with LPS from Porphyromonas gingivalis (a TLR2 activor) or Escherichia coli (a TLR4 activator) PU.1 translocated to the cell nucleus but this was prevented by VIP (Fig. 4). We also showed that subsequent expression of a downstream gene target of PU.1 (monocyte colony stimulating factor receptor; Zhang et al. 1994) was not upregulated (Foster et al. 2007) and that upregulation of both TLR2 and TLR4 was significantly impaired (Foster et al. 2007; Fig. 5). Results which also indicated the inhibitory effect of PU.1 by VIP were observed by decreased, LPS-induced, differentiation of monocytes to macrophages (Foster et al. 2007). This discovery was repeated by studies using a TNBS-induced mouse model of human colitis, which showed that VIP decreased PU.1 binding to DNA and that mutation of PU.1 prevented the inhibitory effect of VIP on TLR4 upregulation (Arranz et al. 2008b), although inhibition of NFкB by VIP in the murine model may also have had an important effect.
Fig. 4.
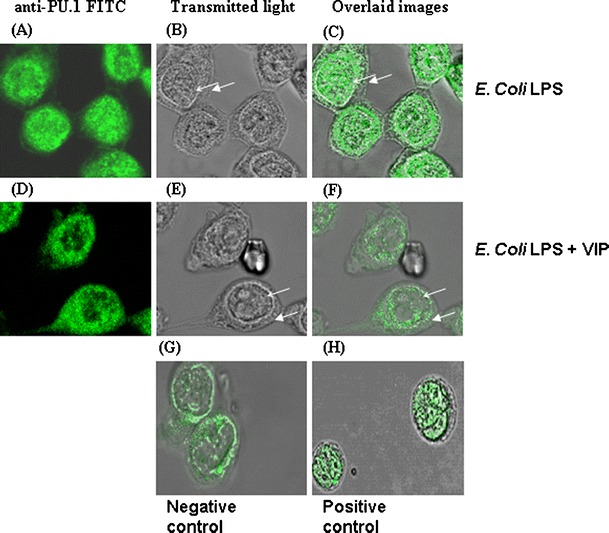
VIP inhibits nuclear translocation of the transcriptional regulator PU.1 in LPS-stimulated human THP1 monocytes. Cells were stimulated with E. coli LPS (100 ng/ml; a–c), E. coli LPS + VIP (10−8M; d–f) for 90 min. Images in the left column show localization of PU.1, images in centre column show transmitted light views, and images in the right column are overlaid images. Nuclear translocation of PU.1 is evident in THP1 cells stimulated with E. coli LPS (a–c) but is inhibited when cells are cocultured with E. coli LPS + VIP (d–f). Unstimulated monocytic THP1 cells (g) demonstrated a perinuclear localization of PU.1 (overlay image of anti-PU.1 FITC and transmitted light image). Cells stimulated with PMA (1 μg/ml) for 90 min (positive control cells) demonstrated PU.1 nuclear translocation (overlay image of anti-PU.1 FITC and transmitted light image; h). All results obtained are representative of results obtained on more than three separate occasions. Scale bar 10 μm. Closed arrows cell membrane, open arrows nuclear membrane. Data is representative of results obtained from five replicate experiments
Fig. 5.
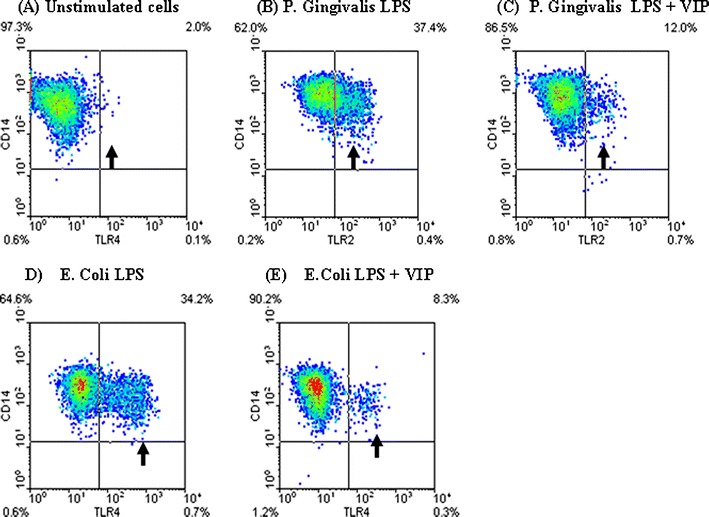
VIP inhibits TLR2 and TLR4 upregulation in LPS-stimulated human THP1 monocytes. a Unstimulated THP1 cells cultured for 24 h following conversion to monocytes by vitamin D3 (monocytic THP1 cells); b monocytic THP1 cells cultured with P. Gingivalis W50 LPS (100 ng/ml) for 24 h with a subsequent 35% increase in TLR2high expressing cell population; c monocytic THP1 cells cultured with P. gingivalis W50 LPS (100 ng/ml) and VIP (10−8 M) for 24 h, showing a 10% increase in TLR2high expressing cell population (25% reduction due to VIP). d monocytic THP1 cells cultured with E. coli 0111:B4 LPS (100 ng/ml) for 24 h with a subsequent 32% increase in TLR4high expressing cell population; c monocytic THP1 cells cultured with E. coli 0111:B4 LPS (100 ng/ml) and VIP (10−8 M) for 24 h, showing a 6% increase in TLR4high expressing cell population (26% reduction due to VIP). Arrows highlight CD14high/TLR2high and CD14high/TLR4high population. Data is representative of results obtained from 10 replicate experiments
Conclusion
It is clear that VIP has therapeutic potential in diverse inflammatory diseases (many of which have not been included in this review). The broad immunomodulatory effect of VIP is due to the inhibition of activity of key transcriptional regulators which transcribe an array of inflammatory proteins. How and in what context VIP can be used has still to be elucidated in many cases but ongoing and future studies may enhance current therapies or even provide therapies for diseases where none as yet exist.
Acknowledgements
The authors would like to thank Mr. Scott Hulme for technical assistance. This work was supported by funding from the BBSRC and MRC.
Conflict of Interest
The authors have no conflict of interest.
References
- Abad C, Martinez C, Juarranz MG, Arranz A, Leceta J, Delgado M, Gomariz RP. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn’s disease. Gastroenterology. 2003;124:961–971. doi: 10.1053/gast.2003.50141. [DOI] [PubMed] [Google Scholar]
- Abad C, Juarranz Y, Martinez C, Arranz A, Rosignoli F, García-Gómez M, Leceta J, Gomariz RP. cDNA array analysis of cytokines, chemokines, and receptors involved in the development of TNBS-induced colitis: homeostatic role of VIP. Inflamm Bowel Dis. 2005;11:674–684. doi: 10.1097/01.MIB.0000171872.70738.58. [DOI] [PubMed] [Google Scholar]
- Abate C, Curran T. Encounters with Fos and Jun on the road to AP-1. Semin Cancer Biol. 1990;1:19–26. [PubMed] [Google Scholar]
- Abreu MT, Fukata M, Arditi M. TLR signaling in the gut in health and disease. J Immunol. 2005;174:4453–4460. doi: 10.4049/jimmunol.174.8.4453. [DOI] [PubMed] [Google Scholar]
- Arranz A, Juarranz Y, Leceta J, Gomariz RP, Martínez C. VIP balances innate and adaptive immune responses induced by specific stimulation of TLR2 and TLR4. Peptides. 2008a;29:948–956. doi: 10.1016/j.peptides.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Arranz A, Androulidaki A, Zacharioudaki V, Martinez C, Margioris AN, Gomariz RP, Tsatsanis C. Vasoactive intestinal peptide suppresses toll-like receptor 4 expression in macrophages via Akt1 reducing their responsiveness to lipopolysaccharide. Mol Immunol. 2008b;45:2970–2980. doi: 10.1016/j.molimm.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Campbell RM, Scanes CG. Evolution of the growth hormone-releasing factor (GRF) family of peptides. Growth Regul. 1992;2:175–191. [PubMed] [Google Scholar]
- Chartrel N, Wang Y, Fournier A, Vaudry H, Conlon MJ. Frog vasoactive intestinal peptide and galanin: primary structures and effects on pituitary adenylate cyclise. Endocrinology. 1995;136:3079–3086. doi: 10.1210/en.136.7.3079. [DOI] [PubMed] [Google Scholar]
- Chorny A, Delgado M. Neuropeptides rescue mice from lethal sepsis by down-regulating secretion of the late-acting inflammatory mediator high mobility group box 1. Am J Pathol. 2008;172:1297–1307. doi: 10.2353/ajpath.2008.070969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorny A, Gonzalez-Rey E, Fernandez-Martin A, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory dendritic cells that prevent acute graft-versus-host disease while maintaining the graft-versus-tumor response. Blood. 2006;107:3787–3794. doi: 10.1182/blood-2005-11-4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophe JM, Waelbroeck M, Chatelain P, Robberecht P. Heart receptors for VIP, PHI and secretin are able to activate adenylate cyclise and to mediate ionotropic and chronotropic effects. Species variations and physiology. Peptides. 1984;5:341–353. doi: 10.1016/0196-9781(84)90232-8. [DOI] [PubMed] [Google Scholar]
- D’Amato M, Beurme FA, Lefebvre RA. Comparison of the effect of vasoactive intestinal polypeptide and non-adrenergic non-cholinergic neurone stimulation in the cat gastric fundus. Eur J Pharmacol. 1988;152:71–82. doi: 10.1016/0014-2999(88)90837-0. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. VIP and PACAP inhibit antigen induced apoptosis of mature T lymphocytes by inhibiting FasL expression. J Immunol. 2000a;164:1200–1210. doi: 10.4049/jimmunol.164.3.1200. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Inhibition of IFN-induced Jak1-STAT1 activation in macrophages by VIP and PACAP. J Immunol. 2000;165:3051–3057. doi: 10.4049/jimmunol.165.6.3051. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase activating polypeptide inhibit expression of Fas ligand in activated T lymphocytes by regulating c-Myc, NFκB, NF-AT, and early growth factors 2/3. J Immunol. 2001a;166:1028–1040. doi: 10.4049/jimmunol.166.2.1028. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit nuclear factor-κB-dependent gene activation at multiple levels in the human monocytic cell line THP-1. J Biol Chem. 2001;276:369–380. doi: 10.1074/jbc.M006923200. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Neuroprotective effect of vasoactive intestinal peptide (VIP) in a mouse model of Parkinson’s disease by blocking microglial activation. FASEB J. 2003;17:944–946. doi: 10.1096/fj.02-0799fje. [DOI] [PubMed] [Google Scholar]
- Delgado M, Munoz-Elias EJ, Kan Y, Gozes I, Fridkin M, Brenneman DE, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit tumor necrosis factor alpha transcriptional activation by regulating nuclear factor-kB and cAMP response element-binding protein/c-Jun. J Biol Chem. 1998;273:31427–31436. doi: 10.1074/jbc.273.47.31427. [DOI] [PubMed] [Google Scholar]
- Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D. VIP and PACAP inhibit IL-12 production in LPS-stimulated macrophages. Subsequent effect on IFN gamma synthesis by T cells. J Neuroimmunol. 1999a;96:167–181. doi: 10.1016/S0165-5728(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide prevent inducible nitric oxide synthase transcription in macrophages by inhibiting NF-kappa B and IFN regulatory factor 1 activation. J Immunol. 1999b;162:4685–4696. [PubMed] [Google Scholar]
- Delgado M, Martinez C, Pozo D, Calvo JR, Leceta J, Ganea D, Gomariz R. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activation polypeptide (PACAP) protect mice from lethal endotoxemia through the inhibition of TNF-alpha and IL-6. J Immunol. 1999c;162:1200–1205. [PubMed] [Google Scholar]
- Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med. 2001;7:563–568. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- Delgado M, Reduta A, Sharma V, Ganea D. VIP/PACAP oppositely affects immature and mature dendritic cell expression of CD80/CD86 and the stimulatory activity for CD4(+) T cells. J Leukoc Biol. 2004;75:1122–1130. doi: 10.1189/jlb.1203626. [DOI] [PubMed] [Google Scholar]
- Delgado M, Varela N, Gonzalez-Rey E. Vasoactive intestinal peptide protects against beta-amyloid-induced neurodegeneration by inhibiting microglia activation at multiple levels. Glia. 2008;56:1091–1093. doi: 10.1002/glia.20681. [DOI] [PubMed] [Google Scholar]
- Delneste Y, Herbault N, Galea B, Magistrelli G, Bazin I, Bonnefoy JY, Jeannin P. Vasoactive intestinal polypeptide synergizes with TNF-α in inducing human dendritic cell maturation. J Immunol. 1999;163:3071–3075. [PubMed] [Google Scholar]
- Du B-H, Eng J, Hulmes JD, Chang M, Pan EY-C, Yalow RS. Guinea pig has a unique mammalian VIP. Biochem Biophys Res Commun. 1985;128:1093–1098. doi: 10.1016/0006-291X(85)91052-6. [DOI] [PubMed] [Google Scholar]
- El Zein N, Badran B, Sariban E. VIP differentially activates beta2 integrins, CR1, and matrix metalloproteinase-9 in human monocytes through cAMP/PKA, EPAC, and PI-3K signaling pathways via VIP receptor type 1 and FPRL1. J Leukoc Biol. 2008;83:972–981. doi: 10.1189/jlb.0507327. [DOI] [PubMed] [Google Scholar]
- Fields PI, Swanson RV, Haidaris CG, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster N, Hulme SD, Barrow PA. Induction of antimicrobial pathways during early-phase immune response to Salmonella spp. in murine macrophages: gamma interferon (IFN-gamma) and upregulation of IFN-gamma receptor alpha expression are required for NADPH phagocytic oxidase gp91-stimulated oxidative burst and control of virulent Salmonella spp. Infect Immun. 2003;71:4733–4741. doi: 10.1128/IAI.71.8.4733-4741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster N, Cheetham J, Taylor JJ, Preshaw PM. VIP inhibits Porphyromonas gingivalis LPS-induced immune responses in human monocytes. J Dent Res. 2005a;84:999–1004. doi: 10.1177/154405910508401106. [DOI] [PubMed] [Google Scholar]
- Foster N, Hulme SD, Barrow PA. Inhibition of IFN-gamma-stimulated proinflammatory cytokines by vasoactive intestinal peptide (VIP) correlates with increased survival of Salmonella enterica serovar typhimurium phoP in murine macrophages. J Interferon Cytokine Res. 2005b;25:31–42. doi: 10.1089/jir.2005.25.31. [DOI] [PubMed] [Google Scholar]
- Foster N, Hulme SD, Barrow PA. Vasoactive intestinal peptide (VIP) prevents killing of virulent and phoP mutant Salmonella typhimurium by inhibiting IFN-gamma stimulated NADPH oxidative pathways in murine macrophages. Cytokine. 2006;36:134–140. doi: 10.1016/j.cyto.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Foster N, Lea SR, Preshaw PM, Taylor JJ. Pivotal advance: vasoactive intestinal peptide inhibits up-regulation of human monocyte TLR2 and TLR4 by LPS and differentiation of monocytes to macrophages. J Leukoc Biol. 2007;81:893–903. doi: 10.1189/jlb.0206086. [DOI] [PubMed] [Google Scholar]
- Ganea D, Delgado M. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) as modulators of both innate and adaptive immunity. Crit Rev Oral Biol Med. 2002;13:229–237. doi: 10.1177/154411130201300303. [DOI] [PubMed] [Google Scholar]
- Gomariz RP, Arranz A, Abad C, Torroba M, Martinez C, Rosignoli F, Garcia-Gómez M, Leceta J, Juarranz Y. Time-course expression of Toll-like receptors 2 and 4 in inflammatory bowel disease and homeostatic effect of VIP. J Leukoc Biol. 2005;78:491–502. doi: 10.1189/jlb.1004564. [DOI] [PubMed] [Google Scholar]
- Gozes I, Brenneman DE. A new concept in the pharmacology of neuroprotection. J Mol Neurosci. 2000;14:61–68. doi: 10.1385/JMN:14:1-2:061. [DOI] [PubMed] [Google Scholar]
- Gozes I, Bardea A, Reshef A, Zamostiano R, Zhukovsky S, Rubinraut S, Fridkin M, Brenneman DE. Neuroprotective strategy for Alzheimer disease: intranasal administration of a fatty neuropeptide. Proc Natl Acad Sci USA. 1996;93:427–432. doi: 10.1073/pnas.93.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grider JR, Rivier JR. Vasoactive intestinal peptide (VIP) as transmitter of inhibitory motor neurones of the gut: evidence from the use of selective VIP antagonists and VIP antiserum. J Pharmacol Exp Ther. 1990;253:738–742. [PubMed] [Google Scholar]
- Groisman EA, Saier MH. Salmonella virulence: new clues to intramacrophage survival. Trends Biochem Sci. 1990;15:30–33. doi: 10.1016/0968-0004(90)90128-X. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Cañas I, Juarranz Y, Santiago B, Arranz A, Martinez C, Galindo M, Payá M, Gomariz RP, Pablos JL. VIP down-regulates TLR4 expression and TLR4-mediated chemokine production in human rheumatoid synovial fibroblasts. Rheumatology. 2006;45:527–532. doi: 10.1093/rheumatology/kei219. [DOI] [PubMed] [Google Scholar]
- Haehnel V, Schwarzfischer L, Fenton MJ, Rehli M. Transcriptional regulation of the human Toll-like receptor 2 gene in monocytes and macrophages. J Immunol. 2002;168:5629–5637. doi: 10.4049/jimmunol.168.11.5629. [DOI] [PubMed] [Google Scholar]
- Harfi I, D’Hondt S, Corazza F, Sariban E. Regulation of human polymorphonuclear leukocytes functions by the neuropeptide pituitary adenylate cyclase-activating polypeptide after activation of MAPKs. J Immunol. 2004;173:4154–4163. doi: 10.4049/jimmunol.173.6.4154. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, Wank SA, Waschek JA. International union of pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- Henning RJ, Sawmiller DR. Vasoactive intestinal peptide: cardiovascular effects. Cardiovasc Res. 2001;49:27–37. doi: 10.1016/S0008-6363(00)00229-7. [DOI] [PubMed] [Google Scholar]
- Herrera JL, Fernández-Montesinos R, González-Rey E, Delgado M, Pozo D. Protective role for plasmid DNA-mediated VIP gene transfer in non-obese diabetic mice. Ann NY Acad Sci. 2006;1070:337–341. doi: 10.1196/annals.1317.041. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Shigemoto R, Mori K, Takahashi K, Nagata S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992;8:811–819. doi: 10.1016/0896-6273(92)90101-I. [DOI] [PubMed] [Google Scholar]
- Itoh N, Obata K, Yanaihara N, Okamoto H. Human preprovasoactive intestinal polypeptide contains a novel PHI-27-like peptide, PHM-27. Nature. 1983;304:547–549. doi: 10.1038/304547a0. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Keino H, Kezuka T, Takeuchi M, Yamakawa N, Hattori T, Usui M. Prevention of experimental autoimmune uveoretinitis by vasoactive intestinal peptide. Arch Ophthalmol. 2004;122:1179–1184. doi: 10.1001/archopht.122.8.1179. [DOI] [PubMed] [Google Scholar]
- Kojima M, Ito T, Oono T, Hisano T, Igarashi H, Arita Y, Kawabe K, Coy DH, Jensen RT, Nawata H. VIP attenuation of the severity of experimental pancreatitis is due to VPAC1 receptor-mediated inhibition of cytokine production. Pancreas. 2005;30:62–70. [PubMed] [Google Scholar]
- Korkmaz OT, Tunçel N, Tunçel M, Oncü EM, Sahintürk V, Celik M. Vasoactive intestinal peptide (VIP) treatment of Parkinsonian rats increases thalamic gamma-aminobutyric acid (GABA) levels and alters the release of nerve growth factor (NGF) by mast cells. J Mol Neurosci. 2010;41:278–287. doi: 10.1007/s12031-009-9307-3. [DOI] [PubMed] [Google Scholar]
- Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123:398–410. doi: 10.1111/j.1365-2567.2007.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laburthe M, Couvineau A. Molecular pharmacology and structure of VPAC receptors for VIP and PACAP. Regul Pept. 2002;108:165–173. doi: 10.1016/S0167-0115(02)00099-X. [DOI] [PubMed] [Google Scholar]
- Laburthe M, Rousset M, Boissard C, Chevalier G, Zweibaum A, Rosselin G. Vasoactive intestinal peptide: a potent stimulator of adenosine 3′:5′-cyclic monophosphate accumulation in gut carcinoma cell lines in culture. Proc Natl Acad Sci USA. 1978;75:2772–2775. doi: 10.1073/pnas.75.6.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Marquez ML, O’Dorisio MS, Karacay B. Vasoactive intestinal peptide (VIP) receptor type 2 (VPAC2) is the predominant receptor expressed in human thymocytes. Ann NY Acad Sci. 2000;921:45–54. doi: 10.1111/j.1749-6632.2000.tb06950.x. [DOI] [PubMed] [Google Scholar]
- Lara-Marquez M, O’Dorisio M, O’Dorisio T, Shah M, Karacay B. Selective gene expression and activation-dependent regulation of vasoactive intestinal peptide receptor type 1 and type 2 in human T cells. J Immunol. 2001;166:2522–2530. doi: 10.4049/jimmunol.166.4.2522. [DOI] [PubMed] [Google Scholar]
- Lodde BM, Baum BJ, Tak PP, Illei G. Experience with experimental biological treatment and local gene therapy in Sjogren’s syndrome: implications for exocrine pathogenesis and treatment. Ann Rheum Dis. 2006;65:1406–1413. doi: 10.1136/ard.2006.052761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: incidence, prevalence and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- Lutz EM, Sheward WJ, West KM, Morrow JA, Fink G, Harmar AJ. The VIP2 receptor: molecular characterisation of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 1993;334:3–8. doi: 10.1016/0014-5793(93)81668-P. [DOI] [PubMed] [Google Scholar]
- MacKenzie CJ, Lutz EM, McCulloch DA, Mitchell R, Harmar AJ. Phospholipase C activation by VIP1 and VIP2 receptors expressed in COS 7 cells involves a pertussis toxin-sensitive mechanism. Ann NY Acad Sci. 1996;26:579–584. doi: 10.1111/j.1749-6632.1996.tb17523.x. [DOI] [PubMed] [Google Scholar]
- Miller SI, Mekalanos JJ. Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J Bacteriol. 1990;172:2485–2490. doi: 10.1128/jb.172.5.2485-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra UK, Pizzo SV. Coordinate regulation of forskolin-induced cellular proliferation in macrophages by protein kinase A/cAMP-response element-binding protein (CREB) and Epac1-Rap1 signaling: effects of silencing CREB gene expression on Akt activation. J Biol Chem. 2005;280:38276–38289. doi: 10.1074/jbc.M507332200. [DOI] [PubMed] [Google Scholar]
- Misra UK, Kaczowka S, Pizzo SV. The cAMP-activated GTP exchange factor, Epac1 upregulates plasma membrane and nuclear Akt kinase activities in 8-CPT-2-O-Me-cAMP-stimulated macrophages: Gene silencing of the cAMP-activated GTP exchange Epac1 prevents 8-CPT-2-O-Me-cAMP activation of Akt activity in macrophages. Cell Signal. 2008;8:1459–1470. doi: 10.1016/j.cellsig.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morice AH, Sever PS. Vasoactive intestinal peptide as a bronchodilator in severe asthma. Peptides. 1986;7:279–280. doi: 10.1016/0196-9781(86)90202-0. [DOI] [PubMed] [Google Scholar]
- Musikacharoen T, Matsuguchi T, Kikuchi T, Yoshikai Y. NF-κ and STAT5 play important roles in the regulation of mouse Toll-like receptor 2 gene expression. J Immunol. 2001;166:4516–4524. doi: 10.4049/jimmunol.166.7.4516. [DOI] [PubMed] [Google Scholar]
- Nagata A, Tanaka T, Minezawa A, Poyurovsky M, Mayama T, Suzuki S, Hashimoto N, Yoshida T, Suyama K, Miyata A, Hosokawa H, Nakayama T, Tatsuno I. cAMP activation by PACAP/VIP stimulates IL-6 release and inhibits osteoblastic differentiation through VPAC2 receptor in osteoblastic MC3T3 cells. J Cell Physiol. 2009;221:75–83. doi: 10.1002/jcp.21831. [DOI] [PubMed] [Google Scholar]
- Nam J, Aguda BD, Rath B, Agarwal S. Biomechanical thresholds regulate inflammation through the NF-kappaB pathway: experiments and modeling. PLoS ONE. 2009;4:e5262. doi: 10.1371/journal.pone.0005262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson A. Structure of the vasoactive intestinal octacosapeptide from chicken intestine. The amino acid sequence. FEBS Lett. 1975;60:322–326. doi: 10.1016/0014-5793(75)80740-X. [DOI] [PubMed] [Google Scholar]
- Nishizawa M, Hayakawa Y, Yanaihara N, Okamoto H. Nucleotide sequence divergence and functional constraint in VIP mRNA evolution between human and rat. FEBS Lett. 1995;183:55–59. doi: 10.1016/0014-5793(85)80953-4. [DOI] [PubMed] [Google Scholar]
- Racusen LC, Binder HJ. Adrenergic interaction with ion transport across colonic mucosa: role of both alpha and beta adrenergic agonists. In: Binder HJ, editor. Mechanisms of intestinal secretion. Alan Liss Inc: New York; 1977. pp. 201–215. [Google Scholar]
- Rawlings SR, Hezareh M. Pituitary adenylate cyclase-activating polypeptide (PACAP) and PACAP/vasoactive intestinal polypeptide receptors: actions on the anterior pituitary gland. Endocr Rev. 1996;17:4–29. doi: 10.1210/edrv-17-1-4. [DOI] [PubMed] [Google Scholar]
- Rehli M. Of mice and men: species variation of Toll-like receptor variation. Trends Immunol. 2002;23:375–378. doi: 10.1016/S1471-4906(02)02259-7. [DOI] [PubMed] [Google Scholar]
- Rehli M, Poltorak A, Schwarzfischer L, Krause SW, Andreesen R, Beutler B. PU.1 and interferon consensus sequence-binding protein regulate the myeloid expression of the human Toll-like receptor 4 gene. J Biol Chem. 2000;275:9773–9781. doi: 10.1074/jbc.275.13.9773. [DOI] [PubMed] [Google Scholar]
- Robberecht P, Waelbroeck M, Camus JC, Neef P, Coy D, Christophe J. Effects of HIS1 modifications on the ability of vasoactive intestinal peptide to stimulate adenylate cyclase from rat and human tissues. Peptides. 1984;5:877–881. doi: 10.1016/0196-9781(84)90110-4. [DOI] [PubMed] [Google Scholar]
- Said SI, Rosenberg RN. Vasoactive intestinal polypeptide: abundant immunoreactivity in neuronal cell lines and normal nervous tissues. Science. 1976;192:907–908. doi: 10.1126/science.1273576. [DOI] [PubMed] [Google Scholar]
- Salomon R, Couvineau A, Rouyer-Fessard C, Voisin T, Lavallée D, Blais A, Darmoul D, Laburthe M. Characterization of a common VIP-PACAP receptor in human small intestinal epithelium. Am J Physiol. 1993;264:294–300. doi: 10.1152/ajpendo.1993.264.2.E294. [DOI] [PubMed] [Google Scholar]
- Segre GV, Goldring SR. Receptors for secretin, calcitonin, parathyroid hormone (PTH)/PTH-related peptide, glucagon-like peptide 1, growth hormone-releasing hormone, and glucagon belong to a newly discovered G-protein-linked receptor family. Trends Endocrinol Metab. 1993;4:309–314. doi: 10.1016/1043-2760(93)90071-L. [DOI] [PubMed] [Google Scholar]
- Sharma V, Delgado M, Ganea D. Granzyme B, a new player in activation-induced cell death, is down-regulated by vasoactive intestinal peptide in Th2 but not Th1 effectors. J Immunol. 2006;176:97–110. doi: 10.4049/jimmunol.176.1.97. [DOI] [PubMed] [Google Scholar]
- Shivdasani RA, Orkin SH. The transcriptional control of hematopoiesis. Blood. 1996;87:4025–4039. [PubMed] [Google Scholar]
- Smalley SG, Barrow PA, Foster N. Immunomodulation of innate immune responses by vasoactive intestinal peptide (VIP): its therapeutic potential in inflammatory disease. Clin Exp Immunol. 2009;157:225–234. doi: 10.1111/j.1365-2249.2009.03956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunçel N, Sener E, Cerit C, Karasu U, Gürer F, Sahintürk V, Bayçu C, Ak D, Filiz Z. Brain mast cells and therapeutic potential of vasoactive intestinal peptide in a Parkinson’s disease model in rats: brain microdialysis, behavior, and microscopy. Peptides. 2005;26:827–836. doi: 10.1016/j.peptides.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Wang Y, Conlon JM. Nueroendocrine peptides (NPY, GRP, VIP, somatostatin) from the brain and stomach of the alligator. Peptides. 1993;14:573–579. doi: 10.1016/0196-9781(93)90147-9. [DOI] [PubMed] [Google Scholar]
- Yang X-J, Ogyzko VV, Nishikawa J, Howard BH, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- Zhang DE, Hetherington CJ, Chen HM, Tenen DG. The macrophage transcription factor PU.1 directs tissue-specific expression of the macrophage colony-stimulating factor receptor. Mol Cell Biol. 1994;14:373–381. doi: 10.1128/mcb.14.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]


