Abstract
Epithelial cells lining the adult colon do not normally express gastrin-releasing peptide (GRP) or its receptor (GRPR). In contrast, GRP/GRPR can be aberrantly expressed in human colorectal cancer (CRC) including Caco-2 cells. We have previously shown that GRPR activation results in the up-regulation of HP1β, an epigenetic modifier of gene transcription. The aim of this study was to identify the genes whose expression is altered by HP1β subsequent to GRPR activation. We determined HP1β binding positions throughout the genome using chromatin immunoprecipitation followed by massively parallel DNA sequencing (ChIP-seq). After exposure to GRP, we identified 9,625 genomic positions occupied by HP1β. We performed gene microarray analysis on Caco-2 cells in the absence and presence of a GRPR specific antagonist as well as siRNA to HP1β. The expression of 97 genes was altered subsequent to GRPR antagonism, while the expression of 473 genes was altered by HP1β siRNA exposure. When these data were evaluated in concert with our ChIP-seq findings, 9 genes showed evidence of possible altered expression as a function of GRPR signaling via HP1β. Of these, genomic PCR of immunoprecipitated chromatin demonstrated that GRPR signaling affected the expression of IL1RAPL2, FAM13A, GBE1, PLK3, and SLCO1B3. These findings provide the first evidence by which GRPR aberrantly expressed in CRC might affect tumor progression.
Keywords: Bombesin, Metastasis
Introduction
Gastrin-releasing peptide (GRP) is a 27 amino acid peptide hormone that acts via a specific 7 transmembrane-spanning G protein coupled receptor. While GRP and the GRP receptor (GRPR) are not normally expressed by epithelial cells lining the colon, both can be aberrantly expressed in colorectal cancer (CRC) (Carroll et al. 1999; Jensen et al. 2008). Although GRP acts as a modest mitogen in a variety of cancer cell lines when studied in vitro, the data from in vivo studies are less clear and do not necessarily suggest that this peptide hormone acts as a clinically significant growth factor [reviewed in (Jensen et al. 2001)]. Yet few studies have been performed to identify the mechanisms whereby GRP alters CRC behavior independently of its modest ability to increase cell proliferation.
We previously used a proteomics approach to attempt to identify the mediators of GRP’s actions in CRC (Ruginis et al. 2006). In that study, we used two-dimensional gel electrophoresis followed by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry to identify proteins whose expression was increased as a result of GRP signaling. One of the proteins so identified was a member of the heterochromatin associated protein family, which we recently showed was heterochromatin protein 1β (Tell et al. 2011).
HP1 proteins localize to centric heterochromatin, telomeres, and specific sites within euchromatin [reviewed in (Dialynas et al. 2008)] and act to modulate gene transcription (Grewal and Jia 2007). Although HP1 proteins primarily repress gene transcription in normal tissues by interacting with methylated histones, they can also activate gene transcription, particularly in malignancy (Grewal and Jia 2007). In the few cancers studied, however, the in vitro data regarding HP1 function has been conflicting. For instance, HP1α is down-regulated in invasive breast cancer cell lines as compared to those that are not invasive (Kirschmann et al. 2000). Increasing HP1α expression in cells that normally express little of this protein decreased invasiveness, whereas decreasing the expression of this protein by RNAi in cells otherwise replete with HP1α increased their invasiveness (Norwood et al. 2006). Similarly, various HP1 isoforms have been shown to correlate with decreased invasiveness of other cancer cell lines including those from papillary thyroid (Wasenius et al. 2003), melanoma (Nishimura et al. 2006), ovarian (Maloney et al. 2007), and embryonal brain cancers (Pomeroy et al. 2002).
In contrast, HP1 has also been suggested to have deleterious effects in cancer cells. For instance, whereas no HP1 isoforms are detected in adult neutrophils, all three isoforms are up-regulated in the granulocytes of patients with acute myeloid leukemia and chronic myeloid leukemia (Dialynas et al. 2008). A single study has shown that HP1γ is up-regulated in human CRC, and that inhibiting this isoform’s expression in CRC cell lines decreases tumor cell growth (Takanashi et al. 2009). Hence, it appears that HP1 proteins can have variable effects in different cancers.
With our identification of a specific ligand—gastrin-releasing peptide—altering the expression of a specific HP1 isoform—HP1β—it is now possible to determine what genes are regulated by this signaling pathway in colon cancer. In this study, we show that GRP-induced expression of HP1β decreases the expression of but one gene, but increases the expression of four genes. Based on what is known about these five proteins, our data provide an important window into possible mechanisms whereby aberrantly expressed GRP/GRPR alter CRC behavior, as well as identifies potential therapeutic targets for the treatment of this type of cancer.
Methods
Materials
Caco-2 cells (with non-functional p53) were all obtained from ATCC (Rockville, MD) and maintained as recommended. RNA Stat-60, isopropanol, chloroform, and DEPC water were all purchased from Fisher Biosciences (Pittsburgh, PA). SiRNA targeted to the relevant mRNA was obtained from Ambion (Austin, TX) and used as directed. Affymetrix human U133 Microarray Analysis Chips were purchased from Affymetrix (Santa Clara, CA). Anti-HP1Hsβ is a rabbit polyclonal antibody directed between residues 150 and the C terminus of the human, mouse, and marsupial protein and was purchased from AbCAM (Cambridge, MA). Anti-RNA polymerase II is a mouse polyclonal antibody directed against the synthetic peptide YSPTSPPS purchased through Active Motif (Carlsbad, CA). RC-30965 was obtained from Sigma Aldrich (St. Louis, MO).
RNA isolation
Total RNA was isolated from the relevant cells using RNA-Stat 60 according to the manufacturer’s specifications. Chloroform was added to solutions before being centrifuged at 12,000×g for 15 min at 4°C. The upper aqueous layer was retrieved and mixed with isopropanol and subsequently centrifuged at 12,000×g for 10 min at 4°C. The pellet was washed with 75% ethanol, centrifuged at 7,500×g for 5 min at 4°C, air-dried, and resuspended in water. mRNA was isolated using Qiagen (Valencia, CA) and Invitrogen (Carlsbad, CA) kits according to manufacturers’ instructions.
Microarray analysis
After confirmation of sample quality as described above, RNA samples were hybridized using a human U133A Microarray Chip. Array data were analyzed with Dchip, a model-based method for expression analysis (http://www.Dchip.org). The minimum expression was rounded up to 10, the average of noise in our hybridization experiments. Samples were separated into two replicates (Antagonist, CBX1 siRNA, and Control) done at similar time points with stock matched reagents.
Real time RT-PCR
Real time PCR was carried out on cDNA using the Applied Biosystems Fast7500 Sequencer (Carlsbad, Ca) in order to confirm the knockdown of the target genes. Taqman real time PCR primers from Applied Biosystems were used along with the Applied Biosystems Gene Expression Master Mix. Samples were quantified using a Nanodrop ND-1000 Spectrophotometer (Wilmington, DE) and diluted accordingly. Each sample was further diluted in a stepwise fashion and loaded into 96 well plates along with the reaction reagents. Each experimental run was load controlled against the 18S ribosomal subunit.
Chromatin immunoprecipitation
CaCo-2 cells were sonicated with a Fisher Sonic Dismembrator 60 (Pittsburgh, Pa) for three 20-s pulses interspersed with one minute rest times, followed by immunoprecipitation using the ChIP-it Express Kit (Active Motif, Carlsbad, Ca). The ChIP-It control kit “Human” was used as positive control. The positive control antibody used was a mouse monoclonal antibody targeted against the synthetic peptide YSPTSPPS corresponding to RNA polymerase II. Positive control primers were designed to target GAPDH, creating a 166-bp product upon PCR. The forward primer for GAPDH was 5′-TAC TAG CGG TTT TAC GGG CG-3′. The reverse was 5′-TCG AAC AGG AGG AGC AGA GAG CGA-3′. For immunoprecipitation of HP1β, a rabbit polyclonal antibody directed to amino acids 61–100 of the protein was used (Santa Cruz Biotechnologies, Santa Cruz, CA). Following immunoprecipitation, genomic DNA was isolated using a Qiagen DNA Micro Kit (Valencia, Ca). We used the gene for ARHGAP9 as a positive control for HP1β chromatin immunoprecipitation since this gene showed strong alteration in expression without showing evidence of altered expression subsequent to altered GRPR signaling, as determined by microarray, indicating that HP1β was likely binding in the vicinity of this gene. PCR primers (Invitrogen, Carlsbad, Ca) targeting the gene ARHGAP9 were 5′-GCA GTC CCA TGC ACA AGA T-3′ (forward) and 5′-TGA GTG GAT TAA CCC CTG CT-3′ (reverse).
Chromatin immunoprecipitation sequencing
Samples for HP1β and IGG negative control were prepared via ChIP. Sequencing was performed using the Oligonucleotide Ligation and Detection (SOLiD) next generation sequencer (Applied Biosystems, Foster City, CA). Sequencing was carried out with 12 million 35 base pair reads. Sequence alignment was performed using the BOWTIE aligner (Langmead et al. 2009), modified for color space reads. Experimental samples were compared against the negative control using a Poisson Distribution as assigned by the MACS aligner (Zhang et al. 2008). These reads were converted to .BED format and uploaded to the UCSC Genome Browser for peak identification.
Genomic PCR of chromatin immunoprecipitated DNA
Genomic DNA was immunoprecipitated after exposure to antibodies directed against HP1β or H3K9, the latter employed because methylated lysine 9 is a known binding target for HP1β (Bannister et al. 2001); as a control, immunoprecipitation was also performed using antibodies to IgG. As a further control, the same immunoprecipitations experiments were performed against cells whose ability to express GRPR was eliminated by siRNA as previously described (Tell et al. 2011). DNA was then subject to PCR with primers targeting genes determined to be both expressed differentially during microarray analysis and displaying a ChIP Sequencing Peak in the local area. Primer constructs were created using Invitrogen Oligoperfect Primer Generator. The sequences for each primer were as follows: SLC0B13 (forward): 5′-ATG ACC CAA ATG CCT GAA-3′; (reverse): 5′-TGG AGA AAA GGG AAC GCT-3′. IL1RAPL2 (forward): 5′-TAA TTG CCA CCG ACT TCT CC-3′; (reverse): 5′-TGA AGG TAC TTC CCC TGT GG-3′. GBE1 (forward): 5′-GCA CTC TGG AGG TGA GAA GG-3′; (reverse) 5′-AGA ATG CGC TGT GTT GTC TG-3′. PLOD2 (forward): 5′-ATG AAT TTT GGC ACC GTG A-3′; (reverse): 5′-AGC CTT GCT TCT TCC GTT TT-3′. FAM13A1 (forward): 5′-CAT TGG ACC AGC CAG TTT C-3′; (reverse): 5′-CCA AGG ACA GTG GGT TCT GT-3′. PLK3 (forward): 5′-CCT CTG GAA GAC TGC TGA CC-3′; (reverse): 5′-CTC ACG AGG GCA AAC TTC TC-3′. CFHR1P (forward): 5′-TGG AGT GCA ATG GTG TGA TT-3′; (reverse) 5′-GAG TTC GAG ACC AGC CTG AC-3′. CFH (forward): 5′-TTC TTG AAG AGC AGT CTT TTG G-3′; (reverse) 5′-AGG AAA GCA AAC CTC CTC CA-3′.
Results
Effect of GRP/GRPR signaling via HP1β as determined by microarray analysis
We previously showed that GRP/GRPR signaling in colon cancer cell lines altered the protein level of HP1β (Ruginis et al. 2006; Tell et al. 2011). Since HP1β is an epigenetic modifier of gene transcription, we next determined which genes had altered expression as a result of both GRPR activation and HP1β expression. To do this, we isolated RNA from GRP/GRPR-expressing Caco-2 cells alone or from cells that had been exposed to the GRPR specific antagonist RC-3095 or siRNA directed to HP1β. We exposed cells to siRNA for 72 h and antagonist for 20 h, time points we previously have shown to down-regulate HP1β under either condition (Tell et al. 2011). Control RNA and RNA extracted from the treated cells was then exposed to the U133A Microarray Chip, as described in “Methods”. Using a cut-off of at least a twofold change in expression, GRPR antagonism resulted in the up-regulation of 32 genes while siRNA directed to HP1β resulted in the up-regulation of 164 genes. However, only nine genes were up-regulated in response to treatment with both reagents (Fig. 1a; Table 1).
Fig. 1.
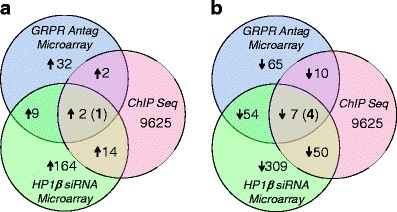
Venn diagram showing the genes up-regulated (a) and down-regulated (b) in response to treatment with siRNA directed to GRPR or HP1β, and the genomic positions occupied by HP1β as determined by ChIP-seq
Table 1.
Genes identified by microarray, ChIP-Seq, and genomic PCR as down-regulated subsequent
| Gene ID | ChIPSeq detected | gPCR confirmed | RT-PCR (fold change) | Pubmed ID | Chromo | Gene description | Antag vs. control (fold change) | HP1β siRNA vs. control (fold change) |
|---|---|---|---|---|---|---|---|---|
| IL1RAPL2 | Yes | Yes | 5.6 ± 1.8 | 26,280 | X | Interleukin 1 receptor accessory protein-like 2 | 4.8 | 5.4 |
| HSF2BP | Yes | No | n.r. | 11,077 | 21 | Heat shock transcription factor 2 binding protein | 2.3 | 3.2 |
| BOP1 | No | No | n.r. | 23,246 | 3 | Block of proliferation 1 | 3.2 | 4.0 |
| CYP27B1 | No | No | n.r. | 1,594 | 12 | Cytochrome P450, family 27, subfamily B, polypeptide 1 | 4.0 | 5.4 |
| KIR2DL5A | No | No | n.r. | 57,292 | 19 | Killer cell immunoglobulin-like receptor, two domains, long cytoplasmic | 4.8 | 6.4 |
| TNFRSF11B | No | No | n.r. | 4,982 | 8 | TNFRSF11B tumor necrosis factor receptor superfamily, member 11b | 3.4 | 2.6 |
| TNP2 | No | No | n.r. | 7,142 | 16 | Transition protein 2 | 3.1 | 4.0 |
| UTP20 | No | No | n.r. | 27,340 | 12 | TP20, small subunit (SSU) processome component, homolog | 3.2 | 3.7 |
| WWP2 | No | No | n.r. | 11,060 | 16 | WW domain containing E3 ubiquitin protein ligase 2 | 3.6 | 4.6 |
Antag: GRPR Specific Antagonist RC-3095. Chromo: Chromosome on which gene is located
In contrast, and again using a cut-off of a greater than twofold change in expression, GRPR antagonism resulted in the down-regulation of 65 genes, while siRNA directed to HP1β resulted in the down-regulation of 309 genes. Of these, 54 genes were down-regulated in response to both treatments (Fig. 1b; Table 2).
Table 2.
Genes identified by microarray, ChIP-Seq, and genomic PCR as up-regulated subsequent to GRPR signaling via HP1β
| Gene ID | ChIP-Seq detected | gPCR confirmed | RT-PCR (fold change) | Pubmed ID | Chromo | Gene description | Antag vs. control (fold change) | HP1β siRNA vs control (fold change) |
|---|---|---|---|---|---|---|---|---|
| FAM13A | Yes | Yes | 2.4 ± 0.6 | 10,144 | 4 | Family with sequence similarity 13, member A | 2.4 | 5.5 |
| GBE1 | Yes | Yes | 3.3 ± 0.7 | 2,632 | 3 | Glucan (1,4-alpha-), branching enzyme 1 | 2.8 | 4.2 |
| PLK3 | Yes | Yes | 2.8 ± 0.7 | 1,263 | 1 | Polo-like kinase 3 | 2.0 | 2.6 |
| SLCO1B3 | Yes | Yes | 4.2 ± 2.12 | 28,234 | 12 | Solute carrier organic anion transporter family, member 1B3 | 2.0 | 3.5 |
| PLOD2 | Yes | No | n.r. | 5,352 | 3 | Procollagen lysine, 2-oxoglutarate 5-dioxygenase 2 | 2.2 | 5.2 |
| ACSF2 | No | No | n.r. | 80,221 | 17 | Acyl-CoA synthetase family member 2 | 2.1 | 2.3 |
| AKAP7 | No | No | n.r. | 9,465 | 6 | A kinase (PRKA) anchor protein 7 | 5.0 | 14.1 |
| APOL1 | No | No | n.r. | 8,542 | 22 | apolipoprotein L, 1 | 2.0 | 3.0 |
| ARL14 | No | No | n.r. | 80,117 | 3 | ADP-ribosylation factor-like 14 | 2.4 | 4.6 |
| BCL3 | No | No | n.r. | 602 | 19 | B-cell CLL/lymphom a 3 | 3.4 | 3.5 |
| BDH2 | No | No | n.r. | 56,898 | 4 | 3-hydroxybutyrate dehydrogenase, type 2 | 2.0 | 3.1 |
| BNIP3L | No | No | n.r. | 665 | 8 | BCL2/adenovirus E1B 19 kDa interacting protein 3-like | 2.4 | 6.0 |
| CCDC64 | No | No | n.r. | 92,558 | 12 | Coiled-coil domain containing 64 | 2.1 | 3.0 |
| CDKN1A | No | No | n.r. | 12,575 | 17 | Cyclin dependent kinase inhibitor 1A (P21) | 2.5 | 4.8 |
| CDKN1C | No | No | n.r. | 1,028 | 11 | Cyclin dependent kinase inhibitor 1 C (p57, Kip2) | 3.3 | 7.6 |
| CLIC3 | No | No | n.r. | 9022 | 9 | Chloride intracellular channel 3 | 2.7 | 3.0 |
| CYP3A5P2 | No | No | n.r. | 79,424 | 7 | Cytochrome P450, family 3, subfamily A, polypeptide 5 pseudogene 2 | 2.2 | 2.4 |
| DUSP6 | No | No | n.r. | 1,848 | 12 | Dual specificity phosphatase 6 | 2.1 | 2.5 |
| ENO2 | No | No | n.r. | 2,026 | 12 | Enolase 2 (gamma, neuronal) | 3.0 | 4.9 |
| FABP1 | No | No | n.r. | 2,168 | 2 | Fatty acid binding protein 1, liver | 3.2 | 2.8 |
| FOSL1 | No | No | n.r. | 8,061 | 11 | FOS-like antigen 1 | 3.0 | 2.2 |
| FSCN1 | No | No | n.r. | 6,624 | 7 | Fascin homolog 1, actin-bundling protein (Strongylocent rotus purpuratus) | 3.1 | 3.8 |
| FXYD3 | No | No | n.r. | 5,349 | 19 | FXYD domain containing ion transport regulator 3 | 2.4 | 4.1 |
| GAL3ST1 | No | No | n.r. | 9,514 | 22 | Galactose-3-Osulfotransferase 1 | 2.0 | 2.9 |
| GEM | No | No | n.r. | 2,669 | 8 | GTP binding protein overexpressed in skeletal muscle | 3.8 | 5.2 |
| GPR87 | No | No | n.r. | 53,836 | 3 | G protein coupled receptor 87 | 5.3 | 2.5 |
| HMGCS2 | No | No | n.r. | 3,158 | 1 | 3-hydroxy-3-methylglutaryl-CoA synthase 2 (mitochondrial) | 2.6 | 2.6 |
| HRH1 | No | No | n.r. | 3,269 | 3 | Histamine receptor H1 | 2.3 | 2.9 |
| KDM4B | No | No | n.r. | 23,030 | 19 | Lysine (K)-specific demethylase 4B | 2.4 | 2.9 |
| LAMA3 | No | No | n.r. | 3,909 | 18 | Laminin, alpha 3 | 2.4 | 2.1 |
| LDLR | No | No | n.r. | 3,949 | 19 | Low density lipoprotein receptor | 2.1 | 2.7 |
| LGALS1 | No | No | n.r. | 3,956 | 22 | Lectin, galactosidebinding, soluble, 1 | 2.2 | 2.5 |
| MAFF | No | No | n.r. | 23,764 | 22 | v-maf musculoaponeurotic fibrosarcoma oncogene homolog F | 2.0 | 3.8 |
| NAB2 | No | No | n.r. | 4,665 | 12 | NGFI-A binding protein 2 (EGR1 binding protein 2) | 2.1 | 3.2 |
| NDRG1 | No | No | n.r. | 10,397 | 8 | N-myc downstream regulated 1 | 5.7 | 18.5 |
| NDUFA4L2 | No | No | n.r. | 56,901 | 12 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 4-like 2 | 9.8 | 9.2 |
| P4HA1 | No | No | n.r. | 5,033 | 10 | Prolyl 4-hydroxylase, alpha polypeptide I | 3.2 | 7.9 |
| PDE9A | No | No | n.r. | 5,152 | 21 | Phosphodiesterase 9A | 2.0 | 2.5 |
| PER2 | No | No | n.r. | 8,864 | 2 | Period homolog 2 (Drosophila) | 2.5 | 3.5 |
| PHLDA1 | No | No | n.r. | 22,822 | 12 | Pleckstrin homology-like domain, family A, member 1 | 2.2 | 3.9 |
| PTK6 | No | No | n.r. | 5,753 | 20 | PTK6 protein tyrosine kinase 6 | 2.2 | 2.4 |
| PTPRH | No | No | n.r. | 5,794 | 19 | Protein tyrosine phosphatase, receptor type, H | 2.1 | 2.3 |
| PTPRR | No | No | n.r. | 5,801 | 12 | Protein tyrosine phosphatase, receptor type, R | 6.5 | 12.0 |
| RARRES1 | No | No | n.r. | 5918 | 3 | Retinoic acid receptor responder (tazarotene induced) 1 | 2.4 | 3.8 |
| RHOF | No | No | n.r. | 54,509 | 12 | Ras homolog gene family, member F (in filopodia) | 2.1 | 3.1 |
| SCARF1 | No | No | n.r. | 8,578 | 17 | Scavenger receptor class F, member 1 | 2.5 | 4.2 |
| SGK1 | No | No | n.r. | 6,446 | 6 | Serum/glucoco rticoid regulated kinase 1 | 2.1 | 3.2 |
| SERPINB9 | No | No | n.r. | 5,272 | 6 | Serpin peptidase inhibitor, clade B (ovalbumin), member 9 | 2.5 | 3.8 |
| SLC11A2 | No | No | n.r. | 4,891 | 12 | Solute carrier family 11 8(proton-coupled divalent metal ion) | 3.0 | 3.7 |
| SPAG4 | No | No | n.r. | 6,676 | 20 | Sperm associated antigen 4 | 3.5 | 4.8 |
| TFF2 | No | No | n.r. | 7,032 | 21 | Trefoil factor 2 | 2.3 | 2.5 |
| ZNF274 | No | No | n.r. | 10,782 | 19 | Zinc finger protein 274 | 3.1 | 3.2 |
Identification of HP1β-regulated genes by ChIP-Seq
Since HP1β is an epigenetic modifier of gene transcription, we performed chromatin immunoprecipitation against genomic DNA obtained from GRP/GRPR-expressing Caco-2 cells in order to identify where this protein was binding in the genome. After genomic DNA extraction and sonication, rabbit polyclonal antibody directed to amino acids 61–100 of HP1β was used to immunoprecipitate DNA actively binding this protein. The DNA sequences were then processed in an Applied Biosystems SOLiD sequencer, generating 12 million 35 base pair reads. In this fashion, we determined that GRPR signaling resulted in 9,625 genomic positions occupied by HP1β.
Combining these data with that obtained by microarray analysis (above), we determined that two genes occupied by HP1β were up-regulated in response to the experimental treatment and that seven genes were down-regulated in response to this same treatment (Fig. 1; Tables 1 and 2). This was performed in order to detect a direct linkage between HP1β induced mRNA alterations and the binding of the HP1β protein along the nucleosome. In this manner, those genes directly affected by changes in HP1β binding were readily determined. Hence, these data suggested that GRPR signaling via HP1β might be down-regulating two genes and up-regulating the expression of seven genes. We next confirmed whether this was the case by performing genomic PCR for these genes on immunoprecipitated DNA.
Genomic and real time PCR
We isolated DNA from GRP/GRPR-expressing Caco-2 cells. Immunoprecipitation was performed after exposing the DNA to antibodies directed against HP1β H3K9 (methylated lysine 9 is a known binding target for HP1β) or IgG (as control). The same immunoprecipitation experiments were performed on Caco-2 cells that had been exposed to GRPR siRNA for 72 h, conditions that we have previously shown completely ablates GRPR expression (Tell et al. 2011). DNA was then subject to PCR with intron-exon spanning primers targeting the genes listed in Tables 1 and 2. Of the two genes potentially down-regulated subsequent to GRPR signaling via HP1β, gPCR was successful only for interleukin 1 receptor accessory protein-like 2 (IL1RAPL2) (Fig. 2). In contrast, of the seven genes potentially up-regulated subsequent to GRPR signaling via HP1β, gPCR was successful for 4: family with sequence similarity 13, member A (FAM13A), glucan (1,4-alpha-), branching enzyme 1 (GBE1), procollagen lysine, 2-oxoglutarate 5-dioxygenase 2 (SLCO1B3), and polo-like kinase 3 (PLK3).
Fig. 2.
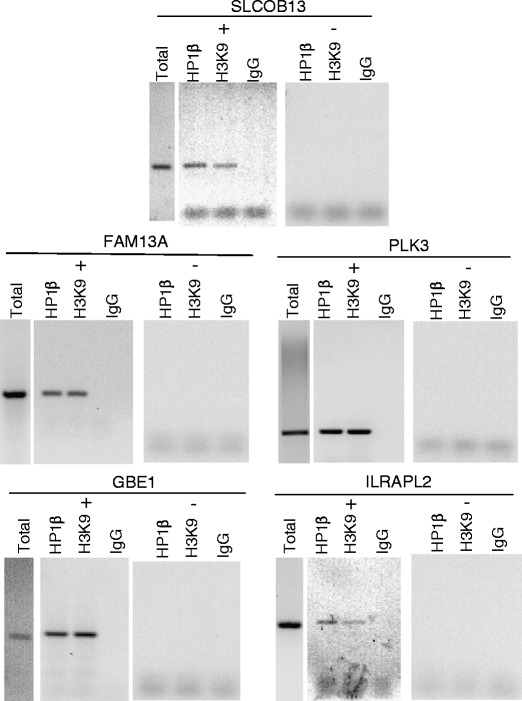
Genomic PCR for genes identified by microarray and ChIP-seq as regulated by GRPR signaling via HP1β. Genomic DNA was extracted from both wild-type Caco-2 cells (+) and cells that had been exposed to GRPR siRNA for 72 h (−) and immunoprecipitated after exposure to antibodies directed against HP1β, H3K9, or IGG. DNA was then subject to PCR with primers targeting genes determined to be both expressed differentially during microarray analysis and displaying a ChIP Sequencing Peak in the local area
Finally, we confirmed that GRPR signaling actually altered mRNA expression for these six genes by performing real time PCR. To do this, we quantified the relevant RNA in GRP/GRPR-expressing Caco-2 cells and compared that to what was expressed in cells exposed to GRPR siRNA. GRPR signaling decreased IL1RAPL2 expression 5.6 ± 1.8-fold (means ± SEM, n = 3); and increased the expression of FAM13A 2.4 ± 0.6-fold, GBE1 3.3 ± 0.7-fold, PLK3 2.8 ± 0.7-fold, and SLCO1B3 4.2 ± 2.12-fold (Tables 1 and 2).
Discussion
HP1 consists of a family of evolutionarily conserved proteins that act to epigenetically alter gene expression. In cancer, the role of HP1 is not clear: although proteins in this family appear largely to protect against tumor cell aggressiveness and metastasis (Kirschmann et al. 2000; Pomeroy et al. 2002; Norwood et al. 2004, 2006), worsened outcomes can also be associated with enhanced HP1 expression (Dialynas et al. 2008; Takanashi et al. 2009). Likewise, a number of studies have found that GRP/GRPR expression can have both deleterious as well as beneficial effects when these proteins are aberrantly expressed in a variety of malignancies [reviewed in (Jensen et al. 2008)]. Consistent with this dichotomous background, the results of this study demonstrate that GRP/GRPR signaling via HP1β can alter the expression of yet other proteins that might be expected to either improve or worsen the outcome of patients with CRC. We show that GRPR-induced up-regulation of HP1β results in the increased expression of FAM13A, GBE1, PLK3, and SLCO1B3 and the decreased expression of IL1RAPL2. Each will be reviewed in turn.
FAM13 (family with sequence similarity 13) was isolated from within a cluster of genes coding for extracellular matrix proteins involved in integrin–receptor interactions (Cohen et al. 2004). Genes within this cluster are involved in bone, mammary gland lobuloalveolar structures, and kidney function, suggesting that FAM13 likewise might be involved in the regulation of tissue architecture. This of interest since we have previously suggested that GRP/GRPR act as morphogens in CRC [reviewed in (Jensen et al. 2001)], promoting a better-differentiated tumor phenotype as they reprise their role in normal gut organogenesis (Carroll et al. 2002). Although there are no data relating to FAM13 expression in colon or colon cancer, it might be that this protein when expressed might be involved in GRP/GRPR promotion improved tumor differentiation, a marker of improved patient outcome.
GBE1 [glucan (1,4-alpha-), branching enzyme 1] deficiency results in glycogen storage disease type IV due to the accumulation of amylopectin-like polysaccharide causing cell swelling and death. More recently, it has been noted that this protein might also act as a morphogen, at least in the context of normal cardiac development (Lee et al. 2010). In cancer, decreased GBE1 levels are noted in ovarian cancer (Birch et al. 2008) and longer survival observed in patients with cervical cancer whose tumors expressed high levels of this protein (Lando et al. 2009). Although nothing is known about GBE1 in colon cancer, GRP/GRPR-induced up-regulation of this protein in this tumor type might be expected to improve patient outcome.
PLK3 (polio-like kinase 3) is one of four isoforms in a family of serine/threonine kinases (Johnson et al. 2007). Although different PLK isoforms in cancer have different phenotypes, PLK3 appears to promote cell cycle arrest, apoptosis, and in selected cancers, act as a tumor suppressor (Dai et al. 2002; Pellegrino et al. 2010). Furthermore, PLK3 knock-out mice develop tumors in various organs at advanced age (Yang et al. 2008), although none were noted arising in the colon. Again, GRP/GRPR-induced expression of PLK3 in CRC would thus be expected to benefit the host.
SLCO1B3 (organic anion transporting polypeptide 1B3; also known as Organic anion transporting polypeptide 1B3 or OATP1B3) is a member of membrane influx transporters that normally regulate the uptake of endogenous compounds, but which are also important in mediating drug absorption [reviewed in (Kalliokoski and Niemi 2009)]. SLCO1B3 is particularly important in regulating the uptake of taxanes such as the chemotherapeutic drug paclitaxel (Smith et al. 2005), and thus its expression has positively correlated with prognosis in a variety of malignancies including breast (Muto et al. 2007) and prostate (Hamada et al. 2008). Yet, SLCO1B3 also promotes bile acid uptake in the colon, and which in a recent study was shown to activate cyclooxygenase-2 gene transcription (Oshio et al. 2008), in turn known to promote tumor cell proliferation. Indeed, SLCO1B3 over-expression enhances the survival of human colon cancer cell lines that harbor wild-type (i.e., not mutated) p53. Hence, the impact of SLCO1B3 on human colon cancers, expressed subsequent to GRPR signaling via HP1β, remains to be elucidated.
Finally, we showed that GRPR signaling via HP1β resulted in the down-regulation of IL1RAPL2 (interleukin 1 receptor accessory protein-like 2). The function of this protein is not well understood but appears to be important in the function of the central nervous system, with mutations contained therein associated with autism (Piton et al. 2008).
Overall, then, our findings do not unambiguously demonstrate what effect GRPR signaling via HP1β might have on patients with colon cancer. FAM13, GBE1, and PLK3 all have been previously shown to be involved in processes that might be expected to improve patient outcome; whereas SLCO1B3 might be expected to worsen patient outcome. In contrast, there are no previously published data for IL1RAPL2 for any malignancy. To determine what effect any of these proteins have in CRC in the context of GRPR signaling will therefore require additional studies.
Acknowledgments
Conflicts of interest
There are no financial or personal conflicts of interest regarding the work contained within this manuscript.
Glossary
- GRP
Gastrin-releasing peptide
- GRPR
GRP receptor
- HP
Heterochromatin protein
Footnotes
Grant support
This work was supported by a VA Merit Review award (to RV Benya).
References
- Birch AH, Quinn MC, et al. Transcriptome analysis of serous ovarian cancers identifies differentially expressed chromosome 3 genes. Mol Carcinog. 2008;47:56–65. doi: 10.1002/mc.20361. [DOI] [PubMed] [Google Scholar]
- Carroll RE, Matkowskyj KA, et al. Aberrant expression of gastrin-releasing peptide and its receptor by well differentiated colon cancers in humans. Am J Physiol. 1999;276:G655–G665. doi: 10.1152/ajpgi.1999.276.3.G655. [DOI] [PubMed] [Google Scholar]
- Carroll RE, Matkowskyj KA, et al. Contribution of gastrin-releasing peptide and its receptor to villus development in the murine and human gastrointestinal tract. Mech Dev. 2002;113:121–130. doi: 10.1016/S0925-4773(02)00032-1. [DOI] [PubMed] [Google Scholar]
- Cohen M, Reichenstein M, et al. Cloning and characterization of FAM13A1-a gene near a milk protein QTL on BTA6: evidence for population-wide linkage disequilibrium in Israeli Holsteins. Genomics. 2004;84:374–383. doi: 10.1016/j.ygeno.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Dai W, Liu T, et al. Down-regulation of PLK3 gene expression by types and amount of dietary fat in rat colon tumors. Int J Oncol. 2002;20:121–126. [PubMed] [Google Scholar]
- Dialynas GK, Vitalini MW, et al. Linking heterochromatin protein 1 (HP1) to cancer progression. Mutat Res. 2008;647(1–2):13–20. doi: 10.1016/j.mrfmmm.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal SI, Jia S. Heterochromatin revisited. Nat Rev Genet. 2007;8(1):35–46. doi: 10.1038/nrg2008. [DOI] [PubMed] [Google Scholar]
- Hamada A, Sissung T, et al. Effect of SLCO1B3 haplotype on testosterone transport and clinical outcome in caucasian patients with androgen-independent prostatic cancer. Clin Cancer Res. 2008;14:3312–3318. doi: 10.1158/1078-0432.CCR-07-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JG, Carroll RE, et al. The case for gastrin-releasing peptide acting as a morphogen when it and its receptor are aberrantly expressed in cancer. Peptides. 2001;22:689–699. doi: 10.1016/S0196-9781(01)00380-1. [DOI] [PubMed] [Google Scholar]
- Jensen RT, Battey JF, et al. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol Rev. 2008;60(1):1–42. doi: 10.1124/pr.107.07108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EF, Stewart KD, et al. Pharmacological and functional comparison of the polo-like kinase family: insight into inhibitor and substrate specificity. Biochemistry. 2007;46:9551–9563. doi: 10.1021/bi7008745. [DOI] [PubMed] [Google Scholar]
- Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009;158:693–705. doi: 10.1111/j.1476-5381.2009.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, Kouzarides T. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–124. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- Kirschmann DA, Lininger RA, et al. Down-regulation of HP1Hsalpha expression is associated with the metastatic phenotype in breast cancer. Cancer Res. 2000;60(13):3359–3363. [PubMed] [Google Scholar]
- Lando M, Holden M, et al. Gene dosage, expression, and ontology analysis identifies driver genes in the carcinogenesis and chemoradioresistance of cervical cancer. PLos Genet. 2009;5(11):e1000719. doi: 10.1371/journal.pgen.1000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, et al. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YC, Chang CJ, et al. Glycogen-branching enzyme deficiency leads to abnormal cardiac development: novel insights into glycogen storage disease IV. Hum Mol Genet. 2010;20(3):455–465. doi: 10.1093/hmg/ddq492. [DOI] [PubMed] [Google Scholar]
- Maloney A, Clarke PA, et al. Gene and protein expression profiling of human ovarian cancer cells treated with the heat shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Cancer Res. 2007;67(7):3239–3253. doi: 10.1158/0008-5472.CAN-06-2968. [DOI] [PubMed] [Google Scholar]
- Muto M, Onogawa T, et al. Human liver-specific organic anion transporter-2 is a potent prognostic factor for human breast carcinoma. Cancer Sci. 2007;98:1570–1576. doi: 10.1111/j.1349-7006.2007.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, Hirokawa YS, et al. Reduced heterochromatin protein 1-beta (HP1beta) expression is correlated with increased invasive activity in human melanoma cells. Anticancer Res. 2006;26(6B):4349–4356. [PubMed] [Google Scholar]
- Norwood LE, Grade SK, et al. Conserved properties of HP1(Hsalpha) Gene. 2004;336(1):37–46. doi: 10.1016/j.gene.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Norwood LE, Moss TJ, et al. A requirement for dimerization of HP1Hsalpha in suppression of breast cancer invasion. J Biol Chem. 2006;281(27):18668–18676. doi: 10.1074/jbc.M512454200. [DOI] [PubMed] [Google Scholar]
- Oshio H, Abe T, et al. Peroxisome proliferator-activated receptor alpha activates cyclooxygenase-2 gene transcription through bile acid transport in human colorectal cancer cell lines. J Gastroenterol. 2008;43:538–549. doi: 10.1007/s00535-008-2188-3. [DOI] [PubMed] [Google Scholar]
- Pellegrino R, Calvisi DF, et al. Oncogenic and tumor suppressive roles of polo-like kinases in human hepatocellular carcinoma. Hepatology. 2010;51:857–868. doi: 10.1002/hep.23467. [DOI] [PubMed] [Google Scholar]
- Piton A, Michaud JL, et al. Mutations in the calcium-related gene IL1RAPL1 are associated with autism. Hum Mol Genet. 2008;17:3965–3974. doi: 10.1093/hmg/ddn300. [DOI] [PubMed] [Google Scholar]
- Pomeroy SL, Tamayo P, et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415(6870):436–442. doi: 10.1038/415436a. [DOI] [PubMed] [Google Scholar]
- Ruginis TA, Taglia L, et al. Consequence of gastrin-releasing peptide receptor activation in a human colon cancer cell line: a proteomic approach. J Proteome Res. 2006;5:1460–1468. doi: 10.1021/pr060005g. [DOI] [PubMed] [Google Scholar]
- Smith NF, Acharya MR, et al. Identification of OATP1B3 as a high-affinity hepatocellular transporter of paclitaxel. Cancer Biol Ther. 2005;4:815–818. doi: 10.4161/cbt.4.8.1867. [DOI] [PubMed] [Google Scholar]
- Takanashi M, Oikawa K, et al. Heterochromatin protein 1gamma epigenetically regulates cell differentiation and exhibits potential as a therapeutic target for various types of cancers. Am J Pathol. 2009;174(1):309–316. doi: 10.2353/ajpath.2009.080148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tell R, Rivera CA, et al. Gastrin-releasing peptide signaling alters colon cancer invasiveness via heterochromatin protein 1Hsβ. Am J Pathol. 2011;178(2):672–678. doi: 10.1016/j.ajpath.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasenius VM, Hemmer S, et al. Hepatocyte growth factor receptor, matrix metalloproteinase-11, tissue inhibitor of metalloproteinase-1, and fibronectin are up-regulated in papillary thyroid carcinoma: a cDNA and tissue microarray study. Clin Cancer Res. 2003;9(1):68–75. [PubMed] [Google Scholar]
- Yang Y, Bai J, et al. Polo-like kinase 3 functions as a tumor suppressor and is a negative regulator of hypoxia-inducible factor-1 alpha under hypoxic conditions. Cancer Res. 2008;68:4077–4085. doi: 10.1158/0008-5472.CAN-07-6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu T, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]


