Abstract
Acute intermittent hypoxia [AIH; 3, 5-min episodes; 35–45 mmHg arterial Po2 (PaO2)] elicits serotonin-dependent phrenic long-term facilitation (pLTF), a form of phrenic motor facilitation (pMF) initiated by Gq protein-coupled metabotropic 5-HT2 receptors. An alternate pathway to pMF is induced by Gs protein-coupled metabotropic receptors, including adenosine A2A receptors. AIH-induced pLTF is dominated by the serotonin-dependent pathway and is actually restrained via inhibition from the adenosine-dependent pathway. Here, we hypothesized that severe AIH shifts pLTF from a serotonin-dependent to an adenosine-dependent form of pMF. pLTF induced by severe (25–30 mmHg PaO2) and moderate (45–55 mmHg PaO2) AIH were compared in anesthetized rats, with and without intrathecal (C4) spinal A2A (MSX-3, 130 ng/kg, 12 μl) or 5-HT receptor antagonist (methysergide, 300 μg/kg, 15 μl) injections. During severe, but not moderate AIH, progressive augmentation of the phrenic response during hypoxic episodes was observed. Severe AIH (78% ± 8% 90 min post-AIH, n = 6) elicited greater pLTF vs. moderate AIH (41% ± 12%, n = 8; P < 0.05). MSX-3 (28% ± 6%; n = 6; P < 0.05) attenuated pLTF following severe AIH, but enhanced pLTF following moderate AIH (86% ± 26%; n = 8; P < 0.05). Methysergide abolished pLTF after moderate AIH (12% ± 5%; n = 6; P = 0.035), but had no effect after severe AIH (66 ± 13%; n = 5; P > 0.05). Thus severe AIH shifts pLTF from a serotonin-dependent to an adenosine-dependent mechanism; the adenosinergic pathway inhibits the serotonergic pathway following moderate AIH. Here we demonstrate a novel adenosine-dependent pathway to pLTF following severe AIH. Shifts in the mechanisms of respiratory plasticity provide the ventilatory control system greater flexibility as challenges that differ in severity are confronted.
Keywords: plasticity, breathing, spinal cord, serotonin, adenosine
breathing cannot cease for more than the briefest periods, or life will not continue. At the same time, breathing must be continuously adjusted throughout life to maintain homeostasis of arterial blood gases. Several control strategies exist to maintain respiratory homeostasis, including feedback, feedforward, and adaptive control strategies (i.e., plasticity; 38).
Plasticity is a hallmark of the neural system controlling breathing (39). One well-known model of respiratory plasticity is phrenic long-term facilitation (pLTF), a long-lasting increase in phrenic motor output elicited by acute exposure to intermittent hypoxia [AIH; historically, arterial Po2 (PaO2) of 35–45 mmHg] (1, 5). pLTF is relatively insensitive to details of the experimental protocol such as the level of hypoxia (30–70 mmHg PaO2; 14, 29) or the duration of hypoxic episodes (29, 42). However, pLTF does require episodic vs. continuous hypoxia (5). Considerable progress has been made toward an understanding of cellular/synaptic mechanisms giving rise to pLTF (10, 28, 39). In fact, we recently realized that multiple distinct cellular pathways give rise to long-lasting phrenic motor facilitation (pMF; a more general term that includes AIH-induced pLTF; 9).
AIH-induced pLTF requires spinal serotonin (5-HT2) receptor activation (3, 15, 22) and episodic spinal 5-HT receptor activation is sufficient to elicit pMF without hypoxia (25, 26). We refer to this mechanism as the “Q” pathway since it is initiated by the Gq-protein-coupled 5-HT2 receptor (7). Downstream signaling includes activation of protein kinase C (11, M. J. Devinney and G. S. Mitchell, unpublished observations) and new protein synthesis (3). Specifically, new synthesis of brain-derived neurotrophic factor (BDNF) and activation of its high-affinity receptor, tropomyosin-related kinase B (TrkB), are necessary and sufficient for AIH-induced pLTF (2).
Another pathway to pMF is termed the “S” pathway (9), because it is induced by Gs protein-coupled metabotropic adenosine (A2A; 17) or 5-HT7 receptors (21). Downstream signaling cascades include protein kinase A and new synthesis of an immature TrkB isoform (17). Although we initially suspected that the Q and S pathways would summate to produce pLTF, we now know that the S pathway actually attenuates the Q pathway via cross-talk inhibition during normal AIH-induced pLTF. Thus pretreatment with spinal A2A receptor antagonists enhances AIH-induced pLTF (20).
Hypoxia induces ATP, adenosine, and/or adenine nucleotide release from neurons and/or glia in the central nervous system (18, 30, 43, 49); however, hypoxia is also reported to decrease adenosine release (8, 13). If extracellular ATP is increased, it will be converted to adenosine by ectonucleotidases, increasing the extracellular adenosine concentration (41). Since severe hypoxia could potentially lead to greater ATP/adenosine release from neurons and/or glia, we hypothesized that severe AIH (PaO2 25–30 mmHg) would shift pLTF from predominantly serotonin-dependent toward predominantly adenosine-dependent mechanisms. We tested this hypothesis in anesthetized and ventilated rats with intrathecal catheters in the cervical spinal cord, thus enabling adenosine and serotonin receptor antagonist administration near the phrenic motor nucleus (3, 20).
MATERIALS AND METHODS
Experimental Groups
Experiments were performed with adult (3–4 mo) male Sprague-Dawley rats (Harlan Colony 218a; Indianapolis, IN). All experimental protocols were approved by the University of Wisconsin Animal Care and Use committee. Each rat group received one of nine treatments: 1) severe acute intermittent hypoxia (AIH; PaO2 25–30 mmHg; n = 6); 2) moderate AIH (PaO2 45–50 mmHg; n = 8); 3) vehicle (artificial cerebral spinal fluid; aCSF) without AIH (i.e., time control; n = 6); 4) severe AIH + the A2A receptor antagonist MSX-3 (n = 6); 5) moderate AIH + MSX-3 (n = 8); 6) MSX-3 without AIH (n = 7); 7) severe AIH + the broad spectrum serotonin receptor antagonist methysergide (n = 5); 8) moderate AIH + methysergide (n = 6); and 9) methysergide without AIH (n = 5). These experimental groups enabled us to test the specific hypotheses that 1) severe AIH elicits an enhanced form of pLTF; 2) severe AIH-induced pLTF is adenosine (not serotonin) dependent; and 3) moderate AIH-induced pLTF is serotonin (not adenosine) dependent.
Experimental Preparation
Anesthesia was induced with isoflurane in an induction chamber and then maintained initially via nose cone (3.5% isoflurane in 50% O2, balance N2). Rats were tracheotomized to enable artificial ventilation (Rodent Respirator, model 683, Harvard Apparatus, Holliston, MA; tidal volume = 2.5 ml). After surgical preparations were complete, rats were converted to urethane anesthesia over 15–20 min (1.8 g/kg iv). The adequacy of anesthesia was tested before protocols commenced and immediately after the protocol was complete; adequacy of anesthetic depth was assessed as the lack of pressor or respiratory neural response to a toe pinch. After conversion to urethane anesthesia, rats were given a continuous intravenous infusion (1.5–6 ml·kg−1·h−1) of a 1:2:0.13 or 1:3:0.6 mixture of 6% hetastarch in 0.9% sodium chloride, lactated Ringer's solution, and 8.4% sodium bicarbonate to maintain fluid and acid-base balance. To monitor end-tidal Pco2 (PetCO2), a flow-through carbon dioxide analyzer was used with sufficient response time to measure PetCO2 in rats (Capnogard, Novametrix, Wallingford, CT). PetCO2 was maintained at 40–45 mmHg throughout surgery. A bilateral vagotomy was performed (midcervical) to prevent entrainment of respiratory neural activity with the ventilator.
To monitor blood pressure and draw blood samples for blood-gas analysis (ABL-800 Flex, Radiometer; Westlake, OH), the femoral artery was cannulated (polyethylene catheter PE50, Intramedic). Blood pressure was monitored with a pressure transducer (Gould, P23ID) and blood samples (0.2–0.4 ml) were drawn for blood gas analysis. Blood gases were assessed during baseline before and after drug delivery, during each of the three hypoxic episodes, and at 15, 30, 60, and 90 min post-AIH. A catheter was placed in the femoral vein (polyethylene PE50, Intramedic) or tail vein (24G × ¾ gauge iv catheter, Surflo). A rectal thermometer (Fisher Scientific, Pittsburgh, PA) was used to monitor body temperature and maintained (37.5 ± 1°C) with a heated surgical table.
The left phrenic nerve was isolated (dorsal approach), cut distally, desheathed, and then covered with a saline-soaked cotton ball until protocols commenced. Laminectomy was performed at cervical level 2 (C2) for all rats except those exposed to moderate and severe hypoxia without drugs. A small incision was made in the dura and a soft silicone catheter (2 Fr; Access Technologies, Skokie, IL) was inserted caudally 3–4 mm until the tip rested approximately over the C4 segment to deliver drugs near the phrenic motor nucleus. The catheter was attached to a 50-μl Hamilton syringe filled with drug or vehicle solutions as described below. Once rats were converted to urethane anesthesia, a minimum of 1 h was allowed before the experimental protocol commenced. After the neurophysiological protocol was complete, all animals were euthanized by urethane overdose.
Neurophysiological Measurements
Rats were paralyzed using pancuronium bromide for neuromuscular blockade (2.5 mg kg iv). Mineral oil was placed in the cavity and the left phrenic nerve was placed on a bipolar silver electrode to record nerve activity. Phrenic neural signals were amplified (10,000×), band-pass filtered (100–10,000 Hz, model 1800, A-M Systems, Carlsborg, WA), rectified, and integrated (Paynter filter, time constant 50 ms, MA-821, CWE, Ardmore, PA). Integrated phrenic nerve bursts were digitized (8 kHz) and analyzed using WINDAQ data-acquisition system (DATAQ Instruments, Akron, OH).
To begin a protocol, the apneic threshold was determined by lowering PetCO2 until phrenic nerve activity ceased for ∼1 min. The recruitment threshold was then determined by slowly increasing the PetCO2 until phrenic nerve activity resumed (1). PetCO2 was raised to ∼2 mmHg above the recruitment threshold and approximately 15–20 min were allowed to establish stable neural activity (i.e., baseline). Another arterial blood sample was drawn ∼5–10 min after drug delivery and throughout the protocol; arterial Pco2 (PaCO2) was maintained ±1.5 mmHg of baseline levels by adjusting inspired CO2 or ventilator rate.
Rats receiving spinal injections received vehicle (12 μl aCSF; in mM: 120 NaCl, 3 KCl, 2 CaCl2, 2 MgCl2, 23 NaHCO3, 10 glucose, pH 7.3–7.45), 10 μM MSX-3 (12 μl), or 20 mM methysergide maleate (15 μl). MSX-3 was stored as 2 mM, 5-μl aliquots. For experiments, 995 μl aCSF was added to give a final concentration of 10 μM (130 ng/kg; Sigma Aldrich); drug solutions were delivered slowly over 1 min, 5 min prior to the first hypoxic episode (or equivalent time for control groups) as described previously (17, 20). Methysergide maleate (20 mM; 300 μg/kg; Sigma Aldrich) was prepared on the day of experiments; the drug was delivered slowly over 1.5- min, ∼10–15 min prior to the first hypoxic episode as previously described (3). Methysergide was given ∼10–15 min prior to the first hypoxic episode instead of 5 min like MSX-3 because methysergide is reported to increase baseline phrenic nerve activity whereas MSX-3 is not (3, 20). However, baseline activity was not significantly affected by methysergide in the present study (not shown). Control rats that did not receive AIH were injected with either vehicle (aCSF, 12 μl) 5–10 min prior, MSX-3 (12 μl) 5 min prior, or methysergide (15 μl) 10–15 min prior to initiating an experimental protocol.
Acute intermittent hypoxia was achieved by delivering 3, 5-min episodes of isocapnic (±1.5 mmHg) hypoxia separated by 5-min intervals of baseline oxygen levels (∼51% inspired O2; PaO2 ≥ 150 mmHg). Moderate hypoxia was achieved by maintaining PaO2 between 45 and 55 mmHg, whereas severe hypoxia was 25–30 mmHg. PaO2 was verified by blood-gas analysis during each hypoxic episode. Following the third hypoxic episode, all animals were returned to baseline inspired O2 levels and maintained for the duration of the experiment.
Data Analyses
Integrated phrenic nerve burst amplitude was averaged over 1-min bins at each experimental time point (baseline, 15, 30, 60, and 90 min). When comparing phrenic nerve burst voltage at baseline between treatment groups, a one-way ANOVA design was used. Phrenic nerve burst amplitude was normalized as a percent change from baseline activity. Respiratory frequency was also normalized to baseline and expressed as an absolute difference in bursts per minute. For all statistical comparisons within and between treatment groups for nerve amplitude, respiratory frequency and blood gases (15-, 30-, 60-, and 90-min time points), a two-way ANOVA with a repeated-measures design was used. pLTF was determined as when time points were significantly different from baseline within a group in addition to comparison to the relevant time control experiment. Hypoxic episodes were compared within groups with a two-way ANOVA with a repeated-measures design. Phrenic responses between hypoxic episodes and among groups were analyzed using a two-way ANOVA with a two-factor arrangement and repeated-measures design. Individual comparisons were made using the Fisher's least significant difference post hoc test (SigmaPlot version 12.0; Systat Software, San Jose, CA). All differences between groups were considered significant if P < 0.05, and all values are expressed as means ± 1 SE.
RESULTS
Blood Gases and Mean Arterial Pressures
During hypoxic episodes.
Although arterial Pco2 (PaCO2) during hypoxic episodes differed between three treatment groups (moderate + MSX-3, severe + methysergide, and moderate + methysergide), PaCO2 was regulated within 1.5 mmHg of its baseline value in all groups studied (Table 1). Thus changes in integrated phrenic nerve burst amplitude with time could not be attributed to differences in chemoreceptor feedback occurring following intermittent hypoxia. In all but one instance, PaO2 was successfully maintained within the target range for severe (25–30 mmHg) and moderate (45–55 mmHg) AIH (Table 1). Mean arterial pressure (MAP) did not differ within groups, but MAP was significantly lower during severe vs. moderate AIH (Table 1).
Table 1.
Arterial Pco2, Po2, and MAP during hypoxic episodes
| PaCO2, mmHg |
PaO2, mmHg |
MAP, mmHg |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Experimental Groups | HX1 | HX2 | HX3 | HX1 | HX2 | HX3 | HX1 | HX2 | HX3 |
| Severe | 49.5 ± 1.9 | 47.8 ± 1.6bc | 47.4 ± 2.5 | 29 ± 1de | 31.8 ± 4de | 27 ± 1de | 78 ± 9de | 71 ± 7bde | 63 ± 8bde |
| Severe + MSX-3 | 47.0 ± 1.6 | 46.9 ± 1.5 | 45.6 ± 1.9 | 30 ± 0.5d | 28 ± 1d | 28 ± 1d | 70 ± 7d | 58 ± 8bd | 53 ± 8bd |
| Severe + methysergide | 53.0 ± 1.3a | 52.7 ± 2.4a | 50.5 ± 1.5ab | 30 ± 1d | 30 ± 1d | 28 ± 1d | 65 ± 4d | 52 ± 2bd | 61 ± 8d |
| Moderate | 47.9 ± 0.9 | 47.5 ± 1.3 | 47.4 ± 1.0 | 54 ± 3d | 47 ± 2bd | 50 ± 2d | 106 ± 8 | 98 ± 7 | 90 ± 6b |
| Moderate + MSX-3 | 47.2 ± 0.4 | 49.2 ± 0.6bd | 48.7 ± 1.6d | 52 ± 2ae | 51 ± 1ae | 52 ± 1ae | 89 ± 5ae | 82 ± 5a | 81 ± 5ae |
| Moderate + methysergide | 50.4 ± 0.4 | 51.0 ± 0.7 | 52.8 ± 0.9be | 50 ± 1cd | 50 ± 1cd | 49 ± 1cd | 89 ± 10cd | 83 ± 3cd | 63 ± 9bdef |
| Vehicle (−AIH) | 46.8 ± 1.0 | 46.8 ± 1.5 | 47.0 ± 1.3 | 327 ± 6 | 313 ± 7b | 314 ± 3b | 108 ± 8 | 108 ± 6 | 102 ± 5 |
| MSX-3 (−AIH) | 45.4 ± 1.4 | 44.8 ± 1.2 | 44.6 ± 1.1 | 332 ± 4 | 331 ± 4 | 330 ± 4 | 102 ± 4 | 96 ± 4 | 105 ± 5 |
| Methysergide (−AIH) | 51.8 ± 2.0 | 51.4 ± 1.2 | 50.1 ± 1.4 | 332 ± 8 | 322 ± 8 | 314 ± 8bf | 119 ± 8 | 114 ± 8 | 113 ± 9 |
All values are expressed as means ±1 SE. HX1, HX2 and HX3 designate hypoxic episodes 1, 2, and 3, respectively; MAP, mean arterial pressure; AIH, acute intermittent hypoxia. Experimental groups include severe or moderate hypoxia, or normoxia. Rats received either a vehicle or drug treatment: MSX-3, an adenosine 2A receptor antagonist, or methysergide, a broad-spectrum serotonin receptor antagonist.
Significant difference from severe + MSX-3.
Significant difference from HX1 in same treatment group.
Significant difference from severe + methysergide.
Significant difference from appropriate time control group (−AIH).
Significant difference from moderate AIH alone.
Significant difference from HX2 in same treatment group. Differences were considered significant if P < 0.05.
Baseline and 90-min posthypoxia.
Measurements of PaCO2 and PaO2 during baseline conditions were similar in all experimental groups (Table 2). In all groups receiving spinal injections, PaCO2 was regulated within 1.5 mmHg of baseline values throughout the protocol, and PaO2 was maintained above 150 mmHg at all times posthypoxia (Table 2). MAP was similar among groups at baseline (except for those rats treated with methysergide; see below) and at 90 min posthypoxia (Table 2). Thus differences in PaCO2, PaO2, or MAP regulation among groups cannot account for differential pMF responses. However, methysergide administration significantly lowered MAP at baseline and compared with AIH alone at 90 min in both the severe and moderate AIH groups. In methysergide-treated groups, it is possible, but unlikely, that decreased MAP modified AIH-induced phrenic motor facilitation since 1) Bach and Mitchell (1) observed that MAP changes of 20 mmHg from control cause little consistent change in respiratory activity in rats; and 2) Neverova et al. (40a) found that larger blood pressure changes due to blood withdrawal had no major effect on LTF in the hypoglossal nerve. Thus it is difficult to argue that a decrease in MAP of 16–25 mmHg post-methysergide had a major impact on the results of this study, although we do not rule out slight modifications in pLTF based on differences in blood pressure regulation. Regardless, such an effect would not alter the fundamental conclusions of this study.
Table 2.
Arterial Pco2, Po2, and MAP during baseline and 90 min posthypoxia
| PaCO2, mmHg |
PaO2, mmHg |
MAP, mmHg |
||||
|---|---|---|---|---|---|---|
| Experimental Groups | Baseline | 90 min | Baseline | 90 min | Baseline | 90 min |
| Severe | 49.2 ± 1.7 | 49.2 ± 1.6 | 280 ± 34abcd | 323 ± 3e | 127 ± 9cd | 125 ± 7cd |
| Severe + MSX-3 | 47.5 ± 1.1 | 47.1 ± 1.1 | 335 ± 3 | 298 ± 26ae | 109 ± 6 | 113 ± 6 |
| Severe + methysergide | 51.7 ± 1.2ab | 51.5 ± 1.3ab | 335 ± 7 | 326 ± 5 | 107 ± 10 | 91 ± 14be |
| Moderate | 47.5 ± 0.8 | 47.4 ± 0.9 | 336 ± 4 | 329 ± 4 | 122 ± 6 | 117 ± 5 |
| Moderate + MSX-3 | 48.6 ± 0.5c | 49.0 ± 0.4c | 330 ± 7 | 324 ± 6 | 103 ± 7a | 99 ± 5a |
| Moderate + methysergide | 52.1 ± 0.7ab | 52.3 ± 0.7ab | 333 ± 9 | 321 ± 3 | 108 ± 9 | 83 ± 8ae |
| Vehicle (−AIH) | 46.9 ± 1.3 | 47.0 ± 1.1 | 329 ± 7 | 322 ± 3 | 110 ± 7 | 101 ± 8 |
| MSX-3 (−AIH) | 45.1 ± 1.5 | 45.2 ± 1.4 | 331 ± 3 | 326 ± 3 | 101 ± 4 | 106 ± 7 |
| Methysergide (−AIH) | 51.2 ± 1.7 | 50.7 ± 2.0 | 326 ± 7 | 324 ± 3 | 119 ± 9 | 94 ± 10e |
Values are expressed as means ±1 SE. Significant difference from
moderate AIH alone,
severe + MSX-3,
appropriate time control group (−AIH)),
severe + methysergide, and
from baseline. Differences were considered significant if P < 0.05.
Progressive Augmentation of the Short-Term Hypoxic Phrenic Response
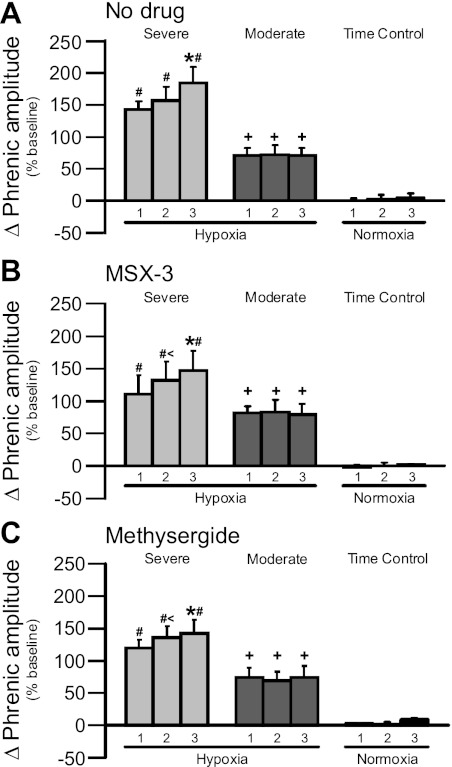
Progressive augmentation has been characterized as a progressive increase in the magnitude of the short-term hypoxic ventilatory (or phrenic) response from the initial to the final episode of an intermittent hypoxia protocol (31, 44). Although progressive augmentation is not generally observed in phrenic activity of rats receiving moderate AIH (1, 17, 19), all severe AIH-treated groups exhibited significant progressive augmentation in this study. Within each group, there was a range in the degree of progressive augmentation (3 of 17 animals did not display progressive augmentation); however, overall averages of hypoxic episodes 2 and 3 were significantly greater than hypoxic episode 1. This phenomenon did not result from variable phrenic baseline discharge as there were no significant differences between AIH-treated groups (1.4 ± 0.1 V for severe AIH only, 1.7 ± 0.3 V for moderate AIH only, 1.9 ± 0.3 V for severe AIH + MSX-3, 1.5 ± 0.3 V for moderate AIH + MSX-3, 1.8 ± 0.3 V for severe AIH + methysergide, 1.7 ± 0.4 V for moderate AIH + methysergide; P > 0.05; data not shown). Thus the short-term hypoxic phrenic response was greater when comparing hypoxic episodes 2 and 3 to episode 1 in rats receiving severe AIH without intrathecal drugs (143 ± 12%, 156 ± 21%, and 184 ± 24% for hypoxic episodes 1, 2, and 3, respectively, n = 6, Fig. 1A). In rats receiving severe AIH with intrathecal drugs, progressive augmentation was also evident (MSX-3: 111 ± 28%, 132 ± 29%, and 147 ± 31%, n = 6, Fig. 1B; methysergide: 120 ± 12%, 136 ± 17%, and 143 ± 21%, n = 5, Fig. 1C; P < 0.05 for episodes 2 and 3 vs. episode 1 in all severe AIH groups). Progressive augmentation during severe AIH was not due to changes in chemoreceptor feedback since neither PaO2 nor PaCO2 exhibited systematic changes that could account for this behavior (Table 1). Hypoxic episodes for all severe AIH-treated groups (i.e., with or without MSX-3 or methysergide pretreatment) were significantly greater than corresponding hypoxic episodes in moderate AIH-treated rats (Fig. 1, A–C; P < 0.05). As reported previously, progressive augmentation was not observed in any of the moderate AIH-treated groups (no drugs: 71 ± 11%, 72 ± 15%, and 71 ± 11% for episodes 1–3, respectively, n = 8, Fig. 1A; MSX-3: 82 ± 10%, 83 ± 19%, and 79 ± 16%, n = 8, Fig. 1B; methysergide: 74 ± 16%, 69 ± 14%, and 74 ± 18%, n = 6, Fig. 1C; P > 0.05 for all groups).
Fig. 1.
Progressive augmentation of the short-term hypoxic phrenic response during severe (light gray bars) but not moderate (dark gray bars) hypoxic episodes or time control groups (normoxia; black bars) where 1, 2, and 3 represent hypoxic or normoxic episodes 1, 2, and 3. In A, the hypoxic response after no drug was administered and during severe acute intermittent hypoxia (AIH) progressively increases with each episode. [Asterisk (*) indicates 3 is significantly greater than 1 and 2]. Neither intrathecal MSX-3 (B) nor methysergide (C) alters this effect, demonstrating that progressive augmentation is independent of spinal serotonin or A2A receptor activation. Moderate AIH did not elicit progressive augmentation, similar to previous reports from our laboratory. There were no changes in time control experiments. Plus (+) indicates a greater response relative to time control experiments at equivalent times. (#) indicates that 1, 2, and 3 during moderate AIH and time control experiments are significantly lower than severe 1, 2, and 3, respectively. (<) indicates 2 is significantly higher than 1 in B and C. Values are means ± 1 SE; significance is P < 0.05.
In time control rats without AIH, there were no detectable increases in phrenic activity (relative to baseline there were no significant differences between groups for baseline phrenic discharge: 1.7 ± 0.2 V for controls where no drug was given, 1.3 ± 0.3 V for MSX-3 time controls, and 2.0 ± 0.4 volts for methysergide time controls; data not shown) at times corresponding to hypoxic episodes in AIH protocols (no drug: 0.6 ± 2%, 2 ± 7%, and 5 ± 7%, n = 6, Fig. 1A; MSX-3: −2 ± 3%, 0.4 ± 5%, and 2 ± 1%, n = 7, Fig. 1B; methysergide: 3 ± 1%, 1 ± 5%, and 9 ± 3%, n = 5, Fig. 1C; P > 0.05 for all groups). Typical phrenic neurograms during experimental protocols for each treatment group are illustrated in Figs. 2–4 (no drugs: Fig. 2; MSX-3: Fig. 3; methysergide: Fig. 4).
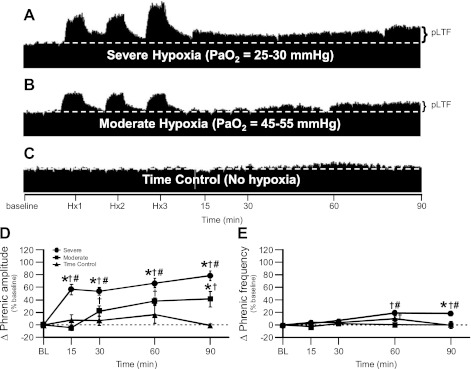
Fig. 2.
Severe AIH elicits enhanced phrenic long-term facilitation (pLTF) vs. pLTF induced by moderate AIH. A–C: representative traces of compressed, integrated phrenic neurograms during severe and moderate AIH compared with rats treated as time controls and not exposed to AIH (white, dashed line in each trace indicates baseline). In A, rats exposed to severe AIH exhibit enhanced pLTF. In B, rats exposed to moderate AIH exhibit typical pLTF. In C, time control rats not exposed to AIH do not exhibit time-dependent changes in phrenic motor output. D: phrenic burst amplitude (percentage change from baseline) in severe (n = 6; circles) and moderate AIH (n = 8; squares) protocols compared with rats treated as time controls (n = 6; triangles) and not exposed to AIH. pLTF is significantly elevated following severe AIH at 15, 30, 60, and 90 min post-hypoxia compared with baseline (BL; †), rats treated with moderate AIH (#) and rats treated as time controls (*) (all P < 0.05). pLTF is significantly elevated following moderate AIH at 30, 60, and 90 min post-hypoxia compared with BL (†) and rats treated as time controls at 90 min post-hypoxia (*) (P < 0.05). E. Phrenic frequency (percentage change from baseline) in severe (circles) and moderate AIH (squares) protocols compared with rats treated as time controls (triangles) and not exposed to AIH, where phrenic frequency was scaled the same as phrenic burst amplitude to enable direct comparisons of magnitude. Minimal LTF in phrenic nerve burst frequency is seen with severe AIH at 60 and 90 min posthypoxia compared with BL (†), rats treated with moderate AIH (#) and only at 90 min post-hypoxia compared with rats treated as time controls (*) (all P < 0.05). Minimal LTF in phrenic nerve burst frequency is seen with moderate AIH only at 60 min posthypoxia compared with BL (†) (P < 0.05).
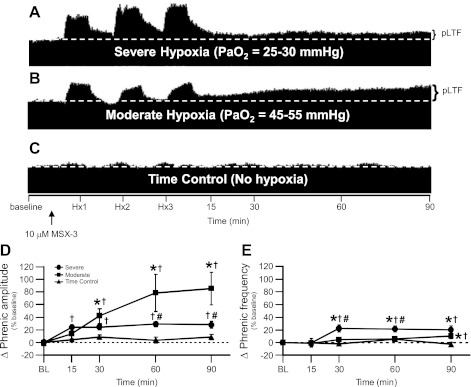
Fig. 3.
Severe, not moderate, AIH-induced pLTF is significantly attenuated by pretreatment with 10 μM MSX-3. A–C: representative traces of compressed, integrated phrenic neurograms during severe and moderate AIH compared with rats treated as time controls and not exposed to AIH after 10 μM MSX-3 spinal delivery (white, dashed line in each trace indicates baseline and black arrow indicates time of 10 μM MSX-3 delivery which was ∼5 min before the first hypoxic episode in each group). In A, rats exposed to severe AIH after spinal MSX-3 exhibit attenuated pLTF. In B, rats exposed to moderate AIH after spinal MSX-3 exhibit pronounced pLTF. In C, time control rats not exposed to AIH after spinal MSX-3 do not exhibit time-dependent changes in phrenic motor output. D: phrenic burst amplitude (percentage change from baseline) in severe (n = 6; circles) and moderate AIH (n = 8; squares) protocols compared with rats treated as time controls (n = 7; triangles) and not exposed to AIH after 10 μM MSX-3 spinal delivery. pLTF is significantly elevated by moderate AIH after 10 μM MSX-3 at 15, 30, 60, and 90 min posthypoxia compared with BL (†), and at 30, 60, and 90 min posthypoxia compared with rats treated as time controls (*) (all P < 0.05). pLTF following severe AIH after 10 μM MSX-3 is significantly attenuated compared with moderate AIH at 60 and 90 min posthypoxia (#) (P < 0.05) and is significantly increased at 30, 60, and 90 min posthypoxia compared with BL (†) (P < 0.05). E: phrenic frequency (percentage change from baseline) in severe (circles) and moderate AIH (squares) protocols compared with rats treated as time controls (triangles) and not exposed to AIH after 10 μM MSX-3 spinal delivery, where phrenic frequency was scaled the same as phrenic burst amplitude to enable direct comparisons of magnitude. Minimal LTF in phrenic nerve burst frequency is seen with severe AIH at 30, 60, and 90 min posthypoxia compared with BL (†) and rats treated as time controls (*), but only at 30 and 60 min posthypoxia compared with moderate AIH (#) (all P < 0.05). Minimal LTF in phrenic nerve burst frequency is seen with moderate AIH at 90 min posthypoxia compared with BL (†) and rats treated as time controls (*) (P < 0.05).
Fig. 4.
Moderate, not severe, AIH-induced pLTF is significantly inhibited by 20 mM methysergide. A–C: representative traces of compressed, integrated phrenic neurograms during severe and moderate AIH compared with rats treated as time controls and not exposed to AIH after 20 mM methysergide spinal delivery (white, dashed line in each trace indicates baseline and black arrow indicates time of 20 mM methysergide delivery which was ∼10 min before the first hypoxic episode in each group). In A, enhanced pLTF (after severe AIH) is not altered in rats pretreated with spinal methysergide. In B, rats exposed to moderate AIH after spinal methysergide delivery exhibit abolished pLTF. In C, time control rats not exposed to AIH after spinal methysergide delivery do not exhibit time-dependent changes in phrenic motor output. D: phrenic burst amplitude (percentage change from baseline) in severe (n = 5; circles) and moderate AIH (n = 6; squares) protocols compared with rats treated as time controls (n = 5; triangles) and not exposed to AIH after 20 mM methysergide spinal delivery. pLTF is significantly increased by severe AIH after 20 mM methysergide delivery at 15, 30, 60, and 90 min posthypoxia compared with BL (†), at 15, 60 and 90 min posthypoxia compared with rats treated with moderate AIH (#) and at 60 and 90 min posthypoxia compared with rats treated as time controls (*) (all P < 0.05). Notice that phrenic amplitude in rats treated with moderate AIH after 20 mM methysergide is not significantly different from BL or from rats treated as time controls; thus moderate AIH induced pLTF is inhibited by 20 mM methysergide. E: phrenic frequency (percentage change from baseline) in severe (circles) and moderate AIH (squares) protocols compared with rats treated as time controls (triangles) and not exposed to AIH after 20 mM methysergide spinal delivery, where phrenic frequency was scaled the same as phrenic burst amplitude to enable direct comparisons of magnitude. Minimal LTF in phrenic nerve burst frequency is only seen with severe AIH at 60 min posthypoxia compared with BL (†), (P < 0.05).
Severe AIH Elicits Greater pLTF vs. Moderate AIH
Previously studied protocols of moderate AIH (35–45 mmHg) elicited a pLTF of 50–60%, at 60 min posthypoxia (3, 4, 27, 50, 20). Here, we investigated AIH-induced pLTF in two different ranges of hypoxia: 1) severe (25–30 mmHg) or 2) more moderate hypoxia (45–55 mmHg) than in previous studies (see Figs. 2–4 for examples of typical phrenic neurograms). The moderate AIH protocol used here elicited smaller pLTF than historical reports, although we did not investigate the same range of AIH (PaO2 35–45 mmHg); in the present study, pLTF was 41 ± 12% baseline at 90 min post-AIH (P < 0.05; n = 8; Fig. 2, B and D). Severe AIH elicited greater pLTF vs. moderate AIH (Fig. 2, A and D) (pLTF: 78 ± 8% baseline at 90 min post-AIH; P < 0.05; n = 6). Time control experiments without AIH did not exhibit changes in phrenic burst amplitude at any time point (−0.7 ± 3% baseline at 90 min; P > 0.05; n = 6) (Fig. 2, C and D).
In this experimental preparation, AIH-induced frequency LTF is typically smaller than pLTF in burst amplitude (4). Both severe AIH-treated rats and animals treated as time controls exhibited significantly greater frequencies at 60 min post-AIH compared with their respective baselines (8 ± 2 bursts/min for severe at 60 min post-AIH, n = 6; 4 ± 2 bursts/min for vehicle- or time control-treated rats at 60 min post-AIH, n = 6; expressed as a percent change from baseline in Figs. 2E and Fig. 5C; P < 0.05), whereas severe AIH-treated animals also had greater frequency LTF at 90 min post-AIH compared with baseline and time control rats (8 ± 2 bursts/min for severe at 90 min post-AIH, n = 6; −0.1 ± 2 bursts/min for vehicle- or time control-treated rats at 90 min post-AIH, n = 6; expressed as a percent change from baseline in Fig. 2E and 5C; P < 0.05). Severe AIH-induced frequency LTF was also significantly greater than in moderate AIH-treated animals at 60 and 90 min posthypoxia, (−0.1 ± 0.8 bursts/min at 60 min post-AIH and −0.5 ± 3 bursts/min for moderate at 90 min post-AIH, n = 8; expressed as a percent change from baseline in Figs. 2E and 5D; P < 0.05).
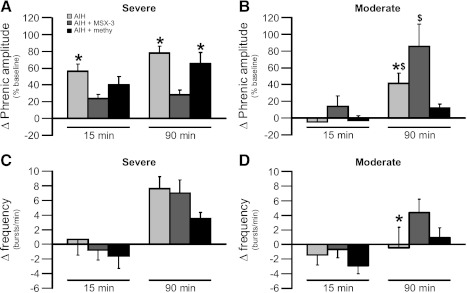
Fig. 5.
Direct comparison of the change in phrenic burst amplitude (percent baseline) and frequency (bursts/min) following severe (A: amplitude; C: frequency) and moderate AIH (B: amplitude; D: frequency) protocols at 15 and 90 min posthypoxia where light gray bars represent AIH only, dark gray bars represent AIH + MSX-3, and black bars represent AIH + methysergide. In A, phrenic amplitude after severe AIH. At 15 and 90 min posthypoxia, pLTF is significantly higher after severe AIH compared with severe AIH + MSX-3 (*). pLTF after severe AIH + methysergide is significantly higher compared with severe AIH + MSX-3 (*) only at 90 min posthypoxia. Values are means ± 1 SE; significance is P < 0.05. In B, phrenic amplitude after moderate AIH. At 90 min posthypoxia, pLTF is significantly higher after moderate AIH compared with moderate AIH + methysergide ($) and is lower compared with moderate AIH + MSX-3 (*). pLTF after moderate AIH + MSX-3 is significantly higher vs. moderate AIH + methysergide ($). Values are means ± 1 SE; significance is P < 0.05. In C, phrenic frequency after severe AIH. There are no significant differences between groups at either 15 or 90 min posthypoxia. Values are means ± 1 SE. In D, phrenic frequency after moderate AIH. Phrenic burst frequency was significantly increased only at 90 min posthypoxia when comparing moderate AIH vs. moderate AIH + MSX-3 (*). Values are means ± 1 SE; significance is P < 0.05.
Severe AIH-Induced pLTF is Adenosine Dependent
Enhanced pLTF following severe AIH requires (spinal) adenosine receptor activation since spinal delivery of the A2A receptor antagonist MSX-3 significantly attenuated severe AIH-induced pLTF. Severe AIH + MSX-3 elicited a pLTF lower than severe AIH alone at all time points (28 ± 6% baseline at 90 min post-AIH; P < 0.05; n = 6; Figs. 3, A and D, and 5A). This attenuated response was still significantly greater than baseline and MSX-3 time controls without AIH at all time points (8 ± 4% baseline at 90 min post-AIH; P < 0.05; n = 7; Fig. 3, C and D). Despite significant pLTF attenuation, severe AIH following MSX-3 still caused significant pLTF at the 15-min time point (24 ± 5% baseline at 15 min; P < 0.05; Figs. 3, A and D, and 5A) vs. MSX-3 time controls without AIH (4 ± 2% baseline at 15 min; P < 0.05; Fig. 3, C and D). Collectively, these data demonstrate that spinal A2A receptor activation is necessary for full expression of severe AIH-induced pLTF. Last, severe AIH-induced frequency LTF was present but small with spinal MSX-3, and was significantly greater than baseline and time-control rats treated with spinal MSX-3 at 30, 60, and 90 min post-hypoxia (Fig. 3E; P < 0.05).
Spinal A2A Receptors Constrain Moderate AIH-Induced pLTF
Spinal A2A antagonists enhanced pLTF following moderate AIH (20). Spinal A2A receptor antagonists enhanced pLTF in this range of PaO2, presumably by relieving cross-talk inhibition of pLTF (20). Here we confirmed that spinal pretreatment with MSX-3 before a more moderate protocol of AIH also enhanced pLTF from 30 to 90 min post-hypoxia (moderate AIH + MSX-3: 86 ± 26% baseline at 90 min; n = 8; moderate AIH alone: 41 ± 12% baseline at 90 min post-AIH; P < 0.05; Figs. 3, B and D, and 5B). This enhanced pLTF following MSX-3 treatment and moderate hypoxia is similar in magnitude to pLTF elicited by severe AIH alone (Fig. 5). MSX-3 time controls without AIH did not affect phrenic motor output at any time point (8 ± 4% baseline at 90 min; P > 0.05; n = 7; Fig. 3, C and D).
Moderate AIH-induced frequency LTF was also present but small with spinal MSX-3; frequency was significantly greater than baseline and time-controls rats treated with spinal MSX-3 only at 90 min post-hypoxia (4 ± 2 bursts/min for moderate + MSX-3, n = 8; −0.7 ± 1 bursts/min for MSX-3 time controls, n = 7; expressed as a percent change from baseline in Fig. 3E; P < 0.05). Severe AIH-induced frequency LTF with spinal MSX-3 was significantly larger than moderate AIH + MSX-3-treated animals at 30 (8 ± 2 bursts/min for severe + MSX-3, n = 6; 2 ± 1 bursts/min for moderate + MSX-3, n = 8; expressed as a percent change from baseline in Fig. 3E; P < 0.05) and 60 min post-hypoxia (8 ± 2 bursts/min for severe + MSX-3, n = 6; 2 ± 2 bursts/min for moderate + MSX-3, n = 8; expressed as a percent change from baseline in Fig. 3E; P < 0.05). Last, moderate AIH-induced frequency LTF was significantly greater in rats treated with MSX-3 than with no drug at 90 min post-hypoxia (4 ± 2 bursts/min for moderate + MSX-3 and −0.5 ± 3 bursts/min for moderate with no drug; expressed as a percent change from baseline in Fig. 5D; P < 0.05)
Severe AIH-Induced pLTF Does Not Require Spinal Serotonin Receptor Activation
Moderate AIH-induced pLTF requires spinal serotonin receptor activation (3); specifically, pLTF is blocked by intrathecal injection of the broad spectrum serotonin receptor antagonist methysergide maleate (3, 20). Spinal delivery of methysergide, prior to the more moderate AIH protocol used here, also blocked pLTF at all time points (12 ± 5% baseline at 90 min; P < 0.05; n = 6) vs. moderate AIH alone (Figs. 4, B and D, and 5B); this response was not different from methysergide time controls without AIH (8 ± 6% baseline, P = 0.79 at 90 min, n = 5; Fig. 4, C and D). There were no significant differences in burst frequency for moderate AIH + methysergide-treated animals (Fig. 4E; P > 0.05).
In stark contrast, pLTF induced by severe AIH does not require spinal serotonin receptor activation. Severe AIH-induced pLTF with spinal methysergide pretreatment was not significantly different from the response shown in Fig. 2D; pLTF was significant at all time points post-AIH (66 ± 13% baseline at 90 min post-AIH; P < 0.05 vs. baseline and methysergide time controls without AIH; not different from severe AIH alone at all points; P > 0.05; Figs. 4, A and C, and 5A). An untested assumption is that the dose of methysergide used was sufficient to block serotonin-dependent pLTF during severe AIH, although we ensure that this is the case. The same dose blocks powerful activation of phrenic motor neurons elicited by pharmacological activation of serotonin receptors (40). Regardless, our data demonstrate that mechanisms of pLTF differed markedly between moderate (serotonergic) and severe (adenosinergic) AIH protocols. Last, severe AIH-induced frequency LTF was present but very small with spinal methysergide and was significantly different from baseline at 60 min post-hypoxia (Fig. 4E; P < 0.05).
DISCUSSION
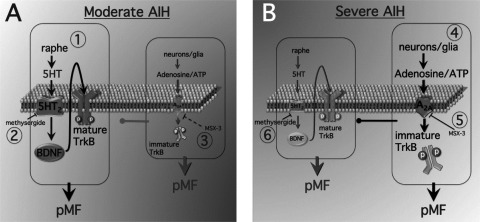
Here we demonstrate for the first time that AIH-induced pLTF arises from distinct mechanisms depending on the severity of hypoxia during AIH episodes. pLTF and ventilatory LTF in rodents have typically been studied after moderate AIH (35–45 mmHg PaO2; 1, 14, 4, 32). This model of pLTF results predominantly from a serotonin-dependent mechanism referred to as the Q pathway to phrenic motor facilitation (pMF; 9; Fig. 6), since spinal Gq protein-coupled metabotropic serotonin receptors are necessary (3) and sufficient (26) to elicit the response. A distinct cellular mechanism giving rise to pMF is initiated by Gs protein-coupled metabotropic receptors, such as the adenosine 2A (A2A; 17) or serotonin type 7 receptor (21). This is referred to as the S pathway to pMF (9; Fig. 6). Contrary to our initial expectations, these unique pathways to pMF interact in a complex way: cross-talk inhibition (20). Thus, when the S pathway is blocked with A2A or 5-HT7 receptor antagonists in the cervical spinal cord, moderate AIH-induced pLTF (i.e., the Q pathway) is amplified, likely due to a higher 5-HT2 receptor abundance and a slightly higher affinity of these receptors for serotonin (vs. 5-HT7) (20; Hoffman and Mitchell, unpublished observations; Figs. 3, B and D, and 5B).
Fig. 6.
Working model for moderate (A) vs. severe AIH (B)-induced pLTF, a form of phrenic motor facilitation (pMF). A: moderate AIH. 1) During moderate hypoxia, we postulate that classical pLTF is triggered via the “Q” pathway (5-HT2 receptor dependent). The dominant “Q pathway” to pMF during moderate hypoxia likely occurs from new BDNF synthesis and subsequent activation of its high-affinity receptor, mature TrkB. At the same time, activation of A2A receptors (Gs coupled) via adenosine elicits the less dominant “S pathway” to pMF. We suggest that new TrkB synthesis (immature form) within phrenic motor neurons normally results in a constraint on the “Q” pathway during moderate hypoxia. 2) Inhibition of 5HT receptors via methysergide inhibits moderate AIH-induced pMF. 3) MSX-3 spinal delivery inhibits A2A receptors and results in enhanced pMF, most likely as a result of a constraint on the “Q” pathway being relieved. B: severe AIH. 4) During severe hypoxia, we postulate that pMF is triggered via the “S” pathway. The “S” pathway likely occurs from severe AIH causing release of adenosine/ATP from neurons/glia and subsequently activating A2A receptors to cause new TrkB synthesis (immature form) within phrenic motor neurons and elicit pMF. We suggest that at the same time, the “Q” pathway is inhibited due to inhibition from the “S” pathway. 5) MSX-3 spinal delivery before severe AIH inhibits A2A receptors and results in attenuated pMF. 6) Methysergide spinal delivery before severe AIH does not affect severe AIH-induced pMF; thus the “Q” pathway is not constraining the “S” pathway during severe AIH.
Since multiple pathways to pMF exist, it is important to understand conditions under which each pathway is utilized, particularly since mutual inhibition suggests that only one pathway is used at a time. An animal may shift from one mechanism to another with changes in the nature, the duration, or the severity of the inducing stimulus. Here, we investigated intact rats exposed to differing severities of AIH to test the hypothesis that pLTF would shift from a predominantly serotonergic (Q) to a predominantly (S) adenosinergic mechanism of pMF. Specifically, we predicted that severe AIH shifts the balance toward A2A receptor-induced pMF. This hypothesis was based on literature reports that 1) hypoxia induces ATP/adenosine/adenine release from neurons and astrocytes (43, 49, 18, 30); 2) ATP is converted to adenosine via endonucleotidases (41); and 3) adenosine elicits pMF by activating A2A receptors, presumably on nearby phrenic motor neurons (17). Further, severe hypoxia results in a release of both adenosine and serotonin in the brain stem of anesthetized cats (45). Thus greater serotonin release during severe AIH may elicit more relative activation of the less abundant and lower affinity 5-HT7 (S) receptor, further constraining 5-HT2 (Q) receptor-induced pLTF (25).
In support of our hypothesis, we demonstrate that 1) severe AIH elicits greater pLTF vs. moderate hypoxia; 2) pLTF elicited by severe AIH is attenuated by intrathecal administration of a selective A2A receptor antagonist, much in contrast to its effects on moderate AIH-induced pLTF (enhancement); and 3) pLTF following moderate hypoxia is blocked by intrathecal administration of the broad-spectrum serotonin receptor antagonist methysergide, whereas this same treatment has no effect on severe AIH-induced pLTF. Collectively, these observations suggest that severe AIH shifts pLTF from a serotonergic toward an adenosinergic mechanism.
Progressive Augmentation During Severe Intermittent Hypoxia
An unanticipated observation was that, in contrast to moderate AIH, severe AIH elicits progressive augmentation of the short-term hypoxic phrenic response. Although progressive augmentation of the phrenic hypoxic response has been described previously (44), it has not been observed in anesthetized rats exposed to moderate AIH, as confirmed here. The mechanism of progressive augmentation remains unknown, although it does not require activation of either spinal A2A or serotonin receptors (Fig. 1). Other, as yet unknown, mechanisms associated with severe AIH must contribute to this response.
Enhanced pLTF After Severe AIH
Since electrical activation of carotid chemoafferent neurons without hypoxia (12, 19, 24, 35) and moderate AIH both elicit 5-HT2 receptor-dependent pLTF (15, 36), the predominant mechanism of pLTF in these conditions appears to be the Q pathway (9). Without chemoafferent neuron activation, sustained severe hypoxia exerts direct effects on CNS neurons and glia, leading to pMF via alternate, serotonin-independent mechanisms (e.g., 16, 34). In carotid denervated rats (6), or in rats with pharmacologically suppressed peripheral chemoreceptors (48), the hypoxic phrenic response is reduced to <30% of normal, yet considerable pLTF is still observed (∼60–70% of normal). The mechanism of this residual pLTF is not yet known, although it may reflect serotonin-independent, adenosine 2A receptor-dependent mechanisms (48). Carotid denervation may shift the balance from a serotonin-dependent Q, to adenosine-dependent S mechanism of pMF, similar to our present observations with severe intermittent hypoxia. Although carotid denervation is not a normal biological state, it may provide proof of principle that shifts may occur in the predominance of Q vs. S pathways to AIH-induced pLTF.
We previously reported that AIH-induced pLTF correlates with the magnitude of the short-term hypoxic phrenic response in meta-analyses of multiple studies (4, 14). On the other hand, there was no correlation between the PaO2 during AIH and pLTF in the range of 30–60 mmHg. Here, we extend the range of these meta-analyses to 25 mmHg, giving a more direct comparison between moderate vs. severe AIH. Overall, our observation of greater pLTF following severe vs. moderate AIH is not inconsistent with earlier reports from our laboratory in this same experimental preparation. However, greater pLTF after severe AIH and the transition from the “Q” to the “S” pathway may have a threshold between the 25- to 30-mmHg range vs. the 30- to 60-mmHg range previously studied.
The larger hypoxic phrenic response during severe (vs. moderate) AIH is predictable based on the shape of the carotid chemoreceptor response to hypoxia (23). Thus the greater pLTF following severe AIH may have resulted from the greater hypoxic phrenic response per se. On the other hand, suppression (48) or elimination (6) of carotid chemoreceptor inputs during moderate AIH greatly attenuates the hypoxic phrenic response, with proportionately less effect on pLTF. Thus we suggest that these are independent events, and that the (spinal) adenosine-dependent pLTF following severe AIH arises from direct effects of hypoxia in the CNS vs. greater carotid chemoreceptor activation (or short-term hypoxic phrenic response).
pLTF Following Moderate AIH is Constrained by A2A Receptor Activation
Although pLTF elicited by AIH in the range of 35–45 mmHg requires spinal serotonin receptor activation (3), it is constrained by A2A receptor activation (20), even though A2A receptor activation alone elicits pMF (17). Here we confirm that moderate AIH-induced pLTF requires spinal serotonin receptor activation (Figs. 4, B and D, and 5B) and is enhanced by spinal administration of an A2A receptor antagonist (Figs. 3, B and D, and 5B). Thus our results are consistent with reports of cross-talk inhibition between Gs-and Gq-dependent pathways (33, 46, 47). Additional data from our laboratory suggest that the S pathway constrains the Q pathway due to activation of protein kinase A, which phosphorylates and inhibits NADPH oxidase-dependent reactive oxygen species formation (20; Hoffman and Mitchell, unpublished observations).
Severe AIH-Induced pLTF is Adenosine (Not Serotonin) Dependent
Here, we demonstrate a novel mechanism leading to an enhanced form of pLTF. This mechanism is revealed by increasing the hypoxic severity during AIH. We conclude that severe AIH shifts the system from predominant serotonergic to predominant adenosinergic mechanisms of pMF. This shift may arise from greater ATP/adenosine/adenine release from neurons and astrocytes during hypoxic episodes (43, 49, 18, 30), thereby increasing extracellular adenosine levels (41).
Previous studies of sustained moderate (>35 Torr) vs. severe (<26 Torr) hypoxia in chemodenervated cats (16) demonstrate 1) mild sustained hypoxia elicited pMF, whereas 2) severe hypoxia caused prolonged adenosine-dependent phrenic inhibition (16). In their study, pMF following moderate hypoxia required brain regions rostral to the brain stem, whereas prolonged inhibition following sustained severe hypoxia required the rostral mesencephalon (34). Phrenic inhibition following sustained severe hypoxia may have arisen from A1 receptor activation, in contrast to our study, where severe intermittent hypoxia may have released less adenosine, eliciting pMF via A2 receptor activation. Indeed, greater relative A2A receptor activation on near-phrenic motor neurons is expected to elicit pMF (17). The studies of Millhorn and colleagues (16, 34) were performed using sustained hypoxia in glomectomized cats, and, thus, we remain uncertain concerning the differential impact of sustained vs. intermittent hypoxia, carotid denervation, and/or species.
Although the (adenosine-dependent) pLTF observed following severe AIH is phenotypically similar to (serotonin-dependent) pLTF following moderate AIH, there are some possible differences to consider: 1) pLTF is robust immediately following severe AIH (and appears to be ramping up in the intervals between hypoxic episodes), but only slowly develops following moderate AIH (Fig. 2); and 2) pLTF following severe AIH may or may not be pattern sensitive, as has been shown for moderate AIH (5). In fact, since adenosine accumulation would be equal or greater following severe sustained (vs. intermittent) hypoxia, pattern-insensitive pLTF may arise (see above). This possibility has not yet been explored.
Conclusions
In this report, we demonstrate that rats use different pathways to pMF depending on severity of intermittent hypoxia inducing the respiratory plasticity. Thus multiple cellular cascades, each leading to pMF (9), interact in complex and interesting ways, providing a range of potential responses in the face of changing physiological conditions or the onset of disease. A detailed understanding of cellular/synaptic mechanism(s) giving rise to pMF in different circumstances may help guide the development of novel therapeutic strategies for severe ventilatory control disorders, including obstructive sleep apnea and respiratory insufficiency following cervical spinal injury or during motor neuron disease (37).
GRANTS
Research support was provided by National Institutes of Health Grants NS-057778 and HL-080209. N. L. Nichols and E. A. Dale were supported by the National Institutes of Health National Research Service Award Postdoctoral Fellowship (T32-HL-007654).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.L.N., E.A.D., and G.S.M. conception and design of research; N.L.N. and E.A.D. performed experiments; N.L.N. analyzed data; N.L.N., E.A.D., and G.S.M. interpreted results of experiments; N.L.N. prepared figures; N.L.N. drafted manuscript; N.L.N., E.A.D., and G.S.M. edited and revised manuscript; N.L.N., E.A.D., and G.S.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Peter Crump for assistance with statistical analyses and Kalen Nichols for assistance with blood-gas analysis.
REFERENCES
- 1. Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7: 48–55, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22: 6239–6246, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baker-Herman TL, Mitchell GS. Determinants of frequency long-term facilitation following acute intermittent hypoxia in vagotomized rats. Respir Physiol Neurobiol 162: 8–17, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol 529: 215–219, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bavis RW, Mitchell GS. Intermittent hypoxia induces phrenic long-term facilitation in carotid-denervated rats. J Appl Physiol 94: 399–409, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Bockaert J, Claeysen S, Bécamel C, Dumuis A, Marin P. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res 326: 553–572, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Dale N, Pearson T, Frenguelli BG. Direct measurement of adenosine release during hypoxia in the CA1 region of the rat hippocampal slice. J Physiol 526: 143–155, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol 669: 225–230, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feldman JL, Neverova NV, Saywell SA. Modulation of hypoglossal motoneuron excitability by intracellular signal transduction cascades. Respir Physiol Neurobiol 147: 131–143, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Fregosi RF, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J Physiol 477: 469–479, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frenguelli BG, Llaudet E, Dale N. High-resolution real-time recording with microelectrode biosensors reveals novel aspects of adenosine release during hypoxia in rat hippocampal slices. J Neurochem 86: 1506–1515, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol 121: 135–146, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Fuller DD, Zabka A, Baker TL, Mitchell GS. Physiological and genomic consequences of intermittent hypoxia. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol 90: 2001–2006, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Gallman EA, Millhorn DE. Two long-lasting central respiratory responses following acute hypoxia in glomectomized cats. J Physiol 395: 333–347, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci 28: 2033–2042, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gourine AV, Llaudet E, Dale N, Spyer KM. Release of ATP in the ventral medulla during hypoxia in rats: role in hypoxic ventilatory response. J Neurosci 25: 1211–1218, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol Regul Integr Comp Physiol 265: R811–R819, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Hoffman MS, Golder FJ, Mahamed S, Mitchell GS. Spinal adenosine 2A receptor inhibition enhances phrenic long term facilitation following acute intermittent hypoxia. J Physiol 588: 255–266, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoffman MS, Mitchell GS. Spinal 5-HT7 receptor activation induces long-lasting phrenic motor facilitation. J Physiol 589: 1397–1407, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kinkead R, Mitchell GS. Time-dependent hypoxic ventilatory responses in rats: effects of ketanserin and 5-carboxamidotryptamine. Am J Physiol Regul Integr Comp Physiol 277: R658–R666, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Kumar P. Sensing hypoxia in the carotid-body: from stimulus to response. Essays Biochem 43: 43–60, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Integrated phrenic responses to carotid afferent stimulation in adult rats following perinatal hyperoxia. J Physiol 500: 787–796, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. MacFarlane PM, Satriotomo I, Windelborn JA, Mitchell GS. NADPH oxidase activity is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. J Physiol 587: 1931–1942, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. MacFarlane PM, Vinit S, Mitchell GS. Serotonin 2A and 2B receptor-induced phrenic motor facilitation: differential requirement for spinal NADPH oxidase activity. Neuroscience 178: 45–55, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. MacFarlane PM, Mitchell GS. Respiratory long-term facilitation following intermittent hypoxia requires reactive oxygen species formation. Neuroscience 152: 189–197, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol 92: 27–37, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Mahamed S, Mitchell GS. Simulated apneas induce serotonin-dependent respiratory long-term facilitation in rats. J Physiol 586: 2171–2181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martín ED, Fernández M, Perea G, Pascual O, Haydon PG, Araque A, Ceña V. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia 55: 36–45, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Mateika JH, Narwani G. Intermittent hypoxia and respiratory plasticity in humans and other animals: does exposure to intermittent hypoxia promote or mitigate sleep apnoea? Exp Physiol 94: 279–296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mateika JH, Sandhu KS. Experimental protocols and preparations to study respiratory long-term facilitation. Respir Physiol Neurobiol 176: 1–11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meszaros JG, Gonzalez AM, Endo-Mochizuki Y, Villegas S, Villarreal F, Brunton LL. Identification of G protein-coupled signaling pathways in cardiac fibroblasts: cross talk between G(q) and G(s). Am J Physiol Cell Physiol 278: C154–C162, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Millhorn DE, Eldridge FL, Kiley JP, Waldrop TG. Prolonged inhibition of respiration following acute hypoxia in glomectomized cats. Respir Physiol 57: 331–340, 1984 [DOI] [PubMed] [Google Scholar]
- 35. Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol 41: 87–103, 1980a [DOI] [PubMed] [Google Scholar]
- 36. Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol 42: 171–188, 1980b [DOI] [PubMed] [Google Scholar]
- 37. Mitchell GS. Respiratory plasticity following intermittent hypoxia: a guide for novel therapeutic approaches to ventilatory control disorders. In: Genetic Basis for Respiratory Control Disorders, edited by Gaultier C. New York: Springer, 2007, p. 291–306 [Google Scholar]
- 38. Mitchell GS, McCrimmon DR, Feldman JL, Baker-Herman Respiration TL. In: Encyclopedia of Neuroscience, edited by Squire LR. Oxford: Academic, 2009, p. 121–130 [Google Scholar]
- 39. Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol 94: 358–374, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Mitchell GS, Sloan HE, Jiang C, Miletic V, Hayashi F, Lipski J. 5-Hydroxytryptophan (5-HTP) augments spontaneous and evoked phrenic motoneuron discharge in spinalized rats. Neuro Letters 141: 75–78, 1992 [DOI] [PubMed] [Google Scholar]
- 40a. Neverova NV, Saywell SA, Nashold LJ, Mitchell GS, Feldman JL. Episodic stimulation of alpha-1 adrenoreceptors induces protein kinase C-dependent persistent changes in motoneuronal excitability. J Neurosci 27: 4435–4442, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parkinson FE, Xiong W, Zamzow CR. Astrocytes and neurons: different roles in regulating adenosine levels. Neurol Res 27: 153–160, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Peng YJ, Prabhakar NR. Reactive oxygen species in the plasticity of respiratory behaviour elicited by chronic intermittent hypoxia. J Appl Physiol 94: 2342–2349, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Phillis JW, O'Regan MH, Perkins LM. Adenosine 5'-triphosphate release from the normoxic and hypoxic in vivo rat cerebral cortex. Neurosci Lett 151: 94–96, 1993 [DOI] [PubMed] [Google Scholar]
- 44. Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Richter DW, Schmidt-Garcon P, Pierrefiche Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J Physiol 514: 567–578, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roy AA, Nunn C, Ming H, Zou MX, Penninger J, Kirshenbaum LA, Dixon SJ, Chidiac P. Upregulation of endogenous RGS2 mediates cross-desensitization between Gs and Gq signaling in osteoblasts. J Biol Chem 281: 32684–32693, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Ryzhov S, Goldstein AE, Biaggioni I, Feoktistov I. Cross-talk between G(s)- and G(q)-coupled pathways in regulation of interleukin-4 by A(2B) adenosine receptors in human mast cells. Mol Pharmacol 70: 727–735, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Sibigtroth CM, Mitchell GS. Carotid chemoafferent activity is not necessary for all phrenic long-term facilitation following acute intermittent hypoxia. Resp Phys Neurobiol 176: 73–79, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wallman-Johansson A, Fredholm BB. Release of adenosine and other purines from hippocampal slices stimulated electrically or by hypoxia/hypoglycemia. Effect of chlormethiazole. Life Sci 55: 721–728, 1994 [DOI] [PubMed] [Google Scholar]
- 50. Wilkerson JE, Satriotomo I, Baker-Herman TL, Watters JJ, Mitchell GS. Okadaic acid-sensitive protein phosphatases constrain phrenic long-term facilitation after sustained hypoxia. J Neurosci 28: 2949–2958, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]