Abstract
Intermittent hypoxia (IH) conditioning minimizes neurocognitive impairment and stabilizes brain mitochondrial integrity during ethanol withdrawal (EW) in rats, but the mitoprotective mechanism is unclear. We investigated whether IH conditioning protects a key mitochondrial enzyme, cytochrome c oxidase (COX), from EW stress by inhibiting mitochondrially directed apoptotic pathways involving cytochrome c, Bax, or phosphor-P38 (pP38). Male rats completed two cycles of a 4-wk ethanol diet (6.5%) and 3 wk of EW. An IH program consisting of 5–10 bouts of 5–8 min of mild hypoxia (9.5–10% inspired O2) and 4 min of reoxygenation for 20 consecutive days began 3 days before the first EW period. For some animals, vitamin E replaced IH conditioning to test the contributions of antioxidant mechanisms to IH's mitoprotection. During the second EW, cerebellar-related motor function was evaluated by measuring latency of fall from a rotating rod (Rotarod test). After the second EW, COX activity in cerebellar mitochondria was measured by spectrophotometry, and COX, cytochrome c, Bax, and pP38 content were analyzed by immunoblot. Mitochondrial protein oxidation was detected by measuring carbonyl contents and by immunochemistry. Earlier IH conditioning prevented motor impairment, COX inactivation, depletion of COX subunit 4, protein carbonylation, and P38 phosphorylation during EW. IH did not prevent cytochrome c depletion during EW, and Bax content was unaffected by EW ± IH. Vitamin E treatment recapitulated IH protection of COX, and P38 inhibition attenuated protein oxidation during EW. Thus IH protects COX and improves cerebellar function during EW by limiting P38-dependent oxidative damage.
Keywords: cerebellum, cytochrome coxidase, ethanol withdrawal, vitamin E
intermittent hypoxia (IH) conditioning is characterized by repeated exposures to moderately hypoxic atmospheres, interspersed with periods of breathing normal air (33). Recent preclinical research has examined IH as a noninvasive treatment for neurological disorders, including stroke (20) and glutamate excitotoxicity (32). IH is also used to model sleep apnea (14), although the hypoxia episodes typically are more frequent and intense than those of therapeutic hypoxia (45). Indeed, therapeutic IH exemplifies the principle of cross adaptation (36), wherein developing resistance to one stress (i.e., IH) confers resistance to other stresses (e.g., ischemia and EW). For instance, conditioning of rats by repeated exposure to moderate IH stress increased myocardial tolerance to severe ischemic stress (37). In this model, we have previously demonstrated that IH conditioning mitigates brain damage associated with the abrupt termination of chronic alcohol consumption (25).
Although early studies have demonstrated the protective effect of IH (36, 46), only just recently have the underlying mechanisms been widely studied (33). Among potential mechanisms, mitochondrial mechanisms appear to be a principal target of IH. This view is based on the fact that mitochondria require O2 to fulfill their principal function of generating ATP. Cytochrome c oxidase (COX), the terminal enzyme complex (IV) of the mitochondrial respiratory chain, catalyzes the transfer of electrons from cytochrome c to oxygen, thereby helping to maintain the electrochemical H+ gradient across mitochondrial membranes, the driving force for ATP synthesis. Considering COX's pivotal role in ATP production, it is not surprising that COX deficiency has been implicated in neurodegenerative illnesses. In a study by Cardoso et al. (4), mitochondria isolated from platelets of Alzheimer's patients showed a 15% decrease in COX activity. COX deficiency reportedly increased oxidant generation in a cellular model of aging (55). Ethanol exposure oxidatively modified liver COX through the lipid peroxidation product, malondialdehyde (7, 55). Jaatinen et al. (19) reported in rats a relationship between COX and EW, such that EW provoked a loss of COX activity, especially in older animals. Since EW imposes profound oxidative stress in the brain (21), COX inactivation in Jaatinen et al.'s study may be attributed to the pro-oxidant nature of EW.
Several molecules directly or indirectly associate with COX to maintain mitochondrial and cellular integrity. Cytochrome c transfers electrons from respiratory complex III to COX (complex IV), and release of the cytochrome c from the intermembrane space into the cytosol is a pivotal event in the mitochondrial apoptosis cascade. Bax is a cytosolic protein that enters mitochondria on an apoptotic insult, subsequently releasing cytochrome c. Similarly, activation of P38 has been implicated in apoptotic cascades. Sustained or hyperactivation of P38 has been found in a variety of pathological conditions (1, 12, 16). P38 is also known to be a stress-responsive protein because its active phosphorylated form (pP38) is increased on stress, like EW (26). As such, there is ample opportunity for cross talk between COX and apoptotic molecules, especially under the pro-oxidant conditions of EW.
This study sought to define mitochondrial mechanisms by which IH conditioning protects the brain from EW. To test the hypothesis that IH protects COX from prooxidant EW by suppressing the P38 pathway, we determined whether IH protects COX through antioxidant mechanisms and whether the antioxidant activity is associated with P38 inhibition. The study focused on cerebellum because of its susceptibility to ethanol and EW toxicity (15, 23, 44).
METHODS
Chemicals.
Analytic grade reagents were purchased from Sigma Aldrich (St. Louis, MO), Cell Signaling Technology (Danvers, MA), Santa Cruz Biotechnology (Santa Cruz, CA), and Mitosciences (Eugene, OR). Diet ingredients were obtained from Research Organics (Cleveland, OH) or MP Biomedicals (Irvine, CA). HT22 cells, a murine hippocampal cell line, were the generous gift of Dr. David Schubert (Salk Institute, San Diego, CA).
Animals.
Male Sprague-Dawley rats (Charles River, Wilmington, MA), age 3 mo at the beginning of the study, were housed individually at controlled temperature (22–25°C) and humidity (55%), with ad libitum access to water and a 12-h light/12-h dark cycle. All animal experimentation was conducted in accordance with the Guide to the Care and Use of Laboratory Animals [DHHS Publication No. (NIH) 85-23, revised 1996, Office of Science and Health Reports, DRR/NIH, Bethesda, MD 20205] and was approved by the University of North Texas Health Science Center Animal Care and Use Committee.
Ethanol and dextrin control diets.
Figure 1 summarizes the ethanol consumption-withdrawal regimen. Rats were assigned to one of four groups (5–7 rats/group) according to diet (ethanol vs. control dextrin) and IH treatment (IH vs. non-IH sham). Thus the four groups were dextrin sham, dextrin + IH, EW sham, and EW + IH. Rats in the EW group consumed a liquid diet containing 6.5% (wt/vol) ethanol (22). Control animals were fed a liquid diet with dextrin isocalorically substituted for ethanol. Each liter of these diets contained 42 g of pulverized casein, 0.6 g of l-methionine, 2.1 g of vitamin mixture, 7.3 g of mineral mixture, 25 g of sucrose, 3 g of xanthum gum, 0.4 g of choline bitartrate, 1 g of Celufil cellulose, 10.5 g of corn oil, and either 56 g of ethanol or 75 g of dextrin in aqueous suspension. One hundred milliliters of fresh diet was placed in each cage daily for 4 wk. On the morning after the 4-wk diet, the diet bottle was removed to initiate EW. Animals were fed conventional chow pellets for 3 wk. This regimen of 4-wk ethanol/dextrin diet and 3-wk conventional diet was repeated once more to model repeated EW.
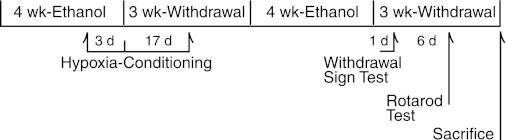
Fig. 1.
Ethanol intoxication-withdrawal program. Young adult male rats completed two cycles of consuming control dextrin or 6.5% ethanol diet for 4 wk, followed by 3 wk of ethanol withdrawal (EW). Intermittant hypoxia (IH) conditioning was applied during the last 3 days of the first ethanol diet and the first 17 days of subsequent EW. Rats were tested for overt signs of acute EW 24 h after the termination of the second ethanol diet, and recovered for 6 days before undergoing 5 days of Rotarod testing. Rats were killed at 3 wk of the second EW period, and cerebelli were harvested for biochemical analyses.
Intermittent hypoxia conditioning program.
The IH conditioning program described by Zong et al. (57) was applied every morning for the last 3 days of ethanol diet and for the first 17 days of EW. This schedule targets mainly EW rather than the ethanol-exposure phase. In addition, initiating IH conditioning a few days before EW allows animals to avoid stress associated with a new procedure that may complicate interpretation of data obtained during EW. Rats were hypoxia- or sham-conditioned in custom-made 267-liter acrylic chambers. These chambers are large enough to accommodate multiple rat cages and to allow the atmosphere within the chamber to be adjusted quickly and precisely by using compressed gas. The IH program consisted of brief (5–10 min) hypoxic exposures (5–8 bouts/day) with intervening 4-min reoxygenation periods (56). Fractional inspired O2 (FiO2) in the chamber was monitored with a precision O2 sensor (Alpha Omega Instruments model 2000). Compressed N2 was introduced into the chamber to lower O2 content to the prescribed value within 90 s. Reoxygenation was achieved by opening the top of the chamber. Non-IH groups underwent sham conditioning protocols in which compressed air instead of N2 was introduced to maintain FiO2 at 21%. The rats exhibited no discomfort or distress during the IH or sham conditioning sessions.
Vitamin E administration.
Some animals received vitamin E (275 mg·kg−1·day−1 po) (35) instead of IH conditioning to test whether an antioxidant administration mimics IH protection. Vitamin E was given by a gavage method for 20 consecutive days corresponding to the IH conditioning program, i.e., the last 3 days of ethanol diet and the first 17 days of the first EW cycle.
Evaluation of EW signs.
Behavioral manifestations of EW excitotoxicity were assessed at 24 h of the second EW cycle. The behavioral signs were scored based on the following criteria modified from the method of Goldstein and Pal (17): 1) vocalization, urination, and defecation on handling (score: 0–3); 2) tail rigidity when the tail was drawn between the rater's fingers (0–3); 3) frequency and severity of tremor during handling (0–3); 4) startle (twitching, jumping, or freezing) observed for 15 s after auditory stimulus (0–3); 5) convulsion during and after handling (0 or 1); 6) spontaneous seizure (0 or 2); and 7) death (0 or 10). The sum of the seven scores equaled each animal's total score. Scoring was done by two evaluators blinded to each rat's treatment. Averaged scores from the two evaluators were used for statistical analysis. This test was conducted in a designated room to limit auditory stimulation.
Rotarod assay of motor performance.
A modified Rotarod test of running coordination (18) was used to assess cerebellar damage at the behavioral level. The accelerating Rotarod is a motor-driven treadmill (Omnirotor, Omnitech Electronics, Columbus, OH) with a rotor consisting of a horizontally mounted, 7-cm diameter nylon cylinder positioned 35.5 cm above a padded surface. The Rotarod surface was made of knurled Perspex to provide an adequate grip, which prevents animals from slipping off the rod. The cylinder was separated into four wide compartments, each accommodating one rat, and was set to accelerate from 4 to 75 rpm at a rate of 0.5 rpm·s2. Acceleration continued until either 75 rpm was reached or the last animal was unable to perform the running response and had fallen to the padded surface. When each rat landed on the surface, a photosensitive switch was tripped and the timer for that compartment stopped. The elapsed time was recorded in tenths of a second as the measure of performance on each trial. Because rats experience stress associated with hyperexcitatory signs during the first few days of EW, which can affect coordination, Rotarod testing began 7 days after the second ethanol or dextrin diet. Each rat underwent 5 consecutive days of three trials per day with 10-min intervals between trials. The first 2 days served to habituate the rats to the Rotarod test, and the results from days 3–5 were taken for statistical analysis.
Tissue extraction.
Rats were humanely killed under anesthesia [xylazine (20 mg/kg, ip) and ketamine (100 mg/kg, ip)] at 3 wk of the second EW period. Cerebelli containing the vermis were harvested and divided in half. Hemi-cerebelli were weighed and homogenized at 4°C in phosphate-buffered saline containing protease (EMD Bioscience, San Diego, CA) and phosphatase inhibitor cocktails and PMSF. An aliquot was combined with an equal volume of lysis buffer [0.5 M Tris·HCl (pH 6.8), 4.4% wt/vol SDS, 20% vol/vol glycerol, 2% vol/vol 2-mercaptoethanol], heated (95°C, 5 min), and sonicated (10 s). Protein concentrations in the extracts were measured by Bradford assay (Bio-Rad, Hercules, CA) (3).
Isolation of mitochondria.
Protein contents of cytochrome c and Bax and the carbonylation of mitochondrial proteins were studied in mitochondria isolated from cerebellum by conventional differential centrifugation with slight modifications (30). Cerebelli were dissected, rinsed, rapidly homogenized in ice-cold isolation buffer (320 mM sucrose, 1 mM dipotassium ethylene diamine tetraacetic acid, 10 mM Tris·HCl), and centrifuged at 1,330 g for 10 min at 4°C. The supernatant was decanted, and the pellet was resuspended in 0.5 volume of the original isolation buffer and centrifuged again. The two supernatants were combined and centrifuged at 21,200 g for 10 min. The resulting pellet was resuspended in 12% Percoll solution (GE Healthcare Bio-Sciences, Uppsala, Sweden) and centrifuged at 6,900 g for 10 min. The resulting soft pellet was washed once with isolation buffer and again centrifuged at 6,900 g for 10 min to obtain the mitochondria-enriched pellet. Protein concentration was measured by Bradford assay (Bio-Rad) (3).
COX activity.
Total COX activity was measured spectrophotometrically using isolated mitochondria (7). Briefly, reduced cytochrome c was prepared by mixing cytochrome c and ascorbic acid in potassium phosphate buffer. COX activity was taken as the rate of ferrocytochrome c oxidation to ferricytochrome c, detected as the decrease in absorbance at 550 nm.
Immunoblotting.
Whole cell lysates from cerebelli containing the vermis were used to measure the protein content of P38 and COX subunit IV. Samples containing 30 μg of protein were resolved by SDS-PAGE on 10% cross-linked gels and then electrophoretically transferred onto PVDF (polyvinylidene fluoride) membranes. Nonspecific binding was blocked with 5% fat-free milk. Blots were washed in phosphate-buffered solution containing 0.05% Tween 20 and then probed overnight with rabbit polyclonal antibody against pP38 (Cell Signaling, Danvers, MA) at 1:500 dilution or mouse monoclonal antibodies (Mitosciences, Eugene, OR) against COX (1:1,000 dilution) or Bax (1:500 dilution). Next, blots were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Bands were detected using Western blotting luminescence (UVP, Upland, CA) and quantified by an image densitometer. Voltage-dependent anion channel 1 (VDAC1) was used as a positive mitochondrial loading control; band densities of cytochrome c and Bax were normalized to VDAC1 densities. β-Actin was used as a loading control for all other immunoblot assays in which the usage of whole tissue lysates does not obscure the accurate detection of target proteins (e.g., P38 and COX subunit IV).
Mitochondrial protein carbonyl content.
Dinitrophenylhydrazine (DNPH) derivatization was carried out as described previously (25); samples prepared in the absence of DNPH served as controls (56). To 1 ml of cerebellar mitochondrial homogenate, 0.2 ml of 10 mM DNPH in 2 N HCl was added. HCl (2 N, 0.2 ml) was added to another 1 ml of homogenate to provide a blank. Mixtures were incubated for 60 min at room temperature. The protein was precipitated with an equal volume of 20% trichloroacetic acid and was washed three times with ethanol-ethyl acetate (1:1 vol/vol). The final precipitate was dissolved in 2 ml of 6 M guanidine hydrochloride (pH 2.3), and insoluble debris was removed by centrifugation. Absorbance of DNP-protein adducts was measured at 360 nm (ε = 22 mM−1·cm−1; Ref. 23) in a Beckman DU 640 spectrophotometer. Carbonyl contents were expressed as nmol carbonyl/mg protein.
HT22 cell culture.
HT22 cells were maintained as described previously (39). The HT22 line was originally selected from HT4 cells that were immortalized from primary hippocampal neurons using a temperature-sensitive small virus-40 T antigen. HT22 cells were grown in DMEM, supplemented with 10% charcoal-stripped fetal bovine serum (HyClone, Logan, UT) and gentamicin (50 mg/ml) at 37°C in an atmosphere containing 5% CO2 and 95% air. HT22 cells were plated into Petri dishes (Greiner Bio-One, Monroe, NC) at ∼106 cells per dish to extract sufficient amounts of protein for the assays. The following day, the cells were exposed to 0, 50, or 100 mM ethanol for 24 h in parafilm-sealed dishes and then incubated for 4 h with ethanol-free DMEM medium to model EW stress. This 24-h ethanol exposure, 4-h washout cycle was repeated to model a second ethanol withdrawal. Stock solution of the P38 inhibitor SB203580 was prepared in dimethyl sulfoxide and applied to cells at 100 nM concentration during the first 4 h postethanol. Vehicle control cells were exposed to dimethyl sulfoxide without SB203580.
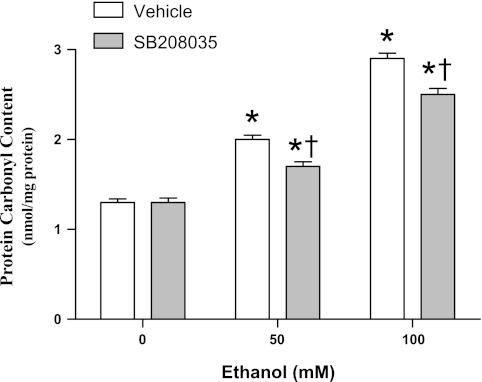
Assessment of protein carbonyl contents in HT22 cells.
Carbonyl contents were measured according to the method of Levine et al. (31). Mitochondria were isolated from HT22 cells collected at the end of the second ethanol washout and centrifuged at 2,000 g for 5 min. The resulting pellet was treated with 100 μl of 2 N HCl and then incubated for 1 h at room temperature in the dark. After trichloroacetic acid (20%) was added, the pellets were incubated on ice for 10 min and centrifuged for 5 min at 10,000 g. The pellets were washed, sonicated, centrifuged again for 10 min at 10,000 g, resuspended in 6 M guanidine hydrochloride (pH 2.3), dissolved overnight, and the absorbance measured at 360 nm. Results are expressed as nanomol carbonyls/mg protein. No effects of dimethyl sulfoxide on protein carbonyl contents or cell viability were detected in vehicle-treated control cultures (data not shown).
Statistical analysis.
All numerical data are expressed as means ± SE. Results of EW signs, Rotarod test, COX activity, protein carbonyl contents, and immunoblots were analyzed by single-factor (diet or treatment) ANOVA. Protein carbonyl contents in HT22 cells were analyzed by two-factor (ethanol concentration; SB203580 or vehicle) ANOVA. When ANOVA detected statistically significant treatment effects, post hoc Tukey's tests were performed to identify specific differences between groups. P values of <0.05 were taken to indicate statistical significance.
RESULTS
Body weight and ethanol consumption.
Rats assigned to the dextrin, EW, and EW + IH groups weighed 319 ± 6, 301 ± 3, and 311 ± 3 g, respectively, at the beginning of the 14-wk diet program, and 467 ± 12, 434 ± 16, and 436 ± 17 g, respectively, at the end of the program (P = not significant). Animals assigned to the EW and EW + IH groups drank similar amounts of ethanol diet: 80.3 ± 0.9 and 81.1 ± 2 ml during the first diet cycle, and 81.7 ± 1 and 82.0 ± 1 ml during the second diet cycle, respectively. Thus IH conditioning altered neither body weight nor the amount of ethanol intake.
Behavioral signs of EW.
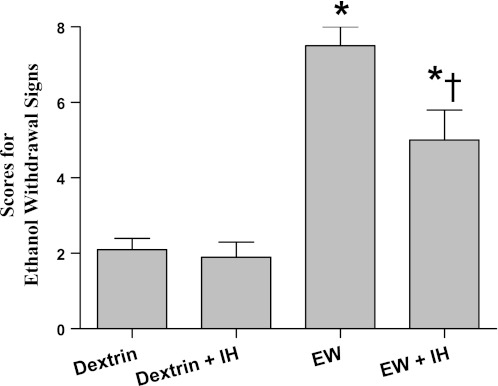
Overt behavioral signs of EW were evaluated 24 h after the second 4-wk ethanol diet, i.e., 5 wk after the completion of IH conditioning (Fig. 2). Single-factor ANOVA revealed treatment effects on the severity of EW signs [F(3,42) = 25; P < 0.001]. These signs were evident in all EW rats but were less severe in IH-conditioned than nonconditioned rats (P = 0.006). Thus IH conditioning mitigated the behavioral manifestations of EW hyperexcitation for at least 5 wk.
Fig. 2.
Behavioral signs of EW. Rats were evaluated for overt signs of EW 24 h after termination of the second ethanol diet. Behavioral signs of acute EW were attenuated by antecedent IH conditioning. Values are means ± SE for 7–10 rats/group. *Significant difference vs. respective dextrin group (P < 0.005). †Significant difference vs. EW (P = 0.006).
Rotarod tests of motor function.
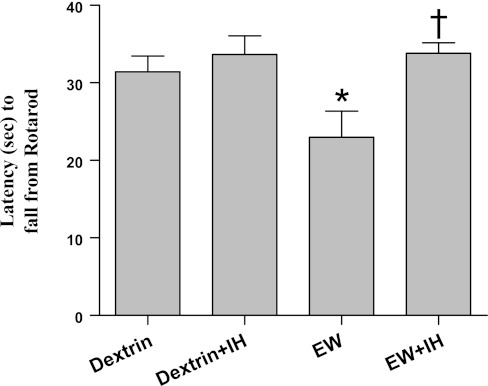
The cerebellum-dependent behavior movement and balance were evaluated by the Rotarod test (Fig. 3). As previously reported (23), rats in the EW group performed this test poorly, falling from the rotating rod sooner than dextrin rats (P = 0.047) [F(3,38) = 5; P = 0.007 by treatment]. IH-conditioned EW rats were able to remain on the rotating rod longer than nonconditioned EW rats (P = 0.016), indicating a protective effect of IH on cerebellar function. There was no significant difference in the Rotarod performance of IH vs. non-IH conditioned dextrin rats.
Fig. 3.
Motoric capacity. Motor function was evaluated by Rotarod testing on days 9–11 of the second EW or the corresponding postdextrin period. Rats were tested three times (three trials) per day with a 10-min interval between trials. EW rats fell from a rotating rod more quickly than dextrin controls (*P = 0.047). IH conditioning during the first EW period prevented motoric impairment during the second EW period (†P = 0.016 vs. EW). Values are means ± SE for 7–10 rats/group.
Cytochrome oxidase.
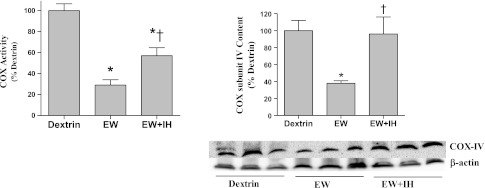
Total COX activity fell 70% in EW vs. dextrin-diet rats (P < 0.001) but did not differ from that of dextrin-diet rats after IH conditioning [F(3,15) = 24; P < 0.001] (Fig. 4, left). COX subunit IV was particularly vulnerable to EW in our previous study (32). Accordingly, the content of COX subunit IV was examined by immunoblot, revealing a 62% decrease in nonconditioned EW rats but no decrement in IH-conditioned EW rats [F(2,10) = 12; P = 0.002] (Fig. 4, right). In dextrin-diet rats, IH conditioning did not significantly alter COX activity or COX subunit IV content (data not shown). Thus IH protects COX from EW, at least in part by preventing subunit IV depletion.
Fig. 4.
Cytochrome c oxidase content. COX activity (left) and COX subunit IV protein content (right) were measured by spectrophotometry and immunoblot, respectively, in cerebelli obtained at 3 wk of the second EW period. Loading control for immunoblots was β-actin. Values are means ± SE for 4–6 rats/group. *Significant difference vs. dextrin control (P < 0.005). †Significant difference vs. EW (P < 0.01).
Cytochrome c and Bax.
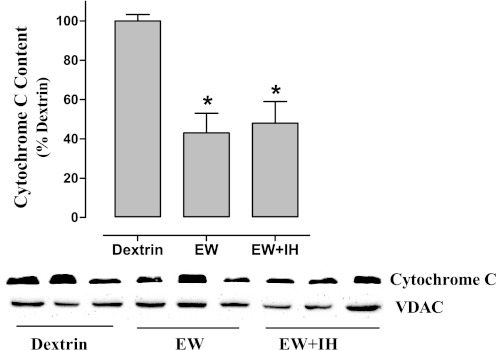
Apoptotic stimuli, including mitochondrial accumulation of Bax, provoke mitochondrial release of cytochrome c. Thus it might be expected that cytochrome c is depleted, whereas Bax is increased by EW in mitochondria in a manner prevented by IH. However, although cytochrome c content was indeed lower in cerebellar mitochondria of EW diet vs. dextrin control rats [F(2,15) = 8.3; P = 0.004], IH conditioning did not prevent this loss (Fig. 5). Furthermore, the mitochondrial content of Bax was not altered under any condition tested (data not shown). These results indicate that neither cytochrome c nor Bax is involved in IH protection of COX.
Fig. 5.
Cytochrome c content. Mitochondria were isolated from cerebelli harvested at 3 wk of the second EW period for immunoblot measurements of cytochrome c content. Values are means ± SE for 6 rats/group. Voltage-dependent anion channel1 (VDAC1) was used as a positive loading control. *Significant difference vs. dextrin (P < 0.005).
Mitochondrial protein oxidation.
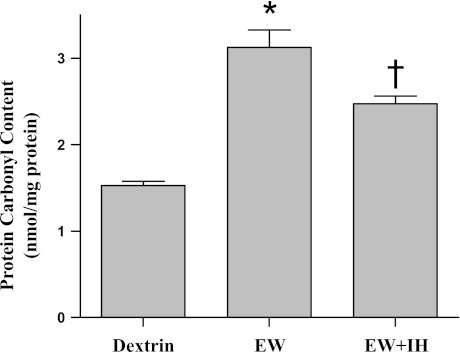
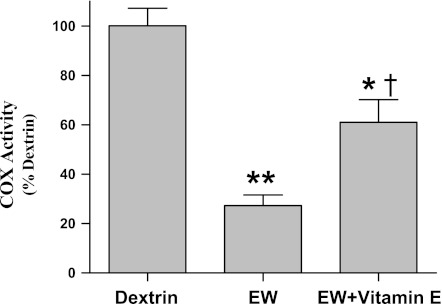
Whether IH preserves COX by suppressing pro-oxidant effects of EW was tested by experiments to determine whether 1) IH protects against oxidative damage to mitochondrial proteins and 2) an antioxidant vitamin E treatment recapitulates IH protection of COX. As previously reported (25), more severe carbonylation of mitochondrial proteins was detected during EW (P < 0.001) vs. dextrin control, but antecedent IH conditioning blunted (P = 0.02) this manifestation of oxidative stress [F(2,27) = 137; P < 0.001] (Fig. 6). The antioxidant vitamin E mimicked the protective effect of IH on COX; thus vitamin E treatment partially restored COX activity during EW toward the dextrin control value [F(2,17) = 37; P < 0.001] (Fig. 7). These data indicate that IH counteracts pro-oxidant EW, and this effect contributes to IH's protection of COX.
Fig. 6.
Mitochondrial protein carbonylation. Mitochondria were isolated at 3 wk of the second EW period. DNPH derivatization was carried out to measure protein carbonyl contents. Values are means ± SE for 7–10 rats/group. *Significant difference vs. dextrin (P < 0.001). †Significant difference vs. EW (P = 0.02).
Fig. 7.
Vitamin E and COX activity. Vitamin E (275 mg·kg−1·day−1) was administered by gavage the last 3 days of the first ethanol diet and the first 17 days of the first EW period. COX was extracted from cerebelli taken from rats killed at 3 wk of the second EW period, and its activity was measured by a colorimetric assay. Values are means ± SE of 7–10 rats/group. Significant difference vs. dextrin: *P = 0.026; **P < 0.001. †Significant difference vs. EW (P = 0.002).
P38 phosphor-activation.
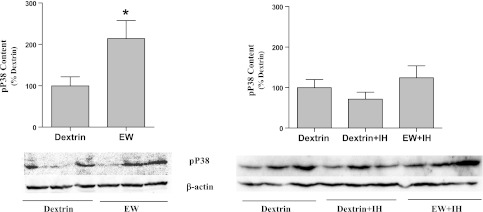
Oxidative stress and P38 activity are interrelated, such that P38 inhibition attenuates reactive O2 species (ROS) generation (6) and ROS activate the P38 signaling pathway (53). Based on these reports, we hypothesized that IH counteracts P38 activation, thereby mitigating protein oxidation. We tested this hypothesis by first examining whether protein oxidation is associated with P38 activation and then examining whether IH prevents P38 activation. As shown in Fig. 8, EW sharply increased phosphor-activation of P38 vs. dextrin control [F(1,8) = 6; P = 0.038]. In contrast, IH conditioning suppressed P38 hyper-phosphorylation during EW (Fig. 8). In cultured neuronal-lineage HT22 cells, the P38 inhibitor SB203580 attenuated the pro-oxidant effect of EW on mitochondrial proteins [F(1,18) = 58, P < 0.001 by SB203580 treatment; F(2,18) = 757, P < 0.001 by ethanol concentration] (Fig. 9). Collectively, these findings indicate that IH conditioning can moderate P38-dependent oxidative damage.
Fig. 8.
P38 phosphorylation. Phosphorylated P38 (pP38) was extracted from cerebelli taken from rats killed at 3 wk of the second EW period and detected by immunoblot. Values (means ± SE, 3/group) are expressed as percentages of the mean value of the dextrin control group. Loading control was β-actin. *Significant difference vs. dextrin (P < 0.01).
Fig. 9.
P38 and protein carbonyls in HT22 cells. HT22 cells were subjected to two cycles of 24 h of ethanol (50 or 100 mM) exposure + 4 h of ethanol washout. P38 inhibitor SB203580 (200 nM) or vehicle was added at the beginning of the first ethanol washout period; 4 h later, mitochondria were isolated to measure protein carbonyl content. Values are means ± SE of 4 experiments/group. *Significant difference vs. 0 mM ethanol (P < 0.05). †Significant difference vs. vehicle (P < 0.001).
DISCUSSION
The principal findings of this study are that IH conditioning applied primarily during EW protects the key mitochondrial enzyme COX from EW inactivation and prevents the severe motor impairment that accompanies EW. These favorable effects of IH conditioning are at least partially attributable to limiting P38-mediated oxidative stress. Importantly, the IH protection is still robust several weeks after the completion of IH conditioning, suggesting that IH evokes a persistent defense mechanism against EW insults.
Massive release of the excitatory neurotransmitter glutamate is considered to be the dominant mechanism of EW-induced excitotoxicity. However, substantial experimental evidence implicates mitochondrial dysfunction in the pathogenesis of EW neurotoxicity. For example, mitochondrial Ca2+ overload resulted in death of spinal neurons (50), and ATP production was impaired in brain mitochondria obtained from ethanol-withdrawn animals (Jung ME, unpublished observations). EW impairment of mitochondria is particularly marked in the cerebellum and other vulnerable brain regions. Indeed, the cerebellum is reported to be among the brain areas most vulnerable to ethanol/EW insults (40, 43). Cerebellar mitochondria isolated from ethanol-withdrawn rats showed more severe membrane swelling than mitochondria from the cortex or hippocampus (25). Our present findings confirm the vulnerability of cerebellar mitochondria to EW that parallels the behavioral manifestations of injured cerebellum (Fig. 3).
Among mitochondrial components, COX was examined as a target of IH protection. COX, which catalyzes the reduction of O2 to H2O at the terminal step of mitochondrial respiration, has also been described as an O2 sensor (41). Indeed, COX is a critical enzyme in maintaining brain health, and COX activity in brain mitochondria has been shown to decrease during aging (49) and in Alzheimer's disease. Accordingly, we hypothesized that IH conditioning protects mitochondria from EW through mechanisms involving COX. Catalytic activity and content of COX were sharply decreased in cerebellar mitochondria of untreated ethanol-withdrawn rats, but antecedent IH conditioning prevented COX depletion. This protection of COX is concordant with an earlier report (46) where male and female rats subjected to simulated intermittent high-altitude hypoxia (5 km, 8 h/day, 5 days/wk, 32 exposures) showed an increase in COX activity in the right ventricular myocardium. However, the decrease in the content of COX subunit IV is somewhat divergent from our previous observation. EW decreased the activity of COX, but it did not significantly alter the content of COX subunit IV in cerebella of female rats (24). This discrepancy may at least partially be attributed to differences of males (present study) vs. females. In fact, a similar phenomenon was observed in cytochrome c: EW decreased mitochondrial cytochrome c in the cerebellum of male rats (Fig. 5) but not in female rats (27). This suggests that the cerebellar mitochondria of females are more tolerant to EW stress than those of males. In support of greater mitochondrial stress tolerance in females vs. males, Du et al. (13) reported that starvation suppressed mitochondrial respiration less severely in neurons of females than males. The interesting sex-related differences in EW susceptibility of brain mitochondria merit further study. Collectively, the present study provides additional support that preservation of mitochondrial COX is pivotal to IH's salutary effects in brain.
Several mechanisms can possibly explain how IH protects COX. Cytochrome c, which transfers electrons from respiratory complex III to complex IV (COX), normally resides in the mitochondrial intermembrane space. Apoptotic insults, such as accumulation of Bax in mitochondria, provoke the release of cytochrome c into cytosol. Therefore, we tested whether the EW-induced inactivation of COX requires the depletion of its substrate (cytochrome c) on the overexpression of mitochondrial Bax. Although EW did indeed deplete mitochondrial cytochrome c, it did not alter Bax content. In fact, the lack of an EW effect on Bax in these young male rats agrees with our laboratory's previous finding in young female rats (27). Moreover, IH conditioning failed to prevent the loss of mitochondrial cytochrome c during EW. These findings support two possibilities: 1) cytochrome c depletion does not necessarily require excessive Bax in mitochondria; 2) cytochrome c depletion is unlikely to mediate IH protection of COX.
Ethanol withdrawal-induced oxidative stress has been widely studied in humans and animals. Alcohol-dependent patients and animals showed increased oxidative markers such as malondialdehyde or free hydroxyl radicals (21, 34, 52). Brain tissues of ethanol-withdrawn female rats showed enhanced lipid peroxidation, which correlated with behavioral impairment (23). The increase in oxidative markers accompanied inactivation of the antioxidant enzymes glutathione synthetase and superoxide dismutase (52). These studies show EW to be a prooxidant stimulus capable of triggering a cascade of adverse events culminating in neurobehavioral impairment.
Regarding the relationship between COX and oxidative stress, Ungvari et al. (49) reported that COX activity fell in aged blood vessels and that free radical production increased; furthermore, siRNA suppression of COX resulted in the excessive generation of mitochondrial ROS. Similarly, the inactivation of COX during EW coincided with an increase in mitochondrial superoxide formation (24, 27). All these studies substantiate the proposed link between COX and oxidative stress. COX deficit may precipitate opening of mitochondrial permeability transition pores, causing membrane potential collapse and loss of ATP (2, 25, 30). Neuronal injury and death triggered by these adverse events may contribute to behavioral impairment.
By impeding electron transfer within the respiratory chain, depletion of mitochondrial cytochrome c creates a pro-oxidant imbalance in redox status, rendering mitochondrial proteins vulnerable to oxidative stress. Oxidized proteins no longer function properly, thereby compromising mitochondrial integrity. Intriguingly, IH conditioning is capable of mitigating mitochondrial accumulation of ROS as well as oxidative protein damage (25). Accordingly, we tested the hypothesis that IH conditioning maintains redox balance of mitochondria, thereby protecting COX, by 1) examining the ability of IH conditioning to minimize oxidative damage to mitochondrial proteins and 2) testing whether an antioxidant, vitamin E, can protect COX in a manner similar to IH conditioning. These experiments confirmed the hypothesis: IH prevented EW-induced mitochondrial protein oxidation, and vitamin E attenuated COX inactivation.
Suppression of the protein kinase P38 could mediate IH's neuroprotection. P38 is abundantly expressed in neurons and mediates synaptic plasticity, memory formation, and gene regulation (9, 48). The activation of P38 is required for brain-derived neurotrophic factor production in brain (28). However, excessive P38 activation is neurotoxic. Glutamate-induced neuronal death is reportedly mediated through the P38 signaling pathway in a manner inhibited by the P38 inhibitor SB203580 (5, 8, 47). We (25) and others (29) implicated P38 signaling in the neurotoxic effects of ethanol exposure and EW by demonstrating SB203580 attenuated ethanol or EW-induced death of HT22 cells. Acute ethanol treatment activated P38 (38) and augmented endotoxin-induced P38 phosphorylation in human monocytes (11). In particular, the massive generation of ROS during EW was attributed to P38 activation (26). Reciprocally, oxidative stress activates P38; thus neurotoxic amyloid-β increased the concentration of H2O2, which subsequently activated P38 (51). The cause-and-effect relationship between oxidative stress and P38 phosphor-activation led us to hypothesize that IH interferes with the oxidative pathway of P38, thereby protecting COX. Two separate experiments confirmed this hypothesis: IH prevented the hyperactivation of P38, and P38 inhibition attenuated protein oxidation during EW. Collectively, IH's protection for COX may be the culmination of well orchestrated regulatory effects of IH on P38-associated oxidative insult and on subsequent COX damage.
The present study revealed neuroprotection 2 mo after animals completed IH conditioning, indicating that IH produces a persistent protection. It is possible that the protective effects of IH are secondary to cellular adaptations occurring during or soon after IH conditioning. Alternatively, IH may exert a delayed protection that gradually develops as an adaptive response. Similar to IH protection in this study, hypoxia preconditioning lessened ischemic brain damage in mice (10, 54). Rybnikova et al. (42) showed that repeated exposures to mild hypoxia increased neuronal tolerance to subsequent severe hypoxia. However, these studies examined short-term effects observed within 3 days after IH conditioning, whereas this study demonstrated protection up to 2 mo after the completion of IH conditioning. Thus the present results are the first to show that IH exerts persistent brain protection from EW insults. In our laboratory's previous study (25), IH's protection was more prominent against brain damage induced by EW than by ethanol per se. Accordingly, we applied IH conditioning mainly during EW (the last 17 days of the 20-day IH program) to examine mechanisms specific to EW. By targeting EW, this IH regimen may have protected neuronal components that are particularly vulnerable to EW insults. Preserving those components might have subsequently influenced behavioral and molecular outcomes. Although we were unable to identify molecular mechanisms that are directly responsible for the behavioral protection, these findings provide new insight regarding the mechanisms of IH protection against EW involving COX and P38-dependent redox events.
GRANTS
This work was supported by grant no. AA-018747 (to M. E. Jung) from the National Institute of Alcohol Addiction and Alcoholism (NIAAA). Postdoctoral stipend support for X. Ju was provided by NIAAA grants AA-018747 and AA-015982.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.J., R.T.M., H.F.D., and M.E.J. conception and design of research; X.J., D.B.M., and M.E.J. performed experiments; X.J., R.T.M., D.B.M., and M.E.J. analyzed data; X.J., R.T.M., H.F.D., D.B.M., and M.E.J. interpreted results of experiments; X.J., D.B.M., and M.E.J. prepared figures; X.J. and M.E.J. drafted the manuscript; X.J., R.T.M., H.F.D., D.B.M., and M.E.J. approved the final version of the manuscript; R.T.M., H.F.D., D.B.M., and M.E.J. edited and revised the manuscript.
REFERENCES
- 1. Barca O, Costoya JA, Senaris RM, Arce VM. Interferon-beta protects astrocytes against tumour necrosis factor-induced apoptosis via activation of p38 mitogen-activated protein kinase. Exp Cell Res 314: 2231–2237, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Bishopric NH, Andreka P, Slepak T, Webster KA. Molecular mechanisms of apoptosis in the cardiac myocyte. Curr Opin Pharmacol 1: 141–150, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 4. Cardoso SM, Proenca MT, Santos S, Santana I, Oliveira CR. Cytochrome c oxidase is decreased in Alzheimer's disease platelets. Neurobiol Aging 25: 105–110, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Chaparro-Huerta V, Flores-Soto ME, Gudino-Cabrera G, Rivera-Cervantes MC, Bitzer-Quintero OK, Beas-Zarate C. Role of p38 MAPK and pro-inflammatory cytokines expression in glutamate-induced neuronal death of neonatal rats. Int J Dev Neurosci 26: 487–495, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Chaves MM, Costa DC, de Oliveira BF, Rocha MI, Nogueira-Machado JA. Role PKA and p38 MAPK on ROS production in neutrophil age-related: lack of IL-10 effect in older subjects. Mech Ageing Dev 130: 588–591, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Chen J, Robinson NC, Schenker S, Frosto TA, Henderson GI. Formation of 4-hydroxynonenal adducts with cytochrome c oxidase in rats following short-term ethanol intake. Hepatology 29: 1792–1798, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Chen RW, Lu XC, Yao C, Liao Z, Jiang ZG, Wei H, Ghanbari HA, Tortella FC, Dave JR. PAN-811 provides neuroprotection against glutamate toxicity by suppressing activation of JNK and p38 MAPK. Neurosci Lett 422: 64–67, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Crews FT, Braun CJ. Binge ethanol treatment causes greater brain damage in alcohol-preferring P rats than in alcohol-nonpreferring NP rats. Alcohol Clin Exp Res 27: 1075–1082, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Dong JW, Zhu HF, Zhu WZ, Ding HL, Ma TM, Zhou ZN. Intermittent hypoxia attenuates ischemia/reperfusion induced apoptosis in cardiac myocytes via regulating Bcl-2/Bax expression. Cell Res 13: 385–391, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Drechsler Y, Dolganiuc A, Norkina O, Romics L, Li W, Kodys K, Bach FH, Mandrekar P, Szabo G. Heme oxygenase-1 mediates the anti-inflammatory effects of acute alcohol on IL-10 induction involving p38 MAPK activation in monocytes. J Immunol 177: 2592–2600, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Du J, Yang S, Wang Z, Zhai C, Yuan W, Lei R, Zhang J, Zhu T. Bone morphogenetic protein 6 inhibit stress-induced breast cancer cells apoptosis via both Smad and p38 pathways. J Cell Biochem 103: 1584–1597, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Du L, Hickey RW, Bayir H, Watkins SC, Tyurin VA, Guo F, Kochanek PM, Jenkins LW, Ren J, Gibson G, Chu CT, Kagan VE, Clark RS. Starving neurons show sex difference in autophagy. J Biol Chem 284: 2383–2396, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fletcher EC, Lesske J, Behm R, Miller CC, 3rd, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol 72: 1978–1984, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci USA 93: 4765–4769, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Giordano G, Klintworth HM, Kavanagh TJ, Costa LG. Apoptosis induced by domoic acid in mouse cerebellar granule neurons involves activation of p38 and JNK MAP kinases. Neurochem Int 52: 1100–1105, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goldstein DB, Pal N. Alcohol dependence produced in mice by inhalation of ethanol: grading the withdrawal reaction. Science 172: 288–290, 1971 [DOI] [PubMed] [Google Scholar]
- 18. Hamm RJ, Pike BR, O'Dell DM, Lyeth BG, Jenkins LW. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma 11: 187–196, 1994 [DOI] [PubMed] [Google Scholar]
- 19. Jaatinen P, Riikonen J, Riihioja P, Kajander O, Hervonen A. Interaction of aging and intermittent ethanol exposure on brain cytochrome c oxidase activity levels. Alcohol 29: 91–100, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Jones NM, Bergeron M. Hypoxic preconditioning induces changes in HIF-1 target genes in neonatal rat brain. J Cereb Blood Flow Metab 21: 1105–1114, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Jung M, Gatch BM, Simpkins JW. The role of female sex steroids in chronic ethanol/ethanol withdrawal toxicity: potential mechanisms of estrogenic protection: review article. Exp Biol Med (Maywood) 230: 8–22, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Jung ME, Yang SH, Brun-Zinkernagel AM, Simpkins JW. Estradiol protects against cerebellar damage and motor deficit in ethanol-withdrawn rats. Alcohol 26: 83–93, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Jung ME, Rewal M, Perez E, Wen Y, Simpkins JW. Estrogen protects against brain lipid peroxidation in ethanol-withdrawn rats. Pharmacol Biochem Behav 79: 573–586, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Jung ME, Agarwal R, Simpkins JW. Ethanol withdrawal posttranslationally decreases the activity of cytochrome c oxidase in an estrogen reversible manner. Neurosci Lett 416: 160–164, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jung ME, Simpkins JW, Wilson AM, Downey HF, Mallet RT. Intermittent hypoxia conditioning prevents behavioral deficit and brain oxidative stress in ethanol-withdrawn rats. J Appl Physiol 105: 510–517, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jung ME, Ju X, Simpkins JW, Metzger DB, Yan LJ, Wen Y. Ethanol withdrawal acts as an age-specific stressor to activate cerebellar P38 kinase. Neurobiol Aging 32: 2266–2278, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jung ME, Ju X, Metzger DB, Simpkins JW. Ethanol withdrawal hastens the aging of cytochrome c oxidase. Neurobiol Aging 33: e21–e32, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Katoh-Semba R, Kaneko R, Kitajima S, Tsuzuki M, Ichisaka S, Hata Y, Yamada H, Miyazaki N, Takahashi Y, Kato K. Activation of p38 mitogen-activated protein kinase is required for in vivo brain-derived neurotrophic factor production in the rat hippocampus. Neuroscience 163: 352–361, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Ku BM, Lee YK, Jeong JY, Mun J, Han JY, Roh GS, Kim HJ, Cho GJ, Choi WS, Yi GS, Kang SS. Ethanol-induced oxidative stress is mediated by p38 MAPK pathway in mouse hippocampal cells. Neurosci Lett 419: 64–67, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Lee SD, Kuo WW, Lin JA, Chu YF, Wang CK, Yeh YL, Wang SG, Liu JY, Chang MH, Huang CY. Effects of long-term intermittent hypoxia on mitochondrial and Fas death receptor dependent apoptotic pathways in rat hearts. Int J Cardiol 116: 348–356, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186: 464–478, 1990 [DOI] [PubMed] [Google Scholar]
- 32. Liu Y, Ji ES, Xiang S, Tamisier R, Tong J, Huang J, Weiss JW. Exposure to cyclic intermittent hypoxia increases expression of functional NMDA receptors in the rat carotid body. J Appl Physiol 106: 259–267, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manukhina EB, Downey HF, Mallet RT. Role of nitric oxide in cardiovascular adaptation to intermittent hypoxia. Exp Biol Med (Maywood) 231: 343–365, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Marotta F, Reizakovic I, Tajiri H, Safran P, Ideo G. Abstinence-induced oxidative stress in moderate drinkers is improved by bionormalizer. Hepatogastroenterology 44: 1360–1366, 1997 [PubMed] [Google Scholar]
- 35. McDonald SR, Sohal RS, Forster MJ. Concurrent administration of coenzyme Q10 and alpha-tocopherol improves learning in aged mice. Free Radic Biol Med 38: 729–736, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Meerson FZ, Krasikov SI, Chavkin II, Bikbulatov MS, Tverdokhlib VP. Reversal of withdrawal injuries of the heart and liver by adaptation to intermittent hypoxia when discontinuing ethanol in chronically alcoholized animals. Kardiologiia 32: 78–82, 1992 [PubMed] [Google Scholar]
- 37. Neckar J, Ostadal B, Kolar F. Myocardial infarct size-limiting effect of chronic hypoxia persists for five weeks of normoxic recovery. Physiol Res 53: 621–628, 2004 [PubMed] [Google Scholar]
- 38. Norkina O, Dolganiuc A, Shapiro T, Kodys K, Mandrekar P, Szabo G. Acute alcohol activates STAT3, AP-1, and Sp-1 transcription factors via the family of Src kinases to promote IL-10 production in human monocytes. J Leukoc Biol 82: 752–762, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Perez E, Liu R, Yang SH, Cai ZY, Covey DF, Simpkins JW. Neuroprotective effects of an estratriene analog are estrogen receptor independent in vitro and in vivo. Brain Res 1038: 216–222, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Ramadoss J, Lunde ER, Chen WJ, West JR, Cudd TA. Temporal vulnerability of fetal cerebellar Purkinje cells to chronic binge alcohol exposure: ovine model. Alcohol Clin Exp Res 31: 1738–1745, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Ripamonti M, Vigano A, Moriggi M, Milano G, von Segesser LK, Samaja M, Gelfi C. Cytochrome c oxidase expression in chronic and intermittent hypoxia rat gastrocnemius muscle quantitated by CE. Electrophoresis 27: 3897–3903, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Rybnikova E, Sitnik N, Gluschenko T, Tjulkova E, Samoilov MO. The preconditioning modified neuronal expression of apoptosis-related proteins of Bcl-2 superfamily following severe hypobaric hypoxia in rats. Brain Res 1089: 195–202, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Santucci AC, Cortes C, Bettica A, Cortes F. Chronic ethanol consumption in rats produces residual increases in anxiety 4 months after withdrawal. Behav Brain Res 188: 24–31, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Schweinsburg BC, Taylor MJ, Alhassoon OM, Videen JS, Brown GG, Patterson TL, Berger F, Grant I. Chemical pathology in brain white matter of recently detoxified alcoholics: a 1H magnetic resonance spectroscopy investigation of alcohol-associated frontal lobe injury. Alcohol Clin Exp Res 25: 924–934, 2001 [PubMed] [Google Scholar]
- 45. Serebrovskaya TV, Manukhina EB, Smith ML, Downey HF, Mallet RT. Intermittent hypoxia: cause of or therapy for systemic hypertension? Exp Biol Med (Maywood) 233: 627–650, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Stieglerova A, Drahota Z, Houstek J, Milerova M, Pelouch V, Ostadal B. Activity of cytochrome c oxidase in the right and left ventricular myocardium of male and female rats exposed to intermittent high altitude hypoxia. Ann NY Acad Sci 874: 269–277, 1999 [DOI] [PubMed] [Google Scholar]
- 47. Suh HW, Kang S, Kwon KS. Curcumin attenuates glutamate-induced HT22 cell death by suppressing MAP kinase signaling. Mol Cell Biochem 298: 187–194, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem 76: 1–10, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Ungvari Z, Labinskyy N, Gupte S, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol 294: H2121–H2128, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Urushitani M, Nakamizo T, Inoue R, Sawada H, Kihara T, Honda K, Akaike A, Shimohama S. N-methyl-d-aspartate receptor-mediated mitochondrial Ca2+ overload in acute excitotoxic motor neuron death: a mechanism distinct from chronic neurotoxicity after Ca2+ influx. J Neurosci Res 63: 377–387, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Valles SL, Borras C, Gambini J, Furriol J, Ortega A, Sastre J, Pallardo FV, Vina J. Oestradiol or genistein rescues neurons from amyloid beta-induced cell death by inhibiting activation of p38. Aging Cell 7: 112–118, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Vallett M, Tabatabaie T, Briscoe RJ, Baird TJ, Beatty WW, Floyd RA, Gauvin DV. Free radical production during ethanol intoxication, dependence, and withdrawal. Alcohol Clin Exp Res 21: 275–285, 1997 [PubMed] [Google Scholar]
- 53. Wang Y, Zhang SX, Gozal D. Reactive oxygen species and the brain in sleep apnea. Respir Physiol Neurobiol 174: 307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Westberg JA, Serlachius M, Lankila P, Penkowa M, Hidalgo J, Andersson LC. Hypoxic preconditioning induces neuroprotective stanniocalcin-1 in brain via IL-6 signaling. Stroke 38: 1025–1030, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Xin MG, Zhang J, Block ER, Patel JM. Senescence-enhanced oxidative stress is associated with deficiency of mitochondrial cytochrome c oxidase in vascular endothelial cells. Mech Ageing Dev 124: 911–919, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Yan LJ, Levine RL, Sohal RS. Oxidative damage during aging targets mitochondrial aconitase. Proc Natl Acad Sci USA 94: 11168–11172, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zong P, Setty S, Sun W, Martinez R, Tune JD, Ehrenburg IV, Tkatchouk EN, Mallet RT, Downey HF. Intermittent hypoxic training protects canine myocardium from infarction. Exp Biol Med (Maywood) 229: 806–812, 2004 [DOI] [PubMed] [Google Scholar]