Abstract
Synaptic activity can modify expression of neurotrophins, which influence the development of neuronal circuits. In the newborn rat, early hyperoxia silences the synaptic activity and input from the carotid body, impairing the development and function of chemoreceptors. The purpose of this study was to determine whether early hyperoxic exposure, sufficient to induce hypoplasia of the carotid body and decrease the number of chemoafferents, would also modify neurotrophin expression within the nucleus tractus solitarii (nTS). Rat pups were exposed to hyperoxia (fraction of inspired oxygen 0.60) or normoxia until 7 or 14 days of postnatal development (PND). In the carotid body, hyperoxia decreased brain-derived neurotrophic factor (BDNF) protein expression by 93% (P = 0.04) after a 7-day exposure, followed by a decrease in retrogradely labeled chemoafferents by 55% (P = 0.004) within the petrosal ganglion at 14 days. Return to normoxia for 1 wk after a 14-day hyperoxic exposure did not reverse this effect. In the nTS, hyperoxia for 7 days: 1) decreased BDNF gene expression by 67% and protein expression by 18%; 2) attenuated upregulation of BDNF mRNA levels in response to acute hypoxia; and 3) upregulated p75 neurotrophic receptor, truncated tropomyosin kinase B (inactive receptor), and cleaved caspase-3. These effects were not observed in the locus coeruleus (LC). Hyperoxia for 14 days also decreased tyrosine hydroxylase levels by 18% (P = 0.04) in nTS but not in the LC. In conclusion, hyperoxic exposure during early PND reduces neurotrophin levels in the carotid body and the nTS and shifts the balance of neurotrophic support from prosurvival to proapoptotic in the nTS, the primary brain stem site for central integration of sensory and autonomic inputs.
Keywords: peripheral arterial chemoreceptors, hypoxic ventilatory response, brain-derived neurotrophic factor, glial-derived neurotrophic factor, plasticity
plasticity is the persistent change in structure and/or function that occurs in neuronal circuits as a result of previous experiences [for review, see (9, 38, 39)]. Factors that augment or attenuate synaptic activity, especially during critical periods of development, can induce plastic changes in neuronal circuits with long-lasting effects; evidence by the classical experiment, showing visual deprivation in newborn cats, inhibits the development of the visual neuronal network (52). Furthermore, developmental plasticity is commonly described for circuits that are involved in learning and memory, but changes in synaptic input can also modify circuits that are involved in cardiorespiratory control (39, 42). The electrical output from the carotid body modifies breathing in response to changes in oxygen tension, with hypoxia increasing and hyperoxia decreasing the electrical activity from the carotid body, leading to a reflex increase and decrease in ventilation, respectively (23). Additionally, activity from the carotid body during early life is important for the development of stable breathing patterns, as evidenced by the finding that chemodenervation during the first weeks of life causes unstable breathing in most mammalian species [for review, see (22)]. This critical period, the first 2–4 wk of postnatal development (PND), coincides with the increase in responsiveness of the carotid body to changes in oxygen tension (30) and is a time when the carotid body is most vulnerable to plasticity-induced changes mediated by environmental exposures (4, 42). The central respiratory circuit is also developing during this time (53). These findings suggest that chemoafferent activity during early development could provide trophic support that stabilizes the central respiratory network; thus exposures that modify the structure and function of the carotid body during a critical developmental period may also modify the plasticity of the central respiratory network.
Hyperoxia can be used as an experimental tool to silence electrical activity from the carotid body. Acute hyperoxic exposures (up to 120 s) are used to determine the contribution of the carotid body to eupneic breathing (13), and chronic exposures to hyperoxia (weeks–months) are used to determine how hyperoxia affects the development of the structure and function of the carotid body [for review, see (4)]. Whereas it is known that hyperoxic exposure during the 1st month of PND causes hypoplasia of the carotid body (17), reduces the number of unmyelinated nerve fibers in the carotid sinus nerve (17), and blunts hypoxic chemosensitivity (35), less is known about how hyperoxic exposure affects the development of the central respiratory network (36).
Brain-derived neurotrophic factor (BDNF) and glial-derived neurotrophic factor (GDNF) provide trophic support for the development and survival of catecholaminergic neurons and other neuronal circuits by modifying the strength of the synapse (11). BDNF and GDNF are expressed in the carotid body during early development and provide obligatory trophic support for the development of primary chemoafferents, which are catecholaminergic (16). Recently, Dmitrieff et al. (14) reported that hyperoxic exposure for 3 days after birth reduces BDNF by 70%, with no change in GDNF protein levels in the carotid body. Synaptic activity also releases BDNF and GDNF (34, 48), neurotrophins that influence survival of catecholaminergic neurons in the peripheral and central nervous system (41). BDNF signaling through the tropomyosin kinase B (TrkB) receptor is essential for normal development and function of the central respiratory network, and deficits in this signaling cascade contribute to the pathophysiology of Rett syndrome (28), showing marked abnormalities in respiratory control. Whereas it is known that later fetal and early postnatal exposure to hyperoxia causes hypoplasia of the carotid body and reduces the number of dopaminergic neurons in the petrosal ganglion, the purpose of this study was to determine whether early postnatal hyperoxic exposure would modify neurotrophin expression and development of catecholaminergic neurons in the nucleus tractus solitarii (nTS), the primary site for central integration of peripheral sensory and autonomic inputs. We also determined whether hyperoxic exposure for 7 days during early PND would modify expression of BDNF in the nTS in response to an acute hypoxic stimulus.
MATERIALS AND METHODS
All experiments were approved by the Animal Care and Use Committee at The Johns Hopkins University School of Medicine.
Hyperoxic exposure.
Time-dated Sprague-Dawley rats were exposed continuously to hyperoxia [fraction of inspired oxygen (FiO2) 0.60, except for 15 min every 2 days for cage changes] or room air (RA; FiO2 0.21), starting ∼48 h prior to parturition and until 7 or 14 days of age. After delivery, the litters were culled to 10 pups each. Both male and female rat pups were used for these experiments. After hyperoxic exposure for 7 days, rat pups were exposed to RA until postnatal day (p)9–10, whereas a second group was exposed to hyperoxia for 14 days and then exposed to RA until p18–19 or p22–23. Animals were then anesthetized with isoflurane and killed, after which time tissues were removed. Carotid bodies with neuronal connections to the petrosal ganglion were removed from hyperoxic and control (RA) animals. The intact carotid body-petrosal ganglion complex was used to determine ex vivo the effect of hyperoxia on the number of chemoafferent cell bodies retrogradely labeled in the petrosal ganglion. From another set of animals, RNA and protein were extracted from the carotid body to determine the expression of BDNF, GDNF, and neurotrophic receptors using real-time quantitative (q)RT-PCR, ELISA, and Western blot analysis. The nTS was microdissected caudal to the obex, rostrally at the midcollicular level (coordinate 1.3 mm) and caudally at the upper cervical spinal cord (coordinate 1.7 mm). The locus coeruleus (LC) was identified ventromedially to the pedunculus cerebellaris, superior and ventrolaterally to the fourth ventricle and 1.5 mm rostrally to the inferior border of the inferior colliculi, and then microdissected bilaterally from the dorsal wall of the rostral pons. After microdissection of the nTS and LC, tissues were homogenized and assayed for BDNF, GDNF, and corresponding receptors, 17 kDa-cleaved caspase-3 levels (Western blot), and tyrosine hydroxylase (TH). Localization of TH+ cells within the nTS was performed by immunohistochemistry.
Ex vivo retrograde labeling of chemoafferents in the petrosal ganglion.
The number of chemoafferents in the petrosal ganglion was determined by using our organotypic retrograde-labeling method, previously described, with minor modifications (49). Instead of microinjecting 4% rhodamine dextran (3 kD; Molecular Probes, Eugene, OR), the carotid body was fenestrated by puncturing it four to five times with a glass micropipette (10 μm diameter tip). Rhodamine dextran was dissolved in 1 μl sterile water containing 5 μM BDNF and GDNF. The solution was allowed to dry to a viscous consistency and then applied to the tip of a glass micropipette to form a small crystal of rhodamine dextran, which was then applied to the fenestrated carotid body. After incubation for ∼12 h in oxygenated (60% O2, 5% CO2) Ringer's solution, containing 125 mM NaCl, 5 mM KCl, 1 mM NaH2PO4, 1 nM MgSO4, 11 mM glucose, 26 mM NaHCO3, and 2 mM CaCl2 at pH 7.4, fluorescent images of the entire petrosal ganglion (en bloc) were captured with a charged-coupled device camera (Hamamatsu Photonics, K.K., Systems Division, Joko-cho, Hamamatsu City, Japan), attached to a fluorescent microscope (Nikon Eclipse E400, Nikon, Tokyo, Japan), using a filter set for rhodamine (abs/em 555/580) with the aid of iVision Software (Omaha, NE). The fluorescent-labeled cell bodies of chemoafferents in each petrosal ganglion were counted in the whole petrosal ganglion using 10× and 40× water-immersion objectives. The petrosal ganglion is ∼100–150 μ thick. We systematically brought the cells into focus at different depths of field using 10× and 40× water-immersion objectives. Every cell that was labeled was captured, and care was taken not to double count cells. There was excellent concordance between the cell counts obtained at 10× and 40×. Quantification was performed by an experienced personnel blinded to the treatment. The median number of fluorescent-labeled cells in the petrosal ganglion was obtained for tissues removed from animals at p9–10 (7-day exposure), p18–19 (14-day exposure), and p22–23 (14-day exposure, followed by 1 wk of RA).
BDNF, GDNF, TrkB, p75ntr, GFR-α1, and RET receptor gene expression measured with real-time qRT-PCR.
Total RNA was extracted from carotid bodies and nTS from hyperoxic and RA-exposed animals (n = 4–6 litters/area of interest/exposure). The PureLink Micro-to-Midi Total RNA Purification System (Invitrogen, Carlsbad, CA) was used, according to the manufacturer's specifications. Approximately 1 μg total RNA was used to generate cDNA using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). The reverse-transcription protocol included 5 min at 25°C, 30 min at 42°C, and 5 min at 85°C. cDNA was then used to amplify the genes of interest by real-time qRT-PCR using 300 nM of specific primers (Table 1). SYBR Green Supermix (Bio-Rad Laboratories) was used for signal detection by MyiQ PCR thermocycler (Bio-Rad Laboratories). The amplification protocol was 40 cycles of 30 s at 95.0°C; 1 min at 62.0°C (BDNF, GDNF), 62.5°C [TrkB, p75 neurotrophic receptor (p75ntr), GDNF receptor α1 (GFR-α1)], or 63.0°C [rearranged during transfection (RET) receptor]; and 1 min at 72.0°C. Three different housekeeping genes—glucose 6-phosphate dehydrogenase (G6PDH), GAPDH, and β-actin—were evaluated to assess the stability under experimental conditions in two different tissues—carotid body and nTS. With the use of the BestKeeper method (46), we determined that the most stable housekeeping gene following hyperoxic exposure was G6PDH in the carotid body and GAPDH in the nTS. The fold difference in gene expression was corrected to the respective housekeeping gene using the Pfaffl method (45). Melting curves were used to ascertain purity of PCR products.
Table 1.
Primers used for real-time qRT-PCR
| Gene | Direction | Sequence | Size | UniSTS/Reference |
|---|---|---|---|---|
| BDNF | Sense | 5′-TGGCCTAACAATGTTTGCAGAT-3′ | 155 bp | 243609 |
| Antisense | 5′-CCACTCAGAAATTCCTCCTGCT-3′ | |||
| TrkB | Sense | 5′-TGAGGCAATGAGCGCTTTAATA-3′ | 193 bp | 218595 |
| Antisense | 5′-GTCCAGTCTGAGAGAGCTGTGG-3′ | |||
| p75ntr | Sense | 5′-GTAGCCTGCCCCTGACCAA-3′ | 121 bp | 464682 |
| Antisense | 5′-GCCTCGTGGGTAAAGGAGTCT-3′ | |||
| GDNF | Sense | 5′-CAGCCCAGAGAATTCCAGAG-3′ | 182 bp | 143191 |
| Antisense | 5′-TTTTGTCATACATTGTCTCGGC-3′ | |||
| GFR-α1 | Sense | 5′-GCTCCTTAGAAGATGCAGAGGC-3′ | 212 bp | 215729 |
| Antisense | 5′-ACCCTGATCCTAACCCTGACCT-3′ | |||
| RET | Sense | 5′-GCACAGCTCTGCTCTATGTCC-3′ | 188 bp | (14) |
| Antisense | 5′-GAGCTGCTCCCAGGAACTATG-3′ | |||
| G6PDH | Sense | 5′-GAAGCCTGGCGTATCTTCAC-3′ | 162 bp | (40) |
| Antisense | 5′-GTGAGGGTTCACCCACTTGT-3′ | |||
| GAPDH | Sense | 5′-CACGGCAAGTTCAACGGCACAGTCA-3′ | 152 bp | (50) |
| Antisense | 5′-GTGAAGACGCCAGTAGACTCCACGAC-3′ |
qRT-PCR, quantitative RT-PCR; UniSTS, Unified Sequence-Tagged Site database; BDNF, brain-derived neurotrophic factor; TrkB, tropomyosin kinase B receptor; p75ntr, p75 neurotrophic receptor; GDNF, glial-derived neurotrophic factor; GFR-α1, GDNF receptor α1; RET, rearranged during transfection receptor; G6PDH, glucose 6-phosphate dehydrogenase.
BDNF protein expression measured with ELISA in carotid body.
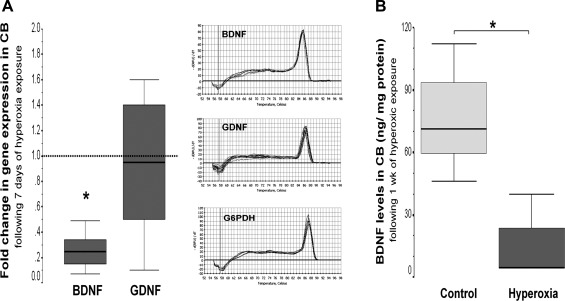
Protein was extracted from carotid bodies to determine BDNF protein expression using ELISA (ChemiKine BDNF Sandwich ELISA Kit, Millipore, Billerica, MA). The range of detection using this method is between 3.9 and 1,000 pg/ml, with an intra-assay variation of ±3.7%. Within 2 wk of freezing at −80°C, tissues were homogenized at 1:40 (w/v), using ice-cold homogenization buffer, prepared in 100 mM Tris-buffered saline (TBS; Tris)/HCl, containing 2% BSA, 1 M NaCl, 4 mM EDTA.Na2, 2% Triton X-100, 0.1% sodium azide, and protease inhibitors: 5 μg/ml aprotinin, 0.5 μg/ml antipain, 157 μg/ml benzamidine, 0.1 μg/ml pepstatin A, and 17 μg/ml PMSF, pH 7.0 (Sigma-Aldrich, St. Louis, MO). A 5-μl aliquot of homogenized tissue was used to determine total protein concentration using the Bradford assay (7). The remaining homogenized tissue was then centrifugated at 14,000 g for 30 min, and clarified supernatant was used for BDNF ELISA. The supernatants were incubated overnight (∼16 h) in the rabbit anti-BDNF polyclonal antibody-coated microplate in duplicate and then exposed to the biotinylated mouse anti-BDNF MAb for 2 h. After exposure to horseradish peroxidase conjugate for 1 h and 3,3′,5,5′-tetramethylbenzidine substrate for 15 min, optical density (OD) at 450 nm was determined using the 640 microplate reader (Bio-Rad Laboratories), and data were reported as absolute values in pg/ml, determined from a standard curve, generated using recombinant BDNF. Absolute values were normalized using protein concentration to final units of ng/mg protein.
Semiquantitative protein expression using Western blot.
The effect of hyperoxia in protein levels for 1) GDNF and receptors (GFR-α1 and RET) in carotid body and nTS; 2) BDNF receptors (p75ntr and full-length TrkB/truncated TrkB) in carotid body, nTS, and LC; and 3) TH, BDNF/pro-BDNF, and 17 kDa-cleaved caspase-3 in the nTS and LC was determined by Western blot analysis. Tissues were microdissected and homogenized in 0.01 M PBS (pH 7.4; Quality Biological, Gaithersburg, MD) at 1:10 (v:v). Homogenized protein (25 μg) was diluted 2:1 (v:v) in loading buffer containing 20% (w/v) glycerol and loaded onto 32% (only for GDNF, BDNF, and cleaved caspase-3) or 15% SDS-PAGE. Protein was transferred to a nitrocellulose membrane, stained with Ponceau S, and blocked with 2.5% nonfat dry milk, with 0.1% Tween-20 in 50 mM TBS (50 mM Tris/HCl and 150 mM NaCl, pH 7.4). Homogenized tissues, described above, were incubated overnight at 4°C with rabbit polyclonal anti-GDNF (sc-328; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal anti-RET (sc-13104), rabbit polyclonal anti-GFR-α1 (sc-10716), mouse monoclonal anti-TrkB (sc-136990 with positive control sc-113925), mouse monoclonal anti-p75ntr (sc-55467), and rabbit polyclonal anti-17 kDa-cleaved caspase-3 (#9661; Cell Signaling Technology, Danvers, MA) primary antibodies at 1:200; rabbit polyclonal anti-BDNF (sc-20981) at 1:500; and mouse monoclonal anti-TH (sc-25265) at 1:1,000. Mouse anti-β-actin MAb (A2228; Sigma-Aldrich) was used at 1:20,000 for protein-loading control. After exposure to each primary antibody, membranes were washed with 5% nonfat dry milk, exposed to goat anti-rabbit or anti-mouse secondary antibody (Bio-Rad Laboratories) at 1:10,000 for 1 h, and developed with enhanced chemiluminescence using the SuperSignal kit (Thermo Scientific, Rockford, IL). To quantify protein immunoreactivity, films were scanned using Adobe Photoshop (Adobe Systems, San Jose, CA), and OD was determined with IP Lab Gel H software (Signal Analytics, Vienna, VA) after subtracting the signal generated by nonspecific binding. Protein levels are expressed as adjusted OD measurements, corrected for loading.
Localizing TH+ immunoreactivity in the nTS.
Animals exposed to hyperoxia or RA for 14 days (n = 4/group) at p22 were anesthetized briefly with isoflurane and transcardially perfused with heparinized 1× PBS (10.6 mM KH2PO4, 56 mM Na2HPO4, and 1.54 M NaCl, pH 7.4), followed by 4% paraformaldehyde in 0.1 M PBS (pH 7.4). Brains were removed, postfixed for 24–48 h in 4% paraformaldehyde, cryoprotected with 30% sucrose in 0.1 M PBS, and sectioned (40 μm) on a vibratome. Tissue sections included the region of the nTS, which is in the caudal brain stem near the area postrema and corresponding to the coordinate 1.3 mm rostral to and 1.7 mm caudal to the obex. Tissue sections were rinsed with 0.1 M PBS, three times for 10 min each, and nonspecific binding was eliminated by incubating sections in a blocking solution containing 5% normal donkey serum (NDS; Santa Cruz Biotechnology) in 0.1 M PBS with 0.3% Triton X-100 for 30 min. Sections were incubated (18–24 h at 4°C) with a mouse monoclonal antiserum against TH (1:2,000; HAB5280 Millipore, Temecula, CA), diluted in PBS with 0.3% Triton X-100 and 2% NDS and then washed with 0.1 M PBS for 30 min. Next, tissue sections were exposed to donkey anti-mouse rhodamine-conjugated secondary antibody (1:250; Jackson ImmunoResearch Laboratories, West Grove, PA) at room temperature for 2 h. Other tissue sections were then mounted and coverslipped with Vectashield fluorescent antifading mounting medium (Vector Laboratories, Burlingame, CA) and observed with a fluorescence microscope (Nikon Eclipse E400, Nikon) using filters for rhodamine (abs/em 555/580). Tissue sections were processed identically, with the exception that the primary antibody was eliminated, and were used as negative controls.
Changes in BDNF gene expression in the nTS following acute hypoxic exposure.
Another set of animals was exposed to RA or hyperoxia (FiO2 0.60) for 7 days (p7), followed by 48 h of RA and then at p9, acutely exposed to hypoxia 8% O2/5% CO2/balanced in N2 or RA for 15 min in a plexiglass chamber. After the exposure, the animals were anesthetized with isoflorane and decapitated, and nTS was microdissected and quickly frozen on dry ice for further processing for changes in gene expression (n = 5/group).
Statistical analysis.
Because of the non-normal distribution of the data, nonparametric statistics with Mann-Whitney U test were used. Results are reported as median with interquartile range (IQR; 25th–75th percentile) and represented as box-and-whisker plots with outliers (boxes symbolize IQR). Bar graphs with unpaired t-test were used only when loading adjustment made the data normally distributed. Significance was assigned by P ≤ 0.05. IBM SPSS Statistics 18.0 software (IBM, Armonk, NY) was used.
RESULTS
Hyperoxic exposure causes carotid body hypoplasia and reduces the number of cell bodies in the petrosal ganglion.
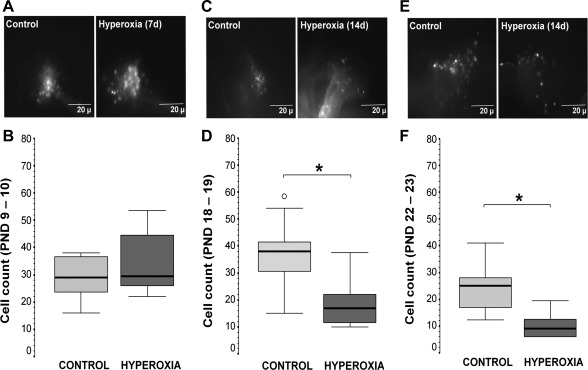
The effect of hyperoxic exposure on the size of the carotid body was only visually determined in our experiments, since it has been previously described by others (6). The carotid bodies of animals exposed to hyperoxia (7 or 14 days) were smaller than those of animals exposed to RA. Erickson et al. (16) also showed that hyperoxic exposure for 4 wk decreased the number of TH+ neurons in the petrosal ganglion and decreased the number of unmyelinated nerve fibers in the carotid sinus nerve. These authors proposed that hyperoxic exposure decreased the number of chemoafferents. With the use of our ex vivo labeling technique, we directly determined the number of chemoafferents by labeling cell bodies of chemoafferents with rhodamine dextran crystals placed in the carotid body; we then counted the number of fluorescently labeled somas in a whole mount of the petrosal ganglion. As shown in the photomicrographs, within the petrosal ganglion, labeled cells and neuronal processes are seen at different ages with the different exposure paradigms (Fig. 1, A, C, and E). Hyperoxic exposure for 7 days did not change the number of chemoafferents (Fig. 1, A and B). However, hyperoxic exposure for 14 days decreased the number of labeled chemoafferents by 55% (P = 0.004 vs. control at p18–19; Fig. 1, C and D) and remained reduced by 64% (P = 0.03 vs. control at p22–23; Fig. 1, E and F), despite return to normoxia for 1 wk following hyperoxic exposure. Thus 2 wk of hyperoxic exposure were sufficient to reduce the number of cell bodies of chemoafferents in the petrosal ganglion, an effect that was not reversed by return to normoxia.
Fig. 1.
Representative photomicrographs of fluorescently labeled cells in the petrosal ganglion (en bloc), which were labeled retrogradely using rhodamine dextran crystals applied to the fenestrated carotid body (CB) from newborn rats after 7-day hyperoxic exposure at postnatal development (PND) postnatal day (p)9–10 (A) and after 14 days exposure at p18–19 (C) and p22–23 (E) and their respective box-and-whisker plots showing the count of chemoafferent neurons in normoxic control (light gray) and hyperoxic (dark gray) animals (B, D, and F). Percentiles [75th and 25th; interquartile range (IQR)] define the upper and lower limits of the box, and the median is represented as the line inside of the box. o, Outliers. *P < 0.05 vs. control; Mann-Whitney U test (n = 7–10/group).
Hyperoxic exposure decreases neurotrophin expression in the carotid body.
Of the neurotrophins examined in the carotid body, hyperoxic exposure for 7 days selectively reduced BDNF gene expression by 76% (P = 0.002 vs. control; Fig. 2A) and protein expression by 93% (P = 0.04 vs. control; Fig. 2B), with no change in GDNF expression (P = 0.44).
Fig. 2.
Box-and-whisker plots showing brain-derived neurotrophic factor (BDNF) and glial-derived neurotrophic factor (GDNF) gene (A) and BDNF protein (B) expression in CB after 7-day hyperoxic exposure. Melting curves show single PCR product in all cases. Percentiles (75th and 25th; IQR) define the upper and lower limits of the box, and the median is represented as the line inside of the box. For gene expression, the box represents the hyperoxic group, whereas the discontinued line represents baseline expression given by normoxic control. G6PDH, glucose 6-phosphate dehydrogenase. *P < 0.05 vs. control group by Mann-Whitney U test (n = 6/group).
Hyperoxic exposure decreases neurotrophin and TH expression in the nTS.
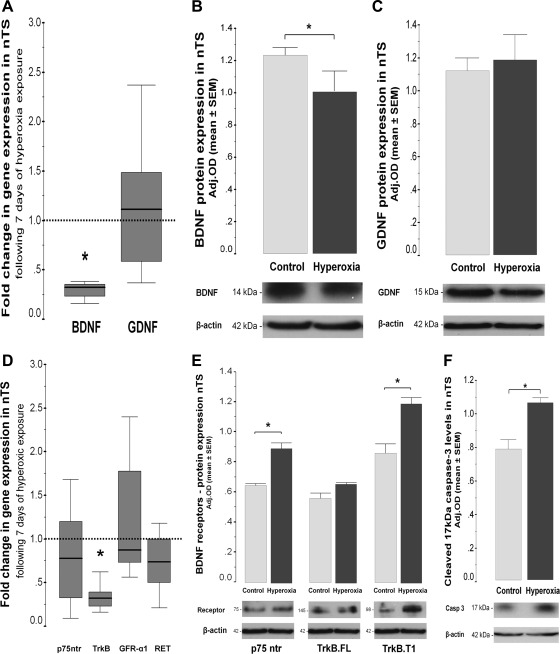
Since the nTS is the central site of integration of the input from carotid chemoreceptors, we determined how hyperoxic exposure modifies the levels of gene and protein expression of BDNF, GDNF, and their corresponding receptors in the nTS using Western blot. Hyperoxia exposure for 7 days decreased BDNF gene expression by 67% (P = 0.01 vs. control normoxia; n = 6/group; Fig. 3A) and protein expression by 18% (P = 0.05; n = 4/group; Fig. 3B), with no changes in pro-BDNF (data not shown). Hyperoxic exposure decreased TrkB gene expression by 68% (P = 0.02 vs. control; n = 6/group; Fig. 3D). Conversely, protein levels of truncated TrkB isoform T1 (∼98 kDa; inactive form of the receptor) increased by 40% (P = 0.002; n = 4/group), whereas the full-length TrkB (∼145 kDa; active form of the receptor) was unchanged (Fig. 3E). Hyperoxia did not affect gene expression for the proapoptotic receptor p75ntr but did increase protein expression by 38% (P = 0.007 vs. control; n = 4/group; Fig. 3E). Lastly, protein levels of 17 kDa-cleaved caspase-3, a marker of apoptosis, increased by 34% in the nTS of hyperoxic-exposed animals (P = 0.01 vs. control normoxia; n = 4; Fig. 3F). Gene and protein expression for GDNF and its receptors (GFR-α1 and RET) was unchanged following 7 days of hyperoxic exposure (Fig. 3, C and D). In the LC, hyperoxic exposure did not alter mRNA or protein levels of: 1) mature BDNF (P = 0.59), 2) pro-BDNF (P = 0.86), 3) full-length TrkB (P = 0.33), 4) truncated TrkB (P = 0.73), or 5) 17 kDa-cleaved caspase-3 (P = 0.66) vs. control normoxia. These data suggest that hyperoxic exposure during the first 7 days of PND shifts the balance of neurotrophic support from prosurvival to proapoptotic, exclusively in the nTS compared with the LC.
Fig. 3.
BDNF and GDNF gene (A) expression, as well as BDNF (B) and GDNF (C) protein expression (Western blot in 32% SDS-PAGE) in nucleus tractus solitarii (nTS) after 7-day hyperoxic exposure. p75 neurotrophic receptor (p75ntr), tropomyosin kinase B (TrkB), GDNF receptor α1 (GFR-α1), and rearranged during transfection (RET) receptor expression is shown (D). p75ntr and full-length (TrkB.FL) and truncated (TrkB.T1) TrkB protein expression in 15% SDS-PAGE is also shown (E). Cleaved, active 17 kDa caspase-3 is shown (F). Gene expression is represented as box-and-whisker plots, 75th and 25th percentiles (IQR) define the upper and lower limits of the box, and the median is represented as the line inside of the box. For gene expression, the box represents the hyperoxic group, whereas the discontinued line represents the baseline expression given by the normoxic control group. *P < 0.05 vs. control group by Mann-Whitney U test (n = 6/group). Bar graphs represent mean ± SE, showing changes in protein expression (Western blot). Optical densitites (ODs) are adjusted (Adj. OD) for loading using β-actin. *P < 0.05 vs. control, unpaired t-test (n = 4/group).
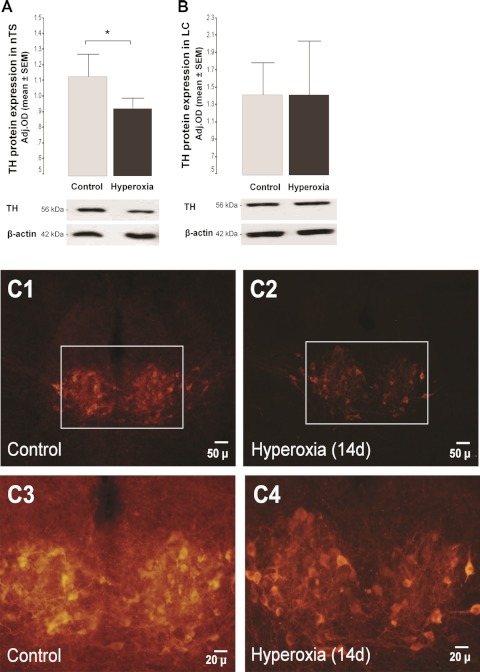
Both the nTS and LC are richly catecholaminergic, and BDNF contributes to the development of peripheral (16) and central (26) catecholaminergic neurons. As outlined above, hyperoxic exposure significantly decreased the expression of BDNF in the nTS of newborn rat pups but did not change the level of expression in the LC. Therefore, we also determined whether hyperoxic exposure affected the development of catecholaminergic neurons exclusively in the nTS. Exposure to hyperoxia for 7 days did not change TH protein expression in either nTS or LC (data not shown); however, in animals exposed to hyperoxia for 14 days, TH protein was reduced by 18% (P = 0.04; Fig. 4A) in the nTS and unchanged in the LC (Fig. 4B). TH+ immunoreactive neurons in the region of the nTS were easily seen in the control animals vs. those exposed to hyperoxia for 14 days (Fig. 4C).
Fig. 4.
Bar graph showing changes in protein expression (Western blot) for tyrosine hydroxylase (TH) in the nTS (A) and locus coeruleus (LC; B) after 14-day hyperoxic exposure, followed by 7–8 days of normoxic exposure. Adj. OD for loading using β-actin. *P < 0.05 (vs. control by unpaired t-test; n = 6/group). Representative sections at the level of the nuclei of nTS from newborn rats at p22, following 14 days of hyperoxic exposure, showing TH+ cells by immunohistochemistry at 2 magnifications (C).
BDNF expression in the nTS following acute hypoxic exposure.
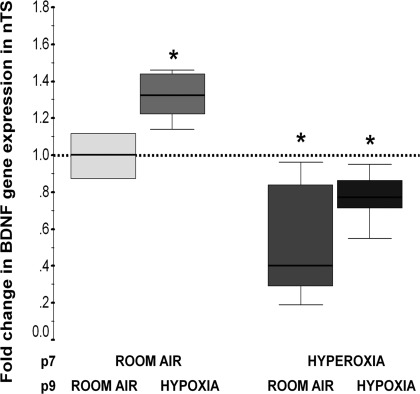
BDNF is released from chemoafferents, which synapse on second-order neurons within the nTS, and we speculate that synaptic activity mediates further BDNF expression. Therefore, we questioned whether an acute hypoxic exposure was associated with an increase in BDNF gene expression in the nTS and how previous hyperoxic exposure during early PND would affect this expression. Acute hypoxia (FiO2 0.08), for 15 min in freely moving rat pups at p9, increased BDNF gene expression by 33% (P = 0.04 vs. control; n = 5/group; Fig. 5) in the nTS of control animals. In contrast, previous hyperoxic exposure (7 days) attenuated the increase in BDNF in the nTS in response to acute hypoxia (Fig. 5).
Fig. 5.
Change in BDNF gene expression following 15 min of hypoxia [fraction of inspired oxygen (FiO2) 0.08] or room air (RA) at p9 in animals previously exposed to normoxia or hyperoxia (FiO2 0.60) for 7 days (until p7). All comparisons are made with animals exposed exclusively to RA and represented as the first box sitting at 1 (discontinued line). Fold change in gene expression relative to exclusive RA (comparison group) is represented as a box-and-whisker plot, with 75th and 25th percentiles (IQR) defining the upper and lower limits of the box and the line inside of the box representing the median. *P < 0.05 vs. control group by Mann-Whitney U test (n = 5/group).
DISCUSSION
Early hyperoxic exposure in newborn rats induces hypoplasia of the carotid body and reduces the number of TH+ neurons in the petrosal ganglion (17). Our findings corroborate and extend the reports of others who have used this experimental model to describe changes in the carotid body after exposure to hyperoxia during early life. We show that 7 days of hyperoxic exposure decreases BDNF expression in the carotid body by 70% without significantly changing the number of chemoafferents in the petrosal ganglion. In addition, hyperoxic exposure for 7 days downregulates BDNF gene and protein expression in the nTS and shifts the balance of the BDNF receptors to a more proapoptotic than prosurvival profile, with no effects on GDNF and GFR-1α receptor or RET gene or protein levels. However, hyperoxic exposure for 14 days does decrease the number of chemoafferents in the petrosal ganglion, an effect that persists after 7 days of return to normoxia. Likewise, hyperoxic exposure for the first 14 days of life decreased TH protein levels and TH+-expressing neurons in the nTS, whereas TH protein expression in the LC was unaffected. Although this paradigm of hyperoxic exposure during early life is known to affect the development of the carotid body and primary sensory neurons in the petrosal ganglion (16), our data are the first to show that hyperoxic exposure also markedly affects BDNF and TH+ expression in the nTS.
Potential mechanisms for hyperoxic-induced changes in the carotid body and petrosal ganglion.
Essentially two hypotheses have been proposed regarding the effect of hyperoxic exposure during this critical period on the plasticity-induced changes in histology and function of the carotid body. These hypotheses are: 1) hyperoxia produces free radical injury, causing greater tissue and cellular damage in younger animals who have fewer protective mechanisms against oxidative stress (32, 44), and 2) hyperoxia silences cellular depolarization, decreasing synaptic activity within the carotid body, potentially impairing its own development and the development of first-order neurons in the petrosal ganglion (24). With regard to the first hypothesis, previous studies from our laboratory, using in vitro preparations of petrosal ganglion neurons exposed to hyperoxia, demonstrate that tissues from younger animals are prone to greater free radical accumulation and neuronal death compared with those from older animals, and these effects were prevented by the use of free radical scavengers, speculating that carotid body hypoplasia and reduced function were secondary to the underdevelopment of antioxidant protection (32). However, this conclusion is still under scrutiny, since Bavis et al. (5) show that treatment with vitamin E or a superoxide dismutase mimetic [manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin pentachloride] does not prevent the detrimental effect of hyperoxic exposure on the carotid body during early PND.
BDNF and GDNF, produced by cells within the carotid body, provide trophic support for development of catecholaminergic neurons in the petrosal ganglion (16, 24). In this study, we explored the hypothesis that hyperoxic exposure reduces neurotrophin levels, specifically BDNF and GDNF, in the carotid body, thereby adversely affecting the development of first-order neurons in the petrosal ganglion. Erickson et al. (17) reported that BDNF levels are reduced in the carotid body of animals exposed to hyperoxia during the first 4 wk of PND. They also found that the number of unmyelinated fibers in the carotid sinus nerve and TH+ neurons in the petrosal ganglion was reduced following 7-day hyperoxic exposure and interpreted these data to be evidence for chemoafferent degeneration (17). For our experiments, we used a more direct approach to determine changes in the number of chemoafferents by using our novel ex vivo technique to retrogradely label these neurons using rhodamine dextran in the carotid body and counting the number of labeled neurons in a whole mount of the petrosal ganglion. We found, with the use of this approach, that 14 days but not 7 days of hyperoxic exposure decrease the number of chemoafferents in the petrosal ganglion. Our findings may in part explain the recent observation that the ablation of hypoxic sensitivity is reversible following 7 days of hyperoxic exposure but is irreversible following 14 days of exposure (3).
Neurotrophins and development of chemoafferents—silent or active trophic support?
Neurotrophins have different roles in modifying neuronal circuits, depending on the stage of development (12). For carotid body chemoreceptors, neurotrophins, specifically BDNF, most likely function as target-derived survival factors for chemoafferents during early development (24). Progenitor cells give origin to sensory neurons, which develop axons that project toward central and peripheral targets. The initial outgrowth of the axons is not neurotrophin dependent, but once the axon reaches its target, they become neurotrophin dependent for a short time (12). The carotid body is a rich source of BDNF, and this expression coincides with target innervation during embryonic development (8). BDNF protein levels in the carotid body peak at embryonic day 16 and then decline such that BDNF expression at birth is undetectable (8). However, in the carotid body, it has been recently reported that BDNF is expressed at p3 (14), and here, we show that BDNF mRNA and protein are abundantly expressed at p9, and its expression is modulated by hyperoxic exposure. Taken together, these data suggest that from late embryonic development to birth, BDNF levels in the carotid body decline, but by 3 days after birth, BDNF expression is again detectable.
At birth, the rise in oxygen tension acutely silences chemoafferent activity, and this is associated with undetectable levels of BDNF in the carotid body (8). However, over the next several days of life, hypoxic chemosensitivity re-emerges and continues to increase during the first several weeks of PND (10). This re-emergence of hypoxic chemosensitivity is associated with upregulation of BDNF expression in the carotid body in the newborn rat (30). BDNF activates TH+ gene transcription (21), potentially explaining the concomitant increase in catecholaminergic expression in the carotid body, which also occurs during the first several weeks of PND (30). It is possible that there is a link between maturation of hypoxic chemosensitivity and re-emergence of BDNF expression in the carotid body and catecholaminergic expression in the carotid body, events that simultaneously occur during the first 2 wk of life. Thus whereas BDNF expression in the carotid body may be responsible for providing “silent” trophic support for axon guidance of chemoafferents during fetal development, with re-emergence of hypoxic chemosensitivity during early PND, BDNF may be providing autocrine support, leading to an increase in transcription of TH+ during the first several weeks of PND. TrkB receptors are expressed on type I cells (51) during this time, supporting this hypothesis.
Neurotrophins released from chemoafferents and development and function of second-order neurons in the nTS.
Although BDNF is expressed in the petrosal ganglion of the fetus, newborn, and adult (8), TrkB receptor expression declines after birth and is not present on chemoafferents in adult animals (54). It has not been shown that BDNF is released within the nTS as a result of chemoafferent stimulation; however, several lines of evidence support a role for BDNF in modulating synaptic activity between chemoafferents and second-order neurons: 1) in response to a depolarizing stimuli, BDNF is released from cultured petrosal ganglion neurons (1); 2) chemoafferents synapse onto a second-order neuron within the nTS (20), and these neurons contain TrkB receptors (2); 3) exogenous BDNF inhibits α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor signaling of dissociated second-order neurons from the nTS (2); 4) exogenous BDNF rescues synaptic dysfunction of second-order neurons in the nTS of methyl CpG-binding protein 2-Null mice, which have reduced levels of BDNF in the nTS (31); and 5) intermittent hypercapnic hypoxia increases BDNF mRNA and protein levels in brain stem nuclei of newborn piglets (43).
Here, we report that BDNF mRNA and protein are expressed in the nTS of newborn rats, and a 7-day hyperoxic exposure during early PND decreases BDNF expression and attenuates its upregulation in response to an acute hypoxic stimulus. A decrease in BDNF expression in the nTS following hyperoxia was greater at the transcriptional than at the translational level, suggesting that further protein decline at later time points postexposure may be expected. Since we used tissue homogenates of the nTS, we do not know the exact source of BDNF within the nTS; however, we suspect that decreased chemoafferent innervation in the hyperoxic-exposed animals accounts for the decreased expression of BDNF mRNA and protein in this region. On the other hand, hyperoxic exposure may induce apoptosis in BDNF-producing neurons in the nTS, similar to what occurs in cortical and subcortical neurons in rats after exposure to FiO2 ≥60% (19). Concurring with these hypotheses, we observed that our paradigm of hyperoxic exposure shifts the balance from prosurvival to proapoptosis signaling in the nTS by increasing: p75ntr; the inactive, truncated form of TrkB (T1) (15); and cleaved caspase-3. This shift to apoptosis signaling was specific to the nTS compared with the LC. We speculate that the absence of monosynaptic excitatory inputs from the carotid body chemoreceptors to the nTS, as a result of hyperoxic exposure, in part, accounts for the differences observed between the nTS and LC.
Effect of hyperoxic exposure on GDNF and receptor expression.
GDNF is another key neurotrophin expressed within the carotid body and through binding to the RET receptor on type I and II cells and chemoafferents, provides autocrine and paracrine support (27, 33), respectively. Although hyperoxic exposure significantly reduced BDNF gene and protein expression in the carotid body and nTS, GDNF protein expression within the nTS was unaffected. Our findings extend the observation of Dmitrieff et al. (14)—that hyperoxic exposure during the first 3 days of PND does not affect GDNF gene or protein expression in the carotid body. These data suggest that GDNF expression is not linked to hyperoxic-induced changes on structure and function in the neural circuit responsible for hypoxic chemosensitivity.
Selective effect of hyperoxic exposure on TH+-producing neurons in the nTS.
Although BDNF and GDNF provide trophic and survival support for TH+ neurons in the central and peripheral nervous systems (25, 29), only BDNF expression is modulated by hyperoxic exposure. Consistent with these findings, we also observe that 7-day hyperoxic exposure reduces TH+ protein expression in neurons in the nTS but not LC (another rich source of catecholaminergic neurons) (47), suggesting that lack of activity from chemoafferents, as a result of hyperoxic exposure, reduces BDNF levels in the nTS and consequently, leads to a decrease of TH+-expressing neurons in the nTS. Whereas not all TH+ neurons in the nTS are second-order neurons for chemoafferents (37), input from peripheral arterial chemoreceptors does modulate activity of TH+ neurons via a polysynaptic pathway (18). BDNF activates TH+ gene transcription in cortical neurons (21), although it is not known if BDNF regulates TH+ expression in catecholaminergic neurons within the nTS. Alternatively, finding fewer TH+ neurons in nTS of hyperoxic animals could result from hyperoxic-induced apoptosis, as discussed previously.
Limitations of the study.
The ex vivo/whole-mount labeling technique has two potential limitations that should be considered: 1) hyperoxic exposure potentially induces an axonopathy, thereby limiting the uptake of the tracer, and 2) counting cells in the whole mount of the petrosal ganglion may have compromised our ability to count the number of labeled neurons accurately. Hyperoxia for 4 wk decreases the number of unmyelinated nerve fibers within the carotid sinus nerve, suggesting that 4 wk of exposure may produce axonopathy (17). We did not determine whether axonopathy was present after 1 or 2 wk of exposure by examining the carotid sinus nerve, but since there was no change in the number of retrogradely labeled cell bodies after 1 wk of exposure, this suggests that axonopathy is not present after 1 wk of exposure. On the other hand, our data show that a 2-wk exposure is sufficient to reduce the number of chemoafferents and perhaps an axonopathy limiting the uptake of the tracer to the soma. Regardless of whether hyperoxia causes direct damage to the soma or disrupts the connection between the soma and the nerve process, both situations would result in abnormal chemoafferents, which do not function properly.
One additional critique of our technique is that we counted cells in a whole mount of the petrosal ganglion instead of serially sectioning the ganglion and using stereography. Since our main focus was to determine the effect of hyperoxic exposure on the neurotrophin expression within the nTS, we used the whole mount of the petrosal ganglion to obtain an estimate as to whether hyperoxia was affecting first-order neurons, thereby increasing the possibility that our paradigm of exposure was modifying inputs into the nTS. We acknowledge that we could have systematically underestimated the number of cells using the whole-mount tissue preparation because of the thickness of the tissue. We improved the accuracy of the technique by capturing the labeled cells at two different magnifications and comparing counts at these magnifications; we minimized bias, because the person who captured the images and counted the cells was unaware of the treatment group. When placed in context with other studies, our findings provide the most direct evidence that hyperoxic exposure for 14 days reduces the number of chemoafferents.
Conclusions.
Hyperoxic exposure during the first several weeks of PND markedly reduces activity from chemoafferents and ablates hypoxic ventilatory responses throughout development in the newborn rat. Here, we show that hyperoxic exposure also decreases BDNF expression and increases markers of apoptosis within the nTS. It is not clear whether our findings are secondary to a direct effect (absence of excitatory input and BDNF support from primary chemoafferents) or a more global, “toxic” effect from hyperoxia on cells and neurons within the nTS. However, these data suggest that global exposure to hyperoxia for the first weeks of PND can have profound effects on the development of peripheral and central circuits that are involved in sensory and autonomic control.
GRANTS
Support for this study was funded by the National Heart, Lung, and Blood Institute Grant RO1HL080725 (to E. B. Gauda) and National Institute of Neurological Disorders and Stroke Grant 5K01NS047422 (to S. M. Johnson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.C-V., C.T., and E.B.G. conception and design of research; R.C-V., A.M., A.R.N., R.A., S.M.J., and E.B.G. performed experiments; R.C-V., A.R.N., F.J.N., and E.B.G. analyzed data; R.C-V., A.M., A.R.N., F.J.N., C.T., R.A., S.M.J., and E.B.G. interpreted results of experiments; R.C-V. prepared figures; R.C-V., A.M., A.R.N., F.J.N., C.T., and E.B.G. drafted manuscript; R.C-V., A.M., A.R.N., F.J.N., C.T., R.A., S.M.J., and E.B.G. edited and revised manuscript; R.C-V., A.M., A.R.N., F.J.N., C.T., R.A., S.M.J., and E.B.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Mrs. Debra Flock, Mr. Devin Mack, Dr. Robert Bennett, Ms. Julia Hinojos, and Ms. Darla Mata for technical and administrative assistance.
REFERENCES
- 1. Balkowiec A, Katz DM. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. J Neurosci 20: 7417–7423, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balkowiec A, Kunze DL, Katz DM. Brain-derived neurotrophic factor acutely inhibits AMPA-mediated currents in developing sensory relay neurons. J Neurosci 20: 1904–1911, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bavis RW, Kim I, Pradhan N, Nawreen N, Dmitrieff EF, Carroll JL, Donnelly DF. Recovery of carotid body O2 sensitivity following chronic postnatal hyperoxia in rats. Respir Physiol Neurobiol 177: 47–55, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bavis RW, Mitchell GS. Long-term effects of the perinatal environment on respiratory control. J Appl Physiol 104: 1220–1229, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Bavis RW, Wenninger JM, Miller BM, Dmitrieff EF, Olson EB, Jr, Mitchell GS, Bisgard GE. Respiratory plasticity after perinatal hyperoxia is not prevented by antioxidant supplementation. Respir Physiol Neurobiol 160: 301–312, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bisgard GE, Olson EB, Jr, Wang ZY, Bavis RW, Fuller DD, Mitchell GS. Adult carotid chemoafferent responses to hypoxia after 1, 2, and 4 wk of postnatal hyperoxia. J Appl Physiol 95: 946–952, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 8. Brady R, Zaidi SI, Mayer C, Katz DM. BDNF is a target-derived survival factor for arterial baroreceptor and chemoafferent primary sensory neurons. J Neurosci 19: 2131–2142, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carroll JL. Developmental plasticity in respiratory control. J Appl Physiol 94: 375–389, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Carroll JL, Bamford OS, Fitzgerald RS. Postnatal maturation of carotid chemoreceptor responses to O2 and CO2 in the cat. J Appl Physiol 75: 2383–2391, 1993 [DOI] [PubMed] [Google Scholar]
- 11. Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol 70: 271–288, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davies AM. The role of neurotrophins during successive stages of sensory neuron development. Prog Growth Factor Res 5: 263–289, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Dejours P. Chemoreflexes in breathing. Physiol Rev 42: 335–358, 1962 [DOI] [PubMed] [Google Scholar]
- 14. Dmitrieff EF, Wilson JT, Dunmire KB, Bavis RW. Chronic hyperoxia alters the expression of neurotrophic factors in the carotid body of neonatal rats. Respir Physiol Neurobiol 175: 220–227, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eide FF, Vining ER, Eide BL, Zang K, Wang XY, Reichardt LF. Naturally occurring truncated TrkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J Neurosci 16: 3123–3129, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Erickson JT, Brosenitsch TA, Katz DM. Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor are required simultaneously for survival of dopaminergic primary sensory neurons in vivo. J Neurosci 21: 581–589, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Erickson JT, Mayer C, Jawa A, Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS, Katz DM. Chemoafferent degeneration and carotid body hypoplasia following chronic hyperoxia in newborn rats. J Physiol 509: 519–526, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Erickson JT, Millhorn DE. Fos-like protein is induced in neurons of the medulla oblongata after stimulation of the carotid sinus nerve in awake and anesthetized rats. Brain Res 567: 11–24, 1991 [DOI] [PubMed] [Google Scholar]
- 19. Felderhoff-Mueser U, Bittigau P, Sifringer M, Jarosz B, Korobowicz E, Mahler L, Piening T, Moysich A, Grune T, Thor F, Heumann R, Buhrer C, Ikonomidou C. Oxygen causes cell death in the developing brain. Neurobiol Dis 17: 273–282, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Finley JC, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res 572: 108–116, 1992 [DOI] [PubMed] [Google Scholar]
- 21. Fukuchi M, Fujii H, Takachi H, Ichinose H, Kuwana Y, Tabuchi A, Tsuda M. Activation of tyrosine hydroxylase (TH) gene transcription induced by brain-derived neurotrophic factor (BDNF) and its selective inhibition through Ca(2+) signals evoked via the N-methyl-d-aspartate (NMDA) receptor. Brain Res 1366: 18–26, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Gauda EB, Carroll JL, Donnelly DF. Developmental maturation of chemosensitivity to hypoxia of peripheral arterial chemoreceptors—invited article. Adv Exp Med Biol 648: 243–255, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev 74: 829–898, 1994 [DOI] [PubMed] [Google Scholar]
- 24. Hertzberg T, Fan G, Finley JC, Erickson JT, Katz DM. BDNF supports mammalian chemoafferent neurons in vitro and following peripheral target removal in vivo. Dev Biol 166: 801–811, 1994 [DOI] [PubMed] [Google Scholar]
- 25. Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24: 677–736, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature 350: 230–232, 1991 [DOI] [PubMed] [Google Scholar]
- 27. Izal-Azcarate A, Belzunegui S, San Sebastian W, Garrido-Gil P, Vazquez-Claverie M, Lopez B, Marcilla I, Luquin MA. Immunohistochemical characterization of the rat carotid body. Respir Physiol Neurobiol 161: 95–99, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Katz DM, Dutschmann M, Ramirez JM, Hilaire G. Breathing disorders in Rett syndrome: progressive neurochemical dysfunction in the respiratory network after birth. Respir Physiol Neurobiol 168: 101–108, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kholodilov N, Yarygina O, Oo TF, Zhang H, Sulzer D, Dauer W, Burke RE. Regulation of the development of mesencephalic dopaminergic systems by the selective expression of glial cell line-derived neurotrophic factor in their targets. J Neurosci 24: 3136–3146, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kholwadwala D, Donnelly DF. Maturation of carotid chemoreceptor sensitivity to hypoxia: in vitro studies in the newborn rat. J Physiol 453: 461–473, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kline DD, Ogier M, Kunze DL, Katz DM. Exogenous brain-derived neurotrophic factor rescues synaptic dysfunction in Mecp2-null mice. J Neurosci 30: 5303–5310, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kwak DJ, Kwak SD, Gauda EB. The effect of hyperoxia on reactive oxygen species (ROS) in rat petrosal ganglion neurons during development using organotypic slices. Pediatr Res 60: 371–376, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Leitner ML, Wang LH, Osborne PA, Golden JP, Milbrandt J, Johnson EM., Jr Expression and function of GDNF family ligands and receptors in the carotid body. Exp Neurol 191, Suppl 1: S68–S79, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Lessmann V. Neurotrophin-dependent modulation of glutamatergic synaptic transmission in the mammalian CNS. Gen Pharmacol 31: 667–674, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Attenuation of the hypoxic ventilatory response in adult rats following one month of perinatal hyperoxia. J Physiol 495: 561–571, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Integrated phrenic responses to carotid afferent stimulation in adult rats following perinatal hyperoxia. J Physiol 500: 787–796, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Massari VJ, Shirahata M, Johnson TA, Gatti PJ. Carotid sinus nerve terminals which are tyrosine hydroxylase immunoreactive are found in the commissural nucleus of the tractus solitarius. J Neurocytol 25: 197–208, 1996 [DOI] [PubMed] [Google Scholar]
- 38. Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: intermittent hypoxia and respiratory plasticity. J Appl Physiol 90: 2466–2475, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol 94: 358–374, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Nunes AR, Monteiro EC, Johnson SM, Gauda EB. Bicarbonate-regulated soluble adenylyl cyclase (sAC) mRNA expression and activity in peripheral chemoreceptors. Adv Exp Med Biol 648: 235–241, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Pascual A, Hidalgo-Figueroa M, Gomez-Diaz R, Lopez-Barneo J. GDNF and protection of adult central catecholaminergic neurons. J Mol Endocrinol 46: R83–R92, 2011 [DOI] [PubMed] [Google Scholar]
- 42. Pawar A, Peng YJ, Jacono FJ, Prabhakar NR. Comparative analysis of neonatal and adult rat carotid body responses to chronic intermittent hypoxia. J Appl Physiol 104: 1287–1294, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peiris TS, Machaalani R, Waters KA. Brain-derived neurotrophic factor mRNA and protein in the piglet brainstem and effects of intermittent hypercapnic hypoxia. Brain Res 1029: 11–23, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Perrone S, Negro S, Tataranno ML, Buonocore G. Oxidative stress and antioxidant strategies in newborns. J Matern Fetal Neonatal Med 23 Suppl 3: 63–65, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30: e36, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol Lett 26: 509–515, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Pickel VM, Joh TH, Reis DJ. Ultrastructural localization of tyrosine hydroxylase in noradrenergic neurons of brain. Proc Natl Acad Sci USA 72: 659–663, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shieh PB, Ghosh A. Molecular mechanisms underlying activity-dependent regulation of BDNF expression. J Neurobiol 41: 127–134, 1999 [PubMed] [Google Scholar]
- 49. Tolosa JN, Cooper R, Myers AC, McLemore GL, Northington F, Gauda EB. Ontogeny of retrograde labeled chemoafferent neurons in the newborn rat nodose-petrosal ganglion complex: an ex vivo preparation. Neurosci Lett 384: 48–53, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Wang GP, Xu CS. Reference gene selection for real-time RT-PCR in eight kinds of rat regenerating hepatic cells. Mol Biotechnol 46: 49–57, 2010 [DOI] [PubMed] [Google Scholar]
- 51. Wang ZY, Bisgard GE. Postnatal growth of the carotid body. Respir Physiol Neurobiol 149: 181–190, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Wiesel TN, Hubel DH. Effects of visual deprivation on morphology and physiology of cells in the cats lateral geniculate body. J Neurophysiol 26: 978–993, 1963 [DOI] [PubMed] [Google Scholar]
- 53. Wong-Riley MT, Liu Q. Neurochemical and physiological correlates of a critical period of respiratory development in the rat. Respir Physiol Neurobiol 164: 28–37, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhuo H, Helke CJ. Presence and localization of neurotrophic receptor tyrosine kinase (TrkA, TrkB, TrkC) mRNAs in visceral afferent neurons of the nodose and petrosal ganglia. Brain Res Mol Brain Res 38: 63–70, 1996 [DOI] [PubMed] [Google Scholar]