Abstract
EMBO J 31 11, 2486–2497 (2012); published online April 17 2012
In this issue of The EMBO Journal, Wilson et al (2012) elegantly discovered an important new axis for intestinal homeostasis and cancer, using an RNAi screen to enhance the RAS-induced multivulva (MUV) phenotype in Caenorhabditis elegans.
Originally identified in their C. elegans screen, the authors subsequently show that conditional deletion of Nrbp1 throughout the adult mouse results in an intestinal progenitor cell phenotype that is associated with increased proliferation and perturbed differentiation (Batlle et al, 2002; Sansom et al, 2004; Finch et al, 2009). These data recapitulate the key features associated with deletion of the adenomatous polyposis coli (Apc) tumour suppressor gene in the murine intestine. APC is the most frequently mutated gene in colorectal cancer in humans, suggesting a common mechanism. The major tumour-suppressive function of Apc is to negatively regulate Wnt signalling and, consistent with this, NRBP1-deficient intestines showed a marked increase in Wnt target genes, including Ccnd1, CD44, Mmp7, Sox9 and Tnfrsf12a. Mechanistically it appears that Nrbp1 negatively regulates Sall4, possibly through an Elongin B–C ubiquitin complex; hence, loss of Nrbp1 allows the upregulation of Sall4, which can then activate Wnt signalling (Figure 1).
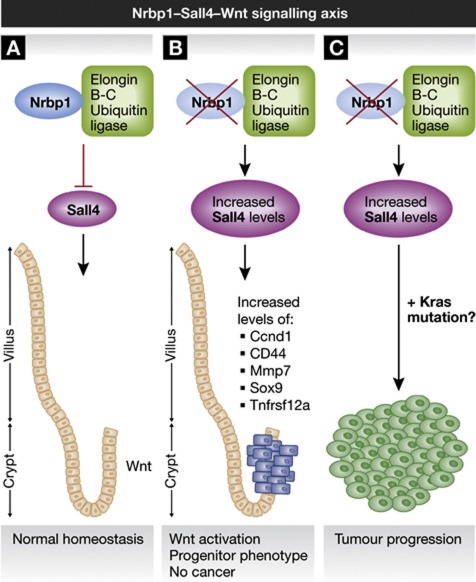
Figure 1.
A novel Nrbp1–Sall4–Wnt signalling axis. (A) Nrbp1 regulates Sall4 levels through its interaction with the Elongin B–C E3 ubiquitin ligase complex, allowing the maintenance of intestinal progenitor cell homeostasis. (B) Loss of Nrbp1 increases Sall4 levels, leading to Wnt activation and an expansion of the crypt progenitor-like cells with aberrant proliferation, differentiation and localisation of cells along the crypt villus axis. (C) Nrbp1 loss following other mutational events such as KRAS drives tumour progression and confers a poor prognosis.
Over the past 5 years, there has been a remarkable progress regarding the identification of stem cell markers in the intestine (Barker et al, 2007). Therefore, one question is whether loss of Nrbp1and downstream Sall4 activation drive a stem-cell-like phenotype in the intestine. In support of this is the marked increase in Lgr5 (the canonical stem cell marker) levels following Nrbp1 loss. Moreover, a recent work by Hobbs et al (2012) showed that Sall4 has essential roles in the maintenance of embryonic germ cells and the differentiation of spermatogonial progenitor cells, and a previous work has shown its requirement for early embryonic development (Warren et al, 2007). However, the finding that long-term deletion of Nrbp1 in the intestine is not tolerated strongly argues against a stem-cell-like phenotype in Nrbp1 cells. Moreover, the main cell type that is produced in the crypts of Nrbp1 are highly proliferative cells that fail to differentiate and therefore are presumably not bona fide stem cells. It is interesting to note that OCT4 overexpression in the murine intestine also drives a progenitor rather than a stem cell phenotype (Hochedlinger et al, 2005) and it is only in the Drosophila intestine when Notch is overexpressed that tumours composed of purely stem cells arise (Hayward et al, 2005). It should be noted that human colorectal cancers retain many features of the intestinal crypt and predominantly consist of progenitor cells, while cancer cells expressing the intestinal stem cell (ISC) signature represent only a fraction of the tumour (Batlle et al, 2002). Given the phenotypic and transcriptome similarities with Apc gene deletion, it is rather surprising that Nrbp1-deficient cells are outcompeted by wild-type cells and crypts cannot grow over longer periods in culture. This highlights that Nrbp1 is unlikely to be an initiating tumour suppressor mutation and would not be able to substitute for Apc gene mutation. One clue to why cells may be lost is the finding that low-level deletion of Nrbp1 can initiate tumourigenesis, suggesting that the presence of surrounding wild-type cells may provide survival advantages to cells lacking Nrbp1.
In contrast to the initiating properties of NRBP1 loss, the data suggesting a cooperative role for Nrbp1 downregulation in cancer appear compelling. Wilson et al (2012) show that Nrbp1 is downregulated in a number of diverse cancer types, including colorectal tissue and lung. It is interesting to note that many cancers show a deregulation of Wnt signalling without loss of the Apc gene or activating mutations in β-catenin. It is possible that Nrbp1 downregulation may contribute to this; thus, future studies correlating Nrbp1 downregulation to Wnt signalling activation in a number of human cancers can provide information on how widespread a function this could be. This may have therapeutic implications, as a number of Wnt signalling inhibitors exist, such as cell surface-blocking antibodies, that are unlikely to work well if Apc is mutated or β-catenin is activated, as there will then be ligand-independent signalling (Ettenberg et al, 2010). In contrast, Wilson et al (2012) show that Nrbp1-deficient cells are still dependent on ligand signalling as removal of R-Spondin, a Wnt-signalling agonist in intestinal cultures, leads rapidly to crypt death.
One prediction from the promotion of the MUV phenotype in C. elegans would be that Nrbp1 downregulation might be associated with tumours that harbour KRAS mutation and loss might cooperate in mouse models carrying KRAS mutation. It is possible that KRAS mutation may overcome this long-term selection against Nrbp1-deficient cells, and it will be of interest to see what extra properties Nrbp1 downregulation confers on KRAS mutant cells. To this end, one could predict that increased levels of Sall4 and Wnt signalling might increase the numbers of cells expressing the stem cell/progenitor cell signature within tumours, a feature conferred by Wnt signalling in a number of tumours (Zheng et al, 2010).
In summary, this study represents the discovery of an exciting novel tumour suppressor axis that plays a key role in intestinal homeostasis and that may be of therapeutic relevance to cancer. As the polyomic and sequencing data continue to proliferate, the validation of these targets remains key. None would have predicted that a screen from promotion of a KRAS phenotype in C. elegans would have implications on intestinal homeostasis. This emphasises the requirement for functional screens and, if strong cooperation exists, physiologically relevant findings for the mammalian/human situation might rapidly emerge.
Footnotes
The authors declare that they have no conflict of interest.
References
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007 [DOI] [PubMed] [Google Scholar]
- Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, Clevers H (2002) Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 111: 251–263 [DOI] [PubMed] [Google Scholar]
- Ettenberg SA, Charlat O, Daley MP, Liu S, Vincent KJ, Stuart DD, Schuller AG, Yuan J, Ospina B, Green J, Yu Q, Walsh R, Li S, Schmitz R, Heine H, Bilic S, Ostrom L, Mosher R, Hartlepp KF, Zhu Z, Fawell S, Yao YM, Stover D, Finan PM, Porter JA, Sellers WR, Klagge IM, Cong F (2010) Inhibition of tumorigenesis driven by different Wnt proteins requires blockade of distinct ligand-binding regions by LRP6 antibodies. Proc Natl Acad Sci USA 107: 15473–15478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch AJ, Soucek L, Junttila MR, Swigart LB, Evan GI (2009) Acute overexpression of Myc in intestinal epithelium recapitulates some but not all the changes elicited by Wnt/beta-catenin pathway activation. Mol Cell Biol 29: 5306–5315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward P, Brennan K, Sanders P, Balayo T, DasGupta R, Perrimon N, Martinez Arias A (2005) Notch modulates Wnt signalling by associating with Armadillo/beta-catenin and regulating its transcriptional activity. Development 132: 1819–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs RM, Fagoonee S, Papa A, Webster K, Altruda F, Nishinakamura R, Chai L, Pandolfi PP (2012) Functional antagonism between Sall4 and Plzf defines germline progenitors. Cell Stem Cell 10: 284–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochedlinger K, Yamada Y, Beard C, Jaenisch R (2005) Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell 121: 465–477 [DOI] [PubMed] [Google Scholar]
- Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, Batlle E, Simon-Assmann P, Clevers H, Nathke IS, Clarke AR, Winton DJ (2004) Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev 18: 1385–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren M, Wang W, Spiden S, Chen-Murchie D, Tannahill D, Steel KP, Bradley A (2007) A Sall4 mutant mouse model useful for studying the role of Sall4 in early embryonic development and organogenesis. Genesis 45: 51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CH, Crombie C, van der Weyden L, Poulogiannis G, Rust AG, Pardo M, Gracia T, Yu L, Choudhary J, Poulin GB, McIntyre RE, Winton DJ, March HN, Arends MJ, Fraser AG, Adams DJ (2012) Nuclear receptor binding protein 1 regulates intestinal progenitor cell homeostasis and tumour formation. EMBO J 31: 2486–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Ying H, Wiedemeyer R, Yan H, Quayle SN, Ivanova EV, Paik JH, Zhang H, Xiao Y, Perry SR, Hu J, Vinjamoori A, Gan B, Sahin E, Chheda MG, Brennan C, Wang YA, Hahn WC, Chin L, DePinho RA (2010) PLAGL2 regulates Wnt signaling to impede differentiation in neural stem cells and gliomas. Cancer Cell 17: 497–509 [DOI] [PMC free article] [PubMed] [Google Scholar]