Abstract
Oxygen is essential for eukaryotic life and is inextricably linked to the evolution of multicellular organisms. Proper cellular response to changes in oxygen tension during normal development or pathological processes, such as cardiovascular disease and cancer, is ultimately regulated by the transcription factor, hypoxia-inducible factor (HIF). Over the past decade, unprecedented molecular insight has been gained into the mammalian oxygen-sensing pathway involving the canonical oxygen-dependent prolyl-hydroxylase domain-containing enzyme (PHD)–von Hippel-Lindau tumour suppressor protein (pVHL) axis and its connection to cellular metabolism. Here we review recent notable advances in the field of hypoxia that have shaped a more complex model of HIF regulation and revealed unique roles of HIF in a diverse range of biological processes, including immunity, development and stem cell biology.
Keywords: development, HIF, hypoxia, immunity, stem cell biology
The canonical HIF pathway
Oxygen-dependent regulation of HIF signalling
Hypoxia-inducible factor (HIF) is a DNA-binding transcription factor that associates with specific nuclear cofactors under hypoxia to transactivate a myriad of genes to trigger various adaptive responses to compromised oxygen tension. HIF is a heterodimer comprising an oxygen-labile α-subunit (HIFα) and a constitutively expressed β-subunit (HIFβ or ARNT). Both subunits are part of the basic Helix–Loop–Helix PER-ARNT-SIM (bHLH-PAS) family of transcription factors. Three genes encoding distinct HIFα isoforms exist in humans: HIF1A, encoding HIF1α; EPAS1, encoding HIF2α; and HIF3A, which is expressed as multiple HIF3α splice variants (Wang et al, 1995; Makino et al, 2002; Maynard et al, 2003). HIF1α, HIF2α, and HIF3α splice variants 1–3 possess an oxygen-dependent degradation domain (ODD) and an N-terminal transactivation domain (NTAD), while HIF1α and HIF2α possess a C-terminal transactivation domain (CTAD; Jiang et al, 1997; Pugh et al, 1997; Gu et al, 1998; Huang et al, 1998; Ema et al, 1999; O'Rourke et al, 1999; Hara et al, 2001; Maynard et al, 2003). In this review, HIF isoforms will be specified where appropriate, while ‘HIFα’ will be used to denote either HIF1α or HIF2α.
Under normoxia (normal oxygen tension), HIFα is hydroxylated on at least one of two conserved proline residues within the ODD (Ivan et al, 2001; Jaakkola et al, 2001; Masson et al, 2001) by prolyl-hydroxylase domain (PHDs)-containing enzymes (Epstein et al, 2001). Hydroxy-HIFα is recognized by the β-domain of von Hippel-Lindau tumour suppressor protein (pVHL) and is subsequently ubiquitylated by the Elongin BC/Cul2/pVHL ubiquitin–ligase complex assembled via the pVHLα domain, thereby marking HIFα for degradation by the 26S proteasome (Maxwell et al, 1999; Ohh et al, 2000). PHDs require oxygen for catalytic activity; thus, prolyl hydroxylation of HIFα is abrogated under hypoxia, allowing HIFα to escape recognition by the pVHL ubiquitin–ligase complex and accumulate in the nucleus. Similarly, biallelic inactivation of VHL has a profound stabilizing effect on HIFα protein levels, thereby implicating pVHL as a predominant HIFα antagonist. Notably, another hydroxylase-domain protein termed factor inhibiting HIF (FIH) participates in the negative regulation of HIFα by hydroxylating asparagine-803 in the CTAD in the presence of oxygen, which sterically inhibits interactions between HIFα and transcriptional coactivators (Lando et al, 2002).
Activation of HIF in hypoxia
Hypoxia is defined in the context of tumours as having an internal partial pressure of oxygen of less than 10–15 mm Hg (Brizel et al, 1999; Khan et al, 2012). In hypoxic conditions or in VHL−/− cells, stabilized HIFα dimerizes with HIFβ before binding to hypoxia-response elements (HREs) comprising a core 5′-[A/G]CGTG-3′ consensus sequence and highly variable flanking sequences in the promoters of HIF-responsive genes (Wenger et al, 2005). FIH-mediated asparagine-803 hydroxylation is also reduced when oxygen is depleted, thereby allowing HIFα to interact with the transcriptional coactivators p300/Creb-binding protein (CBP; Arany et al, 1996). This transcriptional complex transactivates a specific subset of genes that regulate the cellular adaptive response to hypoxia, including, but not limited to, SLC2A1 (glycolysis), VEGFA (angiogenesis), and EPO (erythropoiesis).
Expanding the canonical HIF pathway
SIRT3 is a novel HIF1α antagonist
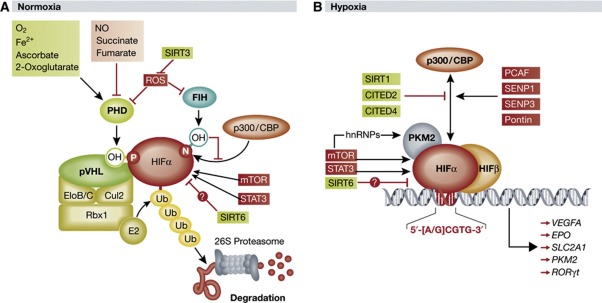
Regulation of HIFα stability via ROS. Oxygen tension and the functional status of pVHL are just two of many factors governing HIFα stability. HIFα protein levels are in part a function of HIFα mRNA stability, which can be negatively regulated by miRNAs (Bruning et al, 2011; Taguchi et al, 2008) and mRNA-destabilizing proteins (Chamboredon et al, 2011). Post-translational modifications (PTMs) of HIFα, such as small ubiquitin-like modifier (SUMO)ylation (Carbia-Nagashima et al, 2007; Cheng et al, 2007) and acetylation (Xenaki et al, 2008; Dioum et al, 2009; Lim et al, 2010), have been reported to affect HIFα stability in a proteasome-dependent manner. PHDs negatively regulate HIFα at the protein level via oxygen-dependent prolyl hydroxylation as described above, but the catalytic activity of PHDs is in turn governed by a variety of inhibitors and/or cofactors in the cellular environment (Figure 1A).
Figure 1.
Expanded model of canonical HIFα regulation. (A) Under normal oxygen tension, HIFα is subject to oxygen-dependent prolyl hydroxylation by PHDs, which allows for substrate recognition and ubiquitylation by pVHL and its associated ubiquitin–ligase complex. Polyubiquitylated HIFα is degraded by the 26S proteasome. The prolyl-hydroxylase activity of PHDs is regulated by a number of intracellular factors, including ROS, which are in turn negatively modulated by SIRT3. Binding of the HIFα coactivator p300/CBP is inhibited by asparaginyl hydroxylation by FIH. HIFα is upregulated at the mRNA level by mTOR and STAT3, while SIRT6 negatively regulates HIFα protein levels. (B) Under low oxygen tension HIFα escapes prolyl hydroxylation by PHDs and associates with nuclear HIFβ. The heterodimer binds to a core consensus sequence at the promoters of HIF-responsive genes, and upon binding to the coactivators p300/CBP and PKM2, initiates transcription. The interaction between HIFα and p300 may be regulated by a variety of factors that sterically impede binding or add/remove PTMs to influence the transcriptional activity of HIFα. See text for details (PHD, prolyl-hydroxylase domain-containing enzyme; NO, nitric oxide; SIRT1/3/6, sirtuin 1/3/6; FIH, factor inhibiting HIF; CBP, Creb-binding protein; OH, hydroxyl group; mTOR, mammalian target of rapamycin; STAT3, signal transducer and activator of transcription 3; ub, ubiquitin moiety; EloB/C, elongins B and C; Cul2, cullin 2; Rbx 1, RING-box protein 1; pVHL, von Hippel-Lindau protein; ROS, reactive oxygen species; HIF, hypoxia-inducible factor; CITED2/4, CBP/p300 interacting transactivator with ED-rich tail 2/4; PCAF, p300/CBP-associated factor; SENP1/3, sentrin-specific protease 1/3; PKM2, pyruvate kinase isoform M2; hnRNPs, heterogeneous nuclear ribonucleoproteins).
In addition to oxygen, PHDs require Fe2+, 2-oxoglutarate, and ascorbate for prolyl-hydroxylase activity (Schofield and Ratcliffe, 2004). In contrast, the enzymatic function of PHDs has been reported to be inhibited by nitric oxide, several metabolic intermediates of the tricarboxylic acid (TCA) cycle such as succinate and fumarate, and reactive oxygen species (ROS; Kaelin and Ratcliffe, 2008). The inverse relationship between prolyl-hydroxylated HIFα and intracellular ROS had been reported by independent groups (Brunelle et al, 2005; Mansfield et al, 2005) prior to the demonstration that peroxide-derived ROS directly inhibited PHD catalytic activity, presumably by oxidizing PHD-bound Fe2+ (Pan et al, 2007). However, the relationship between ROS production and HIFα activity has become increasingly complex as FIH was recently demonstrated to have significantly greater sensitivity to oxidative stress than PHDs (Masson et al, 2012), and respiring mitochondria have been reported to produce significantly lower ROS during hypoxia than under normoxia (Hoffman et al, 2007). Thus, the specific role and relative significance of ROS in mediating the hypoxic response remain unclear.
SIRT3 destabilizes HIF1α in a PHD-dependent manner. The mitochondrial deacetylase sirtuin-3 (SIRT3) was reported to have a tumour suppressor function when SIRT3−/− cells were observed as having aberrant intracellular metabolism, decreased mitochondrial integrity, and a tumourigenic phenotype relative to wild-type cells (Kim et al, 2010). However, the specific signalling axis through which these phenomena were occurring remained unclear. Finley et al (2011a) recently reported that SIRT3 destabilizes HIF1α by inhibiting ROS production, thereby promoting maximal prolyl-hydroxylase activity of PHDs. The net effect of SIRT3-mediated HIFα destabilization was a shift away from glycolytic metabolism and unchecked proliferation. Specifically, SIRT3-knockout mouse embryonic fibroblasts (MEFs) demonstrated increased glucose uptake, lactate production, and glucose-dependent proliferation rates relative to wild-type cells. Gene set enrichment analysis revealed a hypoxic signature in SIRT3-knockout MEFs, prompting an investigation into the role of SIRT3 in HIFα-mediated transactivation of proglycolytic genes. Stable knockdown or knockout of SIRT3 resulted in an intracellular increase in ROS production, which appeared to have a stabilizing effect on HIF1α protein levels under normoxia. This effect was shown to be due at least in part to a decrease in PHD hydroxylase activity, although direct inhibition of PHDs by ROS in this context was not clearly demonstrated. Importantly, stable knockdown of HIF1α in both SIRT3−/− and wild-type MEFs diminished the metabolic differences previously observed between them. Finally, SIRT3 deletions were found to occur in 20% of all human cancers and 40% of all breast and ovarian cancers. Together, these findings confer a specific tumour suppressor function of SIRT3 that counteracts the switch to anaerobic metabolism under normoxia, referred to as the Warburg effect, promoted by ROS generation and HIF1α stabilization.
An independent study supports the conclusion that loss of SIRT3 stabilizes HIF1α, perhaps through ROS production, and also demonstrates that expression of some non-metabolic HIF1α-target genes, such as VEGFA, increases with knockdown of SIRT3 (Bell et al, 2011). This suggests that SIRT3 may oppose additional HIF1α-driven cancer phenotypes beyond the Warburg effect, such as angiogenesis, apoptotic resistance or cellular migration/invasion.
SIRT3 suppresses ROS production. SIRT3 is a predominant mitochondrial deacetylase and is responsible for regulating the activities of a significant subset of mitochondrial proteins, many of which are strongly implicated in cellular metabolism (Lombard et al, 2007). SIRT3 likely suppresses ROS production through several proposed mechanisms, including but not necessarily limited to deacetylation and activation of the antioxidant enzyme superoxide dismutase (SoD; Qiu et al, 2010; Tao et al, 2010), modulation of certain electron transport chain (ETC) components (Ahn et al, 2008; Kim et al, 2010), and activation of several TCA cycle enzymes (Bell and Guarente, 2011). Specifically, deacetylation and activation of isocitrate dehydrogenase 2 (IDH2) by SIRT3 was reported to promote the production of NADPH, which is requisite for proper function of mitrochodrial ROS detoxifying enzymes (Someya and Yu, 2010). In addition, SIRT3 was shown to deacetylate and activate succinate dehydrogenase (SDH), a component of the ETC, which could potentially affect ROS production (Cimen et al, 2010; Finley et al, 2011b). SDH also catalyses the oxidation of succinate to fumarate in the TCA cycle; thus, SIRT3 may negatively regulate intracellular succinate levels (Finley et al, 2011b). Succinate, like ROS, has been reported to inhibit PHDs (Koivunen et al, 2007), which suggests that intracellular accumulation of succinate in SIRT3−/− MEFs contributes to the stabilization of HIF1α via PHD inhibition (Figure 1A) as observed by Finley et al (2011a), although this possibility has yet to be directly addressed.
Sirtuins and the HIF pathway. SIRT3 is not the first sirtuin to be implicated in HIFα regulation (Figure 1B). Sirtuins are NAD+-dependent protein deacetylases and/or ADP-ribosyltransferases known to modulate longevity and metabolism (Imai et al, 2000; Tissenbaum and Guarente, 2001). SIRT1 has been reported to deacetylate both HIF1α (Lim et al, 2010) and HIF2α (Dioum et al, 2009), causing a decrease and, unexpectedly, an increase in transcriptional activity, respectively. SIRT1, in turn, was shown to be upregulated by both HIF1α and HIF2α under hypoxia, which suggests that SIRT1 may play a role in negative- and positive-feedback loops that govern HIFα activity (Chen et al, 2011). In contrast, SIRT6 may negatively regulate HIF1α stability and protein synthesis via yet-defined mechanism, and may also serve as a HIF1α corepressor to impede the induction of HIF1α-responsive genes (Zhong et al, 2010). Thus, SIRT3 is currently the only sirtuin family member that has been demonstrated to destabilize HIF1α through a PHD-dependent mechanism.
PKM2 is a novel HIF1α coactivator
HIFα-mediated transactivation of target genes is modulated by the relative abundance of transcriptional coactivators and/or corepressors, as well as PTMs that affect the physical accessibility of, or affinity between, HIFα and its cofactors (Figure 1B). For example, de-SUMOylation of p300 by sentrin-specific protease (SENP)3 under conditions of oxidative stress enhances binding between p300 and HIF1α, thereby increasing HIF1α transcriptional activity (Huang et al, 2009). Acetylation of lysine-674 on HIF1α by p300/CBP-associated factor (PCAF) also enhances binding between p300 and HIF1α, while deacetylation of the same residue by SIRT1 impedes p300 recruitment and transactivation of HIF1α-target genes (Xenaki et al, 2008; Lim et al, 2010). CBP/p300 interacting transactivator with ED-rich tail (CITED)2 and CITED4 have been shown to bind p300/CBP at the same domain required for binding to HIFα, effectively blocking the HIF1α/p300 interaction and diminishing HIF1α activity (Bhattacharya et al, 1999; Fox et al, 2004). Recently, the chromatin remodelling factor Pontin was demonstrated to be a HIF1α coactivator by mediating the binding between p300 and HIF1α under hypoxia (Lee et al, 2011). Together, these reports demonstrate that the transcriptional activity of HIFα can be efficiently regulated by modulating recruitment of p300 and other cofactors to the HIFα transcriptional complex.
PKM2 enhances HIF1α transcriptional activity. Recently, Luo et al (2011) identified a novel role for the M2 isoform of pyruvate kinase (PKM2), the enzyme that catalyses the final step in glycolysis, as a coactivator of HIF1α and HIF2α (Figure 1B). PKM2 expression was induced by HIF1α, but not HIF2α, suggesting PKM2 and HIF1α participate in a positive-feedback loop that increases their respective activities under hypoxia. PKM2 was demonstrated to associate directly with HIF1α and HIF2α, enhance binding of HIF1α and HIF2α to HREs, and promote HIF1α- and HIF2α-mediated transactivation of hypoxia-inducible genes without affecting total HIFα levels. This effect was purportedly enhanced by prolyl hydroxylation of PKM2 by PHD3, and PHD3 was shown to coprecipitate with exogenous HIF1α/PKM2 complex. While the notion that PHD3, a negative regulator of HIFα protein levels, may be implicated in the positive regulation of HIFα activity is certainly interesting, further study is necessary to more clearly define the function of PHD3 in PKM2-mediated coactivation of HIF1α. Importantly, PKM2 also appeared to bind p300 (although perhaps indirectly), and stable knockdown of PKM2 reduced p300 occupancy of HREs, suggesting PKM2 enhances recruitment of p300 to the HIFα transcriptional complex at the promoters of HIFα-responsive genes. These results indicate that PKM2 promotes a shift toward anaerobic metabolism by augmenting HIFα-mediated transactivation of proglycolytic genes such as LDHA and SLC2A1.
An independent report corroborated the finding that HIFα upregulates PKM2, and that this appears to promote anaerobic metabolism in tumour cells (Sun et al, 2011). It was also suggested that mammalian target of rapamycin (mTOR) upregulates PKM2 via two distinct mechanisms: mTOR-mediated upregulation of HIFα may lead to transactivation of PKM2, while concomitantly, mTOR-mediated upregulation of c-myc may lead to c-myc-mediated upregulation of heterogeneous nuclear ribonucleoproteins (hnRNPs), which are reported to favour splicing of PKM2 mRNA over PKM1 mRNA (David et al, 2010; Sun et al, 2011). Thus, in addition to upregulating HIFα, mTOR may modulate HIFα signalling by upregulating the HIFα coactivator PKM2 (Figure 1B).
Role of PKM2 in the Warburg effect. PKM2 has been previously implicated in the Warburg effect observed in cancer cells (Christofk et al, 2008), although its specific role in promoting aerobic glycolysis has remained unclear. Recent work suggests, perhaps counter-intuitively, that PKM2 promotes glycolysis in tumour cells in a manner that is independent of its biochemical function as a glycolytic enzyme. Phosphorylation of PKM2 at tyrosine-105 (Y105) inhibits its glycolytic activity, and PKM2 was found to be phosphorylated at Y105 in several cancer cell lines (Hitosugi et al, 2009). Furthermore, cells expressing a PKM2 mutant defective in Y105 phosphorylation—that is, a PKM2 mutant possessing greater glycolytic activity than wild-type PKM2—exhibited increased oxidative phosphorylation, decreased lactate production, and slowed tumour growth relative to cells expressing wild-type PKM2 (Hitosugi et al, 2009). These data imply that the enzymatic function of PKM2 actually opposes the Warburg effect. Indeed, Luo et al (2011) demonstrate that a catalytically inactive form of PKM2 promotes anaerobic metabolism via HIFα transactivation as efficiently as wild-type PKM2. Together, these studies conjure a potential model of metabolic reprogramming in tumour cells whereby PKM2 undergoes a functional shift from glycolytic enzyme to HIFα coactivator.
PKM2 as a transcriptional coactivator. PKM2 has also been reported to act as a transcriptional coactivator for β-catenin (Yang et al, 2011). Specifically, activation of epidermal growth factor receptor (EGFR) was shown to trigger nuclear translocation of PKM2, where it appeared to bind phosphorylated β-catenin and subsequently potentiate β-catenin-mediated transactivation of cyclin D1 (Yang et al, 2011). Thus, while PKM2 purportedly promotes glycolysis as a HIFα coactivator (Luo et al, 2011), it may also promote cellular proliferation and cell cycle progression via β-catenin-mediated upregulation of cyclin D1 (Yang et al, 2011). Therefore, the tumour phenotype to which PKM2 contributes may depend upon the specific transcription factor being activated and its particular subset of target genes. Given the findings reported by Yang et al, (2011) and considering that PKM2 must be cytoplasmic to participate in glycolysis, it would seem likely that a nuclear translocation of PKM2 is necessary and precedes coactivation of HIFα. Future work on the role of PKM2 in HIFα-mediated transcription might address this possibility.
Expanding roles of HIF in cell biology
The role of HIFα as a transcription factor in the context of oxygen sensing and tumourigenesis has been extensively characterized. While the aforementioned reports serve to extend our current understanding of HIFα regulation in cell signalling, emerging evidence has implicated a larger role for HIFα in cell biology.
Role of HIF in embryonic development
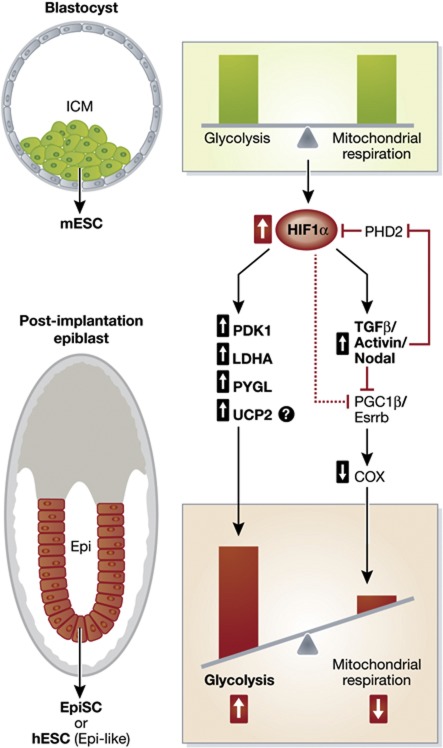
HIF1α drives a metabolic shift to glycolysis during ESC–EpiSC transition. Analogous to the Warburg effect observed in cancer cells, Zhou et al (2012) have recently shown that pluripotent embryonic stem cells (ESCs) also undergo a metabolic shift towards glycolysis during development and this process is regulated by HIF1α. ESCs and epiblast stem cells (EpiSCs) represent two distinct pluripotency stages in embryonic development with unique gene expression profiles and cell morphologies, but a common ability to self-renew and differentiate into diverse cell types (Tesar et al, 2007). Mouse (m)ESCs isolated from the inner cell mass (ICM) of pre-implantation blastocysts represent a naive state, while EpiSCs isolated from the post-implantation epiblast represent a more developmentally advanced or ‘primed’ state (Brook and Gardner, 1997; Evans and Kaufman, 1981; Tesar et al, 2007; Nichols and Smith, 2009). Notably, unlike mESCs, human (h)ESCs, despite their pre-implantation origin, are not considered a naive state and are similar to EpiSCs (Tesar et al, 2007). Recent work by Zhou et al (2012) revealed that EpiSCs and hESCs have lower mitochondrial respiration and higher levels of glycolysis in comparison to mESCs, which switch between both oxidative phosphorylation and glycolysis (Figure 2). Strikingly, inhibition of glycolysis with the glucose analogue 2-deoxy-glucose (2-DG) led to cell death in EpiSCs and hESCs, exemplifying their dependence on this metabolic pathway for survival (Zhou et al, 2012). Similarly, haematopoietic stem cells (HSCs) have been recently shown to utilize glycolysis instead of mitochondrial respiration, suggesting that HIFα may regulate the metabolism of multiple stem cell populations (Simsek et al, 2010).
Figure 2.
HIF1α regulates the metabolic and phenotypic transition from ESC to EpiSC. mESC isolated from the inner cell mass (ICM) of the blastocyst are metabolically and phenotypically distinct from post-implantation EpiSC isolated from the epiblast (Epi) or hESC (Epi-like). HIF1α expression regulates the metabolic and phenotypic transition from ESC to EpiSC. Increased HIF1α expression in EpiSC/hESC promotes a highly glycolytic metabolism via increased expression of glycolytic genes such as PDK1, LDHA, and PYGL and downregulation of mitochondrial COX gene expression via TGFβ/Activin/Nodal signalling.
HIF1α has previously been shown to promote a metabolic switch to glycolysis in response to chronic hypoxia through the transcriptional regulation of key metabolic enzymes such as pyruvate dehydrogenase kinase-1 (PDK1) and lactate dehydrogenase A (LDHA) (Kim et al, 2006; Seagroves et al, 2001). PDK1 phosphorylates and inactivates pyruvate dehydrogenase (PDH), which inhibits the conversion of pyruvate to acetyl coenzyme A (AcCoA) for entry into the citric acid cycle (Semenza, 1996). In addition, HIF1α-mediated upregulation of LDHA increases the conversion of pyruvate to lactate to promote glycolysis (Semenza et al, 1996). Consistent with the reported role of HIF1α in regulating metabolism, Zhou et al (2012) found that HIF1α expression and several HIF-regulated glycolytic genes such as PDK1, LDHA, and PYGL were significantly higher in EpiSCs compared with mESC (Zhou et al, 2012). Furthermore, despite their more developed mitochondria and increased mitochondrial content, EpiSC had lower mitochondrial respiration that was attributed to downregulated expression of mitochondrial cytochrome c oxidase (COX) genes and their regulators peroxisome proliferator-activated receptor-γ coactivitor-1β (PGC-1β) and oestrogen receptor-related receptor-β (Esrrb) (Zhou et al, 2012). HIF1α has previously been shown to downregulate PGC-1β through inhibition of c-Myc transcriptional activity (Zhang et al, 2007), suggesting that HIF1α may actively suppress mitochondrial respiration while promoting glycolysis through the regulation of key metabolic genes (Figure 2). In support of this notion, Zhou et al (2012) demonstrated that overexpression of HIF1α was sufficient to suppress oxidative phosphorylation, enhance glycolysis, and promote the transition from ESC to EpiSC.
Similarly, independent studies have shown that in comparison to differentiated cells, undifferentiated mESC have decreased mitochondrial respiration concomitant with increased glycolytic metabolism, which is necessary to maintain pluripotency (Mandal et al, 2011; Zhang et al, 2011). While Zhang et al suggest that expression of the inner mitochondrial protein, uncoupling protein 2 (UCP2), is responsible for the glycolytic shift in undifferentiated hESC, several reports have demonstrated a positive correlation between HIFα and UCP2 expression (Zhang et al, 2011; Deng et al, 2012). In addition, putative HIF binding sites have been identified in the UCP2 promoter (Oberkofler et al, 2005), suggesting that HIFα may control the expression of UCP2 and several other glycolytic and mitochondrial proteins to promote a universal metabolic shift.
Significance of glycolysis in EpiSC/hESC. Several theories have been proposed to explain the preference for glycolysis in EpiSC/hESC. Although glycolysis is energetically unfavourable in comparison with oxidative phosphorylation (yielding a mere 2 ATP in comparison with 36 ATP per glucose), this metabolic shift helps to promote cell proliferation through increased production of metabolic intermediates for amino acid, protein, and membrane synthesis. This may allow the developing blastocyst to acquire the necessary building blocks for rapid proliferation and differentiation. Second, oxidative phosphorylation is associated with the production of ROS, a known DNA mutagen. Thus, a shift to glycolytic metabolism may be a protective mechanism for the cell to maintain genomic integrity within pluripotent cells. Third, glycolysis promotes extracellular acidification through increased production of lactic acid, which may aid in invasion and implantation of the blastocyst into the uterine wall.
HIF maintains pluripotency in ESC through TGFβ/Activin/Nodal signalling. Analogous to undifferentiated HSCs, which have recently been shown to regulate quiescence and long-term maintenance through the regulation of HIFα expression levels, ESC may maintain pluripotency through a HIF-dependent mechanism (Takubo et al, 2010). TGFβ/Activin/Nodal signalling has previously been shown to drive the ESC to EpiSC transition and maintain pluripotency in EpiSC and hESC by preventing spontaneous differentiation into neuroendoderm (Vallier et al, 2004; James et al, 2005; Camus et al, 2006). Activin B is a direct transcriptional target of HIFα, and hypoxia has recently been shown to promote an ESC to EpiSc transition, suggesting a role for HIFα in not only the metabolic changes, but also the phenotypic changes that occur during ESC development (Wacker et al, 2009; Takehara et al, 2011). In support of these previous findings, Zhou et al (2012) found that inhibition of Activin signalling was sufficient to block the HIF-induced transition from ESC to EpiSC. Furthermore, Activin signalling has been shown to decrease PHD2 and stabilize HIF1α, suggesting that a positive-feedback loop may exist to further amplify signalling (Wiley et al, 2010; Figure 2). In support of this notion, Zhou et al (2012) found that activation of Activin signalling increased both HIF1α expression and glycolytic genes, while downregulating mitochondrial genes. Interestingly, PGC-1β has been shown to be directly downregulated by Activin/Nodal signalling, providing additional evidence to support a role for Activin/Nodal signalling in the active suppression of mitochondrial metabolism (Li et al, 2009). These results infer a novel mechanism by which HIF regulates Activin/Nodal signalling to alter the expression of key metabolic genes, promoting a highly glycolytic metabolism to promote the ESC to EpiSC transition (Figure 2).
Regulation of HIF during early embryonic development. Both the pre-implantation and post-implantation blastocysts exist in the hypoxic environment of the uterus (Fischer and Bavister, 1993). However, Zhou et al (2012) observed considerable differences in the expression level of HIF between these two pluripotent states. Despite the ability of the post-implantation blastocyst to access the vasculature of the surrounding tissue, the EpiSC had higher HIF1α expression. This apparent contradiction warrants further research into the mechanisms underlying HIF expression in pre- and post-implantation ESCs. Does the post-implantation blastocyst experience lower oxygen tension in comparison to the pre-implantation ESC or are there other unique or non-canonical mechanisms of HIF regulation at play during early embryonic development? Earlier work by Fischer and Bavister (1993) demonstrated that hESC utilize glycolysis for metabolism even when removed from the hypoxic environment of the blastocyst. These results indicate that factors other than oxygen tension may regulate HIFα levels (discussed previously) to alter glucose metabolism and promote ESC to EpiSC transition.
The metabolism of HSCs was recently shown to be controlled by the haematopoietic transcription factor, Meis1, which directly activates HIF1α expression (Simsek et al, 2010). These findings suggest that the high expression of HIF1α and shift to glycolytic metabolism is not just a result of the hypoxic environment of the bone marrow, but is tightly regulated by haematopoietic stem-cell–associated transcription factors. Whether or not unique ESC-associated transcription factors regulate HIFα in ESC remains to be determined.
Role of HIF in immunity
Inflamed tissues are often hypoxic as a result of decreased perfusion, oedema, vascular insult and/or influx of oxygen-consuming immune cells or pathogens (Nizet and Johnson, 2009). Thus, the role of HIFα in host immune responses and inflammation has become an important research focus. HIFα expression is stabilized through a variety of mechanisms in a range of immune cell types (Nizet and Johnson, 2009). For instance, recognition of pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS) by toll-like receptors (TLRs) in human monocytes was reported to activate the mitogen-activated protein kinase (MAPK) pathway and ultimately NF-κB, thereby transactivating HIF1A and producing an abundance of HIF1α mRNA and protein under normal oxygen tension (Frede et al, 2006). In addition to canonical stabilization of HIFα protein levels, hypoxia was demonstrated to prohibit PHD-mediated inhibition of IκB kinase-β (IKK-β) (Cummins et al, 2006), thereby leading to activation of NF-κB and subsequent upregulation of HIF1A as demonstrated in murine macrophages (Rius et al, 2008). The mechanism of HIFα stabilization in T cells appears to be analogous to that in myeloid cells, as engagement of T cell receptors (TCRs) by foreign antigens upregulates HIF1α via phosphoinositol-3-kinase (PI3K)/mTOR signalling pathway (Nakamura et al, 2005). Together, these reports demonstrate that stabilization of HIFα in the context of inflammation and immunity can occur through multiple signalling cascades in both normoxic and hypoxic environments.
Following stabilization, HIF1α plays an important role in executing the immune response. Metabolic reprogramming in myeloid cells by HIF1α was shown to increase the production of glycolysis-derived ATP, a critical energy source for macrophage aggregation, invasion, and motility (Cramer et al, 2003). In addition, HIF1α-mediated upregulation of β-integrin was reported to enhance neutrophil adhesion to activated endothelium (Kong et al, 2004). Neutrophil apoptosis was shown to be significantly attenuated in hypoxia by HIF1α (Walmsley et al, 2005) and more recently, PHD3 (Walmsley et al, 2011), although whether this latter effect occurs in a HIF1α-dependent or -independent manner remains unclear. HIFα also contributes to the production of a number of proinflammatory cytokines such as TNFα, interleukin (IL)-1α, IL-1β, IL-6, and IL-12, which are necessary for macrophage activation and phagocytic function, as well as lymphocyte proliferation (Peyssonnaux et al, 2005, 2007; Jantsch et al, 2008; Nizet and Johnson, 2009). In some cases, such as TNFα, HIFα-mediated upregulation occurs as a result of direct transactivation. While the aforementioned studies might suggest that HIFα acts predominantly as a proinflammatory factor, it is worth noting that HIF1α has been demonstrated to have a robust anti-inflammatory effect in hypoxic gastrointestinal mucosa and epithelium as a result of transactivation of specific genes that have a ‘protective-barrier’ effect, such as mucins (Furuta et al, 2001; Comerford et al, 2002; Louis et al, 2006). This effect has also been successfully demonstrated in relevant animal models (Karhausen et al, 2004; Cummins et al, 2008; Robinson et al, 2008). Together, these roles indicate that HIFα depends upon its transcriptional activity in directing the immune response in a wide range of cell types.
HIF1α promotes differentiation of TH17 cells by several distinct mechanisms. Interestingly, HIFα also appears to regulate the differentiation of multiple leukocyte lineages. Hif1a−/− mice exhibit impaired B cell maturation, and HIF1α has been shown to modulate this process by initiating glycolysis at specific stages of B cell differentiation (Kojima et al, 2002, 2010). Similarly, mTOR-mediated upregulation of HIF1α was reported to induce differentiation of naive T cells to proinflammatory TH17 cells, as opposed to anti-inflammatory Treg cells, by promoting glycolysis (Shi et al, 2011). These reports further support the notion that HIF1α contributes to B and T cell differentiation through its role as a transcription factor.
A recent report from Dang et al (2011) demonstrates that the effect of HIF1α on T cell differentiation extends beyond its transcriptional activity. In TH17-skewing conditions (IL-6 with low levels of TGF-β), signal transducer and activator of transcription 3 (STAT3) was shown to transactivate HIF1A. STAT3 was previously reported to positively influence TH17 cell differentiation, presumably by upregulating the key TH17 transcriptional regulator RORγt (Harris et al, 2007), but HIF1α had not been previously implicated in this mechanism. Dang et al (2011) observed decreased expression of TH17 signature genes such as IL-17 in murine Hif1a−/− T cells relative to wild-type T cells, even after culturing in TH17-skewing conditions. In addition, the proportion of Hif1a−/− T cells that stained positive for Foxp3, the predominant transcriptional regulator of Treg cells, was significantly greater than in wild-type T cells. Furthermore, incubation of TH17-skewed wild-type T cells in hypoxia increased the IL-17+ population, but hypoxic treatment had no effect on IL-17 expression in TH17-skewed Hif1a−/− T cells. These results imply that HIF1α supports TH17 cell differentiation by promoting expression of TH17 signature genes.
Dang et al (2011) elucidated the precise mechanism by which HIF1α upregulates IL-17 by demonstrating direct transactivation of RORγt by HIF1α. RORγt then directly induces expression of IL-17A and IL-17R in response to TGF-β and IL-6, thereby promoting differentiation of naive CD4+ T cells to TH17 cells (Ivanov et al, 2006). Thus, these results place HIF1α at a central point in the IL-6/TGF-β–STAT3–RORγt signalling cascade that drives TH17 cell differentiation.
Upregulation of RORγt by HIF1α is dependent upon the transcriptional activity of HIF1α (Figure 3A), a mechanism that is analogous to the aforementioned modes of HIF1α-mediated inflammation and differentiation of lymphocyte lineages. However, one key observation—that RORγt overexpression in Hif1a−/− T cells did not significantly increase the IL-17+ population—suggested that HIF1α might have additional roles in TH17 cell differentiation downstream of RORγt transactivation. Intriguingly, HIF1α and RORγt were shown to directly interact with one another, and induction of IL-17 by HIF1α did not require a functional DNA-binding domain (Dang et al, 2011). In fact, the binding site for RORγt was mapped to amino acids 1–80 on the N terminus of HIF1α, a site that is normally required for HIFβ dimerization and binding to HREs (Jiang et al, 1996; Dang et al, 2011). Dang et al (2011) propose a model (Figure 3B) in which HIF1α transactivates the RORγt gene prior to physically associating with its protein product at the promoters of TH17 signature genes. The purpose of HIF1α in this transcriptional complex appears to be recruitment of p300 through its CTAD, which improves the accessibility of RORγt target gene promoters through p300-mediated acetylation of histones H3 and H4 (Dang et al, 2011).
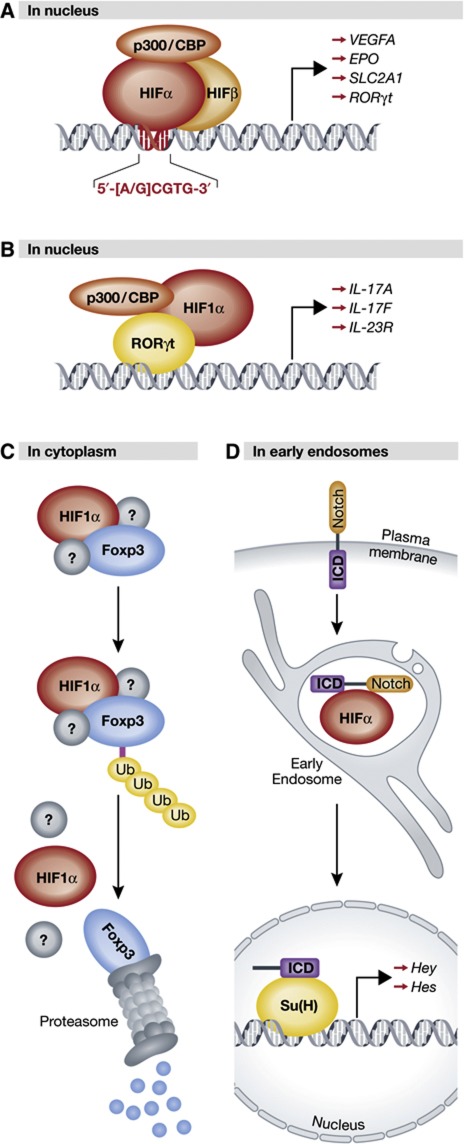
Figure 3.
Canonical and non-canonical regulation by HIFα. (A) Classical DNA-binding transcription factor: HIFα associates with HIFβ and transcriptional coactivators in the nucleus, binds conserved hypoxia-response elements in the DNA sequence of target promoters, and initiates transcription of HIF-target genes. (B) Transcriptional coactivator: HIF1α binds RORδt, but not DNA, at the promoters of RORδt-responsive genes in the nucleus. Recruitment of p300 by HIF1α leads to deacetylation of histones H3 and H4 thereby facilitating transcription. (C) Director for proteasome-mediated degradation: HIF1α binds to Foxp3, presumably in the cytoplasm, and in association with an unidentified degradation complex mediates its ubiquitylation and subsequent proteasomal degradation. (D) Stabilizing force for endocytosed receptors: HIFα (Sima) colocalizes with and stabilizes full-length Notch that has been internalized from the plasma membrane in early endosomes. See text for details (HIF, hypoxia-inducible factor; CBP, Creb-binding protein; IL, interleukin; ub, ubiquitin moiety; ICD, intracellular domain; Su(H), suppressor of Hairless).
Finally, it is proposed that HIF1α actively suppresses Treg differentiation by targeting Foxp3 for ubiquitin-mediated degradation (Figure 3C). While the identification of a possible degradation complex remains to be elucidated, downregulation of Foxp3 by HIF1α appears to be a post-translational, proteasome-dependent phenomenon requiring direct interaction between Foxp3 and the N terminus of HIF1α. Expression of hydroxylation-defective HIF1α mutants, as well as siRNA-mediated knockdown of PHD2, abrogated Foxp3 degradation (Dang et al, 2011), suggesting prolyl hydroxlation of HIF1α necessarily precedes Foxp3 degradation. However, ubiquitylation and degradation of Foxp3 was also shown to significantly increase under hypoxia, a state in which HIF1α levels increase but prolyl hydroxylation of HIF1α by PHD2 is impeded. Therefore, further studies are required to clarify the role of prolyl-hydroxylated HIF1α in Foxp3 degradation.
This work is particularly interesting in that it demonstrates HIF1α promoting TH17 cell differentiation via three unique and functionally distinct roles: a DNA-binding transcription factor, a transcriptional coactivator for RORγt, and a direct mediator of Foxp3 degradation (Figure 3). Notably, all three roles require the same region of HIF1α: the HLH domain, which is necessary for HIF heterodimerization and DNA binding (Jiang et al, 1996), and was shown in this report to bind RORγt and Foxp3. Therefore, it is formally possible that these three functions are mutually exclusive. To better understand the dynamic shifts in HIF1α function in T cell differentiation, it may be informative to elucidate the relative binding affinities of HIFβ, RORγt, and Foxp3 to the HLH domain of HIF1α.
This report also implicates HIF1α in the T cell–mediated inflammatory response. Hif1a−/− T cells failed to develop experimental autoimmune encephalitis in response to myelin oligodendrocyte glycoprotein peptide, whereas wild-type T cells exhibited a robust inflammatory reaction. This observation provides a rationale for investigating the use and efficacy of HIFα inhibitors in the treatment of T cell mediated autoimmune disorders.
Together, these findings outline a positive-feedback mechanism by which TGF-β and IL-6 upregulate HIF1A in a STAT3-dependent manner, leading to transactivation of RORγt and subsequently HIF1α/RORγt/p300-mediated transactivation of TH17 signature genes. Upregulation of IL-17, in addition to HIF1α-mediated degradation of Foxp3, appears to favour TH17 cell differentiation and impede the development of Treg cells, thereby enhancing the inflammatory response. Ultimately, an increase in local inflammation brings about a decrease in oxygen tension, and the resulting stabilization of HIF1α would presumably act to amplify the entire cascade.
Role of HIF in Notch-dependent developmental pathways
HIFα modulates human leukocyte differentiation via canonical and non-canonical pathways, as described above. However, HIFα can induce or suppress the differentiation of a multitude of cell types, including but not limited to adipocytes, medulloblastoma precursor cells, neuroblastoma cells, glioblastoma stem cells, and neural crest progenitors (Jogi et al, 2002; Yun et al, 2002; Sainson and Harris, 2006; Pistollato et al, 2010; Qiang et al, 2012). A number of these reports have implicated Notch, a well-established mediator of cellular differentiation processes, as an important effector.
The Notch receptor is activated upon engagement by a membrane-bound ligand, such as Serrate or Delta, on an adjacent cell (Rebay et al, 1991). Ligand binding triggers proteolytic cleavage of Notch, which is mediated in part by γ-secretase (De Strooper et al, 1999). The cleaved intracellular domain (ICD) of Notch translocates to the nucleus, and upon binding to cofactors such as p300/CBP, Suppressor of Hairless (Su(H)), and/or mastermind (MAML1), transactivates Notch-responsive genes such as HEY and HES (Fortini, 2009).
Collaboration between HIFα and Notch occurs at the intersection between the oxygen-sensing and developmental pathways. Notch has been reported to potentiate HIFα-mediated transactivation of hypoxia-inducible genes in the context of both tumourigenesis and cellular differentiation (Sahlgren et al, 2008; Zheng et al, 2008). Conversely, HIFα has been demonstrated to enhance Notch signalling through several distinct mechanisms. HIF1α has been shown to stabilize Notch ICD in both normoxia and hypoxia in a manner that is independent of its transcriptional activity, although the precise mechanism by which this occurs remains unclear (Gustafsson et al, 2005). Additionally, HIF1α transactivation of anterior pharynx-defective 1A (APH-1A), a component of the γ-secretase complex, has been reported to lead to an increase in intracellular levels of Notch ICD (Wang et al, 2006). HIF1α interaction with Notch at the HEY2 and HES1 promoters has also been reported, and this association augments Notch-mediated transactivation under hypoxia in a manner that appears to depend upon a functional CTAD but does not require DNA binding by HIF1α (Gustafsson et al, 2005; Chen et al, 2010). Interestingly, Notch that is impaired in its ability to bind p300/CBP still retains the ability to transactivate target promoters under hypoxia, suggesting that the function of HIF1α in the Notch transcriptional complex is to recruit p300/CBP (Gustafsson et al, 2005). This is reminiscent of the aforementioned TH17 cell differentiation model, whereby HIF1α recruits p300 to the RORγt transcriptional complex, but does not bind the IL-17 promoter directly to induce transcription. In addition, FIH, another important component of the oxygen-sensing pathway, was reported to negatively regulate the transcriptional activity of Notch, although this effect appeared to be independent of FIH catalytic activity (Coleman et al, 2007; Zheng et al, 2008). Interestingly, FIH has a significantly higher affinity for Notch than for HIF1α (Wilkins et al, 2009); thus, sequestration of intracellular FIH by Notch may mediate transcriptional de-repression of HIF1α (Zheng et al, 2008), thereby delineating another mechanism by which Notch might augment the adaptive response to hypoxia.
Crystal cell development requires direct stabilization of endosomal Notch by HIFα. A recent report from Mukherjee et al (2011) proffers an entirely novel context for HIFα and Notch interaction, with each factor functioning in a starkly non-canonical manner (Figure 3D). Development and differentiation of crystal cells, a Drosophila blood cell subtype that is involved in wound healing, was shown to depend upon stabilization of Notch by HIFα within early endocytic vesicles. The Drosophila HIFα orthologue, Sima, promotes crystal cell expansion in the lymph gland, and was demonstrated to be crucial for the survival of committed crystal cell precursors. Similarly, Notch was shown to be required for initial commitment to the crystal cell lineage as well as expansion, maintenance and survival of committed crystal cell precursors. In addition, cleavage of the Notch ICD was vital for crystal cell survival, indicating an important role for Notch signalling in crystal cell development.
Sima overexpression stabilized Notch protein levels, a finding that is in keeping with previous observations regarding positive regulation of Notch signalling by HIFα. However, rather than observing an increase in nuclear levels of Notch ICD, Mukherjee et al (2011) found that the majority of intracellular Notch colocalized with Sima in early endocytic vesicles within crystal cells. Live endocytic trafficking assays performed on wild-type Drosophila lymph glands revealed that internalization of full-length Notch (Notchfl) from the plasma membrane preceded its rapid degradation in all non-crystal cell types. Intriguingly, activation of Notch in committed crystal cell precursors did not appear to require ligand engagement by either Serrate or Delta, suggesting that Sima-mediated activation of Notch was occurring in a non-canonical, ligand-independent manner. This mechanism seems sensible from an evolutionary standpoint, as Notch signalling in crystal cells appears to be vital for their differentiation and survival, but given that they circulate freely in the bloodstream it is not feasible for them to continuously engage the Notch ligands of neighbouring cells.
The function of Sima in crystal cells did not appear to include transactivation of hypoxia-responsive genes. Overexpression of the Drosophila HIFβ orthologue Tango failed to induce expression of a Sima/Tango-responsive reporter plasmid, and hypoxic treatment only augmented crystal cell expansion. It should be noted, however, that following Notch cleavage and nuclear translocation of ICD there is a possible transcriptional role for Sima in enhancing Notch-mediated transactivation of Notch-target genes through recruitment of, and association with, other cofactors in the Notch transcriptional complex as described previously (Gustafsson et al, 2005; Chen et al, 2010).
Another non-canonical aspect of Sima function in this report involves its unusually high degree of activity in normoxic conditions. Mukherjee et al (2011) demonstrate elevated synthesis of nitric oxide synthase-1 (NOS1) in crystal cells, which appeared necessary for Sima stabilization under normal oxygen tension. This unique feature of crystal cells likely explains why internalized Notch is rapidly degraded in other cell types—intracellular Sima levels may not be sufficiently high under normoxia to stabilize Notch in early endosomes.
Augmentation of Notch signalling by HIF1α was previously shown to require both hypoxic conditions and ligand (Serrate) engagement (Gustafsson et al, 2005). The report by Mukherjee et al (2011) suggests that the sole function of HIFα in crystal cells is augmentation of Notch signalling, and HIFα appears to accomplish this in large part by stabilizing Notch in a ligand-independent manner in a normoxic environment (Mukherjee et al, 2011). Indeed, the proposed role of HIFα in this context represents an entirely new mechanism by which it regulates differentiation and development. Future studies aimed at further characterizing this mechanism should attempt to do so in other species. Drosophila crystal cells are reportedly most similar to mammalian myeloid cells, thus it would be interesting to see if stabilization of Notch by HIFα occurs in the endosomes of myeloid precursors. Additional roles for HIFα in the stabilization of endosomal Notchfl are also worth investigating: Mukherjee et al (2011) report that overexpression of Rab5 suppresses Sima/Notch-mediated crystal cell expansion by increasing endocytic turnover of Notchfl. HIFα has been previously demonstrated to downregulate the Rab5 effector rapabtin-5 (Wang et al, 2009), thereby prolonging the endocytic pathway. However, it is unknown whether HIFα-mediated attenuation of early endosome fusion serves as a secondary mode of Notchfl stabilization.
One important question left in the wake of this study pertains to the apparent lack of HIFα transcriptional activity at HIF-target genes in crystal cells, particularly under hypoxia. Crystal cells are involved in wound healing and mediating the humoral immune response (Meister, 2004; Bidla et al, 2007), and are therefore likely to be presented with significant hypoxic challenge in necrotic or inflamed tissues. Given that HIFα is the key transcriptional regulator of the cellular adaptive response to hypoxia, how do crystal cells overcome the metabolic stresses that inevitably occur in low oxygen tension? One possibility is that they are not meant to. Crystal cells rupture in response to injury or inflammation and release significant amounts of prophenoloxidase, which is crucial for clotting and defence against pathogens (Meister, 2004; Tang et al, 2006; Bidla et al, 2007). Perhaps the lack of an adaptive response to hypoxic challenge is an evolutionarily-developed strategy to ensure vulnerable crystal cells rupture in inflammatory or necrotic conditions. If this is indeed the case, then the mechanism(s) responsible for attenuating the transactivation of HIFα-responsive genes in Drosophila crystal cells under hypoxia warrant future study.
Concluding remarks
Recent work regarding the oxygen-sensing pathway and its predominant effector, HIFα, has expanded our current understanding of HIFα regulation and transactivation, the role of HIFα and underlying genetic aberrations in health and disease, and the multifaceted nature of HIFα in a variety of physiological and pathophysiological contexts. Unexpected new players have entered into the ‘canonical’ model of HIFα regulation and transactivation, and have shown demonstrable influence on the hypoxic response by modulating PHD function or enhancing HIFα transcriptional activity. Furthermore, the traditional dogma regarding the function of HIFα as a hypoxia-inducible, DNA-binding transcription factor is challenged by new reports that demonstrate its capacity as a transcriptional coactivator in developmental and inflammatory contexts, a director for proteasome-mediated degradation, and a stabilizing force for internalized receptors within the endosomes. These notable studies not only provide additional research avenues, but also questions, the answers to which will undoubtedly enhance our appreciation for the increasing complexity and biological relevance of the HIF pathway in humans and other organisms.
Acknowledgments
This work was supported by funds from the Canadian Institutes of Health Research and the Canadian Cancer Society.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T (2008) A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA 105: 14447–14452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany Z, Huang LE, Eckner R, Bhattacharya S, Jiang C, Goldberg MA, Bunn HF, Livingston DM (1996) An essential role for p300/CBP in the cellular response to hypoxia. Proc Natl Acad Sci USA 93: 12969–12973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EL, Emerling BM, Ricoult SJ, Guarente L (2011) SirT3 suppresses hypoxia inducible factor 1alpha and tumor growth by inhibiting mitochondrial ROS production. Oncogene 30: 2986–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EL, Guarente L (2011) The SirT3 divining rod points to oxidative stress. Mol Cell 42: 561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Michels CL, Leung MK, Arany ZP, Kung AL, Livingston DM (1999) Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1. Genes Dev 13: 64–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidla G, Dushay MS, Theopold U (2007) Crystal cell rupture after injury in Drosophila requires the JNK pathway, small GTPases and the TNF homolog Eiger. J Cell Sci 120: 1209–1215 [DOI] [PubMed] [Google Scholar]
- Brizel DM, Dodge RK, Clough RW, Dewhirst MW (1999) Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol 53: 113–117 [DOI] [PubMed] [Google Scholar]
- Brook FA, Gardner RL (1997) The origin and efficient derivation of embryonic stem cells in the mouse. Proc Natl Acad Sci USA 94: 5709–5712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS (2005) Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab 1: 409–414 [DOI] [PubMed] [Google Scholar]
- Bruning U, Cerone L, Neufeld Z, Fitzpatrick SF, Cheong A, Scholz CC, Simpson DA, Leonard MO, Tambuwala MM, Cummins EP, Taylor CT (2011) MicroRNA-155 promotes resolution of hypoxia-inducible factor 1alpha activity during prolonged hypoxia. Mol Cell Biol 31: 4087–4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camus A, Perea-Gomez A, Moreau A, Collignon J (2006) Absence of Nodal signaling promotes precocious neural differentiation in the mouse embryo. Dev Biol 295: 743–755 [DOI] [PubMed] [Google Scholar]
- Carbia-Nagashima A, Gerez J, Perez-Castro C, Paez-Pereda M, Silberstein S, Stalla GK, Holsboer F, Arzt E (2007) RSUME, a small RWD-containing protein, enhances SUMO conjugation and stabilizes HIF-1alpha during hypoxia. Cell 131: 309–323 [DOI] [PubMed] [Google Scholar]
- Chamboredon S, Ciais D, Desroches-Castan A, Savi P, Bono F, Feige JJ, Cherradi N (2011) Hypoxia-inducible factor-1alpha mRNA: a new target for destabilization by tristetraprolin in endothelial cells. Mol Biol Cell 22: 3366–3378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Imanaka N, Griffin JD (2010) Hypoxia potentiates Notch signaling in breast cancer leading to decreased E-cadherin expression and increased cell migration and invasion. Br J Cancer 102: 351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Dioum EM, Hogg RT, Gerard RD, Garcia JA (2011) Hypoxia increases sirtuin 1 expression in a hypoxia-inducible factor-dependent manner. J Biol Chem 286: 13869–13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Kang X, Zhang S, Yeh ET (2007) SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell 131: 584–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC (2008) The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452: 230–233 [DOI] [PubMed] [Google Scholar]
- Cimen H, Han MJ, Yang Y, Tong Q, Koc H, Koc EC (2010) Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry 49: 304–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman ML, McDonough MA, Hewitson KS, Coles C, Mecinovic J, Edelmann M, Cook KM, Cockman ME, Lancaster DE, Kessler BM, Oldham NJ, Ratcliffe PJ, Schofield CJ (2007) Asparaginyl hydroxylation of the Notch ankyrin repeat domain by factor inhibiting hypoxia-inducible factor. J Biol Chem 282: 24027–24038 [DOI] [PubMed] [Google Scholar]
- Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP (2002) Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res 62: 3387–3394 [PubMed] [Google Scholar]
- Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS (2003) HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 112: 645–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, Taylor CT (2006) Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci USA 103: 18154–18159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, Fallon PG, Taylor CT (2008) The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 134: 156–165 [DOI] [PubMed] [Google Scholar]
- Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F (2011) Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell 146: 772–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David CJ, Chen M, Assanah M, Canoll P, Manley JL (2010) HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature 463: 364–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R (1999) A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature 398: 518–522 [DOI] [PubMed] [Google Scholar]
- Deng S, Yang Y, Han Y, Li X, Wang X, Zhang Z, Wang Y (2012) UCP2 inhibits ROS-mediated apoptosis in A549 under hypoxic conditions. PLoS One 7: e30714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, Garcia JA (2009) Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science 324: 1289–1293 [DOI] [PubMed] [Google Scholar]
- Ema M, Hirota K, Mimura J, Abe H, Yodoi J, Sogawa K, Poellinger L, Fujii-Kuriyama Y (1999) Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J 18: 1905–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ (2001) C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43–54 [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292: 154–156 [DOI] [PubMed] [Google Scholar]
- Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, Teruya-Feldstein J, Moreira PI, Cardoso SM, Clish CB, Pandolfi PP, Haigis MC (2011a) SIRT3 opposes reprogramming of cancer cell metabolism through HIF1α destabilization. Cancer Cell 19: 416–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley LW, Haas W, Desquiret-Dumas V, Wallace DC, Procaccio V, Gygi SP, Haigis MC (2011b) Succinate dehydrogenase is a direct target of sirtuin 3 deacetylase activity. PLoS One 6: e23295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Bavister BD (1993) Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J Reprod Fertil 99: 673–679 [DOI] [PubMed] [Google Scholar]
- Fortini ME (2009) Notch signaling: the core pathway and its posttranslational regulation. Dev Cell 16: 633–647 [DOI] [PubMed] [Google Scholar]
- Fox SB, Braganca J, Turley H, Campo L, Han C, Gatter KC, Bhattacharya S, Harris AL (2004) CITED4 inhibits hypoxia-activated transcription in cancer cells, and its cytoplasmic location in breast cancer is associated with elevated expression of tumor cell hypoxia-inducible factor 1alpha. Cancer Res 64: 6075–6081 [DOI] [PubMed] [Google Scholar]
- Frede S, Stockmann C, Freitag P, Fandrey J (2006) Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-kappaB. Biochem J 396: 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, Podolsky DK, Colgan SP (2001) Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med 193: 1027–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu YZ, Moran SM, Hogenesch JB, Wartman L, Bradfield CA (1998) Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3alpha. Gene Expr 7: 205–213 [PMC free article] [PubMed] [Google Scholar]
- Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M (2005) Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell 9: 617–628 [DOI] [PubMed] [Google Scholar]
- Hara S, Hamada J, Kobayashi C, Kondo Y, Imura N (2001) Expression and characterization of hypoxia-inducible factor (HIF)-3alpha in human kidney: suppression of HIF-mediated gene expression by HIF-3alpha. Biochem Biophys Res Commun 287: 808–813 [DOI] [PubMed] [Google Scholar]
- Harris TJ, Grosso JF, Yen HR, Xin H, Kortylewski M, Albesiano E, Hipkiss EL, Getnet D, Goldberg MV, Maris CH, Housseau F, Yu H, Pardoll DM, Drake CG (2007) Cutting edge: an in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol 179: 4313–4317 [DOI] [PubMed] [Google Scholar]
- Hitosugi T, Kang S, Vander HMG, Chung TW, Elf S, Lythgoe K, Dong S, Lonial S, Wang X, Chen GZ, Xie J, Gu TL, Polakiewicz RD, Roesel JL, Boggon TJ, Khuri FR, Gilliland DG, Cantley LC, Kaufman J, Chen J (2009) Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal 2: ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DL, Salter JD, Brookes PS (2007) Response of mitochondrial reactive oxygen species generation to steady-state oxygen tension: implications for hypoxic cell signaling. Am J Physiol Heart Circ Physiol 292: H101–H108 [DOI] [PubMed] [Google Scholar]
- Huang C, Han Y, Wang Y, Sun X, Yan S, Yeh ET, Chen Y, Cang H, Li H, Shi G, Cheng J, Tang X, Yi J (2009) SENP3 is responsible for HIF-1 transactivation under mild oxidative stress via p300 de-SUMOylation. EMBO J 28: 2748–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LE, Gu J, Schau M, Bunn HF (1998) Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA 95: 7987–7992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403: 795–800 [DOI] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr (2001) HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292: 464–468 [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR (2006) The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126: 1121–1133 [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ (2001) Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472 [DOI] [PubMed] [Google Scholar]
- James D, Levine AJ, Besser D, Hemmati-Brivanlou A (2005) TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development 132: 1273–1282 [DOI] [PubMed] [Google Scholar]
- Jantsch J, Chakravortty D, Turza N, Prechtel AT, Buchholz B, Gerlach RG, Volke M, Glasner J, Warnecke C, Wiesener MS, Eckardt KU, Steinkasserer A, Hensel M, Willam C (2008) Hypoxia and hypoxia-inducible factor-1 alpha modulate lipopolysaccharide-induced dendritic cell activation and function. J Immunol 180: 4697–4705 [DOI] [PubMed] [Google Scholar]
- Jiang BH, Rue E, Wang GL, Roe R, Semenza GL (1996) Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem 271: 17771–17778 [DOI] [PubMed] [Google Scholar]
- Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL (1997) Transactivation and inhibitory domains of hypoxia-inducible factor 1alpha. Modulation of transcriptional activity by oxygen tension. J Biol Chem 272: 19253–19260 [DOI] [PubMed] [Google Scholar]
- Jogi A, Ora I, Nilsson H, Lindeheim A, Makino Y, Poellinger L, Axelson H, Pahlman S (2002) Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci USA 99: 7021–7026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG Jr., Ratcliffe PJ (2008) Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 30: 393–402 [DOI] [PubMed] [Google Scholar]
- Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH (2004) Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest 114: 1098–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, Mupparaju S, Hou H, Williams BB, Swartz H (2012) Repeated assessment of orthotopic glioma pO(2) by multi-site EPR oximetry: a technique with the potential to guide therapeutic optimization by repeated measurements of oxygen. J Neurosci Methods 204: 111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, van der Meer R, Nguyen P, Savage J, Owens KM, Vassilopoulos A, Ozden O, Park SH, Singh KK, Abdulkadir SA, Spitz DR, Deng CX, Gius D (2010) SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell 17: 41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Tchernyshyov I, Semenza GL, Dang CV (2006) HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3: 177–185 [DOI] [PubMed] [Google Scholar]
- Koivunen P, Hirsila M, Remes AM, Hassinen IE, Kivirikko KI, Myllyharju J (2007) Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J Biol Chem 282: 4524–4532 [DOI] [PubMed] [Google Scholar]
- Kojima H, Gu H, Nomura S, Caldwell CC, Kobata T, Carmeliet P, Semenza GL, Sitkovsky MV (2002) Abnormal B lymphocyte development and autoimmunity in hypoxia-inducible factor 1alpha -deficient chimeric mice. Proc Natl Acad Sci USA 99: 2170–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H, Kobayashi A, Sakurai D, Kanno Y, Hase H, Takahashi R, Totsuka Y, Semenza GL, Sitkovsky MV, Kobata T (2010) Differentiation stage-specific requirement in hypoxia-inducible factor-1alpha-regulated glycolytic pathway during murine B cell development in bone marrow. J Immunol 184: 154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong T, Eltzschig HK, Karhausen J, Colgan SP, Shelley CS (2004) Leukocyte adhesion during hypoxia is mediated by HIF-1-dependent induction of beta2 integrin gene expression. Proc Natl Acad Sci USA 101: 10440–10445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML (2002) Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science 295: 858–861 [DOI] [PubMed] [Google Scholar]
- Lee JS, Kim Y, Bhin J, Shin HJ, Nam HJ, Lee SH, Yoon JB, Binda O, Gozani O, Hwang D, Baek SH (2011) Hypoxia-induced methylation of a pontin chromatin remodeling factor. Proc Natl Acad Sci USA 108: 13510–13515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Shen JJ, Bournat JC, Huang L, Chattopadhyay A, Li Z, Shaw C, Graham BH, Brown CW (2009) Activin signaling: effects on body composition and mitochondrial energy metabolism. Endocrinology 150: 3521–3529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW (2010) Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell 38: 864–878 [DOI] [PubMed] [Google Scholar]
- Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV Jr., Weissman S, Verdin E, Schwer B (2007) Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 27: 8807–8814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis NA, Hamilton KE, Canny G, Shekels LL, Ho SB, Colgan SP (2006) Selective induction of mucin-3 by hypoxia in intestinal epithelia. J Cell Biochem 99: 1616–1627 [DOI] [PubMed] [Google Scholar]
- Luo W, Hu H, Chang R, Zhong J, Knabel M, O'Meally R, Cole RN, Pandey A, Semenza GL (2011) Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145: 732–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino Y, Kanopka A, Wilson WJ, Tanaka H, Poellinger L (2002) Inhibitory PAS domain protein (IPAS) is a hypoxia-inducible splicing variant of the hypoxia-inducible factor-3alpha locus. J Biol Chem 277: 32405–32408 [DOI] [PubMed] [Google Scholar]
- Mandal S, Lindgren AG, Srivastava AS, Clark AT, Banerjee U (2011) Mitochondrial function controls proliferation and early differentiation potential of embryonic stem cells. Stem Cells 29: 486–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC (2005) Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab 1: 393–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson N, Singleton RS, Sekirnik R, Trudgian DC, Ambrose LJ, Miranda MX, Tian YM, Kessler BM, Schofield CJ, Ratcliffe PJ (2012) The FIH hydroxylase is a cellular peroxide sensor that modulates HIF transcriptional activity. EMBO Rep 13: 251–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ (2001) Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J 20: 5197–5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399: 271–275 [DOI] [PubMed] [Google Scholar]
- Maynard MA, Qi H, Chung J, Lee EH, Kondo Y, Hara S, Conaway RC, Conaway JW, Ohh M (2003) Multiple splice variants of the human HIF-3 alpha locus are targets of the von Hippel-Lindau E3 ubiquitin ligase complex. J Biol Chem 278: 11032–11040 [DOI] [PubMed] [Google Scholar]
- Meister M (2004) Blood cells of Drosophila: cell lineages and role in host defence. Curr Opin Immunol 16: 10–15 [DOI] [PubMed] [Google Scholar]
- Mukherjee T, Kim WS, Mandal L, Banerjee U (2011) Interaction between Notch and Hif-alpha in development and survival of Drosophila blood cells. Science 332: 1210–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Makino Y, Okamoto K, Poellinger L, Ohnuma K, Morimoto C, Tanaka H (2005) TCR engagement increases hypoxia-inducible factor-1 alpha protein synthesis via rapamycin-sensitive pathway under hypoxic conditions in human peripheral T cells. J Immunol 174: 7592–7599 [DOI] [PubMed] [Google Scholar]
- Nichols J, Smith A (2009) Naive and primed pluripotent states. Cell Stem Cell 4: 487–492 [DOI] [PubMed] [Google Scholar]
- Nizet V, Johnson RS (2009) Interdependence of hypoxic and innate immune responses. Nat Rev Immunol 9: 609–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke JF, Tian YM, Ratcliffe PJ, Pugh CW (1999) Oxygen-regulated and transactivating domains in endothelial PAS protein 1: comparison with hypoxia-inducible factor-1alpha. J Biol Chem 274: 2060–2071 [DOI] [PubMed] [Google Scholar]
- Oberkofler H, Iglseder B, Klein K, Unger J, Haltmayer M, Krempler F, Paulweber B, Patsch W (2005) Associations of the UCP2 gene locus with asymptomatic carotid atherosclerosis in middle-aged women. Arterioscler Thromb Vasc Biol 25: 604–610 [DOI] [PubMed] [Google Scholar]
- Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG (2000) Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol 2: 423–427 [DOI] [PubMed] [Google Scholar]
- Pan Y, Mansfield KD, Bertozzi CC, Rudenko V, Chan DA, Giaccia AJ, Simon MC (2007) Multiple factors affecting cellular redox status and energy metabolism modulate hypoxia-inducible factor prolyl hydroxylase activity in vivo and in vitro. Mol Cell Biol 27: 912–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyssonnaux C, Cejudo-Martin P, Doedens A, Zinkernagel AS, Johnson RS, Nizet V (2007) Cutting edge: essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J Immunol 178: 7516–7519 [DOI] [PubMed] [Google Scholar]
- Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V, Johnson RS (2005) HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest 115: 1806–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistollato F, Rampazzo E, Persano L, Abbadi S, Frasson C, Denaro L, D'Avella D, Panchision DM, Della Puppa A, Scienza R, Basso G (2010) Interaction of hypoxia-inducible factor-1alpha and Notch signaling regulates medulloblastoma precursor proliferation and fate. Stem Cells 28: 1918–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh CW, O'Rourke JF, Nagao M, Gleadle JM, Ratcliffe PJ (1997) Activation of hypoxia-inducible factor-1; definition of regulatory domains within the alpha subunit. J Biol Chem 272: 11205–11214 [DOI] [PubMed] [Google Scholar]
- Qiang L, Wu T, Zhang HW, Lu N, Hu R, Wang YJ, Zhao L, Chen FH, Wang XT, You QD, Guo QL (2012) HIF-1alpha is critical for hypoxia-mediated maintenance of glioblastoma stem cells by activating Notch signaling pathway. Cell Death Differ 19: 284–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Brown K, Hirschey MD, Verdin E, Chen D (2010) Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 12: 662–667 [DOI] [PubMed] [Google Scholar]
- Rebay I, Fleming RJ, Fehon RG, Cherbas L, Cherbas P, Artavanis-Tsakonas S (1991) Specific EGF repeats of Notch mediate interactions with Delta and Serrate: implications for Notch as a multifunctional receptor. Cell 67: 687–699 [DOI] [PubMed] [Google Scholar]
- Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M (2008) NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453: 807–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP (2008) Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 134: 145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U (2008) Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA 105: 6392–6397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainson RC, Harris AL (2006) Hypoxia-regulated differentiation: let's step it up a Notch. Trends Mol Med 12: 141–143 [DOI] [PubMed] [Google Scholar]
- Schofield CJ, Ratcliffe PJ (2004) Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol 5: 343–354 [DOI] [PubMed] [Google Scholar]
- Seagroves TN, Ryan HE, Lu H, Wouters BG, Knapp M, Thibault P, Laderoute K, Johnson RS (2001) Transcription factor HIF-1 is a necessary mediator of the pasteur effect in mammalian cells. Mol Cell Biol 21: 3436–3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL (1996) Transcriptional regulation by hypoxia-inducible factor 1 molecular mechanisms of oxygen homeostasis. Trends Cardiovasc Med 6: 151–157 [DOI] [PubMed] [Google Scholar]
- Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A (1996) Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem 271: 32529–32537 [DOI] [PubMed] [Google Scholar]
- Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H (2011) HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med 208: 1367–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, Schneider JW, Zhang CC, Sadek HA (2010) The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 7: 380–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Yu W, Hallowszzz WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA (2010) Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143: 802–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Chen X, Ma J, Peng H, Wang F, Zha X, Wang Y, Jing Y, Yang H, Chen R, Chang L, Zhang Y, Goto J, Onda H, Chen T, Wang MR, Lu Y, You H, Kwiatkowski D, Zhang H (2011) Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc Natl Acad Sci USA 108: 4129–4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Yanagisawa K, Tanaka M, Cao K, Matsuyama Y, Goto H, Takahashi T (2008) Identification of hypoxia-inducible factor-1 alpha as a novel target for miR-17-92 microRNA cluster. Cancer Res 68: 5540–5545 [DOI] [PubMed] [Google Scholar]
- Takehara T, Teramura T, Onodera Y, Hamanishi C, Fukuda K (2011) Reduced oxygen concentration enhances conversion of embryonic stem cells to epiblast stem cells. Stem Cells Dev (advance online publication; doi:10.1089/scd.2011.0322) [DOI] [PubMed] [Google Scholar]
- Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, Shima H, Johnson RS, Hirao A, Suematsu M, Suda T (2010) Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell 7: 391–402 [DOI] [PubMed] [Google Scholar]
- Tang H, Kambris Z, Lemaitre B, Hashimoto C (2006) Two proteases defining a melanization cascade in the immune system of Drosophila. J Biol Chem 281: 28097–28104 [DOI] [PubMed] [Google Scholar]
- Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes McDonald W, Olivier AK, Spitz DR, Gius D (2010) Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell 40: 893–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD (2007) New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448: 196–199 [DOI] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L (2001) Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410: 227–230 [DOI] [PubMed] [Google Scholar]
- Vallier L, Reynolds D, Pedersen RA (2004) Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol 275: 403–421 [DOI] [PubMed] [Google Scholar]
- Wacker I, Sachs M, Knaup K, Wiesener M, Weiske J, Huber O, Akcetin Z, Behrens J (2009) Key role for activin B in cellular transformation after loss of the von Hippel-Lindau tumor suppressor. Mol Cell Biol 29: 1707–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley SR, Chilvers ER, Thompson AA, Vaughan K, Marriott HM, Parker LC, Shaw G, Parmar S, Schneider M, Sabroe I, Dockrell DH, Milo M, Taylor CT, Johnson RS, Pugh CW, Ratcliffe PJ, Maxwell PH, Carmeliet P, Whyte MK (2011) Prolyl hydroxylase 3 (PHD3) is essential for hypoxic regulation of neutrophilic inflammation in humans and mice. J Clin Invest 121: 1053–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T, Sobolewski A, Condliffe AM, Cowburn AS, Johnson N, Chilvers ER (2005) Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med 201: 105–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL (1995) Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 92: 5510–5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Zhang YW, Zhang X, Liu R, Hong S, Xia K, Xia J, Zhang Z, Xu H (2006) Transcriptional regulation of APH-1A and increased gamma-secretase cleavage of APP and Notch by HIF-1 and hypoxia. FASEB J 20: 1275–1277 [DOI] [PubMed] [Google Scholar]
- Wang Y, Roche O, Yan MS, Finak G, Evans AJ, Metcalf JL, Hast BE, Hanna SC, Wondergem B, Furge KA, Irwin MS, Kim WY, Teh BT, Grinstein S, Park M, Marsden PA, Ohh M (2009) Regulation of endocytosis via the oxygen-sensing pathway. Nat Med 15: 319–324 [DOI] [PubMed] [Google Scholar]
- Wenger RH, Stiehl DP, Camenisch G (2005) Integration of oxygen signaling at the consensus HRE. Sci STKE 2005: re12. [DOI] [PubMed] [Google Scholar]
- Wiley M, Sweeney KR, Chan DA, Brown KM, McMurtrey C, Howard EW, Giaccia AJ, Blader IJ (2010) Toxoplasma gondii activates hypoxia-inducible factor (HIF) by stabilizing the HIF-1alpha subunit via type I activin-like receptor kinase receptor signaling. J Biol Chem 285: 26852–26860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins SE, Hyvarinen J, Chicher J, Gorman JJ, Peet DJ, Bilton RL, Koivunen P (2009) Differences in hydroxylation and binding of Notch and HIF-1alpha demonstrate substrate selectivity for factor inhibiting HIF-1 (FIH-1). Int J Biochem Cell Biol 41: 1563–1571 [DOI] [PubMed] [Google Scholar]
- Xenaki G, Ontikatze T, Rajendran R, Stratford IJ, Dive C, Krstic-Demonacos M, Demonacos C (2008) PCAF is an HIF-1alpha cofactor that regulates p53 transcriptional activity in hypoxia. Oncogene 27: 5785–5796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Xia Y, Ji H, Zheng Y, Liang J, Huang W, Gao X, Aldape K, Lu Z (2011) Nuclear PKM2 regulates beta-catenin transactivation upon EGFR activation. Nature 480: 118–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun Z, Maecker HL, Johnson RS, Giaccia AJ (2002) Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell 2: 331–341 [DOI] [PubMed] [Google Scholar]
- Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL (2007) HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 11: 407–420 [DOI] [PubMed] [Google Scholar]
- Zhang J, Khvorostov I, Hong JS, Oktay Y, Vergnes L, Nuebel E, Wahjudi PN, Setoguchi K, Wang G, Do A, Jung HJ, McCaffery JM, Kurland IJ, Reue K, Lee WN, Koehler CM, Teitell MA (2011) UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J 30: 4860–4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Linke S, Dias JM, Gradin K, Wallis TP, Hamilton BR, Gustafsson M, Ruas JL, Wilkins S, Bilton RL, Brismar K, Whitelaw ML, Pereira T, Gorman JJ, Ericson J, Peet DJ, Lendahl U, Poellinger L (2008) Interaction with factor inhibiting HIF-1 defines an additional mode of cross-coupling between the Notch and hypoxia signaling pathways. Proc Natl Acad Sci USA 105: 3368–3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, D’Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, Guimaraes A, Marinelli B, Wikstrom JD, Nir T, Clish CB, Vaitheesvaran B, Iliopoulos O, Kurland I, Dor Y, Weissleder R, Shirihai OS, Ellisen LW, Espinosa JM, Mostoslavsky R (2010) The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell 140: 280–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Choi M, Margineantu D, Margaretha L, Hesson J, Cavanaugh C, Blau AC, Horwitz MS, Hockenbery D, Ware C, Ruohola-Baker H (2012) HIF1α induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition. EMBO J 31: 2103–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]