Abstract
EMBO J 31 11, 2473–2485 (2012); published online April 20 2012
To maintain tissue homeostasis, stem cells must balance self-renewal with differentiation. In some stem cell lineages this process is ‘hard-wired’ by the asymmetric partitioning of determinants at division, such that one stem cell daughter always remains pluripotent and other differentiates. But in a dynamic tissue like the intestinal epithelium, which might need to repair itself following an infection or expand to digest the fall harvest, this balancing act requires more flexibility. Recent studies of intestinal stem cell (ISC) lineages in the fruit fly and mouse provide new insights into how this plasticity is achieved. The mechanisms in these two homologous but rather different organs have remarkable similarities, and so are likely relevant to how stem cell pools are controlled in organs other than the intestine.
Mammalian ISCs reside in the Crypts of Lieberkühn, a protective niche from which they receive signals that maintain their survival, growth, and pluripotency. ISCs are extremely proliferative, generating a constant stream of new progeny that push older cells out of the crypts and away from the niche-specific signalling factors that maintain stemness and promote cell growth and division. Thus, as newborn cells leave the crypts they differentiate. Early labelling and lineage-tracing experiments indicated that each crypt contained a small, relatively fixed number of ISCs, but what controlled their numbers within the niche was not so clear. Models based on asymmetric stem cell divisions have predominated the field, and so researchers searched for proof of such mechanisms in the intestine. They found few clues, however. Using a new lineage-tracing method that genetically marks a cell’s progeny with different-coloured fluorescent tags, Snippert et al (2010) discovered that ISCs in the mouse’s small intestine and colon actually divide symmetrically, and that their decision to differentiate is not coupled to division. Some ISC divisions are duplicative (generating two ISCs), while others are doubly terminal (generating two transient cells committed to differentiate), and still others are functionally asymmetric (generating one ISC and one committed cell; see Figure 1). At the population level, the frequencies of duplicative and terminal divisions (i.e. the ratio of ISC loss to replacement) were found to be essentially equal, explaining how a constant stem cell pool could be maintained (Lopez-Garcia et al, 2010; Snippert et al, 2010). Contrary to early models in the stem cell field, however, Snippert et al showed that the ISC pool undergoes ‘neutral drift’ such that most individual stem cells have a finite lifetime. So much for immortality. To explain the exact balance of ISC loss and duplication, it was postulated that stem cells at the crypt base (the Lgr5+ cells) undergo ‘neutral’ competition for niche signals provided by a limited number of Paneth cells, which thereby define the number of stem cells each crypt can support (Sato et al, 2011). Paneth cells are long-lived secretory cells that reside in the crypt bases, intermingled with ISCs. They express essential regulators of ISC growth and survival, including EGF, TGF-α, Wnt3, and Dll4, and have recently been shown to serve an essential supportive niche role for the ISCs (Sato et al, 2009, 2011). The mechanisms that determine the number of Paneth cells are not yet known, but evidently understanding this is one key to learning exactly how ISC numbers are regulated, at least in the mouse’s small intestine. ISCs in the colonic crypts also undergo neutral drift, but surprisingly, Paneth cells have been reported to be absent from the colon. Thus there may be more than one way to regulate ISC numbers.
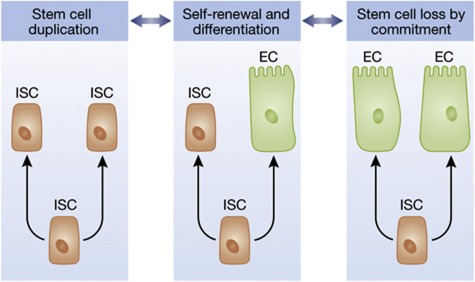
Figure 1.
ISC divisions are most often functionally asymmetric, yielding one differentiated cell (EC: enterocyte) and one new stem cell (middle). But ISCs can also duplicate (left) or be lost when both progeny of a division differentiate. The balance between these behaviours determines whether the intestinal epithelium will grow, shrink, or maintain exact homeostasis. This balance can change, depending on the needs of the organ and organism.
The Drosophila midgut, which is analogous to the mammalian stomach, small intestine, and colon, has recently become a popular system for studying stem cell dynamics. Although this organ is tiny, its structure and cell types are similar to those of the mammalian intestine, as are the stem cell lineage and some key cell-signalling interactions. Drosophila’s ISCs sit basally within the intestinal epithelium and are partitioned into small nests comprised most often of a single ISC and one or two committed, postmitotic, but undifferentiated ISC daughters, termed ‘enteroblasts’ (EB). Initial studies of this system noted that Delta/Notch signalling between the cells within a nest determined which cell would differentiate (the signal receiving cell, or EB) and which would remain pluripotent (the sending cell, or ISC). Simple lineage analysis showed that most ISC divisions were functionally asymmetric, giving rise to one new ISC and one committed EB (Ohlstein and Spradling, 2006, 2007), but when researchers looked for markers of physical asymmetry, such as segregation of Delta (the Notch ligand) during ISC divisions, they found that the two sister cells formed by an ISC division are indistinguishable. This suggested that asymmetry in the ISC lineage might be generated after cell division, by cell-cell interactions in a process often referred to as ‘lateral inhibition’.
Two recent studies using improved lineage-tracing tools and more quantitative approaches now reveal a picture similar to that in the mouse, wherein ISCs can either be duplicated or are lost to differentiation following divisions that are essentially symmetric (O’Brien et al, 2011; de Navascues et al, 2012). Using an exhaustive quantitative approach to lineage and ISC marker analysis, coupled with a mathematical treatment and a simulation, de Navascues et al (2012) demonstrate that just as in the mouse, the fly’s ISCs regularly duplicate or extinguish themselves through symmetric self-renewal or symmetric differentiation. However, the fly’s ISCs generally followed these two paths at equal rates, and so, as in the mouse, the stem cell population was characterized as undergoing neutral drift but nevertheless maintaining a nearly constant number of ISCs as the epithelium turns over.
Flies do not have Paneth cells, but it is noteworthy that the committed EBs adhere tightly to the ISCs using cadherin/catenin-based adherins junctional complexes, and produce an EGFR ligand (Spitz) and a cytokine (Upd) that support ISC growth. Thus, the fly’s EB might function somewhat like the mouse’s Paneth cells, as a limiting niche component that sustains ISCs, and is produced by ISCs. In addition (and also similar to the mouse), it has been proposed that the proximity of an ISC to the visceral muscle, which produces growth and survival factors such as an EGFR ligand (Vein), a Wnt (Wingless), and an insulin-like peptide (dILP3), might help retain stemness or at least ISC survival and maintenance (Lin et al, 2008, 2009; O’Brien et al, 2011), biasing by proximity the fate of ISC daughters after division (Ohlstein and Spradling, 2007).
Although the exact role of these niche factors in the fly is not so well understood, numerous studies of the fly midgut have documented the essential role of Delta/Notch signalling in controlling differentiation following an ISC division. The first studies showed beautifully that Delta produced by the ISC could trigger activation of Notch (the receptor for Delta) in presumptive EBs and thereby drive their differentiation into enterocytes (EC) (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2007). Given this, any factor that tips the balance of lateral inhibition during Notch/Delta signalling between ISC/EB pairs is expected to have a critical impact on ISC numbers in this system (see, e.g. Perdigoto et al, 2011). For instance, de Navascues et al (2012) used partial loss- or gain-of-function alleles of Notch to skew this signalling interaction, and found that they could bias the outcome of ISC divisions either towards duplicative (double ISC) or committed (double EB/EC) fates, respectively. Hence, learning about which natural factors bias the Notch/Delta signalling interaction promises to be one key to understanding the control of stem cell numbers in this system. Indeed, there are already some interesting clues. Several studies suggest that the rate of ISC division is one such biasing factor, since this determines the number of cells in the progenitor cell nests that participate in Delta/Notch interactions. For instance, a strong induction of cytokine/Jak/Stat signalling, which accelerates ISC divisions, appears to be able to transiently increase ISC numbers in the fly’s midgut (Jiang et al, 2009). Cytokine induction is a natural response to enteric infection, and by increasing the stem cell pool it might enhance regeneration following bacterial damage. How cytokine signalling would bias the stem cell lineage is not so clear, but perhaps the ability of the Delta→Notch signalling from ISC to EB to drive commitment is simply outpaced by the rapid accumulation of cells within the nests where the signalling takes place. For example, if a newborn cell were crowed out of the nest it might escape receiving a Delta signal, retain its stemness, and found a new nest of its own.
Especially interesting in this regard is the recent discovery (O’Brien et al, 2011) that increased insulin signalling, a response to feeding that stimulates cell growth and ISC division in the Drosophila gut, can increase the likelihood of functionally symmetric, duplicative ISC divisions. This expands the stem cell pool, and subsequently the size of the whole gut. For a fruit fly, being able to expand its gut in times of abundant food might bring a substantial fitness advantage, since the more the nutrition a female fly can get, the more the number of eggs it can lay. Remarkably, O’Brien et al (2011) also found that starvation, which reduces insulin signalling, had the opposite effect on the fly’s gut: during starvation ISCs were lost by apoptosis and the gut shrank. Although such striking nutritional effects have not yet been demonstrated in a mammal, it is well appreciated that the crypts of Lieberkühn can undergo fission and that this is a likely mechanism for regenerative growth during the repair of, for instance, ulcerous lesions. As in the fly, regenerative growth in the mouse intestine is known to involve growth factor and cytokine signalling. Hence, it would not come as a surprise if these factors also biased ISC lineages and thereby controlled stem cell pools and the size of the epithelium they maintain. Further advances in understanding the rules governing ISC lineages and stem cell numbers promise to be very relevant to regenerative medicine, and might also help explain known interactions between inflammation, nutrition, and stem cell-derived cancers such as colorectal carcinoma.
Acknowledgments
BAE was supported by NIH R01 GM51186, the ERC, and the DKFZ.
Footnotes
The author declares that he has no conflict of interest.
References
- de Navascués J, Perdigoto CN, Bian Y, Schneider MH, Bardin AJ, Martínez-Arias A, Simons BD (2012) Drosophila midgut homeostasis involves neutral competition between symmetrically dividing intestinal stem cells. EMBO J 31: 2473–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA (2009) Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137: 1343–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Xu N, Xi R (2008) Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature 455: 1119–1123 [DOI] [PubMed] [Google Scholar]
- Lin G, Xu N, Xi R (2009) Paracrine unpaired signaling through the JAK/STAT pathway controls self-renewal and lineage differentiation of drosophila intestinal stem cells. J Mol Cell Biol 2: 37–49 [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia C, Klein AM, Simons BD, Winton DJ (2010) Intestinal stem cell replacement follows a pattern of neutral drift. Science 330: 822–825 [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N (2006) Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439: 475–479 [DOI] [PubMed] [Google Scholar]
- O’Brien LE, Soliman SS, Li X, Bilder D (2011) Altered modes of stem cell division drive adaptive intestinal growth. Cell 147: 603–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A (2006) The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439: 470–474 [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A (2007) Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science 315: 988–992 [DOI] [PubMed] [Google Scholar]
- Perdigoto CN, Schweisguth F, Bardin AJ (2011) Distinct levels of Notch activity for commitment and terminal differentiation of stem cells in the adult fly intestine. Development 138: 4585–4595 [DOI] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H (2011) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265 [DOI] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H (2010) Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143: 134–144 [DOI] [PubMed] [Google Scholar]