Abstract
In cytokinesis, there is a lengthy interval between cleavage furrow ingression and abscission, during which the midbody microtubule bundle provides both structural support for a narrow intercellular bridge and a platform that orchestrates the biochemical preparations for abscission. It is currently unclear how the midbody structure is stably maintained during this period. Here, we report a novel role for the ADP-ribosylation factor 6 (ARF6) GTPase in the post-mitotic stabilisation of midbody. Centralspindlin kinesin-6/RhoGAP complex, a midbody component critical for both the formation and function of the midbody, assembles in a sharp band at the centre of the structure in a manner antagonised by 14-3-3 protein. We show that ARF6 competes with 14-3-3 for binding to centralspindlin such that midbodies formed by centralspindlin mutants that can bind 14-3-3 but not ARF6 frequently collapse before abscission. These data indicate a novel mechanism for the regulation of midbody dynamics in which ARF6 protects the compacted centralspindlin assembly from dissipation by 14-3-3.
Keywords: 14-3-3, ARF6, centralspindlin, cytokinesis, midbody
Introduction
At anaphase onset, a microtubule-based structure, the central spindle, is formed between segregating chromosomes (Barr and Gruneberg, 2007; Glotzer, 2009; Douglas and Mishima, 2010). This structure, which consists of the two sets of parallel microtubule bundles with plus-ends overlapped in an interdigitating manner at the centre, is compacted by ingressing cleavage furrow and matures into the midbody. In the zone of overlap (the Flemming body; Zeitlin and Sullivan, 2001; Mollinari et al, 2002), microtubules are embedded in a dense matrix substance, which glues together the two sets of microtubule. After the exit from mitosis, the two daughter cells are kept connected via the midbody until the final separation (abscission). In HeLa cells, the microtubules outside of the Flemming body and the continuity of the daughter cytoplasms are lost about 1 h after the midbody formation (Steigemann et al, 2009; Guizetti et al, 2011) and the dissolution of the intercellular bridge follows these events immediately or after a while, up to several hours (Piel et al, 2001). The midbody is important both as a scaffold for the narrow intercellular bridge and as a platform for biochemical events in preparation for abscission (Otegui et al, 2005; Guizetti and Gerlich, 2010). Failure of cytokinesis by disintegration of the midbody before abscission produces a tetraploid cell that can be the origin of aneuploidy, which is often associated with cancer. Thus, the integrity of the midbody has to be properly preserved until abscission. However, it has been unclear how the post-mitotic midbody is stably maintained for a long time since its assembly.
Centralspindlin is a key factor for the formation and maintenance of the central spindle and the midbody. It is an evolutionarily conserved, stable heterotetramer consisted of MKLP1 kinesin-6 and CYK4 RhoGAP (Mishima et al, 2002; Somers and Saint, 2003). It bundles microtubules both in vitro and in vivo and sharply accumulates to the Flemming body as a component of the midbody matrix depending on its motor activity (Matuliene and Kuriyama, 2002). Centralspindlin recruits various downstream membrane trafficking proteins (Tomas et al, 2004; Gromley et al, 2005; Zhao et al, 2006; Simon et al, 2008) to the centre of the midbody. We have recently discovered that higher-order clustering of centralspindlin is critical for its processive movement along microtubules and efficient microtubule bundling as well as rapid and stable accumulation to the Flemming body (Hutterer et al, 2009). Interestingly, this clustering is inhibited by 14-3-3 protein, which binds to MKLP1 when the second serine residue (S710 of human MKLP1) in the conserved RRSRS motif in the tail domain of MKLP1 is monophosphorylated (Douglas et al, 2010). 14-3-3 sequesters centralspindlin into an inactive, unclustered form. Phosphorylation of the first serine residue (S708 of human MKLP1) by Aurora B kinase (Guse et al, 2005) inhibits 14-3-3 binding and thus releases centralspindlin from the sequestration (Douglas et al, 2010). As Aurora B activity peaks between segregating chromosomes during anaphase (Fuller et al, 2008), regulation of centralspindlin by 14-3-3 and Aurora B kinase provides a mechanism that ensures spatial coupling between chromosome segregation and cytokinesis. However, it has been unclear whether the same mechanism also contributes to the post-mitotic stable maintenance of the midbody.
An endosomal GTPase ADP-ribosylation factor 6 (ARF6), which is a member of the ARF family (Donaldson and Jackson, 2011; Schweitzer et al, 2011), localises to the cleavage furrow and midbody (Schweitzer and D’Souza-Schorey, 2002; Takahashi et al, 2011). Depletion of ARF6 in mammalian cells (Schweitzer and D’Souza-Schorey, 2005) and null mutations in Drosophila (Dyer et al, 2007) cause cytokinesis defects. Interestingly, ARF6, as well as all the other ARF family GTPases examined, binds MKLP1 in a GTP-specific manner at a binding site in the C-terminal tail (Boman et al, 1999; Dyer et al, 2007) that appears to overlap with that for 14-3-3 protein. However, it has remained unclear whether ARF6 colocalises with centralspindlin at the midbody (Fielding et al, 2005) and, more importantly, whether the ARF6–MKLP1 interaction plays an important role in cytokinesis.
In this report, we show that ARF6 competes with 14-3-3 for binding to MKLP1 and thus, when colocalised with centralspindlin at the Flemming body, protects centralspindlin from dissipation by 14-3-3. This provides a novel mechanism for the long-term preservation of the post-mitotic midbody, which is critical for high-fidelity completion of cytokinesis and the maintenance of genome stability.
Results
Centralspindlin is stably maintained at the midbody even after the level of Aurora B kinase declines
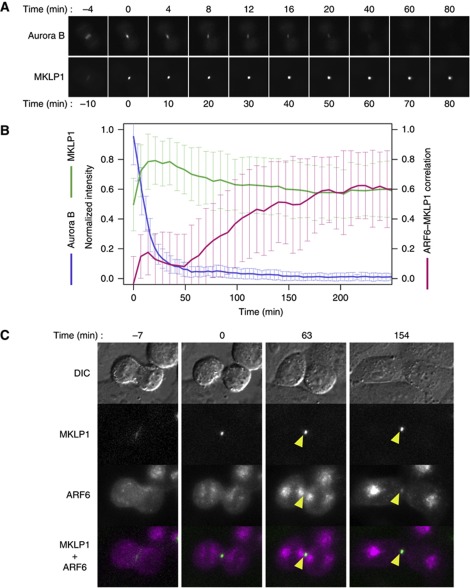
Cytoplasmic Aurora B kinase activity declines after anaphase onset such that it is undetectable 30 min after sister chromatid separation (Fuller et al, 2008). An active form of Aurora B is detected on the midbody but not on the midbody remnant (Steigemann et al, 2009). Signal of GFP-tagged Aurora B kinase at the midbody peaked at midbody formation but continued to decline and almost disappeared within an hour (Figure 1A and B). Consistently with these observations, during mitotic exit, Aurora B-phosphorylated forms (S708-monophosphorylated or S708/S710 double phosphorylated forms) of MKLP1 decrease, while S710-monophosphorylated MKLP1, which can be bound by 14-3-3 protein, increases and appears on the late midbody (Douglas et al, 2010). Although S710-monophosphorylated MKLP1 was not barely detectable right after the completion of the cleavage furrow ingression and the formation of the midbody, it gradually increased concurrently with the decrease of the microtubule signals flanking the Flemming body, which led to the complete disassembly around 70 min after the midbody formation (Supplementary Figures S1 and S2). Nonetheless, steep accumulation of centralspindlin at the Flemming body is stably maintained for a prolonged period (Figure 1A and B) until abscission, which occurs up to a few hours after the furrow completion (Piel et al, 2001), or even after abscission as the midbody remnant (Mishima et al, 2002). This implies that an unknown mechanism protects S710-monophosphorylated centralspindlin from rebinding to 14-3-3 protein, gradually taking over the role of Aurora B during late telophase.
Figure 1.
ARF6 localises to the midbody following the disappearance of Aurora B. (A) Time-lapse observation of HeLa cells stably expressing GFP-tagged MKLP1 or Aurora B during late cytokinesis. (B) Quantification of midbody localisation of MKLP1, Aurora B and ARF6. For MKLP1 (green) and Aurora B (blue), average normalised intensities were plotted. For colocalisation between ARF6 and MKLP1, the local Pearson’s correlation coefficient between mCherry–ARF6 and GFP–MKLP1 (0 for random and 1 for perfect colocalisation) in the 9 × 9 pixel area covering the midbody was plotted (magenta). Error bars indicate the standard deviation. (C) Stills from time-lapse observation of HeLa cells that were cotransfected with GFP–MKLP1 and mCherry–ARF6. Arrowheads indicate their colocalisation at the Flemming body. Bar, 10 μm.
ARF6 colocalises with centralspindlin at the late midbody and competes with 14-3-3 for centralspindlin binding
Molecules that colocalise with centralspindlin at the late midbody are potential candidates to be involved in long-term maintenance of stable midbody. One such molecule is the endosomal GTPase ARF6. To examine the colocalisation of centralspindlin and ARF6 at the late midbody, dual-colour time-lapse imaging of cells expressing MKLP1 N-terminally tagged with green fluorescent protein (GFP–MKLP1) and ARF6 internally tagged (Hall et al, 2008) with a red fluorescent protein (mCherry–ARF6) (Supplementary Figure S3A) was performed (Figure 1C). In early telophase, when MKLP1 accumulated into a distinct focus at the midbody (t=0 min), ARF6 was localised on vesicular compartments that were arranged into four characteristic clusters similar to ‘Golgi twins’ (Gaietta et al, 2006). At this stage, no clear colocalisation of MKLP1 and ARF6 was observed. Inner pairs of ARF6-positive vesicular clusters gradually dispersed. Approximately 1 h after midbody formation, ARF6 began to show colocalisation with MKLP1 at the Flemming body, which gradually increased to a plateau in the subsequent 1–2 h. This colocalisation is not an artefact due to bleed-through of GFP–MKLP1 signal to the mCherry channel since the Flemming body localisation of mCherry–ARF6 was observed even in the absence of GFP–MKLP1 (Supplementary Figure S3B). In contrast, another endosomal GTPase Rab11a (Yu et al, 2007) did not show such colocalisation (Supplementary Figure S3B and C). This ARF6–MKLP1 colocalisation persisted for long periods, even after abscission.
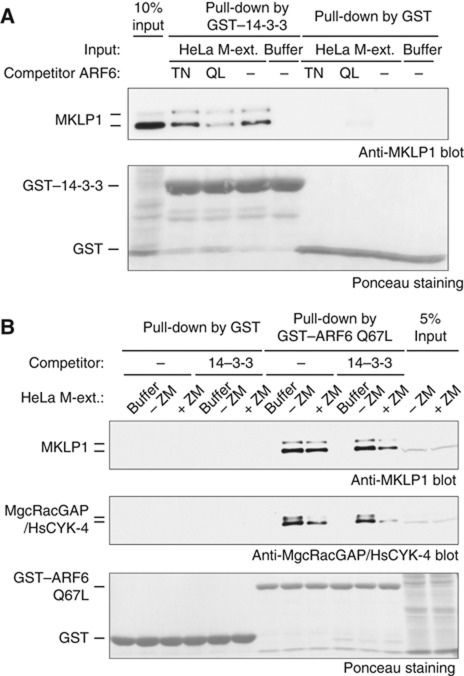
We then examined whether ARF6 can interfere with binding of 14-3-3 to S710-monophosphorylated MKLP1. Endogenous centralspindlin was pulled down by 14-3-3 protein from crude cell extract prepared from mitotic HeLa cells treated with Aurora kinase inhibitor (Douglas et al, 2010). Interestingly, including the GTP-form, but not the GDP-form, of recombinant ARF6 in the assay greatly reduced binding of 14-3-3 to centralspindlin (Figure 2A). In a reciprocal experiment, ARF6 pulled down the centralspindlin holocomplex from the mitotic extract, indicating that ARF6 does not affect the formation of this complex (Figure 2B). Addition of excess 14-3-3 greatly reduced the amount of centralspindlin pulled down, especially when the MKLP1–14-3-3 interaction was enhanced by Aurora inhibitor treatment (Figure 2B). These data indicate that ARF6 and 14-3-3 are mutually exclusive for their interaction with MKLP1. Thus, ARF6 that is locally accumulated to the Flemming body and colocalised with centralspindlin can potentially prevent access of 14-3-3 to centralspindlin.
Figure 2.
14-3-3–MKLP1 and ARF6–MKLP1 interactions are mutually exclusive. (A) Effect of excess ARF6 on the interaction between MKLP1 and 14-3-3 in the crude extract from mitotic HeLa cells treated with an Aurora inhibitor ZM447439, which promotes the MKLP1–14-3-3 interaction. A constitutively active (locked in GTP-form) ARF6 Q67L, but not a GTP-binding deficient T27N mutant, interfered with the MKLP1–14-3-3 interaction. (B) Repression of the ARF6–MKLP1 interaction by excess 14-3-3 in the extracts from mitotic HeLa cells treated with or without ZM447439 (+ZM and −ZM, respectively). Inhibition of Aurora kinase enhanced the interference by 14-3-3.
Separation-of-function mutations in MKLP1 specifically disrupt the interaction with ARF6
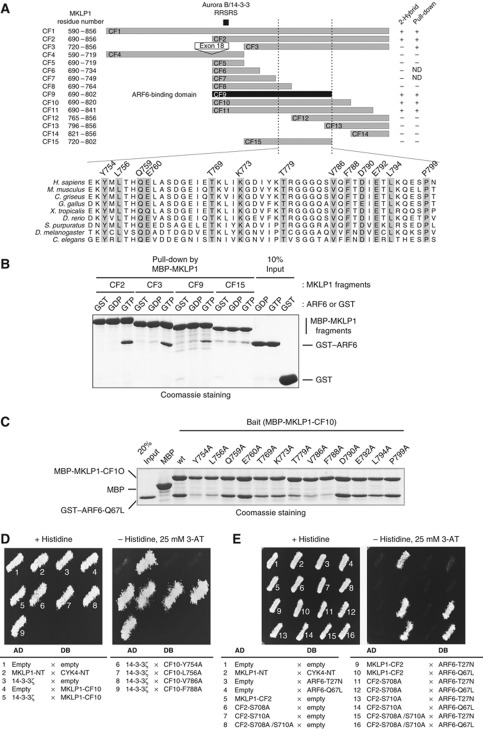
To test whether ARF6 localisation at the Flemming body is dependent on its interaction with MKLP1 and whether this interaction plays a role in stable maintenance of the midbody, we sought to design a separation-of-function mutation of MKLP1 that specifically disrupts the interaction with ARF6 without directly affecting other functions such as 14-3-3 binding. Yeast two-hybrid (Y2H) and in-vitro pull-down analyses with various fragments of MKLP1 identified amino acid 690–802 (CF9 fragment) as the minimum ARF6-binding domain (Figure 3A and B; Supplementary Figure S4A). This region is equivalent to the ARF6-interacting domain on the Drosophila MKLP1 orthologue Pavarotti (Dyer et al, 2007), indicating the evolutionary conservation of this interaction. Interestingly, the minimal ARF6-binding fragment (CF9) contained the AuroraB/14-3-3 regulatory motif in its N-terminal region although this region is dispensable for ARF6-binding in in-vitro pull-down assay (Figure 3B, CF3 fragment).
Figure 3.
ARF6 and 14-3-3 interact with MKLP1 through overlapping, but not identical, regions in the MKLP1 C-terminal tail. (A) Schematic representation of MKLP1 fragments examined for the interaction with ARF6 by Y2H and in-vitro pull-down assays. (B) In-vitro pull-down assay to test ARF6-binding by MKLP1 fragments. GST–ARF6 Q67L (GTP), T27N (GDP) or GST alone (GST) was pulled down by the MKLP1 fragments (CF2, CF3, CF9 or CF15) tagged with maltose-binding protein (MBP) immobilised on amylose beads (New England Biolabs). (C) Alanine substitutions at the conserved residues in the C-terminal half of the minimum ARF6-binding domain and their effects on the MKLP1–ARF6 interaction. ARF6 Q67L was pulled down by the amylose beads immobilised with the MBP-MKLP1-CF10 fragments containing the indicated mutations. (D, E) Y2H assays to test the effects of the MKLP1 mutations in the ARF6-binding domain on the interaction with 14-3-3 (D) and of the mutations in the Aurora B/14-3-3 motif on the interaction with ARF6 (E). Growth on medium lacking histidine and supplemented with 3-aminotriazol (3AT) indicates an interaction between proteins fused to the activation domain (AD) and DNA-binding domain (DB). The mutations at Y754, L756, V786 and F788 specifically disrupt the MKLP1–ARF6 interaction without affecting the MKLP1–14-3-3 interaction while S708 and S710 mutations do not interfere with the MKLP1–ARF6 interaction.
To find ARF6-binding defective point mutations on MKLP1 that do not interfere with 14-3-3 binding, evolutionarily conserved residues in the C-terminal half of ARF6-binding domain (Figure 3A) were scanned by alanine substitution. Mutations on most of the conserved residues affected the interaction with ARF6 as shown by in-vitro pull-down assay (Figure 3C). Similar results were also obtained by Y2H analysis (Supplementary Figure S4B). These mutations did not affect the MKLP1–MgcRacGAP/HsCYK-4 complex formation (Supplementary Figure S5). Importantly, these mutations did not affect binding of MKLP1 to 14-3-3 protein (Figure 3D). Similarly, mutations in the Aurora B/14-3-3 motif did not affect binding of MKLP1 to ARF6 (Figure 3E). These results indicate that ARF6 binding and 14-3-3 binding are primarily independent functions of MKLP1 although they are mutually exclusive.
ARF6–centralspindlin interaction plays an important role in stable maintenance of the midbody during late cytokinesis
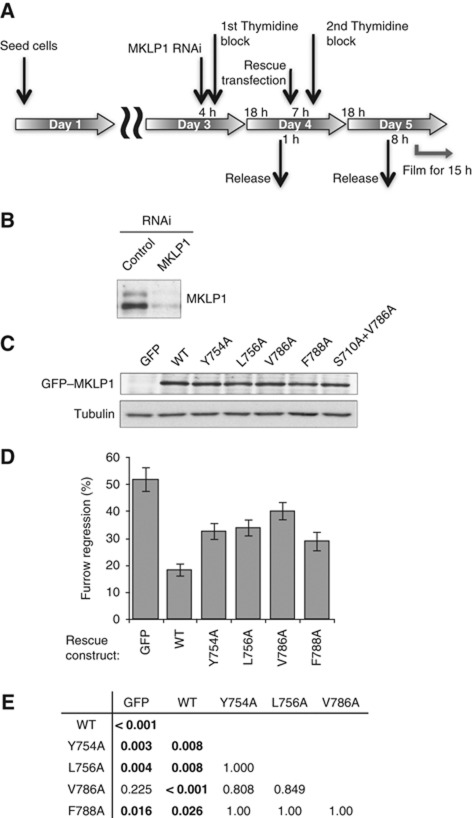
To examine in-vivo function of the MKLP1–ARF6 interaction, we tested the strongest ARF6-binding mutants of MKLP1 (Y754A, L756A, V786A and F788A) in RNAi-based knockdown and rescue assays coupled with cell-cycle synchronisation (Figure 4A). The endogenous MKLP1 was depleted with an siRNA against the 3′-untranslated region (Figure 4B) (Neef et al, 2006) and GFP–MKLP1 constructs were transiently expressed (Figure 4C). Cell division was observed by time-lapse imaging and cytokinesis failure of the cells expressing the transgene were scored (Figure 4D). Approximately 50% of the control cells failed cytokinesis. This defect was rescued to below 20% by expression of wild-type GFP–MKLP1. In contrast, all the ARF6-binding mutants (Y754A, L756A, V786A and F788A) showed significantly weaker rescue (Figure 4E for a table of P-values for multiple comparison), indicating the importance of the MKLP1–ARF6 interaction for successful cytokinesis. Cells expressing GFP–MKLP1-V786A failed cytokinesis almost as frequently as control cells rescued with GFP alone (P=0.225). Importantly, these cytokinesis defects are not due to differences in the level of transgene expression (Figure 4C). This result demonstrates the importance of the MKLP1–ARF6 interaction for successful completion of cytokinesis.
Figure 4.
Defects in the MKLP1–ARF6 interaction cause cytokinesis failure. (A) Schematic of the protocol for knockdown and rescue experiments. (B) Cells were synchronised and transfected with MKLP1 or control siRNA and lysed directly into SDS-loading buffer. Samples were separated using SDS–PAGE and probed for MKLP1 with anti-MKLP1 antibody by western blotting. (C) Cells were transiently transfected with GFP–MKLP1 variants as indicated. Whole cell lysate was probed for GFP–MKLP1 with anti-GFP antibody. (D) Quantitation of cytokinesis failures from live cell recordings of HeLa cells treated with MKLP1 siRNA and transfected with GFP vector or the MKLP1 variants as indicated. The graph shows the percentage of cells that failed cytokinesis averaged from three independent experiments in which at least 100 GFP–MKLP1 or GFP expressing cells were analysed. Error bars indicate the standard deviation. (E) The data of the cytokinesis failure were analysed by generalised linear model for binomial data and P-values after the Tukey correction for multiple comparison are shown.
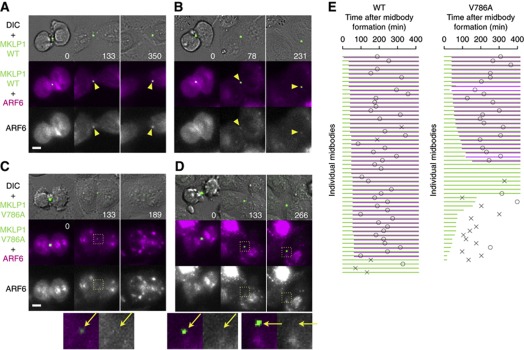
To analyse the details of the cytokinesis defects caused by lack of interaction between MKLP1 and ARF6, we cotransfected mCherry–ARF6 in the MKLP1 knockdown and rescue assay and performed dual-colour time-lapse observation (Figure 5). To examine whether the Flemming body localisation of ARF6 is actually dependent on its interaction with MKLP1, GFP–MKLP1 and mCherry–ARF6 double-positive cells that formed the GFP–MKLP1-positive midbody were analysed. ARF6 localisation to the ‘Golgi twins’-like vesicle compartments was normal in all the cells expressing MKLP1-V786A (Figure 5C and D, 0 min). This is consistent with the lack of colocalisation of MKLP1 and ARF6 during this stage and suggests that their interaction is not essential for the localisation of ARF6 to these vesicles. In contrast, the ARF6 recruitment to the Flemming bodies was often undetectable in the cells expressing MKLP1-V786A (Figure 5C and D, arrows). This provides evidence for the in-vivo interaction between MKLP1 and ARF6 through the C-terminal domain of MKLP1 and indicates the significance of this interaction for the recruitment of ARF6 to the Flemming body in the late midbody stage.
Figure 5.
Defects in the MKLP1–ARF6 interaction cause destabilisation of MKLP1 accumulated at the Flemming body. (A–D) Stills from the knockdown and rescue experiments by cotransfection of mCherry–ARF6 and GFP–MKLP1 constructs. Arrowheads and arrows indicate the Flemming bodies at which colocalisation of mCherry–ARF6 with GFP–MKLP1 was detected or not, respectively. Bar, 10 μm. (E) Schematic summary of the stability of the midbodies and the Flemming body localisation of ARF6. Data are from a single recording representative of triplicate experiments. Green and magenta lines represent the duration at which stable GFP–MKLP1 signal and mCherry–ARF6 colocalisation at the Flemming body were, respectively, detected by visual inspection. Circle and cross indicate the timing of abscission and regression, respectively.
Importantly, in 50% of the cells that failed to recruit ARF6 to the Flemming body, centralspindlin accumulation at the Flemming body was destabilised and lost in later stages, leading to regression of cleavage furrow (Figure 5C and E). This is not due to photo-bleaching since it was not observed with wild-type MKLP1 under the same illumination condition (Figure 5A, B and E). A similar phenotype was also observed in the knockdown and rescue assay without mCherry–ARF6 (data not shown). This supports the hypothesis that the MKLP1–ARF6 interaction might play an important role in the stable maintenance of centralspindlin at the Flemming body. It seems unlikely that the destabilisation of centralspindlin accumulation is the primary cause of the failure of ARF6 recruitment to the Flemming body because ARF6 failed to colocalise with MKLP1 even in some of the cells that could maintain centralspindlin accumulation for up to several hours (Figure 5D and E). Interestingly, these cells showed a significant delay of abscission, indicating that ARF6 at the Flemming body might also play a role in abscission although our method of abscission detection has a limitation that it cannot discriminate between the cells before abscission and the cells after abscission but with the midbody remnant staying at the border of the daughter cells and thus can only set an upper limit on the timing of abscission.
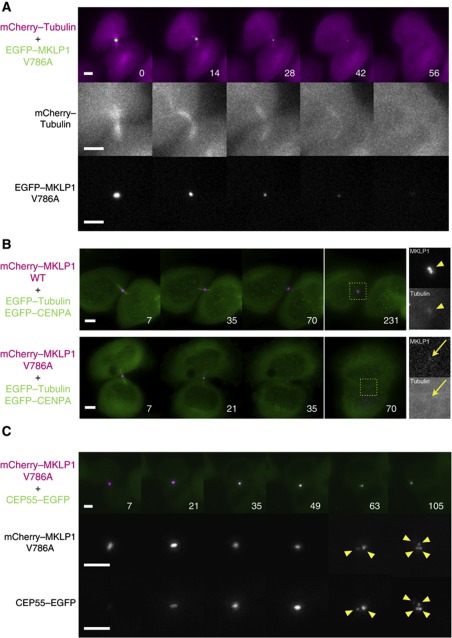
As centralspindlin is essential for the midbody formation, the destabilisation of centralspindlin accumulation at the Flemming body might cause the disintegration of the whole midbody. To test this possibility, we examined the effect of the MKLP1–ARF6 binding defect on the behaviour of other midbody components. When MKLP1-V786A mutant became destabilised earlier than the normal timing of microtubule disassembly, premature disassembly of midbody microtubules associated with the furrow regression was also observed (Figure 6A). In the cells rescued with wild-type MKLP1, a distinct and stable tubulin signal on the Flemming body before abscission or on the midbody remnant after abscission was detected (Figure 6B arrowhead, 9/9 midbody remnants detected by MKLP1). When the MKLP1-V786A signal at the Flemming body was lost, such a distinct tubulin signal became undetectable (Figure 6B arrow, 8/8 cells with decayed MKLP1 signal). CEP55 is a late midbody protein and plays an important role in recruiting ESCRT proteins to the site of abscission (Carlton and Martin-Serrano, 2007; Bastos and Barr, 2010). Similarly to tubulin, when MKLP1-V786A was lost from the midbody, CEP55 signal at the midbody was lost as well (not shown). We also observed fragmentation of CEP55 accumulation at the midbody into multiple smaller pieces in an almost identical manner to that observed for the mutant MKLP1 (Figure 6C, 30/30 midbodies whose fragmentation was detected by MKLP1 signal), which also resulted in the furrow regression. These observations indicate that disappearance of the MKLP1 mutant from the midbody reflects the disintegration of the whole of the Flemming body or the midbody remnant and ARF6–MKLP1 interaction plays an important role in the stable maintenance of the integrity of the midbody.
Figure 6.
Defects in the MKLP1–ARF6 interaction disintegrate the midbody. (A) Stills from the knockdown and rescue experiments with the GFP–MKLP1-V786A mutant performed in the HeLa cells expressing mCherry–tubulin. (B, C) Stills from the knockdown and rescue experiments with the mCherry–MKLP1-V786A mutant performed in the HeLa cells expressing GFP–tubulin and GFP–CENP-A (B) or GFP–CEP55 (C). Arrowheads in (B) indicate the intact midbody remnant with distinct signal for both MKLP1 and tubulin. Arrows indicate the residual MKLP1 signal in the regressing cell lacking a distinct tubulin signal. Arrowheads in (C) indicate the fragments from the disintegrating Flemming body. Bar, 5 μm.
Inhibition of the 14-3-3-centralspindlin interaction suppresses the midbody instability caused by the lack of ARF6–centralspindlin interaction
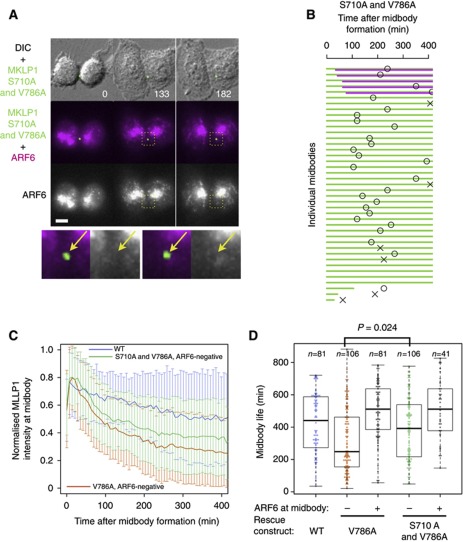
Considering the appearance of MKLP1 S710-monophosphorylation at the Flemming body (Douglas et al, 2010), disintegration of the midbody in V786A mutant cells might be caused by dispersion of centralspindlin by 14-3-3 protein, which targets and solubilises the S710-monophosphorylated form of centralspindlin (Douglas et al, 2010). If this is the case, mutation of S710 to alanine, which prevents 14-3-3 binding, should suppress the midbody destabilisation caused by the mutation of V786 to alanine. As expected, the majority of the cells expressing the S710A and V786A double-mutant MKLP1 did not restore the recruitment of ARF6 to the Flemming body (Figure 7A, arrows). However, the frequency of midbody disintegration was greatly reduced in the cells expressing S710A and V786A double mutant (Figure 7A and B), indicating that, in the absence of the interaction with 14-3-3, the interaction between MKLP1 and ARF6 is not essential for the stability of the Flemming body. This is consistent with the observation that deletion of a region encompassing both the Aurora B/14-3-3 motif and the ARF6-binding domain showed only a relatively mild cytokinesis defect in a knockdown and rescue assay (Matuliene and Kuriyama, 2004). The requirement of this interaction for abscission seems to be rather limited as a majority (83%) of these cells could complete cytokinesis without ARF6–MKLP1 colocalisation at the Flemming body in the absence of the MKLP1–14-3-3 interaction.
Figure 7.
Midbody destabilisation by the lack of MKLP1–ARF6 interaction is suppressed by preventing the MKLP1–14-3-3 interaction. (A) Stills from the knockdown and rescue experiments with the GFP–MKLP1 S710A V786A double mutant cotransfected with mCherry–ARF6. Bar, 10 μm. (B) Schematic summary of the stability of the midbodies and the Flemming body localisation of ARF6. (C) Plots of average normalised intensity of GFP–MKLP1 at the Flemming bodies. Error bars indicate the standard deviation. For the mutants, the data plotted were from the Flemming bodies on which ARF6 localisation was below a threshold (mode of the local Pearson’s correlation coefficient between GFP–MKLP1 and mCherry–ARF6 is below 0.2). (D) Plots of the lifetime of the midbodies defined as the period during which GFP–MKLP1 signal at the individual Flemming body was maintained above 20% of its maximum level. Box and whiskers represent median and quartiles, and maximum and minimum, respectively.
For further quantitative analysis of the stability (Figure 7C and D), the Flemming bodies formed in the cells expressing the GFP–MKLP1 mutants were objectively classified into groups with or without colocalisation of mCherry–ARF6 based on the local Pearson’s correlation coefficient (Figure 1B). Decay of GFP–MKLP1 signal at the ARF6-negative Flemming bodies caused by V786A mutation was suppressed by the additional mutation of S710 to alanine (Figure 7C). The life time of Flemming bodies that failed to recruit ARF6 due to V786A mutation was about half of the wild-type (Figure 7D). This was significantly prolonged by additional S710A mutation (Figure 7D, P=0.024 by Wilcoxon rank sum test). Suppression of V786A-induced midbody disintegration by disrupting the interaction with 14-3-3 indicates that the MKLP1–ARF6 interaction is required to oppose the action of 14-3-3 protein, that is, to protect centralspindlin accumulated at the Flemming body from dissipation by 14-3-3.
Discussion
ARF6 at the post-mitotic midbody protects centralspindlin from dissipation by 14-3-3
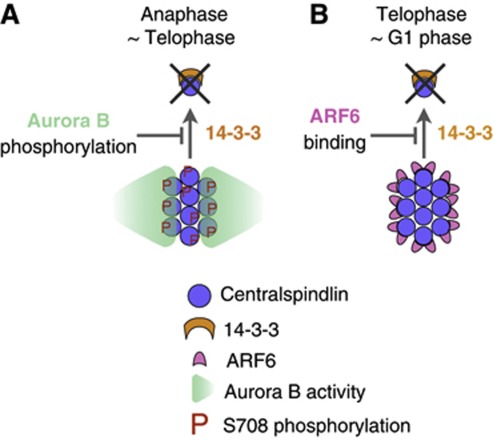
Here, we have revealed that the midbody is potentially a dynamic structure and that a post-mitotic cell stably maintains it by a different strategy from that for the earlier stage of cytokinesis (Figure 8). Centralspindlin has to form clusters for productive interaction with microtubules and to steeply and stably accumulate to the centre of the midbody (Hutterer et al, 2009). 14-3-3 sequesters centralspindlin as an unclustered, inactive form (Douglas et al, 2010). During the process of midbody formation in anaphase and early telophase, when Aurora B activity is still present and forms a local peak at the spindle midzone, Aurora B releases centralspindlin from sequestration by 14-3-3 protein (Douglas et al, 2010), spatially coupling chromosome segregation and cytokinesis (Figure 8A). In late telophase, when Aurora B activity becomes weaker, ARF6 recruited to the Flemming body gradually takes over the centralspindlin-stabilising role of Aurora B and protects centralspindlin from 14-3-3 protein (Figure 8B). Competition by a small GTPase provides a novel scheme of modulation of 14-3-3 activity that might be applicable to a broad range of 14-3-3–target interactions.
Figure 8.
ARF6 protects the post-mitotic midbody from disintegration by 14-3-3. Clustering of centralspindlin, which is antagonised by 14-3-3, is essential for its rapid and sharp accumulation to the central microtubule overlap zone of the central spindle and the midbody. (A) While Aurora B kinase activity is high during anaphase and early telophase, phosphorylation of MKLP1 by Aurora B prevents 14-3-3 from sequestering centralspindlin. (B) When Aurora B activity has post-mitotically declined, MKLP1–ARF6 interaction provides another mechanism to protect centralspindlin clusters from dispersion by 14-3-3.
ARF6 is a member of the ARF family, whose closely related members have distinct yet overlapping functions in multiple aspects of cellular activity. Although localisation to the midbody has not been reported so far for other ARF GTPases, considering the lack of the specificity in MKLP1 binding among ARF family proteins (Boman et al, 1999), other ARFs might also be able to protect centralspindlin from 14-3-3 especially in the absence of ARF6. This would explain the relatively mild phenotypes observed in the early embryonic development of ARF6-knockout mice and in the cultured cells depleted of ARF6 (Schweitzer and D’Souza-Schorey, 2005; Suzuki et al, 2006). To test the role of MKLP1–ARF6 interaction at the post-mitotic midbody from the side of ARF6, novel experimental tools are required that would enable us to shut down the activities of ARF proteins individually and in combination in a temporally controlled manner.
Our finding does not exclude other potential functions of ARF6 at the midbody. It will be interesting to investigate how this distinct role of ARF6 at the Flemming body is coordinated with its other functions such as the regulation of endosomal trafficking (Fielding et al, 2005; Montagnac et al, 2009), which plays critical roles in abscission (Carlton and Martin-Serrano, 2007; Morita et al, 2007; Sagona et al, 2010; Guizetti et al, 2011), in cooperation with other centralspindlin-interacting proteins (Tomas et al, 2004; Fabbro et al, 2005; Zhao et al, 2006; Simon et al, 2008; Bastos and Barr, 2010; Montembault et al, 2010). It has been reported that autophagy-mediated lysosomal degradation contributes to the elimination of midbody remnants after abscission (Pohl and Jentsch, 2009). We speculate that dispersion of centralspindlin by 14-3-3, which can potentially be triggered by activation of ARF6 GTPase, might provide an additional mechanism for this process.
Materials and methods
Antibody reagents
Antibodies against MKLP1 C-terminal peptide, MgcRacGAP/HsCYK-4 and S710-monophosphorylated MKLP1 were described previously (Mishima et al, 2002; Douglas et al, 2010). Commercial antibodies used were: JL-8 mouse monoclonal to GFP (Clontech); DM1α mouse monoclonal to α-tubulin (Sigma); N19 rabbit polyclonal to human MKLP1 (Santa Cruz).
Protein purification
Recombinant human ARF6 and its variants (cDNA provided by H McMahon, MRC Laboratory of Molecular Biology, Cambridge UK) tagged with GST was affinity purified from bacteria using glutathione-Sepharose beads (GE Healthcare) in 10 mM HEPES (pH 7.5), 1 mM EGTA, 1 mM MgCl2, 0.1% (v/v) Triton X-100, 1 mM dithiothreitol (DTT), 10 μg/ml leupeptin, 10 μg/ml pepstatin, 1 mM phenylmethanesulfonyl fluoride and 100 mM NaCl. When required, the bound protein was eluted using the same buffer containing 10 mM reduced L-glutathione (Sigma) or by thrombin digestion to remove GST (Thrombin cleavage-capture kit, Merck Biosciences). Recombinant human 14-3-3ζ tagged with GST was similarly purified using the above buffer containing 250 mM NaCl and the tag cleaved by thrombin digestion. MKLP1 fragments tagged with maltose-binding protein were purified from bacteria using amylose beads (New England Biolabs) in the same buffer as described above.
Cell culture, transfection and RNA interference
HeLa cells were cultured in DMEM (Invitrogen) with 10% fetal bovine serum (FBS) (Invitrogen). HeLa cells stably expressing GFP-tagged MKLP1, Aurora B and CEP55 from their own promoters were previously described (Poser et al, 2008; Hutterer et al, 2009). HeLa cells stably expressing mCherry–tubulin (Kaseda et al, 2012) and both GFP–tubulin and GFP–CENP-A were provided by Dr Andrew McAinsh and Dr Patrick Meraldi, respectively. Cells for time-lapse microscopy were grown on Delta T culture dish (Bioptechs) or Fluorodish (World Precision Instruments). MKLP1 (N-terminal EGFP or mCherry) (Douglas et al, 2010), RAB11a (N-terminal EGFP or mCherry (GenBank: ACF75945.1)), cloned from HeLa cell cDNA library by PCR) and ARF6 (internal mCherry, constructed by replacing CyPET in pEF4-CyPET-ARF6 (Hall et al, 2008) (Addgene) with mCherry) mammalian expression constructs were transfected using Lipofectamine 2000 (Invitrogen) in DMEM containing 5% FBS without antibiotics and the complex was washed off from cells after 3 h. For knockdown and rescue experiments, HeLa cells in Opti-MEM (Invitrogen) were treated with siRNA (100 nM final concentration) targeted to the 3′-UTR of MKLP1 with the sequence 5′-AAGCAGUCUUCCAGGUCAUCUUU-3′ (Neef et al, 2006) using Oligofectamine (Invitrogen). After 4 h, cells were supplemented with 10% FBS and arrested for 18 h with 2.5 mM thymidine. One hour after release from the thymidine arrest, cells were transfected with DNA. The second thymidine arrest (18 h) was started 8 h after the first release. Release from metaphase arrest was performed as described (Petronczki et al, 2007).
Microscopy
Live cell imaging was performed from 8 h after the final release for 15 h at 37°C on a DeltaVision system (Applied Precision) equipped with a × 40 UPlanFLN objective (Olympus) and a Cascade II 512B EM-CCD camera (Photometrics). Z-stacks of 1.5 μm × 10 were recorded at 30 locations every 7 min. For dual-colour observation of ARF6 and Rab11a (Supplementary Figure S3B), 1 μm × 15 z-stacks were recorded with a × 60 PlanApoN objective (Olympus). The time-lapse images were analysed using OMERO (Swedlow et al, 2009) (http://www.openmicroscopy.org/) and ImageJ (Rasband WS, ImageJ, US National Institutes of Health, Bethesda, Maryland, USA; http://imagej.nih.gov/ij/, 1997–2011) software. For presentation of fluorescence image, three (GFP–MKLP1) or five (mCherry–ARF6) z-sections around the focal plane at the centre of the midbody were maximum projected. For quantification of the fluorescence intensity at the midbody, the mean intensity inside a circle of a radius of 2 (MKLP1) or 3 (Aurora B) pixels (1 pixel=0.411 μm) centred at the peak was subtracted by a local background value, that is, the mean intensity in a surrounding ring-like area of an inner radius of 3 (MKLP1) or 4 (Aurora B) pixels and an outer radius of 5 (MKLP1) or 6 (Aurora B) pixels.
Binding experiments
HeLa cells arrested with nocodazole (40 ng/ml) and treated with 2 μM ZM447439 (Tocris Bioscience) were lysed in buffer containing 20 mM PIPES (pH 7), 2 mM EGTA, 2 mM MgCl2, 0.1% (v/v) Triton X-100, 1 mM DTT, 10 μg/ml leupeptin, 10 μg/ml pepstatin, 1 mM phenylmethanesulfonyl fluoride, 20 mM NaF, 5 μM microcystin and 150 mM NaCl and clarified by centrifugation. The crude extract (1 mg/ml total protein) was mixed with glutathione-Sepharose beads (GE Healthcare) coated with GST–ARF6 or GST–14-3-3ζ for 2 h with rotation at 4°C. Beads were washed three times with lysis buffer, and bound proteins were resolved by SDS–PAGE and detected by western blotting. For competition, the lysate was supplemented with 1.5 mg/ml 14-3-3ζ or ARF6. Y2H analysis was performed with the ProQuest Two-Hybrid System (Invitrogen) as described (Douglas et al, 2010).
Supplementary Material
Acknowledgments
We thank J Mason, A Sossick and F Gergely for technical help; AA Hyman and H McMahon for reagents and C Lindon for helpful discussion. We also thank M Douglas, T Davies, M Petronczki, A McAinsh and R Cross for critical comments on the manuscripts and for helpful suggestions. This research was supported by Cancer Research UK programme Grants C19769/A6356 and A11985 (MM), Human Frontier Science Program (AH) and by core infrastructure supports from Cancer Research UK and the Wellcome Trust. Work of IP at the Hyman laboratory was supported by the European Community’s Seventh Framework Programme (FP7/2007–2013) under Grant agreement 241548 (MitoSys Project).
Author contributions: Project was designed by MM and NJ. All the experiments were performed by NJ except for establishment of BAC-transformed cells (IP), live imaging in Figure 1A (AH) and experiments in Figure 6; Supplementary Figures S1, S2 and S5 (MM). Data were analysed by NJ and MM. The manuscript was written by MM and NJ.
Footnotes
The authors declare that they have no conflict of interest.
References
- Barr FA, Gruneberg U (2007) Cytokinesis: placing and making the final cut. Cell 131: 847–860 [DOI] [PubMed] [Google Scholar]
- Bastos RN, Barr FA (2010) Plk1 negatively regulates Cep55 recruitment to the midbody to ensure orderly abscission. J Cell Biol 191: 751–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman AL, Kuai J, Zhu X, Chen J, Kuriyama R, Kahn RA (1999) Arf proteins bind to mitotic kinesin-like protein 1 (MKLP1) in a GTP-dependent fashion. Cell Motility Cytoskeleton 44: 119–132 [DOI] [PubMed] [Google Scholar]
- Carlton JG, Martin-Serrano J (2007) Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science 316: 1908–1912 [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Jackson CL (2011) ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat Rev Mol Cell Biol 12: 362–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas ME, Davies T, Joseph N, Mishima M (2010) Aurora B and 14-3-3 coordinately regulate clustering of centralspindlin during cytokinesis. Curr Biol 20: 927–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas ME, Mishima M (2010) Still entangled: assembly of the central spindle by multiple microtubule modulators. Semin Cell Dev Biol 21: 899–908 [DOI] [PubMed] [Google Scholar]
- Dyer N, Rebollo E, Domínguez P, Elkhatib N, Chavrier P, Daviet L, González C, González-Gaitán M (2007) Spermatocyte cytokinesis requires rapid membrane addition mediated by ARF6 on central spindle recycling endosomes. Development (Cambridge, England) 134: 4437–4447 [DOI] [PubMed] [Google Scholar]
- Fabbro M, Zhou BB, Takahashi M, Sarcevic B, Lal P, Graham ME, Gabrielli BG, Robinson PJ, Nigg EA, Ono Y, Khanna KK (2005) Cdk1/Erk2- and Plk1-dependent phosphorylation of a centrosome protein, Cep55, is required for its recruitment to midbody and cytokinesis. Dev Cell 9: 477–488 [DOI] [PubMed] [Google Scholar]
- Fielding AB, Schonteich E, Matheson J, Wilson G, Yu X, Hickson GR, Srivastava S, Baldwin SA, Prekeris R, Gould GW (2005) Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J 24: 3389–3399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller BG, Lampson MA, Foley EA, Rosasco-Nitcher S, Le KV, Tobelmann P, Brautigan DL, Stukenberg PT, Kapoor TM (2008) Midzone activation of aurora B in anaphase produces an intracellular phosphorylation gradient. Nature 453: 1132–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaietta GM, Giepmans BN, Deerinck TJ, Smith WB, Ngan L, Llopis J, Adams SR, Tsien RY, Ellisman MH (2006) Golgi twins in late mitosis revealed by genetically encoded tags for live cell imaging and correlated electron microscopy. Proc Natl Acad Sci USA 103: 17777–17782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M (2009) The 3Ms of central spindle assembly: microtubules, motors and MAPs. Nat Rev Mol Cell Biol 10: 9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromley A, Yeaman C, Rosa J, Redick S, Chen CT, Mirabelle S, Guha M, Sillibourne J, Doxsey SJ (2005) Centriolin anchoring of exocyst and SNARE Complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell 123: 75–87 [DOI] [PubMed] [Google Scholar]
- Guizetti J, Gerlich DW (2010) Cytokinetic abscission in animal cells. Semin Cell Dev Biol 21: 909–916 [DOI] [PubMed] [Google Scholar]
- Guizetti J, Schermelleh L, Mäntler J, Maar S, Poser I, Leonhardt H, Müller-Reichert T, Gerlich DW (2011) Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science 331: 1616–1620 [DOI] [PubMed] [Google Scholar]
- Guse A, Mishima M, Glotzer M (2005) Phosphorylation of ZEN-4/MKLP1 by Aurora B regulates completion of cytokinesis. Curr Biol 15: 778–786 [DOI] [PubMed] [Google Scholar]
- Hall B, McLean MA, Davis K, Casanova JE, Sligar SG, Schwartz MA (2008) A fluorescence resonance energy transfer activation sensor for Arf6. Anal Biochem 374: 243–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutterer A, Glotzer M, Mishima M (2009) Clustering of centralspindlin is essential for its accumulation to the central spindle and the midbody. Curr Biol 19: 2043–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaseda K, McAinsh AD, Cross RA (2012) Dual pathway spindle assembly increases both the speed and the fidelity of mitosis. Open Biol 1: 12–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuliene J, Kuriyama R (2002) Kinesin-like protein CHO1 is required for the formation of midbody matrix and the completion of cytokinesis in mammalian cells. Mol Biol Cell 13: 1832–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuliene J, Kuriyama R (2004) Role of the midbody matrix in cytokinesis: RNAi and genetic rescue analysis of the mammalian motor protein CHO1. Mol Biol Cell 15: 3083–3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima M, Kaitna S, Glotzer M (2002) Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev Cell 2: 41–54 [DOI] [PubMed] [Google Scholar]
- Mollinari C, Kleman JP, Jiang W, Schoehn G, Hunter T, Margolis RL (2002) PRC1 is a microtubule binding and bundling protein essential to maintain the mitotic spindle midzone. J Cell Biol 157: 1175–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnac G, Sibarita JB, Loubéry S, Daviet L, Romao M, Raposo G, Chavrier P (2009) ARF6 Interacts with JIP4 to control a motor switch mechanism regulating endosome traffic in cytokinesis. Curr Biol 19: 184–195 [DOI] [PubMed] [Google Scholar]
- Montembault E, Zhang W, Przewloka MR, Archambault V, Sevin EW, Laue ED, Glover DM, D'Avino PP (2010) Nessun Dorma, a novel centralspindlin partner, is required for cytokinesis in Drosophila spermatocytes. J Cell Biol 191: 1351–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita E, Sandrin V, Chung HY, Morham SG, Gygi SP, Rodesch CK, Sundquist WI (2007) Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J 26: 4215–4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef R, Klein UR, Kopajtich R, Barr FA (2006) Cooperation between mitotic kinesins controls the late stages of cytokinesis. Curr Biol 16: 301–307 [DOI] [PubMed] [Google Scholar]
- Otegui MS, Verbrugghe KJ, Skop AR (2005) Midbodies and phragmoplasts: analogous structures involved in cytokinesis. Trends Cell Biol 15: 404–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronczki M, Glotzer M, Kraut N, Peters JM (2007) Polo-like kinase 1 triggers the initiation of cytokinesis in human cells by promoting recruitment of the RhoGEF Ect2 to the central spindle. Dev Cell 12: 713–725 [DOI] [PubMed] [Google Scholar]
- Piel M, Nordberg J, Euteneuer U, Bornens M (2001) Centrosome-dependent exit of cytokinesis in animal cells. Science 291: 1550–1553 [DOI] [PubMed] [Google Scholar]
- Pohl C, Jentsch S (2009) Midbody ring disposal by autophagy is a post-abscission event of cytokinesis. Nat Cell Biol 11: 65–70 [DOI] [PubMed] [Google Scholar]
- Poser I, Sarov M, Hutchins JR, Hériché JK, Toyoda Y, Pozniakovsky A, Weigl D, Nitzsche A, Hegemann B, Bird AW, Pelletier L, Kittler R, Hua S, Naumann R, Augsburg M, Sykora MM, Hofemeister H, Zhang Y, Nasmyth K, White KP et al. (2008) BAC TransgeneOmics: a high-throughput method for exploration of protein function in mammals. Nat Methods 5: 409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagona AP, Nezis IP, Pedersen NM, Liestøl K, Poulton J, Rusten TE, Skotheim RI, Raiborg C, Stenmark H (2010) PtdIns(3)P controls cytokinesis through KIF13A-mediated recruitment of FYVE-CENT to the midbody. Nat Cell Biol 12: 362–371 [DOI] [PubMed] [Google Scholar]
- Schweitzer JK, D'Souza-Schorey C (2002) Localization and activation of the ARF6 GTPase during cleavage furrow ingression and cytokinesis. J Biol Chem 277: 27210–27216 [DOI] [PubMed] [Google Scholar]
- Schweitzer JK, D’Souza-Schorey C (2005) A requirement for ARF6 during the completion of cytokinesis. Exp Cell Res 311: 74–83 [DOI] [PubMed] [Google Scholar]
- Schweitzer JK, Sedgwick AE, D’Souza-Schorey C (2011) ARF6-mediated endocytic recycling impacts cell movement, cell division and lipid homeostasis. Semin Cell Dev Biol 22: 39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GC, Schonteich E, Wu CC, Piekny A, Ekiert D, Yu X, Gould GW, Glotzer M, Prekeris R (2008) Sequential Cyk-4 binding to ECT2 and FIP3 regulates cleavage furrow ingression and abscission during cytokinesis. EMBO J 27: 1791–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers WG, Saint R (2003) A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev Cell 4: 29–39 [DOI] [PubMed] [Google Scholar]
- Steigemann P, Wurzenberger C, Schmitz MH, Held M, Guizetti J, Maar S, Gerlich DW (2009) Aurora B-mediated abscission checkpoint protects against tetraploidization. Cell 136: 473–484 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kanai Y, Hara T, Sasaki J, Sasaki T, Kohara M, Maehama T, Taya C, Shitara H, Yonekawa H, Frohman MA, Yokozeki T, Kanaho Y (2006) Crucial role of the small GTPase ARF6 in hepatic cord formation during liver development. Mol Cell Biol 26: 6149–6156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedlow JR, Goldberg IG, Eliceiri KW (2009) Bioimage informatics for experimental biology. Annu Rev Biophys 38: 327–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Takei T, Koga H, Takatsu H, Shin HW, Nakayama K (2011) Distinct roles of Rab11 and Arf6 in the regulation of Rab11-FIP3/arfophilin-1 localization in mitotic cells. Genes Cells 16: 938–950 [DOI] [PubMed] [Google Scholar]
- Tomas A, Futter C, Moss SE (2004) Annexin 11 is required for midbody formation and completion of the terminal phase of cytokinesis. J Cell Biol 165: 813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Prekeris R, Gould GW (2007) Role of endosomal Rab GTPases in cytokinesis. Eur J Cell Biol 86: 25–35 [DOI] [PubMed] [Google Scholar]
- Zeitlin SG, Sullivan KF (2001) Animal cytokinesis: breaking up is hard to do. Curr Biol 11: R514–R516 [DOI] [PubMed] [Google Scholar]
- Zhao WM, Seki A, Fang G (2006) Cep55, a microtubule-bundling protein, associates with centralspindlin to control the midbody integrity and cell abscission during cytokinesis. Mol Biol Cell 17: 3881–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.