Abstract
Cirrhotic patients are immunocompromised with a high risk of infection. Proinflammatory cytokines and hemodynamic circulation derangement further facilitate the development of serious consequences of infections. Other than spontaneous bacterial peritonitis, bacteremia and bacterial infections of other organ systems are frequently observed. Gram-negative enteric bacteria are the most common causative organism. Other bacterial infections, such as enterococci, Vibrio spp., Aeromonas spp., Clostridium spp., Listeria monocytogenes, Plesiomonas shigelloides and Mycobacterium tuberculosis are more prevalent and more virulent. Generally, intravenous third generation cephalosporins are recommended as empirical antibiotic therapy. Increased incidences of gram-positive and drug-resistant organisms have been reported, particularly in hospital-acquired infections and in patients receiving quinolones prophylaxis. This review focuses upon epidemiology, microbiology, clinical features and treatment of infections in cirrhosis other than spontaneous bacterial peritonitis, including pathogen-specific and liver disease-specific issues.
Keywords: Bacterial infection, Cirrhosis
INTRODUCTION
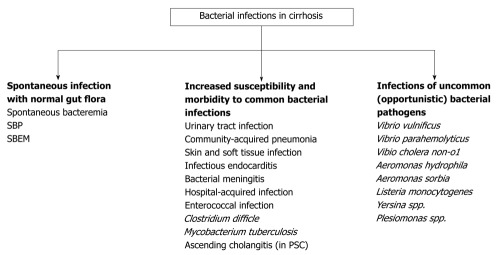
Despite the advancement in medical care for patients with advanced liver disease in the past decades, bacterial infections remain very common and account for significant morbidity and mortality (approximately 30%) in these patients[1,2]. Cirrhosis is an immunocompromised state which predisposes the patient to a variety of infections[3]. Once infection occurs, the proinflammatory cytokines and hemodynamic circulation derangement further facilitate the development of serious consequences of infections such as septic shock, multiple organ failure and death[3]. Bacterial infections are commonly caused by gram-negative enteric bacteria; however, a number of uncommon pathogens are also more frequently observed and more virulent in cirrhotic patients. Moreover, these pathogens can present with various clinical syndromes which may be difficult to recognize. Appropriate preventive measurements have been shown to reduce the risk of overall bacterial infections. Early recognition and prompt management are warranted in order to minimize their complications. The outline of bacterial infection in cirrhotic patients is shown in Figure 1.
Figure 1.
The outline of bacterial infection in cirrhotic patients. SBP: Spontaneous bacterial peritonitis; SBEM: Spontaneous bacterial empyema; PSC: Primary sclerosing cholangitis.
EPIDEMIOLOGY
Bacterial infection is responsible for approximately 30%-50% of deaths in cirrhotic patients[1,3-5]. Compared to a 5%-7% infection rate reported in hospitalized patients in general, those hospitalized with cirrhosis have an infection rate of 32%-34%[6,7] and which may be up to 45% in those with gastrointestinal bleeding[8]. The most common bacterial infections are spontaneous bacterial peritonitis (SBP) (25%-31%), urinary tract infection (UTI) (20%-25%), pneumonia (15%-21%), bacteremia (12%) and soft tissue infection (11%)[7,9]. Approximately 75% of bacterial infections in patients with cirrhosis are caused by gram-negative bacteria, e.g. Escherichia coli, Klebsiella spp., Enterobacter spp., P. aeruginosa, Vibrio spp., Aeromonas spp., whereas gram positive comprise 20.2% and anaerobes only 3.2%[10]. However, in cirrhotic patients who had been hospitalized, received quinolones prophylaxis and had invasive procedures, the risk of gram-positive organisms is more frequently encountered (38%-70%)[7,11,12]. In addition, resistant organisms are isolated in up to 64% of hospital-acquired infection and are associated with poor outcomes[12].
PATHOGENESIS AND CONSEQUENCES OF INFECTION IN CIRRHOSIS
State of immune dysfunction in cirrhosis
Cirrhotic patients are in a multifactorial state of local and systemic immune dysfunction[3]. Porto-systemic shunting allows less bacteria and endotoxins to be cleared by the liver from the portal circulation[1]. Systemic reticuloendothelial system function is also significantly impaired[1,3,13]. Cirrhosis is associated with a decrease in bactericidal activity of phagocytic cells, an impaired opsonic activity and a reduction in complement and protein C levels[1,3,13]. In addition, immunodeficiency state can be further complicated by compelling factors such as skin/mucosal problems, malnutrition, alcohol intake and immunomodulatory therapy (Table 1).
Table 1.
Immune dysfunction in cirrhotic patients
| Natural barriers | Fragile, thin and/or edematous skin Alteration of gastrointestinal motility, mucosal permeability and bacterial flora ↑ Gastrointestinal mucosal ulcerations |
| Hepatic RES activity | Portosystemic shunting Kupffer cells – ↓ number, impaired function |
| Cellular defense mechanisms | RES – ↓ activation, ↓ chemotaxis, ↓ phagocytosis, ↓ production of pro-inflammatory cytokines PMN – ↓ lifespan, ↓ intracellular killing activity, ↓ phagocytosis, ↓ chemotaxis |
| Serum factors | ↓ Complement levels (C3, C4, CH50) ↓ Opsonic activity ↓ Protein C activity |
| Iatrogenic and treatment-related factors | Invasive procedure and catheters Medications: immunosuppressive agents, proton pump inhibitors |
| Other compelling factors | Malnutrition Alcohol drinking |
RES: Reticuloendothelial system; PMN: Polymorphonuclear neutrophil.
Bacterial translocation
Bacterial translocation is defined as the migration of bacteria or bacterial products from the intestinal lumen to mesenteric lymph nodes and other extra-intestinal sites. It has been implicated as the key step in the pathogenesis of SBP and spontaneous bacteremia in cirrhotic patients. The mechanisms of bacterial translocation are complex and not yet completely understood. Immune dysfunction, intestinal bacterial overgrowth and altered intestinal permeability are hypothesized to contribute to the development of bacterial translocation[14]. Gram-negative enteric bacteria, enterococci and other streptococci have been reported to be the most adept at translocating to mesenteric lymph nodes. More recently, it has been linked to the development of the hyperdynamic circulatory state in cirrhosis, characterized by splanchnic and systemic vasodilatation, increased cardiac output and decreased arterial blood pressure[1]. An amplification of bacteria and their products can lead to activation of monocytes, lymphocytes and pro-inflammatory cytokines, which exacerbate the pre-existing hyperdynamic circulation in cirrhosis[1,14].
Systemic inflammatory response syndrome and sepsis in cirrhosis
Cirrhotic patients are prone to develop sepsis, septic shock, sepsis-induced organ failure and death[3,15]. In cirrhosis, bacterial infection is accompanied by an imbalanced cytokine response, which converts responses that are normally helpful against infections into excessive, detrimental inflammation[3,15]. The pathophysiology of the exaggerating inflammatory response in cirrhotic patients has been postulated. In the early stage of sepsis, bacteria and their products, particularly lipopolysaccharides, activate toll-like receptor-4, which induces the release of pro-inflammatory cytokines[5,15]. Nitric oxide (NO), a key mediator contributing to a circulation compromised in septic patients, is markedly released in infected cirrhotic patients[5,15].
A pre-existing hyperdynamic circulatory state predisposes devastating consequences from a sepsis-induced NO and cytokine storm which eventually leads to refractory hypotension, inadequate tissue perfusion, multiorgan failure and death[1,5,15]. Additional factors, such as relative adrenal insufficiency[16], beta-blockers[17], low levels of protein C and high-density lipoprotein, may further adversely complicate the course of sepsis in cirrhosis[3].
Clinical consequences and prognosis of infections in cirrhosis
Bacterial infection in cirrhotic patients is associated with poor clinical outcomes (up to 4-fold mortality)[2]. The mortality rate of sepsis in cirrhotic patients is approximately 26%-44%[2,13].
A recent analytical review of 11 987 cirrhotic patients suggested several clinical predictors of death after infection, such as advanced liver disease, presence of shock and/or organ failure (particularly kidneys), gastrointestinal bleeding, encephalopathy, hepatocellular carcinoma and nosocomial acquisition[2]. Patients who survived a significant episode of infection are still at high risk of death (up to 30%) within 1 year[2].
Acute renal dysfunction following infections has been observed in 27%-34% of patients with advanced cirrhosis[18-20]. Thus, it is a strong independent risk of death in these patients with a 40%-50% mortality rate[2,19,20]. Several risk factors for the development of renal failure in cirrhotic patients with bacterial infections include advanced liver disease[19-21], pre-existing renal insufficiency[21], inadequate circulatory volume[19], low baseline cardiac output[22], lack of resolution of infection[20] and not receiving early albumin infusion[18]. Renal failure that does not respond to albumin infusion in the setting of bacterial infection without septic shock was recently considered hepatorenal syndrome (HRS)[23]. Sepsis-related renal failure and HRS can persistently progress despite the resolution of infection, thus needing further special interventions[18].
Bacterial infections can precipitate a rapid deterioration of liver functions and encephalopathy which is associated with poor short-term prognosis[1,15]. Pulmonary complications are increasingly common in cirrhotic patients. Acute respiratory distress syndrome may develop as a result of exaggerated systemic inflammatory response syndrome (SIRS) in severe sepsis which leads to higher mortality[24]. Aspiration is common in encephalopathic patients. Prognosis of cirrhotic patients who were intubated were dismal, with a 33%-60% mortality rate[25].
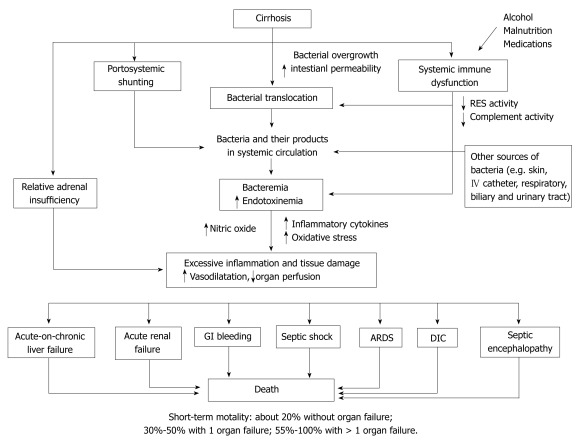
The effects of sepsis on coagulation cascades are more complex in cirrhosis. Patients with advanced cirrhosis are associated with thrombocytopenia and low clotting factors (e.g. factor V, VII, X and prothrombin). The consumption of coagulation factors and the enhanced fibrinolytic activity by sepsis-induced inflammatory cytokines leads to a further worsening of pre-existing coagulation and platelet abnormalities[5,15,26]. Presence of bacterial infection in patients with variceal bleeding is independently associated with failure to control and early recurrent bleeding[27]. Antibiotic prophylaxis in cirrhotic patients with variceal hemorrhage decreases infections, rebleeding and mortality[28] (Figure 2).
Figure 2.
Proposed pathogenesis and consequences of bacterial infections in cirrhotic patients. GI: Gastroenterology; RES: Reticuloendothelial system; ARDS: Acute respiratory distress syndrome; DIC: Disseminated intravascular coagulation.
ORGAN-SPECIFIC INFECTIONS
Urinary tract infection
UTI is the second most common bacterial infection in cirrhosis after SBP[7]. In cirrhotic patients, the prevalence of bacterinuria is 16%-18%, which is twice as frequent as matched controls[29,30] and which may be attributed to increased bladder post-void residual volume in cirrhotic patients[31]. Notably, bacterinuria is not consistently associated with an increased risk of sepsis[29]. Several predisposing factors for UTI have been suggested, including advanced liver disease, urinary catheter and female sex[13,29,30]. As in non-cirrhotic individuals, the common pathogens are gram-negative bacilli and coagulase-negative staphylococcus[29,30]. Treatment with cephalosporins or quinolones is generally effective. Notably, a high prevalence of resistant bacteria has recently been reported, not only in hospital-acquired (69%), but also community-acquired UTI (22%)[32].
Pneumonia
Pulmonary infections are common in cirrhotic patients. The causative organisms of community-acquired pneumonia appear to be the same as in general adults[33]. Compared to non-cirrhotic, cirrhotic patients with community-acquired pneumonia are more frequently associated with bacteremia, multi-lobar involvement, impaired consciousness, renal failure, septic shock and death (overall mortality 7.4% vs 14.4%, P < 0.024)[34]. Excessive alcohol intake can further impair pulmonary host defense and increase the risk of oropharyngeal aspiration[35]. Careful monitoring and empirical treatment with intravenous beta-lactams plus macrolides or intravenous anti-pneumococcal quinolones is recommended[33]. The risk of hospital-acquired pneumonia is increased in cirrhosis, particularly in the setting of gastrointestinal hemorrhage, tracheal intubation and encephalopathy. Thus, it is associated with resistant organisms and dreadful outcomes[7,12]. Empirical antibiotics for cirrhotic patients with hospital-acquired pneumonia should include intravenous anti-pseudomonal cephalosporins, carbapenams or piperacillin-tazobactam, plus ciprofloxacin or levofloxacin, and vancomycin or linezolid[36].
Bacteremia
Bacteremia without particular organ-specific source is increasingly common in cirrhosis and can be arbitrarily divided into 2 entities: (1) primary or spontaneous bacteremia; and (2) secondary bacteremia. True primary bacteremia shares the same initial step of pathogenesis as SBP, whereby bacteria flora in the gut lumen translocate into the bloodstream. It is generally encountered in the setting of advanced cirrhosis and is often caused by gram-negative enteric bacilli, enterococci and Streptococcus spp.[7,11].
Secondary bacteremia, in which pathogens come from an exogenous source, can be observed either in community-acquired settings, such as gastrointestinal bleeding, wound exposure and food-borne, or in healthcare-associated settings, such as transarterial chemoembolization[37], transjugular intrahepatic portosystemic shunt[38], therapeutic endoscopy[39] and intravenous catheters[7]. The causative organisms are largely dependent on the origin of bacteremia. Bacteremia and/or SBP occur in 17%-45% of patients following an episode of gastrointestinal bleeding[8] and, like those patients with primary bacteremia, the causative organisms are typically gram-negative enteric bacteria. Bacteremia associated with invasive procedures is commonly caused by S. aureus and S. epidermidis[7,11]. Notably, methicillin-resistant S. aureus (MRSA) was reported in up to 35% of nosocomial bacteremia in cirrhotic patients[11]. Although relatively uncommon, several case reports and case series have reported cases of severe community-acquired bacteremia in cirrhotic patients caused by Vibrio spp. and Aeromonas spp. without obviously localized infection[40-45]. Previous exposure to flood or seawater, or prior consumption of uncooked seafood may be a clue for diagnosis[40-45]. Intravenous third-generation cephalosporins and/or fluoroquinolones are commonly used as an empirical therapy for community-acquired bacteremia without other risk for specific or resistant pathogens. The use of antipseudomonal and glycopeptide antibiotics should be considered for hospital-acquired infection depending on the local pattern of resistant bacteria.
Spontaneous bacterial empyema
Spontaneous bacterial empyema (SBEM) is the infection of a pre-existing hydrothorax in which pneumonia has been excluded. It has been reported to be present in 10%-20% of hospitalized patients with hepatic hydrothorax[46-48]. The pathogenesis of SBEM, SBP and spontaneous bacteremia are closely interconnected; thus, they share the same types of common pathogens. SBEM can occur either with SBP, through transdiaphragmatic spread, or without SBP, through hematogenous spread (53% associated with SBP, 30% had no clinical ascites and 17% had non-infected ascites)[49]. Therefore, thoracocentesis should be performed when an infection is suspected in cirrhotic patients with ascites and hydrothorax, particularly in those with non-infected ascites. Risk factors for developing SBEM are the presence of SBP, low pleural fluid protein and complement levels, and advanced liver disease[47,50].
The criteria for diagnosis are: (1) pleural fluid polymorphonuclear neutrophil (PMN) ≥ 250 cell/mm3 with a positive culture or ≥ 500 cells/mm3 with a negative culture; and (2) exclusion of parapneumonic infection[46,49]. Notably, culture of pleural fluid should be performed by inoculating 10 mL of pleural fluid into a blood culture bottle at bedside, which is the same as the standard recommendation for SBP[49,51]. Analysis of pleural fluid by reagent strip for leukocyte esterase might be a rapid and easy-to-use tool for the detection of SBEM[52]. Hospital mortality has been reported as 20%-40% in cirrhotic patients with SBEM[46-48]. Treatment with intravenous third-generation cephalosporin should be initiated immediately when pleural fluid PMN ≥ 250 cell/mm3 while awaiting culture result. In cases with slow clinical recovery, a repeat thoracocentesis is suggested to document the treatment response. Chest tube drainage can be harmful in cirrhotic patients with hepatic hydrothorax and should not be used in the treatment of SBEM[49].
Skin and soft tissue infection
Several reasons can contribute to an increased risk of skin and soft tissue infection (SSTI) in cirrhotic patients, such as fragile, thin and edematous skin, poor hygiene standards, malnutrition, frequent hospitalization and invasive procedures. Antibiotic therapy is generally effective for mild cellulitis; however, it is associated with a considerable recurrence rate of 21%[53]. Attention must be paid to severe cellulitis and necrotizing fasciitis that are increasingly reported and often carry a high mortality rate in cirrhotic patients, ranging from 6%-76% depending on the pathogens, extension of disease, presence of hemorrhagic bullae, severity of cirrhosis and management[54-56].
The common causative organisms are gram-positive cocci (S. aureus, beta-hemolytic streptococci) and gram-negative enteric bacteria (occasionally polymicrobial)[54]. Remarkably, the incidence of gram-negative pathogens, such as E. coli, Klebsiella spp., P. aeruginosa, Aeromonas spp., Vibrio spp., has evidently increased in cirrhotic patients[42-45,55-57]. Unlike the general population, necrotizing fasciitis in cirrhotic patients sometimes develops without an obvious portal of entry in the extremities, thereby suggesting a potential alternative pathway of bacterial translocation and bacteremia leading to SSTI[55,56]. In addition, approximately two thirds of cirrhotic patients with necrotizing fasciitis caused by gram-negative pathogens had concurrent bacteremia and/or initially presented with septic shock[55]. The presence of severe pain and/or SIRS out of proportion to the local wound appearance raises the possibility of necrotizing fasciitis. Careful evaluation with a high index of suspicion is mandatory since an early surgical intervention has been shown to reduce morbidity and mortality in necrotizing fasciitis[54-56,58].
There is no specific guideline for the empirical antibiotic therapy for severe SSTI in cirrhosis. Given a high morbidity/mortality and wide-range of possible pathogens in cirrhotic individuals, gram-stained smears from pus and/or infected tissue should be immediately obtained and broad-spectrum antibiotics should be prompt utilized, such as third or fourth generation cephalosporins, amoxicillin-clavulanate, piperacillin-tazobactam and carbapenams. Combination therapy with fluoroquinolones or cloxacillin may be considered if a gram-negative or gram-positive pathogen is highly suspicious, respectively. Empirical treatment is effective in approximately 80% of community-acquired SSTI. Importantly, it is effective in only half of cirrhotic patients with nosocomial SSTI, which is largely due to a higher incidence of MRSA and P. aeruginosa[32].
Endocarditis
Infectious endocarditis (IE) classically occurs in patients with underlying valvular heart disease and prosthetic valves. Interestingly, a recent review of 316 IE cases found that approximately 10% of patients had underlying liver cirrhosis[59]. IE in cirrhotic patients was often observed in those patients who were hospitalized and/or had invasive procedures[7,59]. The common causative organisms are gram-positive such as Streptococci (S. pyogenes, S. agalactiae, S. viridans), S. aureus, S epidemidis and enterococci[59,60]. A minimum of 4-6 wk of antibiotic therapy is recommended. Caution should be taken since the majority of IE cases in cirrhosis are health-care associated and therefore the incidence of drug-resistant pathogens is considerably increased[59,60]. The mortality rate of cirrhotic patients with IE is high (27%-51%) despite treatment, especially in those patients with advanced cirrhosis and staphylococcal infection[59,60].
Meningitis
The incidence of community-acquired bacterial meningitis in the general population is estimated around 5/100 000 adults per year; the majority of these caused by S. pneumoniae and N. meningitides[61]. Several reports suggested that the incidence and the virulence of bacterial meningitis are substantially increased (up to 10-fold) in cirrhotic patients; thus, mortality rate in these patients is approximately 50%-63% and even higher in older patients and those with alcohol-related cirrhosis[62-65]. Cirrhotic patients, compared to non-cirrhotic patients, had a longer duration of symptoms before the time of diagnosis (> 4 d: 32% vs 16%, respectively), less obvious physical signs (nuchal rigidity: 75% vs 92%, respectively); greater incidence of relapse (18% vs 1%, respectively), and increasing incidence of E. coli and L. monocytogenes[62,64].
In the clinical setting of fever with headache and/or alteration of conscious in cirrhotic patients, the possibility of a central nervous system infection should not be overlooked. Neurological examinations are sometimes limited and ambiguous, particularly in the presence of concurrent hepatic encephalopathy. Prompt empirical central nervous system-dosed antibiotics and an appropriate diagnostic approach are key to decrease morbidity and mortality in patients with bacterial meningitis[66]. A combination of vancomycin, third generation cephalosporins and ampicillin is recommended for empirical therapy in cirrhotic patients with bacterial meningitis[66].
PATHOGENS AND THEIR CLINICAL FEATURES
Vibrio spp.
V. vulnificus is a gram-negative, motile, marine bacterium that is endemic in warm coastal water[42]. Exposure to this organism usually occurs through the ingestion of seafood (e.g. shellfish, raw oyster) or inoculation via traumatic injury in marine environments. V. vulnificus infection generally occurs in patients who are elderly or those who are compromised with comorbidities, especially cirrhosis[42,57]. It typically manifests as three clinical features: (1) SSTI: direct inoculation of organism causing wound infection or cellulitis, which generally occurs within 24-48 h after exposure. The lesions are typically painful and associated with rapid evolution to the hemorrhagic bullae and then to necrotic ulcers, necrotizing fasciitis and secondary bacteremia; (2) primary sepsis; and (3) gastrointestinal illnesses characterized by abdominal pain, diarrhea, and vomiting[42,67]. The virulence of V. vulnificus is linked to the availability of iron and its secreting toxin.
Infections from other marine Vibrios also increasingly occur and are associated with poor outcomes in cirrhotic patients[10,58,67-69]. V. cholera non-o1 infection occurs in endemic areas, such as the United States, Mexico, East and Southeast Asia[10,58,67-69]. The route of acquisition and clinical features can mimic V. vulnificus infection. V. parahemolyticus generally causes watery diarrhea, abdominal pain and vomiting.
An early recognition, aggressive treatment of shock and surgical management of SSTI is crucial. Most isolated marine Vibrios are susceptible to third generation cephalosporins, tetracyclines and fluoroquinolones. The combination of cefotaxime and minocycline or fluoroquinolones has been shown to have a synergistic effect against marine Vibrios[10,67,69].
Aeromonas spp.
Aeromonas spp. is a gram-negative, facultative anaerobic bacteria that is ubiquitous in fresh and brackish water. Infections are more frequently encountered in immunocompromised patients, particularly cirrhosis and malignancy[44,45,67,70-72]. A. hydrophila and A. veronii biovar sobria are the most often isolated species from symptomatic patients. Aeromonas bacteremia in cirrhotic patients tends to be monomicrobial, whereas polymicrobial bacteremia (frequently combined with E. coli or Klebsiella spp.) is commonly seen in patients with malignancy[44,72]. Drug of choice for empirical treatment is either intravenous carbapenams or a combination of intravenous third generation cephalosporins and aminoglycosides or fluoroquinolones.
Mycobacterial tuberculosis
The incidence and virulence of tuberculosis (TB) are increased in cirrhotic patients. Extrapulmonary involvement is more frequently observed (11%-31%)[73,74]. TB peritonitis possibly mimics SBP. TB peritonitis occurs in less advanced cirrhosis and its ascites has a lower white blood cell count, higher proportion of mononuclear cells, higher levels of protein and adenosine deaminase (ADA)[75]. More than 50% of TB peritonitis cases in the United States had underlying cirrhosis, especially alcohol-related[76]. Though ADA level is generally helpful in the detection of TB peritonitis, the presence of cirrhosis may reduce its sensitivity to 30%[76-78]. Laparoscopic biopsy sometimes is required for definitive diagnosis by revealing multiple whitish nodules scattered over the peritoneum, lymphocytic inflammation with granulomas and/or acid-fast organisms on the histopathological examination[77,79]. Patients with TB and cirrhosis often respond well to anti-TB treatment but are associated with more treatment-related hepatotoxicity incidence[73,77].
Clostridium difficile
C. difficile infection has recently been recognized as a significant problem in hospitalized cirrhotic patients. US database of over 80 000 patients analysis suggested that C. difficile-associated diarrhea (CDAD) is an independent risk of death in hospitalized cirrhotic patients (OR 1.55, 95% CI: 1.29-1.85)[80]. It is also associated with an increase in the length of hospital stay and hospital cost in these patients. There was no correlation between severity of cirrhosis and the development of CDAD[80] (Table 2).
Table 2.
Individual pathogens and their clinical manifestations in cirrhotic patients
| Pathogens | Common clinical features | Key points |
| E.coli, Klebsiella spp. Enterobacter spp. and other gram-negative enteric bacteria[6,7,9,12,13] | SBP, bacteremia, UTI, biliary tract infection, meningitis | ↑ Incidence of resistant organisms in hospital-acquired infection and in patients taking prophylactic quinolones |
| Plesiomonas shigelloides [10,81,99] | Septicemia, diarrhea, SBP, meningitis, SSTI | ↑ Incidence in hemochromatosis Risk factors: contaminated food and water |
| Vibrio spp. (V. vulnificus, non-o1 V. cholera, V. parahemolyticus)[10,42,43,57,81] | SSTI, bacteremia, gastroenteritis | ↑ Incidence in cirrhosis, particularly hemochromatosis ↑ Virulence; mortality 50%-60% in bacteremic form and about 24% for SSTI Risk factors: contaminated food and seawater |
| Aeromonas spp. (A. hydrophilla, A. sobria)[44,45,67,70-72] | Bacteremia, biliary tract infection, gastroenteritis, SBP, SSTI | ↑ Incidence ↑ Virulence; mortality 20%-60% Risk factors: contaminated food and water |
| Yersinia spp. (Y. enterocolitica, Y. pseodotuberculosis)[10,81] | Bacteremia, SBP, hepatosplenic abscesses | ↑ Incidence in hemochromatosis ↑ Virulence; mortality about 50% in bacteremic form |
| Campylobacter spp. (C. jejuni, C. fetus)[10,100] | Bacteremia, SBP | ↑ Incidence Mortality about 10% in bacteremic form |
| Pateurella multocida[13,101,102] | SSTI, bacteremia, arthritis, meningitis | ↑ Incidence Mortality about 10% in bacteremic form Risk factors: cat and dog bites or scratches |
| Staphylocccus aureus[7,11,13] | Bacteremia, SSTI, endocarditis | ↑ Incidence, particularly in those who are hospitalized and/or had invasive procedure ↑ Incidence of MRSA carriage and infection |
| Streptococcus pneumonia[94,95] | Bacteremia, pneumonia, SBP, SSTI, meningitis | ↑ Incidence of invasive pneumococcal disease ↑ Virulence Vaccination is recommended |
| Streptococcus group B[103,104] | Bacteremia, SBP, SSTI, pneumonia | ↑ Incidence Mortality 10%-25% |
| Clostridium difficile[80] | Antibiotic-associated diarrhea and colitis | ↑ Incidence ↑ Virulence; mortality 14% Risk factors: hospitalization, antibiotics, proton pump inhibitors |
| Clostridium spp. (C. perfringens, C. bifermentans, C. septicum)[13,105] | Bacteremia, SSTI, peritonitis | ↑ Incidence ↑ Virulence; mortality 54%-65% |
| Enterococcus spp (E. faecium, E. faecalis, E. galinarum)[7,11,59,106-109] | SBP, bacteremia, UTI, endocarditis, biliary tract infection | ↑ Incidence, particularly in hospital-acquired infection and in patients taking prophylactic quinolones ↑ Virulence; mortality rate up to one third in bacteremic form and up to 60% in enterococcal peritonitis Pre-transplant VRE colonization (13%-15% from surveillance) is associated with increased morbidity and mortality following liver transplant |
| Listeria monocytogenes[10,64,81] | Bacteremia, meningitis, SBP | ↑ Incidence in cirrhosis, particularly hemochromatosis |
| Mycobacterium tuberculosis (TB)[73,74,77] | Pulmonary TB, extra-pulmonary TB (e.g. peritonitis, disseminated TB) | ↑ Incidence ↑ Virulence; mortality 22%-48% ↑ Extra-pulmonary forms ↑ Risk for multi-drug resistance TB ↑ Risk for anti-TB hepatotoxicity |
SBP: Spontaneous bacterial peritonitis; UTI: Urinary tract infection; SSTI: Skin and soft tissue infection; TB: Tuberculosis.
LIVER DISEASE-SPECIFIC ISSUES
Hemochromatosis
The association of hemochromatosis and certain pathogens has been well described. Several mechanisms have been proposed to explain this association. Iron excess induces oxidative stress which results in organ damage and impairment of immune function[81]. Hepcidin, a central iron-regulatory hormone, has recently been recognized for an immunomodulatory and broad antimicrobial property[82,83]. Inadequate expression and functional impairment of hepcidin in patients with hemochromatosis may connect to the increased susceptibility for infections[82,83]. Hemochromatosis is associated with a decrease in proliferation, migration, phagocytic activity and cytokines secreting ability of the immune cells, thereby, principally impairing cell-mediated immune response[81].
Aside from impaired host defense, the growth and virulence of various organisms are enhanced by a high iron environment[81]. Interestingly, chelation therapy with desferoxamine in patients with hemochromatosis secondary to long-term transfusion may further stimulate the growth of particular bacteria, such as V. vulnificus, Y. enterocolitica, K. pneumonia and S. aureus, which can use it for efficient iron uptake via specific receptors[84,85]. On the other hand, newer iron chelators (deferasirox and deferiprone) do not act as siderophores and therefore may depress the growth of iron-dependent organisms[84,85].
A number of pathogens have been reported to be of increased susceptibility in patients with hemochromatosis, such as E. coli, Vibrio spp. (V. vulnificus, V. cholerae), L. monocytogenes, Yersenia spp. (Y. pseudotuberculosis, Y. enterocolitica), Plesiomonas shigelloides, Mycobacterium tuberculosis, cytomegalovirus and fungi (A. fumigatus, Mucor spp.)[81,86].
Primary sclerosing cholangitis
Patients with primary sclerosing cholangitis (PSC) are susceptible to repeated episodes of bacterial cholangitis, especially after biliary tract manipulation[87,88]. The incidence of cholangitis following endoscopic retrograde cholangiopancreatography (ERCP) is higher in PSC patients (4%-16%) compared to non-PSC patients, particularly in those who had therapeutic ERCP procedures[89]. If cholangitis occurs without biliary intervention, the presence of stones, dominant strictures or cholangiocarcinoma should be considered. Most common causative organisms are gram-negative enteric bacteria and enterococci[90]. Recurrent bacterial cholangitis may benefit from long term antibiotic prophylaxis[87].
PREVENTIVE MEASUREMENTS
All cirrhotic patients should be aware of the risk of infections and contact their physicians instantly when they are febrile or ill. Raw/uncooked foods, close contact to at-risk animals or sick people and wound exposure to flood or seawater should be avoided, particularly in those with advanced liver disease.
Prophylactic antibiotics should be utilized in cirrhotic patients at high risk of developing infection, including gastrointestinal bleeding and those undergoing invasive endoscopic or surgical procedures[28,39]. Long-term prophylaxis for patients with a history of SBP and those who have low ascitic fluid protein (< 1.5 gm/dL) is recommended[51]. On the other hand, overuse of antibiotic prophylaxis can lead to the development of resistant organisms and CDAD[7,80]. The rate of culture-positive infection caused by quinolone-resistant gram-negative bacilli was very high (65%) in patients on long-term norfloxacin prophylaxis[7]. Notably, prophylactic antibiotics are not recommended for routine endoscopy, elective variceal band ligation and abdominal paracentesis[39,51,91].
Immunization against hepatitis A and B viruses, influenza and pneumococcus are recommended in patients with cirrhosis[92,93]. Both cellular and humoral immune responses are suppressed in cirrhotic patients which may be associated with suboptimal early post-vaccination response and loss of long-term immunogenicity[92]. Therefore, a booster dose early during the follow-up is suggested in order to improve the immune response[92]. Cirrhotic patients are able to receive both inactivated and live vaccines according to the current guidelines[92,93]. S. pneumoniae infections are common, more severe and frequently associated with poor outcome in cirrhotic patients[94,95]. Anti-pneumococcal vaccination is recommended with booster injections every 5 years[92]. Incidence of seasonal flu is not evidently increased in cirrhotic patients; however, influenza infection may precipitate hepatic decompensation[92,96]. Influenza vaccine is well-tolerated and clinically effective in cirrhotic patients despite a slightly reduced immunogenicity[97,98].
ACKNOWLEDGMENTS
The authors are grateful to K Rajender Reddy, MD and Alex R Bonnel at the University of Pennsylvania for supportive guidance.
Footnotes
Peer reviewers: Dr. Henning Gronbaek, Medical Department V, Aarhus University Hospital, Norrebrogade 44, Aarhus 8000, Denmark; Krishnan Rajeshwari, Professor, Department of Pediatrics, Maulana Azad Medical College, New Delhi 110002, India
S- Editor Wu X L- Editor Roemmele A E- Editor Wu X
References
- 1.Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28:26–42. doi: 10.1055/s-2008-1040319. [DOI] [PubMed] [Google Scholar]
- 2.Arvaniti V, D'Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–1256, 1256.e1-e5. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:727–738. doi: 10.1016/j.cgh.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 4.Barnes PF, Arevalo C, Chan LS, Wong SF, Reynolds TB. A prospective evaluation of bacteremic patients with chronic liver disease. Hepatology. 1988;8:1099–1103. doi: 10.1002/hep.1840080520. [DOI] [PubMed] [Google Scholar]
- 5.Wong F, Bernardi M, Balk R, Christman B, Moreau R, Garcia-Tsao G, Patch D, Soriano G, Hoefs J, Navasa M. Sepsis in cirrhosis: report on the 7th meeting of the International Ascites Club. Gut. 2005;54:718–725. doi: 10.1136/gut.2004.038679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borzio M, Salerno F, Piantoni L, Cazzaniga M, Angeli P, Bissoli F, Boccia S, Colloredo-Mels G, Corigliano P, Fornaciari G, et al. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001;33:41–48. doi: 10.1016/s1590-8658(01)80134-1. [DOI] [PubMed] [Google Scholar]
- 7.Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, Rodés J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–148. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 8.Bernard B, Grangé JD, Khac EN, Amiot X, Opolon P, Poynard T. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: a meta-analysis. Hepatology. 1999;29:1655–1661. doi: 10.1002/hep.510290608. [DOI] [PubMed] [Google Scholar]
- 9.Caly WR, Strauss E. A prospective study of bacterial infections in patients with cirrhosis. J Hepatol. 1993;18:353–358. doi: 10.1016/s0168-8278(05)80280-6. [DOI] [PubMed] [Google Scholar]
- 10.Brann OS. Infectious complications of cirrhosis. Curr Gastroenterol Rep. 2001;3:285–292. doi: 10.1007/s11894-001-0051-2. [DOI] [PubMed] [Google Scholar]
- 11.Campillo B, Richardet JP, Kheo T, Dupeyron C. Nosocomial spontaneous bacterial peritonitis and bacteremia in cirrhotic patients: impact of isolate type on prognosis and characteristics of infection. Clin Infect Dis. 2002;35:1–10. doi: 10.1086/340617. [DOI] [PubMed] [Google Scholar]
- 12.Merli M, Lucidi C, Giannelli V, Giusto M, Riggio O, Falcone M, Ridola L, Attili AF, Venditti M. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol. 2010;8:979–985. doi: 10.1016/j.cgh.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Christou L, Pappas G, Falagas ME. Bacterial infection-related morbidity and mortality in cirrhosis. Am J Gastroenterol. 2007;102:1510–1517. doi: 10.1111/j.1572-0241.2007.01286.x. [DOI] [PubMed] [Google Scholar]
- 14.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422–433. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 15.Gustot T, Durand F, Lebrec D, Vincent JL, Moreau R. Severe sepsis in cirrhosis. Hepatology. 2009;50:2022–2033. doi: 10.1002/hep.23264. [DOI] [PubMed] [Google Scholar]
- 16.Tsai MH, Peng YS, Chen YC, Liu NJ, Ho YP, Fang JT, Lien JM, Yang C, Chen PC, Wu CS. Adrenal insufficiency in patients with cirrhosis, severe sepsis and septic shock. Hepatology. 2006;43:673–681. doi: 10.1002/hep.21101. [DOI] [PubMed] [Google Scholar]
- 17.Wong F, Salerno F. Beta-blockers in cirrhosis: friend and foe? Hepatology. 2010;52:811–813. doi: 10.1002/hep.23852. [DOI] [PubMed] [Google Scholar]
- 18.Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403–409. doi: 10.1056/NEJM199908053410603. [DOI] [PubMed] [Google Scholar]
- 19.Terra C, Guevara M, Torre A, Gilabert R, Fernández J, Martín-Llahí M, Baccaro ME, Navasa M, Bru C, Arroyo V, et al. Renal failure in patients with cirrhosis and sepsis unrelated to spontaneous bacterial peritonitis: value of MELD score. Gastroenterology. 2005;129:1944–1953. doi: 10.1053/j.gastro.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Fasolato S, Angeli P, Dallagnese L, Maresio G, Zola E, Mazza E, Salinas F, Donà S, Fagiuoli S, Sticca A, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223–229. doi: 10.1002/hep.21443. [DOI] [PubMed] [Google Scholar]
- 21.Terg R, Gadano A, Cartier M, Casciato P, Lucero R, Muñoz A, Romero G, Levi D, Terg G, Miguez C, et al. Serum creatinine and bilirubin predict renal failure and mortality in patients with spontaneous bacterial peritonitis: a retrospective study. Liver Int. 2009;29:415–419. doi: 10.1111/j.1478-3231.2008.01877.x. [DOI] [PubMed] [Google Scholar]
- 22.Møller S, Henriksen JH. Cirrhotic cardiomyopathy. J Hepatol. 2010;53:179–190. doi: 10.1016/j.jhep.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 23.Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–1318. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.TenHoor T, Mannino DM, Moss M. Risk factors for ARDS in the United States: analysis of the 1993 National Mortality Followback Study. Chest. 2001;119:1179–1184. doi: 10.1378/chest.119.4.1179. [DOI] [PubMed] [Google Scholar]
- 25.Thomson SJ, Moran C, Cowan ML, Musa S, Beale R, Treacher D, Hamilton M, Grounds RM, Rahman TM. Outcomes of critically ill patients with cirrhosis admitted to intensive care: an important perspective from the non-transplant setting. Aliment Pharmacol Ther. 2010;32:233–243. doi: 10.1111/j.1365-2036.2010.04341.x. [DOI] [PubMed] [Google Scholar]
- 26.Plessier A, Denninger MH, Consigny Y, Pessione F, Francoz C, Durand F, Francque S, Bezeaud A, Chauvelot-Moachon L, Lebrec D, et al. Coagulation disorders in patients with cirrhosis and severe sepsis. Liver Int. 2003;23:440–448. doi: 10.1111/j.1478-3231.2003.00870.x. [DOI] [PubMed] [Google Scholar]
- 27.Goulis J, Armonis A, Patch D, Sabin C, Greenslade L, Burroughs AK. Bacterial infection is independently associated with failure to control bleeding in cirrhotic patients with gastrointestinal hemorrhage. Hepatology. 1998;27:1207–1212. doi: 10.1002/hep.510270504. [DOI] [PubMed] [Google Scholar]
- 28.Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila FI, Soares-Weiser K, Uribe M. Antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding. Cochrane Database Syst Rev. 2010:CD002907. doi: 10.1002/14651858.CD002907.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cadranel JF, Denis J, Pauwels A, Barbare JC, Eugène C, di Martino V, Poquet E, Medini A, Coutarel P, Latrive JP, et al. Prevalence and risk factors of bacteriuria in cirrhotic patients: a prospective case-control multicenter study in 244 patients. J Hepatol. 1999;31:464–468. doi: 10.1016/s0168-8278(99)80038-5. [DOI] [PubMed] [Google Scholar]
- 30.Rabinovitz M, Prieto M, Gavaler JS, Van Thiel DH. Bacteriuria in patients with cirrhosis. J Hepatol. 1992;16:73–76. doi: 10.1016/s0168-8278(05)80097-2. [DOI] [PubMed] [Google Scholar]
- 31.Bercoff E, Déchelotte P, Weber J, Morcamp D, Denis P, Bourreille J. Urinary tract infection in cirrhotic patients, a urodynamic explanation. Lancet. 1985;1:987. doi: 10.1016/s0140-6736(85)91764-7. [DOI] [PubMed] [Google Scholar]
- 32.Acevedo J, Fernandez J, Castro M, Garcia O, Rodriguez de Lopez C, Navasa M, Gines P, Aroyo V. Current efficacy of recommended empiric antibiotic therapy in patients with cirrhosis and bacterial infection. J Hepatol. 2009;50 Suppl 1:S5. [Google Scholar]
- 33.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Musher DM, Niederman MS, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viasus D, Garcia-Vidal C, Castellote J, Adamuz J, Verdaguer R, Dorca J, Manresa F, Gudiol F, Carratalà J. Community-acquired pneumonia in patients with liver cirrhosis: clinical features, outcomes, and usefulness of severity scores. Medicine (Baltimore) 2011;90:110–118. doi: 10.1097/MD.0b013e318210504c. [DOI] [PubMed] [Google Scholar]
- 35.Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc. 2005;2:428–432. doi: 10.1513/pats.200507-065JS. [DOI] [PubMed] [Google Scholar]
- 36.American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 37.Castells A, Bruix J, Ayuso C, Brú C, Montanyà X, Boix L, Rodès J. Transarterial embolization for hepatocellular carcinoma. Antibiotic prophylaxis and clinical meaning of postembolization fever. J Hepatol. 1995;22:410–415. doi: 10.1016/0168-8278(95)80103-0. [DOI] [PubMed] [Google Scholar]
- 38.DeSimone JA, Beavis KG, Eschelman DJ, Henning KJ. Sustained bacteremia associated with transjugular intrahepatic portosystemic shunt (TIPS) Clin Infect Dis. 2000;30:384–386. doi: 10.1086/313653. [DOI] [PubMed] [Google Scholar]
- 39.Banerjee S, Shen B, Baron TH, Nelson DB, Anderson MA, Cash BD, Dominitz JA, Gan SI, Harrison ME, Ikenberry SO, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67:791–798. doi: 10.1016/j.gie.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 40.Lin CJ, Chiu CT, Lin DY, Sheen IS, Lien JM. Non-O1 Vibrio cholerae bacteremia in patients with cirrhosis: 5-yr experience from a single medical center. Am J Gastroenterol. 1996;91:336–340. [PubMed] [Google Scholar]
- 41.Ko WC, Chuang YC, Huang GC, Hsu SY. Infections due to non-O1 Vibrio cholerae in southern Taiwan: predominance in cirrhotic patients. Clin Infect Dis. 1998;27:774–780. doi: 10.1086/514947. [DOI] [PubMed] [Google Scholar]
- 42.Chiang SR, Chuang YC. Vibrio vulnificus infection: clinical manifestations, pathogenesis, and antimicrobial therapy. J Microbiol Immunol Infect. 2003;36:81–88. [PubMed] [Google Scholar]
- 43.Matsumoto K, Ohshige K, Fujita N, Tomita Y, Mitsumizo S, Nakashima M, Oishi H. Clinical features of Vibrio vulnificus infections in the coastal areas of the Ariake Sea, Japan. J Infect Chemother. 2010;16:272–279. doi: 10.1007/s10156-010-0050-z. [DOI] [PubMed] [Google Scholar]
- 44.Ko WC, Lee HC, Chuang YC, Liu CC, Wu JJ. Clinical features and therapeutic implications of 104 episodes of monomicrobial Aeromonas bacteraemia. J Infect. 2000;40:267–273. doi: 10.1053/jinf.2000.0654. [DOI] [PubMed] [Google Scholar]
- 45.Wang JH, Wang CY, Chi CY, Ho MW, Ho CM, Lin PC. Clinical presentations, prognostic factors, and mortality in patients with Aeromonas sobria complex bacteremia in a teaching hospital: a 5-year experience. J Microbiol Immunol Infect. 2009;42:510–515. [PubMed] [Google Scholar]
- 46.Xiol X, Castellví JM, Guardiola J, Sesé E, Castellote J, Perelló A, Cervantes X, Iborra MJ. Spontaneous bacterial empyema in cirrhotic patients: a prospective study. Hepatology. 1996;23:719–723. doi: 10.1002/hep.510230410. [DOI] [PubMed] [Google Scholar]
- 47.Chen TA, Lo GH, Lai KH. Risk factors for spontaneous bacterial empyema in cirrhotic patients with hydrothorax. J Chin Med Assoc. 2003;66:579–586. [PubMed] [Google Scholar]
- 48.Chen CH, Shih CM, Chou JW, Liu YH, Hang LW, Hsia TC, Hsu WH, Tu CY. Outcome predictors of cirrhotic patients with spontaneous bacterial empyema. Liver Int. 2011;31:417–424. doi: 10.1111/j.1478-3231.2010.02447.x. [DOI] [PubMed] [Google Scholar]
- 49.Alonso JC. Pleural effusion in liver disease. Semin Respir Crit Care Med. 2010;31:698–705. doi: 10.1055/s-0030-1269829. [DOI] [PubMed] [Google Scholar]
- 50.Sese E, Xiol X, Castellote J, Rodríguez-Fariñas E, Tremosa G. Low complement levels and opsonic activity in hepatic hydrothorax: its relationship with spontaneous bacterial empyema. J Clin Gastroenterol. 2003;36:75–77. doi: 10.1097/00004836-200301000-00020. [DOI] [PubMed] [Google Scholar]
- 51.Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087–2107. doi: 10.1002/hep.22853. [DOI] [PubMed] [Google Scholar]
- 52.Castellote J, Lopez C, Gornals J, Domingo A, Xiol X. Use of reagent strips for the rapid diagnosis of spontaneous bacterial empyema. J Clin Gastroenterol. 2005;39:278–281. doi: 10.1097/01.mcg.0000155125.74548.28. [DOI] [PubMed] [Google Scholar]
- 53.Rongey C, Lim NH, Runyon BA. Cellulitis in patients with cirrhosis and edema: an under-recognized complication currently more common than spontaneous bacterial peritonitis. Open Gastroenterol J. 2008;2:24–27. [Google Scholar]
- 54.McHenry CR, Piotrowski JJ, Petrinic D, Malangoni MA. Determinants of mortality for necrotizing soft-tissue infections. Ann Surg. 1995;221:558–563; discussion 563-565. doi: 10.1097/00000658-199505000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee CC, Chi CH, Lee NY, Lee HC, Chen CL, Chen PL, Chang CM, Wu CJ, Ko NY, Tsai MC, et al. Necrotizing fasciitis in patients with liver cirrhosis: predominance of monomicrobial Gram-negative bacillary infections. Diagn Microbiol Infect Dis. 2008;62:219–225. doi: 10.1016/j.diagmicrobio.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 56.Liu BM, Chung KJ, Chen CH, Kung CT, Ko SF, Liu PP, Chang HW. Risk factors for the outcome of cirrhotic patients with soft tissue infections. J Clin Gastroenterol. 2008;42:312–316. doi: 10.1097/MCG.0b013e31802dbde8. [DOI] [PubMed] [Google Scholar]
- 57.Kuo YL, Shieh SJ, Chiu HY, Lee JW. Necrotizing fasciitis caused by Vibrio vulnificus: epidemiology, clinical findings, treatment and prevention. Eur J Clin Microbiol Infect Dis. 2007;26:785–792. doi: 10.1007/s10096-007-0358-5. [DOI] [PubMed] [Google Scholar]
- 58.Tsai YH, Huang TJ, Hsu RW, Weng YJ, Hsu WH, Huang KC, Peng KT. Necrotizing soft-tissue infections and primary sepsis caused by Vibrio vulnificus and Vibrio cholerae non-O1. J Trauma. 2009;66:899–905. doi: 10.1097/TA.0b013e31816a9ed3. [DOI] [PubMed] [Google Scholar]
- 59.Fernández Guerrero ML, González López J, Górgolas M. Infectious endocarditis in patients with cirrhosis of the liver: a model of infection in the frail patient. Eur J Clin Microbiol Infect Dis. 2010;29:1271–1275. doi: 10.1007/s10096-010-0998-8. [DOI] [PubMed] [Google Scholar]
- 60.Hsu RB, Chen RJ, Chu SH. Infective endocarditis in patients with liver cirrhosis. J Formos Med Assoc. 2004;103:355–358. [PubMed] [Google Scholar]
- 61.Schut ES, de Gans J, van de Beek D. Community-acquired bacterial meningitis in adults. Pract Neurol. 2008;8:8–23. doi: 10.1136/jnnp.2007.139725. [DOI] [PubMed] [Google Scholar]
- 62.Pauwels A, Pines E, Abboura M, Chiche I, Levy VG. Bacterial meningitis in cirrhosis: review of 16 cases. J Hepatol. 1997;27:830–834. doi: 10.1016/s0168-8278(97)80320-0. [DOI] [PubMed] [Google Scholar]
- 63.Molle I, Thulstrup AM, Svendsen N, Schonheyder HC, Sorensen HT. Risk and case fatality rate of meningitis in patients with liver cirrhosis. Scand J Infect Dis. 2000;32:407–410. doi: 10.1080/003655400750044999. [DOI] [PubMed] [Google Scholar]
- 64.Cabellos C, Viladrich PF, Ariza J, Maiques JM, Verdaguer R, Gudiol F. Community-acquired bacterial meningitis in cirrhotic patients. Clin Microbiol Infect. 2008;14:35–40. doi: 10.1111/j.1469-0691.2007.01839.x. [DOI] [PubMed] [Google Scholar]
- 65.Barahona-Garrido J, Hernández-Calleros J, Téllez-Avila FI, Chávez-Tapia NC, Remes-Troche JM, Torre A. Bacterial meningitis in cirrhotic patients: case series and description of the prognostic role of acute renal failure. J Clin Gastroenterol. 2010;44:e218–e223. doi: 10.1097/MCG.0b013e3181d88d53. [DOI] [PubMed] [Google Scholar]
- 66.Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, Whitley RJ. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267–1284. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]
- 67.Oliver JD. Wound infections caused by Vibrio vulnificus and other marine bacteria. Epidemiol Infect. 2005;133:383–391. doi: 10.1017/s0950268805003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thamlikitkul V. Vibrio bacteremia in Siriraj Hospital. J Med Assoc Thai. 1990;73:136–139. [PubMed] [Google Scholar]
- 69.Patel NM, Wong M, Little E, Ramos AX, Kolli G, Fox KM, Melvin J, Moore A, Manch R. Vibrio cholerae non-O1 infection in cirrhotics: case report and literature review. Transpl Infect Dis. 2009;11:54–56. doi: 10.1111/j.1399-3062.2008.00339.x. [DOI] [PubMed] [Google Scholar]
- 70.Lau SM, Peng MY, Chang FY. Outcomes of Aeromonas bacteremia in patients with different types of underlying disease. J Microbiol Immunol Infect. 2000;33:241–247. [PubMed] [Google Scholar]
- 71.Choi JP, Lee SO, Kwon HH, Kwak YG, Choi SH, Lim SK, Kim MN, Jeong JY, Choi SH, Woo JH, et al. Clinical significance of spontaneous Aeromonas bacterial peritonitis in cirrhotic patients: a matched case-control study. Clin Infect Dis. 2008;47:66–72. doi: 10.1086/588665. [DOI] [PubMed] [Google Scholar]
- 72.Lay CJ, Zhuang HJ, Ho YH, Tsai YS, Wang LS, Tsai CC. Different clinical characteristics between polymicrobial and monomicrobial Aeromonas bacteremia--a study of 216 cases. Intern Med. 2010;49:2415–2421. doi: 10.2169/internalmedicine.49.4117. [DOI] [PubMed] [Google Scholar]
- 73.Cho YJ, Lee SM, Yoo CG, Kim YW, Han SK, Shim YS, Yim JJ. Clinical characteristics of tuberculosis in patients with liver cirrhosis. Respirology. 2007;12:401–405. doi: 10.1111/j.1440-1843.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 74.Thulstrup AM, Mølle I, Svendsen N, Sørensen HT. Incidence and prognosis of tuberculosis in patients with cirrhosis of the liver. A Danish nationwide population based study. Epidemiol Infect. 2000;124:221–225. doi: 10.1017/s0950268899003593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim NJ, Choo EJ, Kwak YG, Lee SO, Choi SH, Woo JH, Kim YS. Tuberculous peritonitis in cirrhotic patients: comparison of spontaneous bacterial peritonitis caused by Escherichia coli with tuberculous peritonitis. Scand J Infect Dis. 2009;41:852–856. doi: 10.3109/00365540903214264. [DOI] [PubMed] [Google Scholar]
- 76.Hillebrand DJ, Runyon BA, Yasmineh WG, Rynders GP. Ascitic fluid adenosine deaminase insensitivity in detecting tuberculous peritonitis in the United States. Hepatology. 1996;24:1408–1412. doi: 10.1002/hep.510240617. [DOI] [PubMed] [Google Scholar]
- 77.Sanai FM, Bzeizi KI. Systematic review: tuberculous peritonitis--presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther. 2005;22:685–700. doi: 10.1111/j.1365-2036.2005.02645.x. [DOI] [PubMed] [Google Scholar]
- 78.Riquelme A, Calvo M, Salech F, Valderrama S, Pattillo A, Arellano M, Arrese M, Soza A, Viviani P, Letelier LM. Value of adenosine deaminase (ADA) in ascitic fluid for the diagnosis of tuberculous peritonitis: a meta-analysis. J Clin Gastroenterol. 2006;40:705–710. doi: 10.1097/00004836-200609000-00009. [DOI] [PubMed] [Google Scholar]
- 79.Reddy KR, DiPrima RE, Raskin JB, Jeffers LJ, Phillips RS, Manten HD, Schiff ER. Tuberculous peritonitis: laparoscopic diagnosis of an uncommon disease in the United States. Gastrointest Endosc. 1988;34:422–426. doi: 10.1016/s0016-5107(88)71410-8. [DOI] [PubMed] [Google Scholar]
- 80.Bajaj JS, Ananthakrishnan AN, Hafeezullah M, Zadvornova Y, Dye A, McGinley EL, Saeian K, Heuman D, Sanyal AJ, Hoffmann RG. Clostridium difficile is associated with poor outcomes in patients with cirrhosis: A national and tertiary center perspective. Am J Gastroenterol. 2010;105:106–113. doi: 10.1038/ajg.2009.615. [DOI] [PubMed] [Google Scholar]
- 81.Khan FA, Fisher MA, Khakoo RA. Association of hemochromatosis with infectious diseases: expanding spectrum. Int J Infect Dis. 2007;11:482–487. doi: 10.1016/j.ijid.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 82.Vyoral D, Petrak J. Hepcidin: a direct link between iron metabolism and immunity. Int J Biochem Cell Biol. 2005;37:1768–1773. doi: 10.1016/j.biocel.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 83.Ganz T. Hepcidin--a peptide hormone at the interface of innate immunity and iron metabolism. Curr Top Microbiol Immunol. 2006;306:183–198. doi: 10.1007/3-540-29916-5_7. [DOI] [PubMed] [Google Scholar]
- 84.Neupane GP, Kim DM. Comparison of the effects of deferasirox, deferiprone, and deferoxamine on the growth and virulence of Vibrio vulnificus. Transfusion. 2009;49:1762–1769. doi: 10.1111/j.1537-2995.2009.02186.x. [DOI] [PubMed] [Google Scholar]
- 85.Chan GC, Chan S, Ho PL, Ha SY. Effects of chelators (deferoxamine, deferiprone and deferasirox) on the growth of Klebsiella pneumoniae and Aeromonas hydrophila isolated from transfusion-dependent thalassemia patients. Hemoglobin. 2009;33:352–360. doi: 10.3109/03630260903211888. [DOI] [PubMed] [Google Scholar]
- 86.Boelaert JR, Vandecasteele SJ, Appelberg R, Gordeuk VR. The effect of the host's iron status on tuberculosis. J Infect Dis. 2007;195:1745–1753. doi: 10.1086/518040. [DOI] [PubMed] [Google Scholar]
- 87.Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–678. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 88.Mendes F, Lindor KD. Primary sclerosing cholangitis: overview and update. Nat Rev Gastroenterol Hepatol. 2010;7:611–619. doi: 10.1038/nrgastro.2010.155. [DOI] [PubMed] [Google Scholar]
- 89.Bangarulingam SY, Gossard AA, Petersen BT, Ott BJ, Lindor KD. Complications of endoscopic retrograde cholangiopancreatography in primary sclerosing cholangitis. Am J Gastroenterol. 2009;104:855–860. doi: 10.1038/ajg.2008.161. [DOI] [PubMed] [Google Scholar]
- 90.Rerknimitr R, Fogel EL, Kalayci C, Esber E, Lehman GA, Sherman S. Microbiology of bile in patients with cholangitis or cholestasis with and without plastic biliary endoprosthesis. Gastrointest Endosc. 2002;56:885–889. doi: 10.1067/mge.2002.129604. [DOI] [PubMed] [Google Scholar]
- 91.Llach J, Elizalde JI, Bordas JM, Gines A, Almela M, Sans M, Mondelo F, Pique JM. Prospective assessment of the risk of bacteremia in cirrhotic patients undergoing lower intestinal endoscopy. Gastrointest Endosc. 1999;49:214–217. doi: 10.1016/s0016-5107(99)70489-x. [DOI] [PubMed] [Google Scholar]
- 92.Loulergue P, Pol S, Mallet V, Sogni P, Launay O. Why actively promote vaccination in patients with cirrhosis? J Clin Virol. 2009;46:206–209. doi: 10.1016/j.jcv.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 93.Mehta G, Rothstein KD. Health maintenance issues in cirrhosis. Med Clin North Am. 2009;93:901–15, viii-ix. doi: 10.1016/j.mcna.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 94.Choi SH, Park HG, Jun JB, Lee SO, Choi SH, Woo JH, Kim YS. Clinical characteristics and outcomes of pneumococcal bacteremia in adult patients with liver cirrhosis. Diagn Microbiol Infect Dis. 2009;63:160–164. doi: 10.1016/j.diagmicrobio.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 95.Bouza E, Pintado V, Rivera S, Blázquez R, Muñoz P, Cercenado E, Loza E, Rodríguez-Créixems M, Moreno S. Nosocomial bloodstream infections caused by Streptococcus pneumoniae. Clin Microbiol Infect. 2005;11:919–924. doi: 10.1111/j.1469-0691.2005.01260.x. [DOI] [PubMed] [Google Scholar]
- 96.Duchini A, Viernes ME, Nyberg LM, Hendry RM, Pockros PJ. Hepatic decompensation in patients with cirrhosis during infection with influenza A. Arch Intern Med. 2000;160:113–115. doi: 10.1001/archinte.160.1.113. [DOI] [PubMed] [Google Scholar]
- 97.Cheong HJ, Song JY, Park JW, Yeon JE, Byun KS, Lee CH, Cho HI, Kim TG, Kim WJ. Humoral and cellular immune responses to influenza vaccine in patients with advanced cirrhosis. Vaccine. 2006;24:2417–2422. doi: 10.1016/j.vaccine.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 98.Song JY, Cheong HJ, Ha SH, Hwang IS, Kee SY, Jeong HW, Lee CG, Kim WJ. Clinical impact of influenza immunization in patients with liver cirrhosis. J Clin Virol. 2007;39:159–163. doi: 10.1016/j.jcv.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 99.Alcañiz JP, de Cuenca Morón B, Gómez Rubio M, Martínez Albares JL, Garcia Alvarez J. Spontaneous bacterial peritonitis due to Plesiomonas shigelloides. Am J Gastroenterol. 1995;90:1529–1530. [PubMed] [Google Scholar]
- 100.Pigrau C, Bartolome R, Almirante B, Planes AM, Gavalda J, Pahissa A. Bacteremia due to Campylobacter species: clinical findings and antimicrobial susceptibility patterns. Clin Infect Dis. 1997;25:1414–1420. doi: 10.1086/516127. [DOI] [PubMed] [Google Scholar]
- 101.Migliore E, Serraino C, Brignone C, Ferrigno D, Cardellicchio A, Pomero F, Castagna E, Osenda M, Fenoglio L. Pasteurella multocida infection in a cirrhotic patient: case report, microbiological aspects and a review of literature. Adv Med Sci. 2009;54:109–112. doi: 10.2478/v10039-009-0005-8. [DOI] [PubMed] [Google Scholar]
- 102.Tseng HK, Su SC, Liu CP, Lee CM. Pasteurella multocida bacteremia due to non-bite animal exposure in cirrhotic patients: report of two cases. J Microbiol Immunol Infect. 2001;34:293–296. [PubMed] [Google Scholar]
- 103.Jackson LA, Hilsdon R, Farley MM, Harrison LH, Reingold AL, Plikaytis BD, Wenger JD, Schuchat A. Risk factors for group B streptococcal disease in adults. Ann Intern Med. 1995;123:415–420. doi: 10.7326/0003-4819-123-6-199509150-00003. [DOI] [PubMed] [Google Scholar]
- 104.Farley MM. Group B streptococcal disease in nonpregnant adults. Clin Infect Dis. 2001;33:556–561. doi: 10.1086/322696. [DOI] [PubMed] [Google Scholar]
- 105.Chen YM, Lee HC, Chang CM, Chuang YC, Ko WC. Clostridium bacteremia: emphasis on the poor prognosis in cirrhotic patients. J Microbiol Immunol Infect. 2001;34:113–118. [PubMed] [Google Scholar]
- 106.Lee JH, Yoon JH, Kim BH, Chung GE, Myung SJ, Kim W, Kim YJ, Kim EC, Lee HS. Enterococcus: not an innocent bystander in cirrhotic patients with spontaneous bacterial peritonitis. Eur J Clin Microbiol Infect Dis. 2009;28:21–26. doi: 10.1007/s10096-008-0578-3. [DOI] [PubMed] [Google Scholar]
- 107.McBride SJ, Upton A, Roberts SA. Clinical characteristics and outcomes of patients with vancomycin-susceptible Enterococcus faecalis and Enterococcus faecium bacteraemia--a five-year retrospective review. Eur J Clin Microbiol Infect Dis. 2010;29:107–114. doi: 10.1007/s10096-009-0830-5. [DOI] [PubMed] [Google Scholar]
- 108.McNeil SA, Malani PN, Chenoweth CE, Fontana RJ, Magee JC, Punch JD, Mackin ML, Kauffman CA. Vancomycin-resistant enterococcal colonization and infection in liver transplant candidates and recipients: a prospective surveillance study. Clin Infect Dis. 2006;42:195–203. doi: 10.1086/498903. [DOI] [PubMed] [Google Scholar]
- 109.Russell DL, Flood A, Zaroda TE, Acosta C, Riley MM, Busuttil RW, Pegues DA. Outcomes of colonization with MRSA and VRE among liver transplant candidates and recipients. Am J Transplant. 2008;8:1737–1743. doi: 10.1111/j.1600-6143.2008.02304.x. [DOI] [PubMed] [Google Scholar]