Abstract
Caspase 2 was initially identified as a neuronally expressed developmentally down-regulated gene (HUGO gene nomenclature CASP2) and has been shown to be required for neuronal death induced by several stimuli, including NGF (nerve growth factor) deprivation and Aβ (β-amyloid). In non-neuronal cells the PIDDosome, composed of caspase 2 and two death adaptor proteins, PIDD (p53-inducible protein with a death domain) and RAIDD {RIP (receptor-interacting protein)-associated ICH-1 [ICE (interleukin-1β-converting enzyme)/CED-3 (cell-death determining 3) homologue 1] protein with a death domain}, has been proposed as the caspase 2 activation complex, although the absolute requirement for the PIDDosome is not clear. To investigate the requirement for the PIDDosome in caspase-2-dependent neuronal death, we have examined the necessity for each component in induction of active caspase 2 and in execution of caspase-2-dependent neuronal death. We find that both NGF deprivation and Aβ treatment of neurons induce active caspase 2 and that induction of this activity depends on expression of RAIDD, but is independent of PIDD expression. We show that treatment of wild-type or PIDD-null neurons with Aβ or NGF deprivation induces formation of a complex of caspase 2 and RAIDD. We also show that caspase-2-dependent execution of neurons requires RAIDD, not PIDD. Caspase 2 activity can be induced in neurons from PIDD-null mice, and NGF deprivation or Aβ use caspase 2 and RAIDD to execute death of these neurons.
Keywords: β-amyloid, hippocampal neuron, nerve growth factor deprivation, neuronal death, Penetratin1 small-interfering RNA (Pen1-siRNA), PIDDosome, sympathetic neuron
Abbreviations: Aβ, β-amyloid; AS, antisense; biotin-VAD-FMK, biotin-Val-Ala-DL-Asp-fluoromethylketone; CARD, caspase-recruitment domain; CED-3, cell-death determining 3; DD, death domain; ERK, extracellular-signal-regulated kinase; HFIP, 1,1,1,3,3,3 hexafluoro-2-propanol; NGF, nerve growth factor; P1, postnatal day 1; Pen1, Penetratin1; PIDD, p53-inducible protein with a death domain; RAIDD, RIP (receptor-interacting protein)-associated ICH-1 [ICE (interleukin-1β-converting enzyme)/CED-3 homologue 1] protein with a DD; RIP, receptor-interacting protein; RIP1K, RIP kinase 1; SCG, superior cervical ganglia; siRNA, small interfering RNA; TFD, trophic factor deprivation
INTRODUCTION
Although caspase 2 was the second mammalian caspase identified [1,2] much remains to be discovered regarding the regulation and function of this caspase. The initial identification in mouse was by a screen of NEDD (neural-precursor-cell-expressed developmentally down-regulated) genes, suggesting a role for caspase 2 in neuronal development [1]. This role was not substantiated in caspase 2-null mice, which had grossly normal neuronal development [3], but we have shown that these mice had an increase in caspase 9 and Smac (second mitochondrial-derived activator of caspase)/DIABLO [direct IAP (inhibitor of apoptosis)-binding protein with low pI] in the nervous system, providing a potential compensation for loss of caspase 2 [4]. Our previous studies of neuronal death in primary neuronal cultures have shown that caspase 2 is required for death induced by Aβ (β-amyloid) or by NGF (nerve growth factor) deprivation in wild-type neurons [4–6]. Structurally caspase 2 resembles other initiator caspases, with a long pro-domain which contains a CARD (caspase-recruitment domain) sequence, similar to caspase 9, DRONC (Drosophila Nedd2-like caspase) and CED-3 (cell-death determining 3) [7]. Caspases with long pro-domains are activated by proximity-induced dimerization; at endogenous caspase levels dimerization is facilitated by adaptor proteins, such as Apaf-1 (apoptotic protease-activating factor 1) for caspase 9 and ced-4 for ced-3 [8]. For caspase 2 RAIDD {RIP (receptor-interacting protein)-associated ICH-1 [ICE (interleukin-1β-converting enzyme)/CED-3 homologue 1] protein with a DD (death domain)} has been identified as a specific adaptor protein and shown to interact via CARD with caspase 2 [9]. RAIDD was found to interact via its DD with RIP1K (RIP kinase 1), but a role for a caspase 2–RAIDD–RIP1K complex was never confirmed. Subsequent studies have shown that RAIDD can interact with the DD-containing protein PIDD (p53-inducible protein with a DD) via the DD. The PIDDosome, composed of PIDD, RAIDD and caspase 2, was proposed as the activation platform for caspase 2 [10]. This complex was shown to form spontaneously in cell extracts subjected to a temperature shift. PIDD-null mice have been generated, and non-neuronal cells were studied for the response to DNA damage and PIDD was not required [11,12]. Caspase 2 activity was not measured in those studies, but formation of a caspase 2-containing large-molecular-mass complex upon temperature shift was shown to occur independent of PIDD or RAIDD [11]. We have previously shown that RAIDD is required for NGF-deprivation-mediated death of primary cultures of sympathetic neurons [13]; this death paradigm requires caspase 2 [4]. We have also shown that, although DNA-damage-mediated neuronal death is caspase-dependent [14], RAIDD is not required for this death [13]. We now examine the requirement for the PIDDosome in caspase 2 activation and the execution of caspase-2-mediated neuronal death, specifically death induced by Aβ or by NGF deprivation. We show that caspase 2 can be activated and mediate death in the absence of PIDD, but not in the absence of RAIDD. We further show that Aβ treatment and NGF deprivation induce formation of a complex of caspase 2 and RAIDD in wild-type and PIDD-null neurons.
EXPERIMENTAL
For all animal experimentation, Institutional and National guidelines for the care and use of laboratory animals was followed.
Neuronal hippocampal cultures
Primary cultures of hippocampal neurons were established from E18 (embryonic day 18) rat pups of pregnant Sprague–Dawley rats (Charles River Laboratories) or from P1 (postnatal day 1) mice as described previously [15]. Briefly, hippocampi were dissected, dissociated by trituration in serum-free medium and plated on poly-D-lysine (0.1 mg/ml)-coated tissue culture plates. Neurons were plated in a 1:1 mixture of Eagle's MEM (minimal essential medium) and Ham's F12 (Invitrogen) supplemented with glucose (6 mg/ml), putrescine (60 μM), progesterone (20 nM), transferrin (100 μg/ml), selenium (30 nM), penicillin (0.5 units/ml) and streptomycin (0.5 μg/ml) (Sigma) at a density of 300000 cells/ml. Cell were incubated at 37°C in a 5% CO2 atmosphere and treated 7 days post-plating.
Sympathetic neuronal cultures
Primary cultures of dissociated sympathetic neurons were prepared from the SCG (superior cervical ganglia) of P1 wild-type or PIDD-null mice [11] as described previously [4]. Briefly, SCG were removed from the neonates and trypsinized for 45 min at 37°C to remove the capsules. Cells were resuspended in RPMI 1640 medium with 10% horse serum supplemented with mouse NGF (50 ng/ml) and plated on to tissue culture plates coated with collagen. At 1 day after plating, uridine and 5-fluorodeoxyuridine (10 μM each) were added to the cultures and left for 4 days to eliminate non-neuronal cells.
Neuronal PC12 cells
PC12 cells were grown on collagen-coated plates for 7 days in serum-free RPMI 1640 medium containing NGF (100 ng/ml) as described previously [13].
siRNA (small interfering RNA) and AS (antisense)-RNA design
siRNAs were designed according to published design guidelines [16] with dTdT 3′ overhangs. Sequences for the sense strand of the central 19-nt double-stranded region were: siCaspase-2, 5′-GCCAUGCACUCCUGAGUUU-3′; siRAIDD, 5′-CCACAUUCAAGAAAUCAAA-3′; siPIDD, 5′-CCUGGGUGAUGCAGAAACU-3′; and AS-PIDD, 5′-AACACUGCAGCCAUCAC-3′.
siRNA and AS-RNA coupling
siRNA duplexes with a 5′ thiol modification on the sense strand or AS-RNA with a 5′ thiol modification were synthesized and HPLC-purified (Dharmacon) [17,18]. Annealed siRNA duplexes or AS-RNA were resuspended in buffer provided by the manufacturer and treated with an equimolar mixture of TCEP [tris-(2-carboxyethyl)phosphine] at room temperature (23°C) for 1 h. An equimolar ratio of Pen1 (Penetratin1; Q-Biogene) was added, and the mixture was heated to 65°C for 15 min and then incubated at 37°C for 1 h. The yields of the reactions were estimated at 90% by SDS/PAGE using SybrGold (Molecular Probes) (results not shown).
Aβ preparation
Aβ1–42 peptide was purchased from Dr David Teplow (David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, U.S.A.) and was resuspended in 100% HFIP (1,1,1,3,3,3 hexafluoro-2-propanol) at a final concentration of 1 mM. For complete solubilization, the peptide was homogenized using a 250 μl Hamilton syringe with a Teflon plunger. HFIP was removed by evaporation in a SpeedVac, Aβ1–42 was resuspended at a concentration of 5 mM in DMSO and sonicated in a water bath sonicator (Branson Ultrasonics, model 3510) for 10 min. Oligomers were prepared as described previously [19]. Briefly, Aβ1–42 was diluted in PBS+1/10 volume of 2% SDS in H2O to 400 μM. Aβ was incubated for 24 h at 37°C and further diluted to 100 μM in PBS, followed by an 18 h incubation at 37°C.
NGF deprivation
At 7 days after plating, sympathetic neurons were rinsed three times in sterile PBS, and then either re-exposed to medium supplemented with NGF, or deprived of NGF by applying an anti-mouse NGF antibody (Sigma, 1:200). For neuronal PC12 cells, cells were washed five times in serum-free medium without NGF and then either re-exposed to medium with NGF or serum-free medium without NGF.
Neuronal viability
The number of viable cells was determined by quantifying the number of intact nuclei as described previously [5,20]. For hippocampal neurons, culture medium was removed by aspiration and 100 μl of CHAPS lysis buffer [150 nM KC1, 50 mM Hepes, 0.1% CHAPS and a protease inhibitor tablet (pH 7.4)] was added to the well. This solution dissolves cell membranes providing a suspension of intact nuclei. Intact nuclei were quantified using a haemocytometer. Triplicate wells were scored and are reported as means±S.E.M. Significance is calculated by Student's t test. For sympathethic neurons, each culture was scored as numbers of living phase-bright neurons counted in the same field at various times. Three replicate cultures were assessed for each condition, and data are normalized to the number of neurons present in each culture at the time of Aβ1–42 addition or NGF deprivation and are reported as means±S.E.M.
mRNA extraction and real-time PCR
RNA was isolated from cultured neurons using TRIzol® reagent. cDNA was prepared by reverse transcriptase using SuperScript II and oligo(dT) primer (Invitrogen). Primers were designed to amplify a 300–400 base region of the targeted mRNA spanning the siRNA-targeting site for PIDD, RAIDD and CASP2 (HUGO gene symbol for caspase 2). cDNA was added to a reaction mixture (OmniMix HS beads; Cepheid) with SYBR Green (Molecular Probes) together with appropriate primers at 0.5 μM each. Levels of transcripts were analysed using the Cepheid SmartCycler (Fisher) following the manufacturer's specifications. RNA controls were used to ensure that amplification of products did not come from genomic DNA. Melting curve analysis was used to determine the temperature for fluorescence detection. For each mRNA, quantification was made from the linear portion of the amplification curve. TUBB (β-tubulin) and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) were used as internal controls for normalization of the expression of mRNA levels against PIDD, RAIDD and CASP2.
Western blot analysis
Proteins were electrophoretically separated by SDS/PAGE (12% gels) and transferred on to a 0.2 μm nitrocellulose membrane (Whatman) in 2.5 mM Tris/19.2 mM Glycine/20% methanol transfer buffer. The membrane was blocked in Odyssey blocking buffer (LI-COR Biosciences) diluted 1:1 in PBS for 1 h at room temperature and incubated with primary antibodies overnight at 4°C, and with secondary antibody [IRdye 800 (1:25000 dilution; Rockland Immunochemicals) or Alexa Fluor® 680 (1:25000; Molecular Probes)] for 2 h at room temperature. The membrane was washed three times in PBST (PBS containing 0.05% Tween 20) followed by a final wash in PBS. Bands were visualized and quantified using the Odyssey Infrared Imager (LI-COR Biosciences). Primary antibodies used were anti-(caspase 2) (custom-made [6]), anti-RAIDD (Stressgen) and anti-PIDD (Axxora). As a loading control the same membranes were incubated with antibodies against α-tubulin (1:10000; Abcam), ERK (extracellular-signal-regulated kinase) 1 (1:10000; Santa Cruz Biotechnology) or actin (1:10000; Sigma). Levels of immunopositive bands were analysed densitometrically using ImageJ (NIH).
Biotin-VAD-FMK (biotin-Val-Ala-DL-Asp-fluoromethylketone) trapping of active caspases
The biotin-VAD-FMK assay was used to detect the presence of active caspase 2, as described previously [21]. Briefly, rat hippocampal neurons or mouse sympathetic neurons were pre-treated for 2 h with 50 μM biotin-VAD-FMK (MP Biomedical) at 37°C. Cells were treated with 3 μM oligomeric Aβ for the times indicated and were harvested in CHAPS buffer [150 mM KCl, 50 mM Hepes and 0.1% CHAPS (pH 7.4)] supplemented with protease inhibitor cocktail tablets (Complete Mini, Roche). Cells were spun at 15000 g for 10 min at 4°C and the supernatant was collected and boiled for 5 min. Streptavidin–agarose beads were added to the boiled supernatant and incubated in a rotor overnight at 4°C. Beads were spun at 7500 g for 5 min at 4°C, washed five times with PBS, resuspended in sample buffer [1% SDS, 3% glycerol and 20 mM Tris/HCl (pH 6.8)] and boiled for 5 min. Beads in sample buffer were spun at 15000 g for 10 min at 4°C, supernatant was collected and supplemented with 5% 2-mercaptoethanol and samples were loaded on to a 12% PAGE gel for Western blot analysis using an affinity-purified polyclonal anti-(caspase 2) antibody.
Caspase 2 and RAIDD co-immunoprecipitation
Mouse IgG or rabbit IgG magnetized beads (Invitrogen) were pre-coated with an anti-RAIDD antibody, anti-CRADD (caspase 2 and RIPK1 domain-containing adaptor with DD; Abnova) antibody or anti-(caspase 2) (custom-made) antibody respectively for 2 h at 4°C on a rotator. In total, 2 μg of anti-CRADD antibody or 10 μl of anti-(caspase 2) antibody was used per 30 μl of beads. Primary rat hippocampal neurons were treated with Aβ1–42 (3 μM) for 2 or 4 h. Cell lysates were prepared using CHAPS lysis buffer. Lysates (70–120 μg) were loaded on to anti-CRADD antibody pre-coated beads and incubated overnight at 4°C on a rotator. Following overnight incubation the captured proteins were boiled off the beads at 100°C for 5 min. The immunoprecipitated samples, along with inputs, were then subjected to Western blot analysis using affinity-purified polyclonal anti-(caspase 2) or monoclonal anti-CRADD antibodies.
RESULTS
Caspase 2 is activated in neurons by Aβ exposure or NGF deprivation
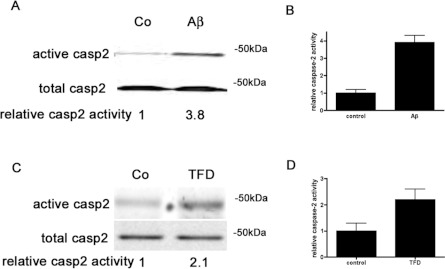
Our laboratory and others have previously described that knock down of caspase 2 protects neurons against Aβ-mediated [5] or NGF deprivation-induced [4,6] cell death. Caspase 2 is an initiator caspase; initiator caspases do not require cleavage for activation [8]. Thus cleavage is not an accurate read-out for activation. To measure caspase 2 activity, we have recently adapted the caspase-affinity ligand method [22] to detect active initiator caspases in neurons and found that 3 h of Aβ treatment induced caspase 2 activation [21]. To confirm and extend these findings, we used this method to examine caspase 2 activity after Aβ and NGF deprivation [TFD (trophic factor deprivation)]. Primary neuronal cultures were treated with 50 μM biotin-VAD-FMK for 2 h and then exposed to death-inducing stimuli, either 3 μM Aβ for hippocampal cultures or anti-NGF antibody to deplete NGF from sympathetic neuronal cultures. After 2 h of treatment with the death stimuli, cultures were harvested and biotin-VAD-FMK–caspase complexes were isolated and active caspase 2 was identified by Western blot analysis (Figure 1). Aβ treatment for 2 h induced an almost 4-fold increase in caspase 2 activity in primary hippocampal neurons (Figures 1A and 1B). After 2 h of NGF withdrawal, caspase 2 activity increased 2-fold in sympathetic neurons compared with basal levels (Figures 1C and 1D). Western blot analysis with anti-caspase 9 or anti-caspase 8 antibodies did not show activation of either of these caspases (results not shown). We have recently shown that another paradigm of neuronal death induces active caspase 9 and caspase 8, supporting the fact that we are able to detect these caspases when they are active [23]. These results imply that both death stimuli, Aβ and NGF withdrawal, activate caspase 2, and that caspase 2 is the critical caspase responsible for neuronal death under these conditions.
Figure 1. Aβ and NGF deprivation induce active caspase 2 in neurons.
(A) Hippocampal neuron cultures were treated with 50 μM biotin-VAD-FMK for 2 h and then with or without 3 μM Aβ for an additional 2 h. Active caspase 2 was pulled down using streptavidin beads and identified by Western blot analysis with a polyclonal anti-(caspase 2) antibody. Representative blots are shown; the experiments were repeated five times. (B) Densitometry of relative caspase 2 activity induced by Aβ treatment from five independent blots. (C) Sympathetic neuron cultures were treated with 50 μM biotin-VAD-FMK for 2 h and then with or without NGF deprivation (TFD) for an additional 2 h. Active caspase 2 was pulled down using streptavidin beads and identified by Western blot analysis with a polyclonal anti-(caspase 2) antibody. Representative blots are shown; the experiments were repeated three times. (D) Densitometry of relative caspase 2 activity induced by TFD from three independent blots. Co, control.
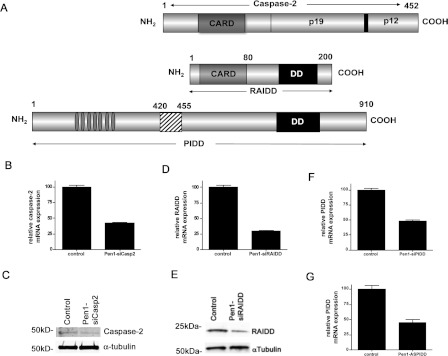
These results led us to study the caspase 2 activation complex in these neurons. The PIDDosome has been proposed to induce caspase 2 activation [10]. This complex has been proposed to form by the interaction between caspase 2 and RAIDD via their CARD and between RAIDD and PIDD via their DDs (a schematic representation of this activation complex, partners and interaction domains is shown in Figure 2A). We have shown previously that AS knockdown of caspase 2 protects neurons from Aβ [5] and from NGF deprivation [6], and that siRNA against RAIDD protects from NGF deprivation [13]. To more fully determine the function of the PIDDosome in caspase-2-mediated neuronal death, we designed siRNA for each component of the complex; we also used the previously published AS-RNA sequence for PIDD [24] and linked it to Pen1. These siRNAs and AS-RNA contain a 5′ thiol modification used to link the oligonucleotide via a disulfide bond to Pen1 [17,18] and their knock-down efficiency was tested by Western blot analysis (Figures 2C and 2E) and by real-time PCR (Figures 2B, 2D, 2F and 2G). All of the siRNA presented here showed a reduction of at least 50% at the mRNA levels and, for caspase 2 and RAIDD, an approximately 70% knock down at the protein level. As expected, siRNA against caspase 2 did not change the expression level of other caspases, and siRNA against PIDD or RAIDD did not change expression levels of any of the caspases (results not shown). We attempted to measure endogenous PIDD using commercially available antibodies, but the PIDD antibodies were found to not be specific for endogenous PIDD (Supplementary Figure S1 at http://www.BiochemJ.org/bj/444/bj4440591add.htm).
Figure 2. Potential components of the caspase 2 activation complex.
(A) Schematic diagram of components of the PIDDosome: caspase 2, RAIDD and PIDD. Potential interaction domains (CARD for caspase 2 and RAIDD, and DD for RAIDD and PIDD) are indicated. (B) Hippocampal neuron cultures were treated with Pen1-siCasp2 (80 nM) for 6 h and analysed by quantitative PCR for CASP2 expression, mRNA was normalized to TUBB mRNA expression. n=3. (C) Hippocampal neuron cultures were treated with Pen1-siCasp2 (80 nM) for 6 h and analysed by Western blotting for caspase 2 protein expression, α-tubulin was used as a loading control. Representative blots are shown; the experiments were repeated three times. (D) Hippocampal neuron cultures were treated with Pen1-siRAIDD (80 nM) for 6 h and analysed by quantitative PCR for RAIDD expression, mRNA was normalized to TUBB mRNA expression. n=3. (E) Hippocampal neuron cultures were treated with Pen1-siRAIDD (80 nM) for 5 h and analysed by Western blotting for RAIDD protein expression, α-tubulin was used as a loading control. Representative blots are shown; the experiments were repeated three times. (F) Hippocampal neuron cultures were treated with Pen1-siPIDD (80 nM) for 6 h and analysed by quantitative PCR for PIDD expression, mRNA was normalized to TUBB mRNA expression. n=3. (G) Hippocampal neuron cultures were treated with Pen1-AS-PIDD (80 nM) for 6 h and analysed by quantitative PCR for PIDD expression, mRNA was normalized to TUBB mRNA expression. n=3.
RAIDD, but not PIDD, is required for caspase-2-dependent neuronal death
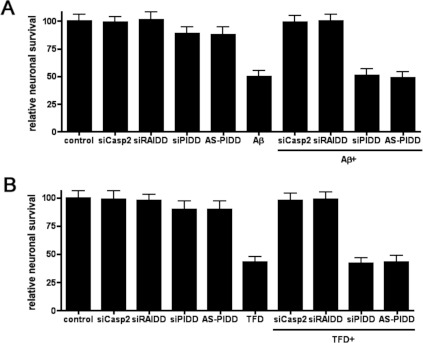
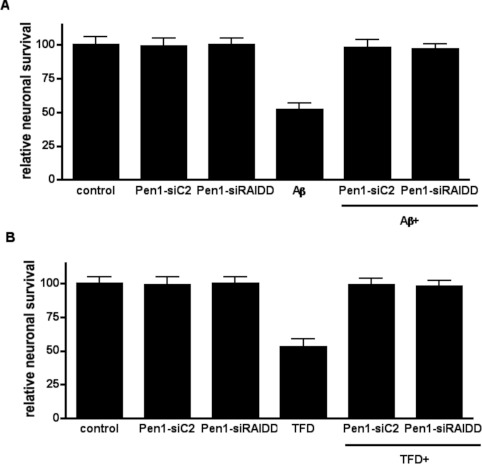
To determine which components of the caspase 2 activation complex were required for neuronal cell death, we investigated the role of each component in neuronal survival using the Pen1-siRNA and Pen1-AS-RNA. We treated hippocampal neurons with Pen1-siRNA against caspase 2, RAIDD, PIDD (siCasp2, siRAIDD and siPIDD) or AS-RNA against PIDD (AS-PIDD) and 3 μM Aβ was added to the neurons. After 24 h of Aβ treatment neuronal survival was quantified. In order to evaluate off-target effects associated with the presence of the siRNA, a set of neurons were left untreated (control), whereas another set of sister neurons were treated exclusively with siRNA. The addition of 3 μM Aβ to the cultures induced, over a 24 h period, 52% cell death compared with the control untreated neurons (Figure 3A). Although the presence of siCasp2 or siRAIDD totally prevented Aβ-induced cell death, siPIDD or AS-PIDD did not abrogate Aβ toxicity. The efficacy of siCasp2 validates the siRNA approach for these studies as it confirms the requirement for caspase 2 that we have previously shown using either AS knockdown or caspase 2-null neurons [5]. The effect of siRAIDD suggests a critical role for RAIDD in Aβ-induced toxicity. None of the siRNAs used in the present study were toxic since the cultures treated only with siRNA showed survival rates similar to those found in control neurons.
Figure 3. RAIDD, not PIDD, is required for caspase-2-dependent neuronal death.
(A) Hippocampal neuron cultures were treated for 2 h with or without the indicated Pen1-oligonucleotides (80 nM) and then treated with or without 3 μM Aβ for 24 h. Relative neuronal survival was quantified and is expressed as the percentage survival relative to control cultures, each point was assayed in triplicate and the experiment was repeated three times. (B) Sympathetic neuron cultures were treated for 2 h with the indicated Pen1-oligonucleotides (80 nM) and then with or without NGF deprivation (TFD) for 24 h. Relative neuronal survival was quantified after 24 h TFD and is expressed as the percentage of neurons remaining relative to the initial number of neurons in each culture, each point was assayed in triplicate and the experiment was repeated three times.
Our previous studies investigating the function of RAIDD in NGF-deprivation-induced neuronal death used a different siRNA sequence and did not use Pen1 to deliver the siRNA [13]. To confirm that the Pen1-siRAIDD was also effective against NGF deprivation, sympathetic neurons were treated with each individual Pen1-siRNA independently or in combination with NGF deprivation, and neuronal survival was measured 24 h after NGF deprivation. Similar to the results found for Aβ, NGF deprivation killed over 50% of the neurons and, as expected, siCasp2 and siRAIDD both prevented NGF-deprivation-induced neuronal death. In contrast, down-regulation of PIDD via siRNA or AS-RNA had no effect. None of the siRNA used were toxic to the sympathetic neurons (Figure 3B). These data confirm our previous data and, taken together, show that RAIDD, and not PIDD, is the critical adaptor molecule responsible for executing caspase-2-mediated neuronal death.
RAIDD is necessary for the activation of caspase 2
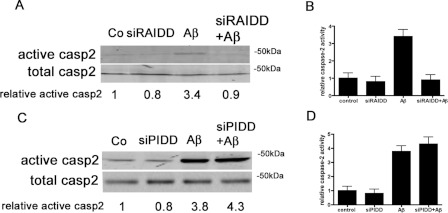
Having shown that RAIDD is required for caspase-2-dependent neuronal death, we now tested whether RAIDD is required for induction of caspase 2 activity. In order to address this question, primary hippocampal neurons were pre-treated with Pen1-siRAIDD for 1 h, treated with 50 μM biotin-VAD-FMK for 2 h prior to Aβ treatment, and then exposed to 3 μM Aβ for 2 h. In a parallel experiment, sister neurons were treated with vehicle (control) or treated with Pen1-siRAIDD or Aβ alone. Samples were harvested and biotin-VAD-FMK–caspase complexes were isolated to detect levels of active caspase 2. Aβ induced an almost a 4-fold increase in the levels of active caspase 2 compared with control cells (Figures 4A and 4B). Pre-treatment of neurons with Pen1-siRAIDD totally prevented Aβ-induced caspase 2 activation. The addition of Pen1 siRAIDD alone did not activate caspase 2. This demonstrates that RAIDD is necessary for caspase 2 activation in neurons. The same blot was re-probed for caspase 9 and for caspase 8 and there was no activation of either of these caspases (results not shown).
Figure 4. RAIDD is required for caspase 2 activation, PIDD is not.
(A) Hippocampal neuron cultures were treated with or without Pen1-siRAIDD (80 nM) for 1 h and then with biotin-VAD-FMK (50 μM) for 2 h prior to Aβ treatment and exposure to 3 μM Aβ or vehicle for 2 h. Active caspase 2 was pulled down using streptavidin beads and identified by Western blot analysis with a polyclonal anti-(caspase 2) antibody. Representative blots are shown; the experiments were repeated three times. (B) Densitometry of relative caspase 2 activity induced by Aβ treatment with and without Pen1-siRAIDD from three independent blots. (C) Hippocampal neuron cultures were treated with or without Pen1-siPIDD (80 nM) for 1 h, then with biotin-VAD-FMK (50 μM) for 2 h, and then with or without 3 μM Aβ for an additional 2 h. Active caspase 2 was pulled down using streptavidin beads and identified by Western blot analysis with a polyclonal anti-(caspase 2) antibody. Representative blots are shown; the experiments were repeated three times. (D) Densitometry of relative caspase 2 activity induced by Aβ treatment with and without Pen1-siRAIDD from three independent blots. Co, control.
PIDD is not required for caspase 2 activation
To determine whether PIDD has a role in the activation of caspase 2, we treated hippocampal neurons with or without Pen1-siPIDD for 1 h, and then added biotin-VAD-FMK for 2 h followed by the addition of Aβ. After a 2 h incubation with Aβ, samples were harvested, biotin-VAD-FMK–caspase complexes were isolated and levels of active caspase 2 were determined by Western blot analysis (Figures 4C and 4D). In accordance with previous results, Aβ resulted in the activation of caspase 2 by almost 4-fold, and Pen1-siPIDD-treated cultures showed similar levels of active caspase 2 to those detected in control cultures. However, the presence of Pen1-siPIDD in conjunction with Aβ did not prevent the activation of caspase 2, demonstrating that the presence of PIDD is not necessary for the activation of caspase 2.
PIDD-null neurons undergo Aβ and NGF-deprivation-mediated caspase-2-dependent death
Because both PIDD and RAIDD have been proposed to be part of the caspase 2 activation complex, and we have shown that PIDD is not essential for caspase 2 activation, we studied whether PIDD-null primary neurons are sensitive to caspase-2-dependent death stimuli, such as Aβ or NGF deprivation. Primary sympathetic neurons from PIDD-null mice were treated with Pen1-siRNA against caspase 2 or RAIDD alone, or in combination with Aβ, and neurons were subjected to survival analysis 24 h after the addition of Aβ into the cultures (Figure 5A). PIDD-null neurons were as sensitive to Aβ as wild-type neurons, showing an approximately 50% neuronal survival after 24 h. As found for wild-type neurons, PIDD-null neurons were totally protected against Aβ toxicity when treated with either Pen1-siCasp2 or Pen1-siRAIDD, each treatment yielding 100% survival of neurons.
Figure 5. Caspase 2 and RAIDD execute Aβ- or NGF-deprivation-induced death of PIDD-null neurons.
(A) Sympathetic neuron cultures from PIDD-null mice were cultured with the indicated Pen1-siRNA (80 nM) and then treated with or without 3 μM Aβ for 24 h. Relative neuronal survival was quantified and is expressed as the percentage survival relative to control cultures. Each point was assayed in triplicate and the experiment was repeated three times. (B) Sympathetic neuron cultures from PIDD-null mice were treated for 2 h with the indicated Pen1-siRNA (80 nM) and then with or without NGF deprivation (TFD) for 24 h. Relative neuronal survival was quantified after 24 h TFD and is expressed as the percentage of neurons remaining relative to the initial number of neurons in each culture. Each point was assayed in triplicate and the experiment was repeated three times.
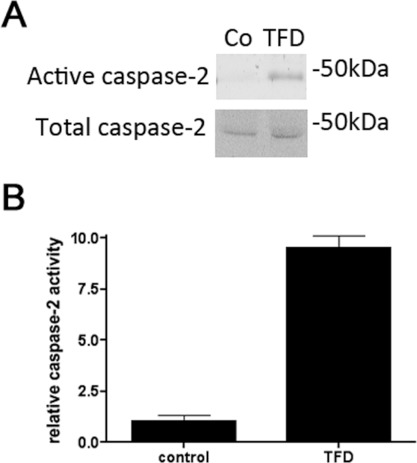
To determine the requirement for PIDD in NGF-deprivation-mediated death, PIDD-null primary sympathetic neurons were cultured and pre-treated for 1 h with Pen1-siRNA against caspase 2 or RAIDD, and then subjected to NGF deprivation. Survival was quantified after 1 day of treatment (Figure 5B). Neurons lacking PIDD were as sensitive as wild-type neurons to NGF deprivation, and siCasp2 and siRAIDD each protected neurons against NGF-deprivation-mediated apoptosis. These results demonstrate that PIDD-null neurons can undergo caspase-2-dependent death under the conditions studied, and that RAIDD is an essential modulator of caspase-2-dependent death. To confirm that there is induction of caspase 2 activity in PIDD-null neurons, PIDD-null neurons were pre-treated for 2 h with biotin-VAD-FMK followed by a 2 h depletion of NGF. Biotin-VAD-FML–caspase complexes were isolated and analysed by Western blotting to detect active caspase 2. NGF depletion for 2 h was able to activate caspase 2 in PIDD-null neurons (Figure 6), in levels comparable with those seen with wild-type neurons (Figure 1). These observations demonstrate that the caspase 2 machinery is intact in the absence of PIDD.
Figure 6. NGF deprivation induces caspase 2 activation in PIDD-null neurons.
(A) Sympathetic neuron cultures from PIDD-null mice were treated with 50 μM biotin-VAD-FMK for 2 h and then with or without NGF deprivation (TFD) for an additional 2 h. Active caspase 2 was pulled down using streptavidin beads and identified by Western blot analysis with a polyclonal anti-(caspase 2) antibody. Representative blots are shown; the experiments were repeated three times. Co, control. (B) Densitometry of relative caspase 2 activity induced by TFD from three independent blots.
Aβ and NGF deprivation induce formation of a complex of RAIDD and caspase 2 in wild-type and PIDD-null neurons
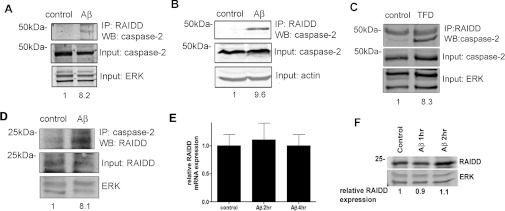
To further study the activation complex for caspase 2, we performed co-immunoprecipitation studies. Hippocampal neurons were treated with Aβ for 1 h, immunoprecipitated with an anti-RAIDD antibody and then immunoblotted for caspase 2 (Figure 7A). There was a more than 8-fold increase in caspase 2 pulled down by the anti-RAIDD antibody following treatment with Aβ for 1 h. To evaluate the requirement for PIDD in formation of this complex, we used hippocampal neurons from PIDD-null mice and showed that Aβ treatment induced a similar induction of the caspase 2–RAIDD interaction (Figure 7B). To determine whether TFD also induced formation of a caspase 2– RAIDD complex, we used neuronal PC12 cells, a model that we have previously shown requires caspase 2 to execute death [6]. We used this model because the sympathetic neuron cultures do not provide enough protein for co-immunoprecipitation analysis. TFD induced formation of the caspase 2–RAIDD complex (Figure 7C). In Figure 7(D) we show that, in hippocampal neurons treated with Aβ, immunoprecipitation with caspase 2 pulls down RAIDD, at a level similar to the reverse immunoprecipitations. There is no increase in RAIDD mRNA (Figure 7E) or protein expression (Figure 7F) in response to Aβ treatment, suggesting that it is the co-localization of caspase 2 and RAIDD that is critical.
Figure 7. A complex of caspase 2 and RAIDD is induced by Aβ or TFD treatment.
(A) Hippocampal neurons were treated with 3μM Aβ for 1 h and harvested for immunoprecipitation with an anti-RAIDD antibody followed by immunoblotting for caspase 2. Input samples were immunoblotted for caspase 2 and ERK. Representative blots are shown; the experiments were repeated three times. Densitometry was used to determine the relative amount of caspase 2 pulled down with the anti-RAIDD antibody. (B) Hippocampal neurons from PIDD-null mice were treated with 3μM Aβ for 4 h and harvested for immunoprecipitation with an anti-RAIDD antibody followed by immunoblotting for caspase 2. Input samples were immunoblotted for caspase 2 and actin. Representative blots are shown; the experiments were repeated three times. Densitometry was used to determine the relative amount of caspase 2 pulled down with the anti-RAIDD antibody. (C) Neuronal PC12 cells were subjected to TFD for 4 h and and harvested for immunoprecipitation with an anti-RAIDD antibody followed by immunoblotting for caspase 2. Input samples were immunoblotted for caspase 2 and ERK. Representative blots are shown; the experiments were repeated three times. Densitometry was used to determine the relative amount of caspase 2 pulled down with the anti-RAIDD antibody. (D) Hippocampal neurons were treated with 3 μM Aβ for 4 h and harvested for immunoprecipitation with an anti-(caspase 2) antibody followed by immunoblotting for RAIDD. Input samples were immunoblotted for RAIDD and ERK. Representative blots are shown; the experiments were repeated three times. Densitometry was used to determine the relative amount of RAIDD pulled down with the anti-(caspase 2) antibody. (E) Sister cultures were treated as described in (A) and RNA was isolated and analysed by quantitative PCR for RAIDD expression, mRNA was normalized to TUBB mRNA expression. n=3. (F) Sister cultures were treated as described in (A) and immunoblotted for RAIDD and ERK. Representative blots are shown; the experiments were repeated three times. Densitometry was used to determine the relative amount of RAIDD. IP, immunoprecipitation; WB, Western blot.
DISCUSSION
The present study shows that both Aβ and NGF deprivation induce caspase 2 activity and caspase-2-dependent neuronal death. We also show that RAIDD is necessary for caspase 2 activity and neuronal death, but PIDD is not necessary. We provide data showing that, in neurons, Aβ treatment induces the formation of a complex containing caspase 2 and RAIDD. Our previous studies have shown the functional requirement for caspase 2 in two paradigms of neuronal death, Aβ [5] and NGF deprivation [4,6]. We now extend those previous studies to the detection of caspase 2 activity after both of these death stimuli. The approach to measuring caspase activity is one that has evolved over the years. Cleavage has been used as an indicator of caspase activation for both effector and initiator caspases. However, it is now clear that initiator caspases do not require cleavage for activation, activation occurs via proximity-induced dimerization [8]. This activation may be followed by autoprocessing, which can enhance caspase activity, as shown for caspase 8 [25,26], or have little effect on caspase activity, as shown for caspase 9 [27]. However, the critical step in activation of caspases with long pro-domains is dimerization, and this is effected through interaction with specific adaptor proteins. Since cleavage is not necessary for activation, assays that measure cleavage do not present a clear measure of initiator caspase activation. In support of this, studies of PIDD-null cells showed that caspase 3 or caspase 7 is responsible for the cleavage of caspase 2, which is not an activation step [11]. Another frequently used measure of caspase activity uses peptide substrates to measure activity in cell lysates. For caspase 2 the substrate is VDVAD (Val-Asp-Val-Ala-Asp), but this substrate is a better substrate for caspase 3 than for caspase 2, thus it is also an inadequate measure of caspase 2 activity [28]. For initiator caspases a method has been adapted that uses a caspase-affinity ligand, biotin-VAD-FMK, to capture active caspases. By pre-treating cells with this reagent the proximal caspase(s) in the death pathway will be captured and all subsequent effects of these caspases in the cells will be inhibited. This approach has been used in non-neuronal cells for a variety of death paradigms [22]. We have previously adapted this for use in primary neurons [21] and in the central nervous system in vivo [23]. Our previous studies of Aβ showed that active caspase 2 could be detected 3 h after treatment of hippocampal cultures with Aβ. We now show that caspase 2 is active within 2 h of Aβ treatment, and that NGF deprivation also induces active caspase 2 within 2 h of deprivation. The induction of caspase 2 activity complements our studies that show the functional requirement for caspase 2 in these paradigms [4–6].
The activation complex of caspase 2 is also unknown. Since we have now shown that both Aβ and NGF deprivation induce caspase 2 activity and require caspase 2 to execute death, we used several approaches to study the requirement for the PIDDosome to activate caspase 2 in neurons. For each member of the PIDDosome we used Pen1-siRNA to achieve significant knockdown of expression of the targeted mRNA/protein. Pen1 provides facilitated non-toxic delivery of siRNA to neurons at a high efficiency. We have previously shown that transfection of siRNA using Lipofectamine™ induced more than 50% death in neuronal cultures [18]. Since we are studying cell death mechanisms it is critical that we are not inducing death by means other than the death stimuli under study. Our previous work has shown that RAIDD is required for NGF-deprivation-mediated death of neurons, but we did not examine the effect of depletion of RAIDD on caspase 2 activity in those studies [13]. In the present study we show that RAIDD is required for induction of caspase 2 activity and for execution of Aβ and NGF deprivation death, thus also extending the requirement for RAIDD to the Aβ paradigm. We also show that a complex of caspase 2 and RAIDD is formed in neurons treated with Aβ or with NGF deprivation.
For PIDD we used multiple approaches to determine its function in these paradigms. An AS-RNA sequence had been used previously by another group [24] and we used this sequence linked to Pen1. We also used an siRNA to knockdown PIDD and achieved significant knockdown of mRNA, but could not measure protein because we found that the commercial antibodies available did not detect endogenous PIDD, since the same proteins were detected in wild-type and PIDD-null brains (Supplementary Figure S1). However, the PIDD-null neurons provided a third approach to determining whether PIDD was required for induction of caspase 2 activity or for execution of Aβ- or NGF-deprivation-mediated death. All of these approaches showed that PIDD was not essential for caspase-2-mediated death. Aβ induced formation of a caspase 2–RAIDD complex in PIDD-null neurons. Active caspase 2 was induced in neurons in which PIDD was knocked down or knocked out. PIDD-null neurons died in response to Aβ or NGF deprivation, and death was abrogated by caspase 2 or RAIDD knockdown.
The requirement for RAIDD in various paradigms of cell death has not been fully delineated. Overexpression of PIDD induces cleavage of caspase 2 and cell death [10]. This death was shown to require RAIDD, but to be only partially dependent on caspase 2 expression [29]. Assay of caspase 2 activity showed that PIDD overexpression induced caspase 2 activity, but not as robustly as overexpression of RAIDD [30]. These results suggest that overexpression of PIDD does require RAIDD and activates caspase 2. Another death paradigm shown to require RAIDD is heat-shock-induced death. In a series of studies the Green laboratory has shown that heat-shock induces caspase 2 activity and requires RAIDD to execute death [22,30]. Other groups have shown for multiple death stimuli that neither RAIDD nor PIDD were required for a variety of death stimuli in non-neuronal cells [11,12]; however, these studies did not show that caspase 2 is required for these death stimuli. As noted above, caspase 2 has been implicated as functioning in several death paradigms on the basis of data showing caspase 2 cleavage or utilizing VDVAD to measure or inhibit caspase 2. However, in the absence of appropriate measures of caspase 2 activity and of specific inhibition of caspase 2, it is not evident that these death stimuli actually require caspase 2, and thus studies of the PIDDosome complex are not revealing without definitive proof that caspase 2 is executing death. In the present study we have utilized a molecular approach to determine that caspase 2 indeed acts as a neuronal executioner in these models, and we also have biochemically measured caspase 2 activity. Our results may suggest that there is a neuron-specific activation complex for caspase 2 which contains RAIDD.
Taken together, the results of the present study show that both Aβ and NGF deprivation induce active caspase 2 in neurons and that RAIDD is required for execution of death by these stimuli, whereas PIDD is not. Thus the entire PIDDosome is not necessary for caspase 2 activity in neurons, whereas caspase 2 and RAIDD are essential and do form a complex when exposed to the death stimulus. Whether another protein is also part of the caspase 2 activation complex in neurons remains to be determined.
Online data
AUTHOR CONTRIBUTION
Elena Ribe, Leonidas Stefanis and Carol Troy conceived the study and designed the experiments. Elena Ribe, Ying Jean, Rebecca Goldstein and Claudia Manzl performed the experiments. Claudia Manzl and Andreas Villunger developed the PIDD-null mice. Elena Ribe, Ying Jean and Carol Troy analysed the data and wrote the paper.
FUNDING
This work was supported by the National Institutes of Health [grant number NS43089 (to C.M.T.)]; the Spanish Ministry of Education and Science (MEC postdoctoral fellowship to E.M.R.); the Austrian Science Fund (to A.V.); and the Tyrolean Science Fund (to C.M.).
References
- 1.Kumar S., Kinoshita M., Noda M., Copeland N. G., Jenkins N. A. Induction of apoptosis by the mouse Nedd2 gene, which encodes a protein similar to the product of the Caenorhabditis elegans cell death gene ced-3 and the mammalian IL-1β-converting enzyme. Genes Dev. 1994;8:1613–1626. doi: 10.1101/gad.8.14.1613. [DOI] [PubMed] [Google Scholar]
- 2.Wang L., Miura M., Bergeron L., Zhu H., Yuan J. Ich-1, an Ice/ced-3-related gene, encodes both positive and negative regulators of programmed cell death. Cell. 1994;78:739–750. doi: 10.1016/s0092-8674(94)90422-7. [DOI] [PubMed] [Google Scholar]
- 3.Bergeron L., Perez G. I., Macdonald G., Shi L., Sun Y., Jurisicova A., Varmuza S., Latham K. E., Flaws J. A., Salter J. C., et al. Defects in regulation of apoptosis in caspase-2-deficient mice. Genes Dev. 1998;12:1304–1314. doi: 10.1101/gad.12.9.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Troy C. M., Rabacchi S. A., Hohl J. B., Angelastro J. M., Greene L. A., Shelanski M. L. Death in the balance: alternative participation of the caspase-2 and -9 pathways in neuronal death induced by nerve growth factor deprivation. J. Neurosci. 2001;21:5007–5016. doi: 10.1523/JNEUROSCI.21-14-05007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Troy C. M., Rabacchi S. A., Friedman W. J., Frappier T. F., Brown K., Shelanski M. L. Caspase-2 mediates neuronal cell death induced by β-amyloid. J. Neurosci. 2000;20:1386–1392. doi: 10.1523/JNEUROSCI.20-04-01386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Troy C. M., Stefanis L., Greene L. A., Shelanski M. L. Nedd2 is required for apoptosis after trophic factor withdrawal, but not superoxide dismutase (SOD1) downregulation, in sympathetic neurons and PC12 cells. J. Neurosci. 1997;17:1911–1918. doi: 10.1523/JNEUROSCI.17-06-01911.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamkanfi M., Declercq W., Kalai M., Saelens X., Vandenabeele P. Alice in caspase land. A phylogenetic analysis of caspases from worm to man. Cell Death Differ. 2002;9:358–361. doi: 10.1038/sj.cdd.4400989. [DOI] [PubMed] [Google Scholar]
- 8.Pop C., Salvesen G. S. Human caspases: activation, specificity, and regulation. J. Biol. Chem. 2009;284:21777–21781. doi: 10.1074/jbc.R800084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan H., Dixit V. M. RAIDD is a new ‘death’ adaptor molecule. Nature. 1997;385:86–89. doi: 10.1038/385086a0. [DOI] [PubMed] [Google Scholar]
- 10.Tinel A., Tschopp J. The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science. 2004;304:843–846. doi: 10.1126/science.1095432. [DOI] [PubMed] [Google Scholar]
- 11.Manzl C., Krumschnabel G., Bock F., Sohm B., Labi V., Baumgartner F., Logette E., Tschopp J., Villunger A. Caspase-2 activation in the absence of PIDDosome formation. J. Cell Biol. 2009;185:291–303. doi: 10.1083/jcb.200811105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim I. R., Murakami K., Chen N. J., Saibil S. D., Matysiak-Zablocki E., Elford A. R., Bonnard M., Benchimol S., Jurisicova A., Yeh W. C., Ohashi P. S. DNA damage- and stress-induced apoptosis occurs independently of PIDD. Apoptosis. 2009;14:1039–1049. doi: 10.1007/s10495-009-0375-1. [DOI] [PubMed] [Google Scholar]
- 13.Wang Q., Maniati M., Jabado O., Pavlaki M., Troy C. M., Greene L. A., Stefanis L. RAIDD is required for apoptosis of PC12 cells and sympathetic neurons induced by trophic factor withdrawal. Cell Death Differ. 2006;13:75–83. doi: 10.1038/sj.cdd.4401690. [DOI] [PubMed] [Google Scholar]
- 14.Park D. S., Morris E. J., Stefanis L., Troy C. M., Shelanski M. L., Geller H. M., Greene L. A. Multiple pathways of neuronal death induced by DNA-damaging agents, NGF deprivation, and oxidative stress. J. Neurosci. 1998;18:830–840. doi: 10.1523/JNEUROSCI.18-03-00830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Troy C. M., Friedman J. E., Friedman W. J. Mechanisms of p75-mediated death of hippocampal neurons: role of caspases. J. Biol. Chem. 2002;277:34295–34302. doi: 10.1074/jbc.M205167200. [DOI] [PubMed] [Google Scholar]
- 16.Elbashir S. M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 17.Troy C. M., Derossi D., Prochiantz A., Greene L. A., Shelanski M. L. Downregulation of Cu/Zn superoxide dismutase leads to cell death via the nitric oxide-peroxynitrite pathway. J. Neurosci. 1996;16:253–261. doi: 10.1523/JNEUROSCI.16-01-00253.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson T. J., Harel S., Arboleda V. A., Prunell G. F., Shelanski M. L., Greene L. A., Troy C. M. Highly efficient small interfering RNA delivery to primary mammalian neurons induces microRNA-like effects before mRNA degradation. J. Neurosci. 2004;24:10040–10046. doi: 10.1523/JNEUROSCI.3643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barghorn S., Nimmrich V., Striebinger A., Krantz C., Keller P., Janson B., Bahr M., Schmidt M., Bitner R. S., Harlan J., et al. Globular amyloid β-peptide oligomer: a homogenous and stable neuropathological protein in Alzheimer's disease. J. Neurochem. 2005;95:834–847. doi: 10.1111/j.1471-4159.2005.03407.x. [DOI] [PubMed] [Google Scholar]
- 20.Rukenstein A., Rydel R. E., Greene L. A. Multiple agents rescue PC12 cells from serum-free cell death by translation- and transcription-independent mechanisms. J. Neurosci. 1991;11:2552–2563. doi: 10.1523/JNEUROSCI.11-08-02552.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tizon B., Ribe E. M., Mi W., Troy C. M., Levy E. Cystatin C protects neuronal cells from amyloid-β-induced toxicity. J. Alzheimers Dis. 2010;19:885–894. doi: 10.3233/JAD-2010-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tu S., McStay G. P., Boucher L. M., Mak T., Beere H. M., Green D. R. In situ trapping of activated initiator caspases reveals a role for caspase-2 in heat shock-induced apoptosis. Nat. Cell Biol. 2006;8:72–77. doi: 10.1038/ncb1340. [DOI] [PubMed] [Google Scholar]
- 23.Akpan N., Serrano-Saiz E., Zacharia B. E., Otten M. L., Ducruet A. F., Snipas S. J., Liu W., Velloza J., Cohen G., Sosunov S. A., et al. Intranasal delivery of caspase-9 inhibitor reduces caspase-6-dependent axon/neuron loss and improves neurological function after stroke. J. Neurosci. 2011;31:8894–8904. doi: 10.1523/JNEUROSCI.0698-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y., Ma W., Benchimol S. Pidd, a new death-domain-containing protein, is induced by p53 and promotes apoptosis. Nat. Genet. 2000;26:122–127. doi: 10.1038/79102. [DOI] [PubMed] [Google Scholar]
- 25.Pop C., Fitzgerald P., Green D. R., Salvesen G. S. Role of proteolysis in caspase-8 activation and stabilization. Biochemistry. 2007;46:4398–4407. doi: 10.1021/bi602623b. [DOI] [PubMed] [Google Scholar]
- 26.Oberst A., Pop C., Tremblay A. G., Blais V., Denault J. B., Salvesen G. S., Green D. R. Inducible dimerization and inducible cleavage reveal a requirement for both processes in caspase-8 activation. J. Biol.Chem. 2010;285:16632–16642. doi: 10.1074/jbc.M109.095083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stennicke H. R., Deveraux Q. L., Humke E. W., Reed J. C., Dixit V. M., Salvesen G. S. Caspase-9 can be activated without proteolytic processing. J. Biol. Chem. 1999;274:8359–8362. doi: 10.1074/jbc.274.13.8359. [DOI] [PubMed] [Google Scholar]
- 28.McStay G. P., Salvesen G. S., Green D. R. Overlapping cleavage motif selectivity of caspases: implications for analysis of apoptotic pathways. Cell Death Differ. 2008;15:322–331. doi: 10.1038/sj.cdd.4402260. [DOI] [PubMed] [Google Scholar]
- 29.Berube C., Boucher L. M., Ma W., Wakeham A., Salmena L., Hakem R., Yeh W. C., Mak T. W., Benchimol S. Apoptosis caused by p53-induced protein with death domain (PIDD) depends on the death adapter protein RAIDD. Proc. Natl. Acad. Sci. U.S.A. 2005;102:14314–14320. doi: 10.1073/pnas.0506475102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouchier-Hayes L., Oberst A., McStay G. P., Connell S., Tait S. W., Dillon C. P., Flanagan J. M., Beere H. M., Green D. R. Characterization of cytoplasmic caspase-2 activation by induced proximity. Mol. Cell. 2009;35:830–840. doi: 10.1016/j.molcel.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.