Abstract
INTRODUCTION
Venous thromboembolism (VTE) prophylaxis has become a major issue for surgeons both in the UK and worldwide. Sev-eral different sources of guidance on VTE prophylaxis are available but these differ in design and detail.
METHODS
Two similar audits were performed, one year apart, on the VTE prophylaxis prescribed for all general surgical inpatients during a single week (90 patients and 101 patients). Classification of patients into different risk groups and compliance in prescribing prophylaxis were examined using different international, national and local guidelines.
RESULTS
There were significant differences between the numbers of patients in high, moderate and low-risk groups according to the different guidelines. When groups were combined to indicate simply ‘at risk’ or ‘not at risk’ (in the manner of one of the guidelines), then differences were not significant. Our compliance improved from the first audit to the second. Patients at high risk received VTE prophylaxis according to guidance more consistently than those at low risk.
CONCLUSIONS
Differences in guidance on VTE prophylaxis can affect compliance significantly when auditing practice, depending on the choice of ‘gold standard’. National guidance does not remove the need for clear and detailed local policies. Making decisions about policies for lower-risk patients can be more difficult than for those at high risk.
Keywords: Audit, Venous thromboembolism, Risk stratification
Venous thromboembolism (VTE) is a major safety issue for patients and for healthcare services worldwide.1,2 The effectiveness of pharmacological and mechanical methods of prophylaxis against VTE has been clearly demonstrated but these have been used inconsistently and inadequately.3–5 In recognition of the widespread professional and public concern about failure to provide appropriate prophylaxis, several national and international organisations have reviewed the published evidence and developed guidelines aimed at reducing the incidence of recognised and unrecognised VTE in hospitalised patients.6–11
In the UK national guidance has been published by the National Institute for Health and Clinical Excellence (NICE), first in 2007 (for surgical inpatients) and more recently in 2010 (for all hospitalised patients).6,11 It provides ‘high level’ guidance for all hospital specialties about assessing patients for any risk of VTE and bleeding, and about who should receive pharmacological or mechanical prophylaxis. However, it leaves more detailed decisions about types and doses of anticoagulants and about types of mechanical prophylaxis to the discretion of individual hospitals and units.
An important issue for consideration when producing local policies for VTE prophylaxis is risk stratification, in other words whether some patients at risk for VTE are at greater risk than others and therefore should receive different doses of anticoagulant drugs or different mechanical methods – typically intermittent pneumatic compression (IPC) rather than graduated compression stockings (GCS).
Auditing the adequacy of VTE prophylaxis has become an important quality-assurance measure. However, the existence of a variety of different guidelines (including a NICE guideline that rightly accommodates flexibility in practice) can make both auditing and interpreting the results of audit problematic. The different sources of guidance make somewhat different recommendations about which patients should receive what prophylaxis. They also stratify the risks of patients in different ways (or not at all). This study examined the effect of using criteria from different guidelines to evaluate practice.
Methods
Two audits were performed one year apart, as follows:
Audit 1 was performed in November 2008. It included all adult general surgical elective and emergency inpatient admissions to the Royal Devon and Exeter NHS Foundation Trust during a single week. Patients were under the care of two upper gastrointestinal, three lower gastrointestinal, three vascular, two breast and four urological consultant surgeons.
The audit was carried out by LH and DR using the medical and nursing notes and prescription charts, each evening while patients were still in hospital. Data collection was done according to a proforma (Fig 1) that specified aspects of VTE risk, what prophylaxis each patient was prescribed and whether this concurred with three guidance documents current at that time: NICE clinical guideline (CG) 46,6 the European consensus (EC) guidelines7 and the Department of Health (DH) risk stratification tool.8 No local guideline was used; the audit was done in part to guide agreement about a cohesive departmental policy.
Figure 1.

Summary of proforma used for auditing venous thromboprophylaxis in general surgery patients
The results of Audit 1 were presented at a departmental meeting and to new trainees, and the intention to carry out a second audit was announced. In addition, a cohesive unit policy was agreed and used as one of the standards in Audit 2.
Audit 2 was performed a year after Audit 1, during November 2009. The methods were the same except that the new local policy (Fig 2) was used instead of the EC guidelines7 (it included similar risk stratification) and a slightly amended proforma was used (Fig 1).
Figure 2.
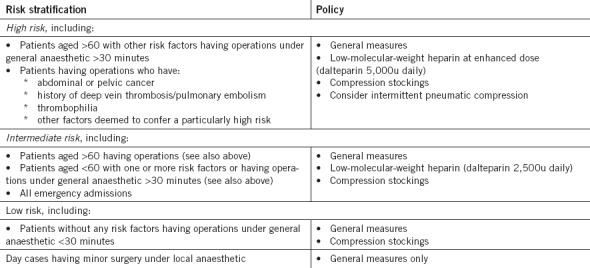
Venous thromboprophylaxis policy for surgical patients (approved by Royal Devon and Exeter NHS Foundation Trust executive in April 2009)
All statistical comparisons were done by chi-square analysis. For the purposes of comparing patients ‘at risk’ (ie with risk factors) or ‘not at risk’ according to NICE CG46, patients in high and moderate-risk groups of the other guidance were combined and those in low/minimal/no-risk groups were combined for 2 × 2 comparison. When comparing classification of patients by guidance that stratified into three levels of risk, an adequate size contingency table was used (for example, a 3 × 2 comparison for risk stratification).
Results
There were 90 patients (61 elective, 29 emergencies) in Audit 1 and 101 patients (41 elective, 60 emergencies) in Audit 2.
Table 1 shows the classification of patients for their levels of VTE risk according to each of the guidelines and the numbers for whom prophylaxis was prescribed correctly or incorrectly (note that the DH tool is only a risk assessment instrument and does not stipulate what prophylaxis patients should receive). Table 2 shows a breakdown of the VTE prophylaxis prescribed; compliance with each guidance was estimated from the number of patients expected to be on each modality of prophylaxis divided by the observed numbers. Tables 1 and 2 illustrate that compliance in giving particular types of prophylaxis was higher for high-risk patients but generally less concordant with guidance for those requiring less or no prophylaxis.
Table 1.
Number and proportion of patients in each risk stratification group and compliance with guidelines
| Assessment tool | Audit 1 | Audit 2 | |||
|---|---|---|---|---|---|
| Patients in group n=90 | Patients on correct prophylaxis | Patients in group n=101 | Patients on correct prophylaxis | ||
| Department of Health risk stratification tool | High risk | 65 (72%) | 75 (74%) | ||
| Moderate risk | 12 (13%) | 8 (8%) | |||
| Low risk | 13 (15%) | 18 (18%) | |||
| NICE CG46 | ‘At risk’ | 67 (74%) | 54/67 (81%) | 81 (80%) | 73/81 (90%) |
| Low risk | 23 (26%) | 3/23 (13%) | 20 (20%) | 2/20 (10%) | |
| European consensus guidelines | High risk | 36 (40%) | 18/36 (50%) | ||
| Moderate risk | 38 (42%) | 24/38 (63%) | |||
| Low risk | 16 (18%) | 4/16 (25%) | |||
| Local guidelines | High risk | 33 (33%) | 22/33 (67%) | ||
| Moderate risk | 66 (65%) | 57/66 (86%) | |||
| Low risk | 2 (2%) | 1/2 (50%) | |||
Table 2.
Number and proportion of patients on specific prophylaxis and compliance with guidelines
| Prophylaxis | Audit 1 | Audit 2 | ||||
|---|---|---|---|---|---|---|
| Patients in group n=90 | Number compliant with NICE CG46 | European consensus guidelines | Patients in group n=101 | Number compliant with NICE CG46 | Local guidelines | |
| No prophylaxis | 7 (8%) | 0/7 (0%) | 0/7 (0%) | 9 (9%) | 3/9 (33%) | 2/9 (22%) |
| Mechanical prophylaxis | 9 (10%) | 5/9 (56%) | 6/9 (67%) | 17 (17%) | 16/17 (94%) | 14/17 (82%) |
| Low dose LMWH alone | 11 (12%) | 5/11 (45%) | 2/11 (18%) | 4 (4%) | 2/4 (50%) | 3/4 (75%) |
| Low dose LMWH and mechanical prophylaxis | 42 (47%) | 27/42 (64%) | 20/42 (48%) | 54 (54%) | 38/54 (70%) | 47/54 (87%) |
| High dose LMWH alone | 3 (3%) | 3/3 (100%) | 3/3 (100%) | 3 (3%) | 3/3 (100%) | 2/3 (67%) |
| High dose LMWH and mechanical prophylaxis | 18 (20%) | 17/18 (94%) | 15/18 (83%) | 14 (14%) | 13/14 (93%) | 12/14 (86%) |
LMWH = low-molecular-weight heparin
Table 3 shows numbers of patients stratified into different level of risk according to different guidelines. There were significant differences in the percentages of patients classified as ‘high risk’ by the DH tool and EC guideline in Audit 1 (72% and 40% respectively, p<0.001), and between the DH tool and local guideline in Audit 2 (74% and 33% respectively, p<0.001). However, when high and moderate-risk groups were combined, then the percentages of patients in these combined groups were only dissimilar in Audit 2 (82% for the DH tool compared to 98% for local guidance). There was a significant difference for combined high + moderate risk between the NICE CG46 and our local guideline in Audit 2 (80% and 98% of patients classified as being ‘at risk’ respectively, p<0.001).
Table 3.
Chi-square analysis of observed differences in risk stratification between the guidelines and comparisons of the audit data; for NICE CG46: ‘high + moderate risk’ = ‘at risk’
| Number of patients in each risk group | X2 | p | ||
|---|---|---|---|---|
| Audit 1 | DH risk stratification tool | EC guidelines | 22.16 | <0.001 |
| DH risk stratification tool | NICE CG46 | 3.47 | 0.06 | |
| EC guidelines | NICE CG46 | 1.60 | 0.21 | |
| Audit 2 | DH risk stratification tool | Local guidelines | 74.59 | <0.001 |
| DH risk stratification tool | NICE CG46 | 0.13 | 0.71 | |
| Local guidelines | NICE CG46 | 16.53 | <0.001 | |
DH = Department of Health; EC = European consensus; NICE CG = National Institute for Health and Clinical Excellence clinical guideline
From the perspective of our local practice, compliance improved significantly from Audit 1 to Audit 2. The overall compliance with NICE CG46 improved from 63% to 74% (p=0.04) and with EC guidelines in Audit 1 compared with (similar) local guidelines in Audit 2 from 51% to 80% (p=0.03).
Discussion
These two audits have shown improvements in our local practice using NICE CG46,6 which for general surgical patients has few substantial differences from NICE CG92, published in January 2010.11 More importantly, they have demonstrated how different our compliance with ‘best practice’ appeared to be when using different sources of guidance as the gold standard (particularly in relation to stratifying patients in any greater detail than ‘at risk’ or ‘not at risk’). These observations raise questions about which gold standard to choose for auditing practice in VTE prophylaxis and about how best to deal with stratification of risk. At the outset, it should be noted that all this focuses on ‘general surgery’ as defined above in the above Methods section and not on other disciplines such as orthopaedic surgery and general medicine.
Our compliance rates in both audits were significantly higher for the NICE CG46 ‘at risk’ criteria (81% and 90%) than for the EC or local guidelines. In other words, we performed quite well in giving prophylaxis to ‘at risk’ patients but less well when judged for our use of the correct prophylaxis for specified levels of increased risk. The percentages of patients who fell into low, moderate and high risk groups differed between the different sources of guidance. Our local discussions have highlighted the fact that patients at lower risk may pose more difficult decisions in policy making than those at high risk.12 This is in part because it may be difficult to be sure which of these patients really are at risk (for example, many fit patients become mobile very rapidly after a hernia repair or scrotal surgery but some do not and this is unpredictable).
In addition, the evidence of benefit from prophylaxis for lower-risk patients is more controversial.13,14 Our local policy, developed after Audit 1, took a particularly risk-averse approach, such that only two patients (2%) were ‘low risk’. This approach was driven by the strong patient safety agenda (both in the UK and internationally) and by other pressures that led the surgeons to agree on a low threshold for using mechanical and/or pharmacological methods of prophylaxis unless there was a good reason not to do so.
In contrast to the low-risk patients, those at particularly high risk are, by and large, easily recognised. Our highest risk patients (those who ought to have been receiving high dose low-molecular-weight heparin (LMWH), most in combination with mechanical prophylaxis) had these prescribed correctly in around 90% of cases, unlike those who required only one modality and/or lower doses of heparin. Especially when patients are at very high risk, the responsible surgeon is likely to make personal judgements about prophylaxis. This is generally accepted as best practice and is implicit in the NICE CG92, which gives recommendations for broad types of prophylaxis rather than any amount of detail about precisely what type of prophylaxis to use in patients at different levels of risk.11,13
This contrasts with the other sources of guidance used in our audits and with the internationally influential guidelines from the American College of Chest Physicians.9,10 We had originally considered using the latter in Audit 1 but decided to confine the guidelines used to three in number, relevant to the UK and Europe.
Uncertainties about the use of different doses of LMWH were in part the stimulus for our audit of practice and development of local guidelines. There is evidence that higher doses are more effective in reducing VTE in general high-risk surgery patients.15–18 However, there is no good evidence that higher doses are more effective for moderate and low-risk patients, and the risk of bleeding may be increased. There are also uncertainties about the relative effectiveness of different types of mechanical prophylaxis depending on level of risk but most specialists seem to favour IPC over GCS for patients at very high risk.10 Making clear local agreements about these methods is vital because ready availability of IPC has costs and organisational consequences greater than the routine use of GCS.
Conclusions
A single national gold standard, in the form of the 2010 NICE CG92 allied with the DH risk assessment tool, is a good foundation for any audit of VTE prophylaxis. However, it does not absolve clinicians from making decisions about the specific types (and doses) of prophylaxis they wish to specify in local policies. When auditing the performance of their clinical teams in prescribing (and giving) prophylaxis, they need to decide whether to take a ‘broad brush’ approach (ie are patients who are ‘at risk’ getting some sort of VTE prophylaxis?) or whether to audit specific prophylactic measures in detail. Based on our experience, these two approaches will give significantly different pictures of the standard of practice.
References
- 1.Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet. 2008;371:387–394. doi: 10.1016/S0140-6736(08)60202-0. [DOI] [PubMed] [Google Scholar]
- 2.Nutescu EA. Assessing, preventing, and treating venous thromboembolism: evidence-based approaches. Am J Health Syst Pharm. 2007;64(11 Suppl 7):S5–S13. doi: 10.2146/ajhp070108. [DOI] [PubMed] [Google Scholar]
- 3.Weitz JI. Unanswered questions in venous thromboembolism. Thromb Res. 2009;123(Suppl 4):S2–S10. doi: 10.1016/S0049-3848(09)70135-5. [DOI] [PubMed] [Google Scholar]
- 4.Anaya DA, Nathens AB. Thrombosis and coagulation: deep vein thrombosis and pulmonary embolism prophylaxis. Surg Clin North Am. 2005;85:1163–1177. doi: 10.1016/j.suc.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Deheinzelin D, Braga AL, Martins LC, et al. Incorrect use of thromboprophylaxis for venous thromboembolism in medical and surgical patients: results of a multicentric, observational and cross-sectional study in Brazil. J Thromb Haemost. 2006;4:1266–1270. doi: 10.1111/j.1538-7836.2006.01981.x. [DOI] [PubMed] [Google Scholar]
- 6.National Institute for Health and Clinical Excellence. CG46 Venous Thromboembolism: Reducing the Risk. London: NICE; 2007. [Google Scholar]
- 7.Prevention and treatment of venous thromboembolism. International Consensus Statement (guidelines according to scientific evidence) Int Angiol. 2006;25:101–161. [PubMed] [Google Scholar]
- 8.Department of Health. Risk Assessment for Venous Thromboembolism. London: DH; 2008. [Google Scholar]
- 9.Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):338S–400S. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 10.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 11.National Institute for Health and Clinical Excellence. CG92 Venous Thromboembolism: Reducing the Risk. London: NICE; 2010. [Google Scholar]
- 12.Roche-Nagle G, Curran J, Bouchier-Hayes DJ, Tierney S. Risk-based evaluation of thromboprophylaxis among surgical inpatients: are low risk patients treated unnecessarily? Ir J Med Sci. 2007;176:169–173. doi: 10.1007/s11845-007-0049-3. [DOI] [PubMed] [Google Scholar]
- 13.Polk HC, Jr, Qadan M. Prevention of venous thromboembolism after elective surgery is better influenced by judgement than by protocols. Br J Surg. 2010;97:1315–1317. doi: 10.1002/bjs.7224. [DOI] [PubMed] [Google Scholar]
- 14.Sweetland S, Green J, Liu B, et al. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. BMJ. 2009;339:b4583. doi: 10.1136/bmj.b4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergqvist D. Low molecular weight heparin for the prevention of venous thromboembolism after abdominal surgery. Br J Surg. 2004;91:965–974. doi: 10.1002/bjs.4639. [DOI] [PubMed] [Google Scholar]
- 16.Bergqvist D, Burmark US, Flordal PA, et al. Low molecular weight heparin started before surgery as prophylaxis against deep vein thrombosis: 2500 versus 5000 XaI units in 2070 patients. Br J Surg. 1995;82:496–501. doi: 10.1002/bjs.1800820421. [DOI] [PubMed] [Google Scholar]
- 17.Wicky J, Couson F, Ambrosetti P, et al. Postoperative deep venous thrombosis (DVT) and low-molecular weight heparin (LMWH) type and dosage. Thromb Haemost. 1993;69:402–403. [PubMed] [Google Scholar]
- 18.ENOXACAN Study Group. Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep vein thrombosis in elective cancer surgery: a double-blind randomized multicentre trial with venographic assessment. Br J Surg. 1997;84:1099–1103. [PubMed] [Google Scholar]


